Abstract
An aerobic Lactobacillus plantarum culture displayed growth stagnation during early growth. Transcriptome analysis revealed that resumption of growth after stagnation correlated with activation of CO2-producing pathways, suggesting that a limiting CO2 concentration induced the stagnation. Analogously, increasing the CO2 gas partial pressure during aerobic fermentation prevented the temporal growth stagnation.
Lactobacillus plantarum is a facultative heterofermentative lactic acid bacterium used worldwide in production of fermented food and feed products, and its natural habitats are anaerobic or microaerobic. Nevertheless, the responses of L. plantarum to aerobic growth conditions and the corresponding oxidative stresses are relevant for a variety of industrial processing conditions (10). Moreover, a potential ability to respire and increase the biomass yield during aerobic growth has been demonstrated for several lactic acid bacteria, including Lactococcus lactis (2, 5). L. plantarum is able to utilize oxygen under certain circumstances, including glucose limitation conditions (6), and the absence of the ubiquitous defensive reaction catalyzed by superoxide dismutase in L. plantarum was found to be compensated for by the capacity of this bacterium to accumulate very high concentrations of intracellular Mn(II) ions (up to 35 mM), which act as a scavenger system for superoxide (1, 8).
In this work an aerobically grown culture showed consistent temporary growth stagnation during the early logarithmic phase. Transcriptome analyses revealed genes that were differentially expressed before and after the growth stagnation, and comprehensive analysis of these differentially expressed genes revealed that resumption of growth after the observed growth stagnation corresponded with the activation of a range of CO2-producing metabolic pathways, suggesting that the growth stagnation was due to CO2 limitation. Correspondingly, it could be shown that modifying the gas supplementation regimen by increasing the CO2 partial pressure relieved growth stagnation, confirming the CO2 limitation hypothesis.
Growth of L. plantarum under aerobic conditions.
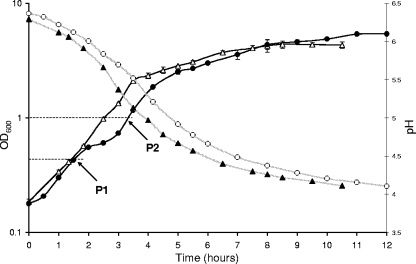
L. plantarum strain WCFS1 (9) was grown aerobically and anaerobically at 37°C in MRS medium (3), and growth was monitored for 12 h (Fig. 1). L. plantarum displayed two phases of logarithmic growth (Fig. 1), which is in agreement with previous observations (4). The final cell density was higher in the aerobic culture (optical density at 600 nm [OD600], 5.5) than in the anaerobic culture (OD600, 4.5). Interestingly, temporary growth stagnation occurred during aerobic fermentation after approximately 2 h, a feature not observed in the anaerobic culture (Fig. 1). Following this stagnation, growth resumed, and the maximum growth rate was comparable to that observed in anaerobic cultures (Fig. 1).
FIG. 1.
Growth characteristics of L. plantarum in batch fermentations under anaerobic conditions (N2 headspace) and under aerobic conditions (air flushed at a rate of 1 volume/min). Symbols: ▵, optical density of the anaerobic culture; ▴, acidification of the anaerobic culture; •, optical density of the aerobic culture; ○, acidification of the aerobic culture. The sampling points for transcriptome analyses are indicated: P1 (OD600, ∼0.4) and P2 (OD600, ∼1.0).
The high growth rates and rapid acidification of the medium in the early growth phase, apparently irrespective of the presence of oxygen, confirmed previously described results (6, 7). Moreover, the significantly altered physiology of aerobically grown L. plantarum cells compared to anaerobically grown cells as a consequence of the presence of oxygen was illustrated by the higher cell density reached in the aerobic culture and the fermentation pattern slightly shifted toward acetate production (6 mM acetate was produced aerobically, and 2 mM acetate was produced anaerobically). However, the growth stagnation observed under aerobic conditions could not be readily explained, and we decided to study this phenomenon further.
