Abstract
This paper reports the development of microencapsulated bacteriophage Felix O1 for oral delivery using a chitosan-alginate-CaCl2 system. In vitro studies were used to determine the effects of simulated gastric fluid (SGF) and bile salts on the viability of free and encapsulated phage. Free phage Felix O1 was found to be extremely sensitive to acidic environments and was not detectable after a 5-min exposure to pHs below 3.7. In contrast, the number of microencapsulated phage decreased by 0.67 log units only, even at pH 2.4, for the same period of incubation. The viable count of microencapsulated phage decreased only 2.58 log units during a 1-h exposure to SGF with pepsin at pH 2.4. After 3 h of incubation in 1 and 2% bile solutions, the free phage count decreased by 1.29 and 1.67 log units, respectively, while the viability of encapsulated phage was fully maintained. Encapsulated phage was completely released from the microspheres upon exposure to simulated intestinal fluid (pH 6.8) within 6 h. The encapsulated phage in wet microspheres retained full viability when stored at 4°C for the duration of the testing period (6 weeks). With the use of trehalose as a stabilizing agent, the microencapsulated phage in dried form had a 12.6% survival rate after storage for 6 weeks. The current encapsulation technique enables a large proportion of bacteriophage Felix O1 to remain bioactive in a simulated gastrointestinal tract environment, which indicates that these microspheres may facilitate delivery of therapeutic phage to the gut.
Bacteriophage therapy, referred to as the therapeutic use of bacteriophage to treat pathogenic bacterial infections, has recently been suggested as a possible alternative to chemical antibiotics. Phages are ubiquitous and plentiful in nature and have the potential to provide solutions to the challenges posed by the emergence of antibiotic-resistant pathogens as they possess unique advantages, such as host specificity, exponential growth, and few side effects (40) compared with common antibiotics. Phage therapy has been tested against zoonotic pathogens in several animals and has been the subject of several reviews (40, 41). Successful applications have been reported including studies which demonstrate the ability of phage therapy to treat Escherichia coli infections in calves and lambs (38) and to reduce both Campylobacter jejuni (28) and Salmonella (3) colonization of broiler chickens. Other studies include the application of phage for the control of Salmonella and Campylobacter on the surface of meat (18).
Salmonella continues to be one of the main causes of food-borne illness worldwide. More than 2,400 serotypes of Salmonella enterica have been identified, and most serotypes are capable of infecting a variety of animal species, including humans (8, 36). There is an increase in occurrence of multidrug-resistant strains of S. enterica, which demands a search for alternative therapeutic agents to chemical antibiotics. Bacteriophage therapy has been studied as a method of treatment for the control of Salmonella (3, 20, 34). In particular, bacteriophage Felix O1, a member of the Myoviridae group, has been found to have a broad host range within the genus Salmonella and thus is an excellent candidate for evaluation of therapeutic applications (47). However, for an effective treatment of intestinal pathogens including Salmonella, phage survival during passage through various animal digestive systems needs to be considered. The viability of orally administered phage may be rapidly reduced under the acidic conditions of the stomach and in the presence of enzymes and other digestive compounds such as bile (22). Without protection, phage might not survive gastric passage and thus not be infective in the intestine (6). Therefore, it is highly necessary to develop an effective delivery system to protect orally administered phage from the harsh gastrointestinal environment en route to the infection site in the intestine. One possible way to protect phages is by encapsulating them in microspheres or microparticles comprised of pH-dependent polymers.
Microencapsulation is defined as a technology of packaging solids, liquids, or gaseous materials in miniature capsules that can release their contents at controlled rates under specific conditions (2). The choice of materials and the methodology for encapsulation are dependent on the active agent in question and the target application (46). Microencapsulation has been applied to enhance the viability of probiotic bacteria during processing and also for targeted delivery to the gastrointestinal tract (26, 35). Entrapment of mammalian cells in physical membranes has been developed based on the promise of its therapeutic usefulness in tissue transplantation (43). In addition, the microencapsulation of viruses has been studied as an effective adjuvant system to induce specific immune responses via mucosal routes (32). Development of oral microencapsulated forms for delivering viral vaccines with spermine-alginate (29, 33), poly(dl-lactide-co-glycolide) (39), and chitosan-bile salt microspheres (25) has been reported in previous studies. However, in terms of the development of a suitable methodology and encapsulation matrix for use with phage, these approaches have some limitations such as the involvement of organic solvents (39), low encapsulation efficiency, and poor protective effects upon exposure to gastric acid (29), which make them unsuitable for microencapsulation of phage for oral delivery. Although patent applications have been submitted for related technologies regarding the use of skim milk and fatty acid (31) or polymethacrylate copolymer (45) for preparation of a dosage form of phage, limited data are shown, especially on the systematic evaluation of the encapsulated products claimed (17).