Changes during the early growth phase of an aerobic culture.
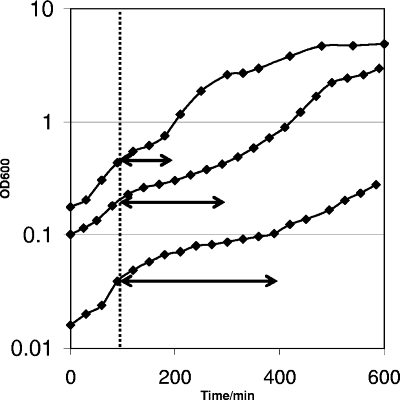
As described above, L. plantarum displayed temporary growth stagnation in the early aerobic growth phase (Fig. 1). Since the precultures used to inoculate the aerobic fermentation cultures were already adapted to aerobic growth, it seems likely that a limitation of the growth medium and not the presence of oxygen per se caused the growth stagnation. Notably, growth stagnation always appeared to occur approximately 100 min after the initiation of fermentation, independent of the inoculum size (Fig. 2). Nevertheless, inoculation at a higher density resulted in shorter stagnation periods, and the duration of the growth stagnation correlated inversely with the inoculum size (Fig. 2). These characteristics suggest that the growth stagnation observed was due to limitation of a specific medium component that was flushed out by aeration during the initial phase of fermentation.
FIG. 2.
Temporary stagnation of growth during aerobic growth and dependence of the duration of the stagnation on inoculum size. The results for three cultures, inoculated at OD600 of 0.2, 0.1, and 0.02, are shown. Clearly, the duration of the stagnation increased with decreasing initial optical density (arrows). Representative graphs for experiments performed in triplicate are shown.
Full-genome transcriptional analyses of aerobic and anaerobic cultures.
To elucidate the processes that caused the growth stagnation in the aerobic culture, genome-wide transcription analyses were performed using amplicon-based DNA microarrays (GEO accession no. GPL6368 [see the supplemental material]). Cells used for transcriptome analyses were harvested before and after the growth stagnation using a quenching method to minimize changes in the transcriptome during harvesting (11). RNA was subsequently isolated, transcriptome experiments were performed, and the results were analyzed as described in the supplemental material.
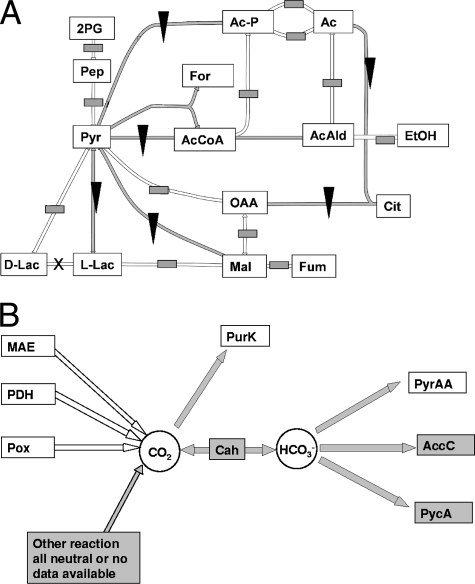
The transcriptome profiles (GEO accession no. GSE10194) obtained before growth stagnation (P1, corresponding to an OD600 of ∼0.4) and after resumption of growth following the stagnation period (P2, corresponding to an OD600 of ∼1.0) (Fig. 1) were projected on a metabolic map using a metabolic model for L. plantarum WCFS1 (13). This projection revealed several pyruvate-associated metabolic pathways that are expressed at a higher level in P2 than in P1, including pyruvate dehydrogenase and malate dehydrogenase pathways (Fig. 3A; see the supplemental material). Interestingly, these pathways include a reaction that produces CO2, while a third CO2-producing pathway that involves pyruvate oxidase displayed a similar trend of expression modulation, albeit with lower significance (P = 0.11) (Fig. 3B; see the supplemental material), suggesting that CO2 production is enhanced in P2 compared to P1. CO2 is essential in purine biosynthesis during production of the intermediate 5-amino-1-ribosylimidazole 5-phosphate. Moreover, the reaction catalyzed by carbamoyl-phosphate synthase, which is involved in pyrimidine and arginine synthesis, requires the dissolved form of carbon dioxide (HCO3−) (Fig. 3B). The data suggest that limiting CO2 concentrations lead to growth stagnation due to their impact on the rates of biosynthesis of nucleotides. Moreover, the data suggest that CO2 is not limiting during the early growth phase (P1), since high growth rates are sustained without induction of the CO2-producing pathways. Time-dependent CO2 limitation could occur by stripping of the CO2 in the medium as a consequence of the high gas flush rate imposed by aerobic conditions. The induction of CO2-producing pathways appears to indicate that the bacterium initiates endogenous CO2 production to compensate for the limiting environmental availability of this compound.
FIG. 3.
(A) Transcriptome data for aerobic P1 versus P2 projected on a metabolic map of pyruvate metabolism of L. plantarum. The filled triangles indicate reactions corresponding to upregulated genes in P2, the gray boxes indicate no significant regulation, and a multiplication sign indicates that no data are available. All reactions were significantly regulated (P < 0.05), except for the pox gene reaction (P = 0.11). Abbreviations: 2PG, 2-phosphoglycerate; Pep, phosphoenolpyruvate; Pyr, pyruvate; L-Lac, l-lactate; D-Lac, d-lactate; Mal, malate; Fum, fumarate; OAA, oxaloacetate; Cit, citrate; EtOH, ethanol; AcAld, acetaldehyde; Ac, acetate; Ac-P, acetyl phosphate; AcCoA, acetyl coenzyme A; For, formate. (B) Carbon dioxide-consuming and -producing reactions in L. plantarum. Enzymes are differentially expressed at time points P1 and P2 in aerobic cultures. The open arrows indicate reactions corresponding to upregulated genes in P2, the gray arrows indicate no significant regulation. All reactions were significantly regulated (P < 0.05), except for the pox gene reaction (P = 0.11). Abbreviations: MAE, malic enzyme; PDH, pyruvate dehydrogenase; Pox, pyruvate oxidase; PurK, phosphoribosylamino-imidazole carboxylase; Cah, carbonate anhydrase; PyrAA, carbamoyl-phosphate synthase; AccC, acetyl-coenzyme A carboxylase; PycA, pyruvate carboxylase.
Improved fermentation of aerobic L. plantarum.
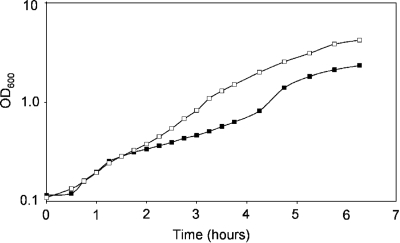
To investigate whether CO2 limitation causes the growth stagnation observed during the early logarithmic growth phase, L. plantarum WCFS1 was cultured aerobically and the culture was flushed with air or with air enriched with 1% CO2. To emphasize the growth stagnation, the culture was inoculated so that the initial OD600 was approximately 0.1 (Fig. 4; also see above). In the culture flushed with normal air, growth stagnation occurred as usual after approximately 100 min (Fig. 4), and due to the lower initial OD600 it lasted for at least 2.5 h. In contrast, the culture flushed with 1% CO2-enriched air displayed no growth stagnation (Fig. 4), confirming that CO2 limitation hampers continuous growth of aerobically cultured L. plantarum, which can readily be compensated for by supplementation with additional CO2.
FIG. 4.