The objective of the present study was to develop an efficient, phage-compatible method for microencapsulation of bacteriophage Felix O1 using a chitosan-alginate-CaCl2 system with the capacity to protect phage from conditions simulating those of the pig gastrointestinal tract and facilitate delivery of viable phage to the pig gut. In this study we evaluated the viability of phage throughout the microencapsulation procedure and following a period of storage time and determined the resistance of microencapsulated phage to simulated conditions of the pig gastrointestinal tract (including gastric juice and bile salts).
Here, we report a process for the microencapsulation of Salmonella bacteriophage Felix O1 using natural polysaccharides including alginate and chitosan. Sodium alginate is an anionic linear polysaccharide composed of alternating blocks of 1,4-linked α-l-guluronic and β-d-mannuronic acid residues. The gelation of alginate polymers is achieved by cross-linking between the carboxylate anions of guluronic acid and the calcium ions (14). To stabilize the ionic gel network and reduce permeability, Ca-alginate microspheres can be coated with chitosan, a polycationic polymer derived from the natural polymer chitin, by electrostatic interactions with the negatively charged alginate acid groups (15). Chitosan-alginate microspheres have been widely used for drug release systems (1) and bacteria immobilization (24, 26) because of their favorable properties, such as mild gelation conditions, biocompatibility, biodegradability, nontoxicity, and pH dependency (15).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacteriophage Felix O1 was obtained from the Felix d'Hérelle Reference Center (Université Laval, Quebec, Canada). S. enterica serovar Typhimurium DT104 (ATCC 700408) was used for propagating and enumerating phage Felix O1. All bacterial cultures were grown on tryptic soy broth (TSB) or tryptic soy agar (TSA) (Difco-BD, Franklin Lakes, NJ) at 37°C. Serovar Typhimurium DT104 was grown and maintained for routine use on TSA plates stored at 4°C. Cells were cultured by picking a single serovar Typhimurium colony from a TSA plate, inoculating 5 ml of TSB, and incubating the culture for 18 to 20 h at 37°C with vigorous shaking. These overnight cultures were then subcultured at a 10−2 dilution into fresh TSB and grown until an optical density at 600 nm of 1.0 was reached.
Bacteriophage preparation and bacteriophage titration.
Phage was propagated on serovar Typhimurium strain DT104 as described previously (47). Crude phage lysates were prepared in TSB liquid culture, filter sterilized by a vacuum-driven, 0.22-μm-pore-size filter (Millipore, Nepean, Ontario, Canada), and stored at 4°C. Purified phage was prepared by a method modified from the method of Sambrook et al. (37). Briefly, crude TSB lysate was clarified by centrifugation at 13,000 × g for 20 min at 4°C, digested with 1 μg ml−1 DNase and RNase (Sigma), and precipitated in the presence of 10% polyethylene glycol 8000 and 1 M NaCl at 4°C overnight. The polyethylene glycol was extracted from the precipitate with chloroform, and the phage suspension was purified by CsCl density gradient centrifugation. The phage pellets were resuspended in SM buffer (0.1% gelatin [wt/vol], 100 mM NaCl, 8 mM MgSO4, 50 mM Tris-HCl, pH 7.5) and stored at 4°C until use. Phage stocks were titrated by serial dilution in SM buffer as previously described (16). Briefly, a log-phase culture of serovar Typhimurium DT104 was diluted 10−1 in TSB and mixed thoroughly, and the suspension was applied to the surface of TSA plates, flooding each plate such that the entire surface was inoculated. Excess bacterial suspension was aspirated, and plates were air dried. Serial 10-fold dilutions of the phage suspension were prepared, and 10 μl of each dilution was spotted, in duplicate, onto an inoculated plate. The plates were incubated at 37°C overnight, and the plaques present on each plate were counted.
Preparation of chitosan-coated Ca-alginate microspheres loaded with bacteriophage Felix O1.
The microencapsulation procedure, a two-stage procedure outlined by Gåserød et al. (14), was modified to make it more suitable for bacteriophage. In brief, Ca-alginate microspheres were first made using an Inotech Encapsulator IER-50 (Inotech Biosystems International, Inc.) by extruding a mixture containing 108 PFU ml−1 bacteriophage suspended in 2.2% (wt/vol) low-viscosity sodium alginate (Sigma-Aldrich, Oakville, Ontario, Canada) solution (in 50 mM Tris-HCl, pH 7.5) into 50 mM CaCl2 while gently stirring with a magnetic bar. The microspheres were prepared using a 300-μm encapsulator nozzle at a frequency of 550 Hz and a voltage of 1.40 kV. The resulting microspheres were allowed to harden in 50 mM CaCl2 solution for 30 min. Then the Ca-alginate microspheres were suspended in a solution of chitosan (0.4%, wt/vol) for 20 min to coat the surface of the alginate microspheres. After coating, the microspheres were rinsed with distilled water, filtered, and either sealed in sterile, conical tubes (wet microspheres) or air dried in the presence of 10% (wt/vol) trehalose (Sigma-Aldrich, Oakville, Ontario, Canada) for up to 30 h in a laminar flow hood at 22°C. Once dried, the microspheres were stored in sealed tubes for 6 weeks at 4°C and 22°C. Wet microspheres were stored at 4°C. Samples of both wet and dried microspheres were collected and then assayed for phage survival at intervals of 1 week. For titration of the microencapsulated phage, dissolution of microspheres was performed to release the phage particles as previously described (27, 48). Briefly, phage-loaded microspheres were dissolved in microsphere-broken solution (MBS) containing 50 mM sodium citrate, 0.2 M sodium bicarbonate, and 50 mM Tris-HCl, pH 7.5, for 10 min with shaking at room temperature. Dried microspheres required rehydration in SM buffer for 15 min at 37°C before dissolution in MBS. The released phage was titrated as described above. Phage titer was expressed in PFU per gram of wet microspheres. The encapsulation efficiency of phage was calculated as follows: encapsulation efficiency = (quantity of phage released from the dissolved microspheres/quantity of phage initially taken to prepare the microspheres) × 100. The results are presented as the mean encapsulation efficiency ± standard deviations (SD) of triplicate microsphere preparations.
Electron microscopy.
Microspheres were examined by scanning electron microscopy for size and surface morphologies at 10 kV (Hitachi S-4500; Hitachi Co. Ltd., Tokyo, Japan). The dried microspheres were mounted on metal grids using double-sided tape and coated with Au/Pd under vacuum.
Samples of microencapsulated phage for transmission electron microscopy (TEM) were prepared as follows: phage-containing microspheres were embedded in LR White for thin sectioning by fixing samples with 2% (vol/vol) glutaraldehyde in 50 mM HEPES buffer, pH 7.2, containing 0.05% (wt/vol) ruthenium red for at least 4 h. After samples were washed with HEPES buffer, the material was postfixed in 2% (wt/vol) osmium tetroxide in the same HEPES buffer containing ruthenium red for another 4 h. After a final wash in HEPES, the specimen was dehydrated through graded alcohols (25 to 100%, vol/vol) and embedded in LR White resin. Ultrathin sections (60 nm) were cut using a Reichert-Young Ultracut ultramicrotome and stained with uranyl acetate and lead citrate. Electron micrographs were taken with a Philips CM10 TEM (Philips Scientifics, Eindhoven, The Netherlands) operating at 100 kV using standard operating conditions.
Stability of free phage Felix O1 at different pH values.
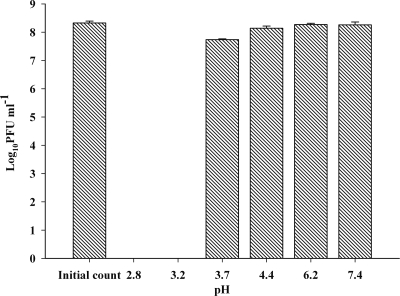
The stability of phage Felix O1 was studied in 0.2% (wt/vol) NaCl solution adjusted with 1 M HCl to pH 2.8, 3.2, 3.6, 4.0, 5.0, and 7.0. A total of 100 μl of phage suspension (in SM buffer) was added to 9.9 ml of prewarmed (37°C) pH-adjusted NaCl solution to give a concentration of about 108 PFU ml−1. After the addition of phage the pHs of the final assay solutions were 2.8, 3.2, 3.7, 4.4, 6.2, and 7.4. These pH values are within the desired range of the pig's gastrointestinal tract. After a 5-min incubation at 37°C, 100-μl aliquots were collected, 10-fold serially diluted, and immediately assayed for phage survival. The experiment was replicated three times, and the results are presented in mean survival rate ± SD.