Elimination of the temporal growth stagnation by increased CO2 level. The culture flushed with air (1 volume/min) showed the typical temporary growth stagnation (▪), whereas growth stagnation did not occur in the culture flushed with air mixed with 1% CO2 (□).
The metabolic model for L. plantarum WCFS1 predicts that bicarbonate is used for the production of purines and pyrimidines (12). The solubility of carbonate at 37°C and at pH <6.5 is low, and intense flushing of media with air leads to a decrease in the carbonate concentration, ultimately causing growth stagnation in cultures flushed with air (Fig. 1) or nitrogen (data not shown). The dependence of growth on the concentration of CO2 or carbonate was confirmed by the resumption of growth when the culture was flushed with air containing 1% additional CO2.
Our analyses indicate the power of postgenomic approaches for discovering bacterial fermentation- or growth-limiting factors. They provide a first step for directed adjustment of fermentation conditions to improve bacterial performance.
Supplementary Material
Acknowledgments
This work was supported by grant IGE1018 from the Dutch IOP-Genomics Program.
Footnotes
Published ahead of print on 6 June 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Archibald, F. S., and I. Fridovich. 1981. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooijmans, R. J., B. Poolman, G. K. Schuurman-Wolters, W. M. de Vos, and J. Hugenholtz. 2007. Generation of a membrane potential by Lactococcus lactis through aerobic electron transport. J. Bacteriol. 189:5203-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Man, J. D., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 4.de Vries, M. C., E. E. Vaughan, M. Kleerebezem, and W. M. de Vos. 2004. Optimising single cell activity assessment of Lactobacillus plantarum by fluorescent in situ hybridisation as affected by growth. J. Microbiol. Methods 59:109-115. [DOI] [PubMed] [Google Scholar]
- 5.Gaudu, P., K. Vido, B. Cesselin, S. Kulakauskas, J. Tremblay, L. Rezaiki, G. Lamberret, S. Sourice, P. Duwat, and A. Gruss. 2002. Respiration capacity and consequences in Lactococcus lactis. Antonie van Leeuwenhoek 82:263-269. [PubMed] [Google Scholar]
- 6.Gotz, F., B. Sedewitz, and E. F. Elstner. 1980. Oxygen utilization by Lactobacillus plantarum. I. Oxygen consuming reactions. Arch. Microbiol. 125:209-214. [DOI] [PubMed] [Google Scholar]
- 7.Gregory, E. M., and I. Fridovich. 1974. Oxygen metabolism in Lactobacillus plantarum. J. Bacteriol. 117:166-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groot, M. N., E. Klaassens, W. M. de Vos, J. Delcour, P. Hols, and M. Kleerebezem. 2005. Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology 151:1229-1238. [DOI] [PubMed] [Google Scholar]
- 9.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kung, L., Jr., R. S. Tung, and K. Maciorowski. 1991. Effect of a microbial inoculant and/or glycopeptide antibiotic on fermentation and aerobic stability of wilted alfalfa silage. Anim. Feed Sci. 35:37-48. [Google Scholar]
- 11.Pieterse, B., R. H. Jellema, and M. J. van der Werf. 2006. Quenching of microbial samples for increased reliability of microarray data. J. Microbiol. Methods 64:207-216. [DOI] [PubMed] [Google Scholar]
- 12.Teusink, B., F. H. van Enckevort, C. Francke, A. Wiersma, A. Wegkamp, E. J. Smid, and R. J. Siezen. 2005. In silico reconstruction of the metabolic pathways of Lactobacillus plantarum: comparing predictions of nutrient requirements with those from growth experiments. Appl. Environ. Microbiol. 71:7253-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teusink, B., A. Wiersma, D. Molenaar, C. Francke, W. M. de Vos, R. J. Siezen, and E. J. Smid. 2006. Analysis of growth of Lactobacillus plantarum WCFS1 on a complex medium using a genome-scale metabolic model. J. Biol. Chem. 281:40041-40048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.