Survival of microencapsulated phage in SGF.
The stability of the microencapsulated phage in gastric conditions was evaluated using simulated gastric fluid (SGF). SGF was comprised of 3.2 mg ml−1 pepsin (Sigma-Aldrich, Oakville, Ontario, Canada) in 0.2% (wt/vol) NaCl at pH 2.0 and 2.4 (44). A total of 160 mg of dried microspheres (equivalent to 1 g of fresh microspheres) was added to test tubes containing 10 ml of prewarmed (37°C) SGF and incubated at 37°C for 0, 5, 15, 30, 60, 90, and 120 min. The incubation was terminated by placing the microspheres in 10 ml of SM buffer (pH 7.5). The experiment was replicated three times, and the results are presented in mean survival rate ± SD.
Stability of free and microencapsulated phage in bile salts.
To determine the effects of bile salts on free and microencapsulated phage, samples of dried microspheres and samples of phage suspension were added to test tubes containing prewarmed simulated bile containing 1% or 2% (wt/vol) porcine bile extract (Sigma-Aldrich, Oakville, ON, Canada) and incubated at 37°C for 1 h and 3 h. For microencapsulated phage, 160 mg of dried microspheres was added to test tubes containing 10 ml of 1% or 2% (wt/vol) prewarmed bile and incubated at 37°C for 1 h and 3 h. Afterward, the beads were collected and washed with SM buffer. Microspheres were dissolved in MBS as described above, and the survival of released phage from microspheres was immediately assayed. For free phage, 100 μl of phage suspension (109 PFU ml−1) was placed into a culture tube with 9.9 ml of simulated bile fluid. After 1 h and 3 h of incubation, 100-μl aliquots were collected, 10-fold serially diluted, and immediately assayed for phage survival. Distilled water (pH 7.0) was used as a control. The experiment was repeated three times, and the results are presented in mean survival rate ± SD.
In vitro release of microencapsulated phage in SIF.
Simulated intestinal fluid (SIF) was prepared as described in the United States Pharmacopoeia (44) and consisted of 10 mg ml−1 pancreatin (Sigma-Aldrich, Oakville, Ontario, Canada) in 50 mM KH2PO4 at pH 6.8. Microsphere samples (200 mg) were added to conical tubes containing 50 ml of prewarmed SIF and incubated at 37°C with shaking at 100 rpm. At predetermined time points, 100 μl of this solution was taken out and immediately assayed for phage titer. The same volume of the fresh medium was added to replace the volume of the withdrawn samples. The cumulative amount of released phage was plotted against time.
Statistical analysis.
Statistical analysis was performed using SPSS version 13.0 for Windows. The significance of differences was evaluated using one-way analysis of variance with a Student-Neuman-Keuls test for multiple comparisons (12). A Student's t test was used to compare the significance of differences in bile tolerance between nonencapsulated and microencapsulated phage. The differences were considered significant at a P value of <0.05.
RESULTS
Microencapsulation of bacteriophage Felix O1.
The influence of the encapsulation process on the stability of bacteriophage was compared to the stability of bacteriophage in the nonencapsulated state. To effectively evaluate the stability of phage encapsulated in microspheres, a chemical method was used to release the phage from alginate-chitosan microspheres under mild conditions. Initial phage titer before encapsulation was adjusted to 8.27 to 8.78 log10 PFU ml−1. A high level of phage loading at 8.48 ± 0.19 log10 PFU g−1 of microspheres was achieved, giving a mean phage encapsulation efficiency of 93.3% ± 5.7%. The average phage titer loss during encapsulation was very low, implying that the phage was efficiently entrapped in the alginate gel matrix without significant detrimental effects on phage viability.
Electron microscopy and morphology.
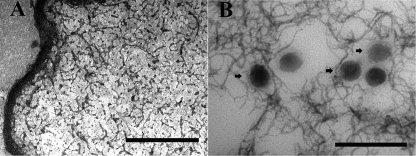
The morphological analysis of the wet microspheres carried out soon after the microencapsulation procedure showed that they were of uniform size and had rounded external surfaces (Fig. 1A). The average size of the wet microspheres was 780 μm, with a range of 740 to 810 μm. After drying, the microspheres were generally spherical with a wrinkled surface and a collapsed center, and the average size decreased to 290 μm (Fig. 1B and C). The physical evidence for incorporation of phage into chitosan-alginate microspheres was demonstrated by TEM (Fig. 2B).
FIG. 1.
Morphology and size of chitosan-alginate microspheres. Optical micrographs of chitosan-alginate microspheres before (A) and after (B) drying. (C) Scanning electron micrograph of external surface structure of air-dried microspheres.
FIG. 2.
TEM images of chitosan-alginate microspheres. (A) Blank microspheres. (B) Microspheres loaded with phage Felix O1. Arrows indicate the cross-section of the phage. Scale bar, 200 nm.
Acid sensitivity of bacteriophage Felix O1.
Free phage Felix O1 was tested for pH stability in 0.2% NaCl solution adjusted to various pH levels (pH 2.8 to 7.0). The results showed that phage Felix O1 was extremely sensitive to acidic environments (Fig. 3). At low pH levels a rapid drop in viability was observed, with an 8-log unit drop in titer after 5 min of incubation below pH 3.7. However, for the same incubation period at pH 3.7, the drop in titer was only 0.6 log units.
FIG. 3.
Effects of pH on the stability of nonencapsulated phage Felix O1. Phage Felix O1 was incubated in 0.2% NaCl adjusted to various pH levels for 5 min at 37°C. Each value in the figure represents the mean ± SD (n = 3). Limit of detection, <102 PFU ml−1.
Resistance of microencapsulated bacteriophage Felix O1 to SGF.
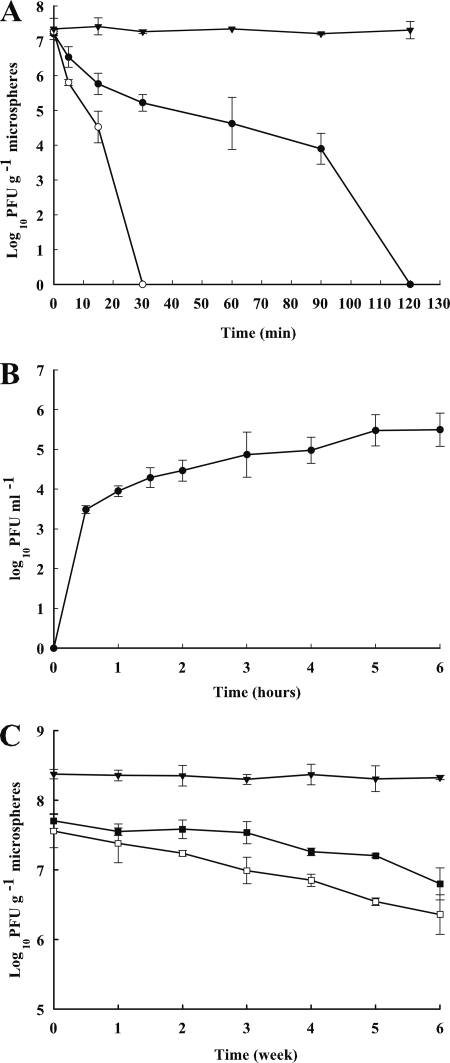
To determine the likelihood of microencapsulated phage Felix O1 surviving passage through the stomach following oral administration, microencapsulated bacteriophage Felix O1 was tested for stability in SGF. Significant protection of bacteriophage Felix O1 by microencapsulation was observed at both pH 2.4 and 2.0. Figure 4A shows the survival of bacteriophage Felix O1 loaded in chitosan-coated alginate microspheres as a function of time after incubation in SGF containing pepsin adjusted to pH 2.0 and 2.4. Following 60 min of incubation at pH 2.4, the viable counts of microencapsulated phage decreased by 2.58 log units, and viable microencapsulated phage was still observed after a 90-min incubation. At pH 2.0, the titer of microencapsulated phage decreased to an undetectable level within 30 min of incubation, while compared to free phage, much higher viability was observed after 5- and 15-min incubations. As Felix O1 was completely inactivated in 0.2% NaCl at pH 2.8 following 5 min of incubation (Fig. 3), the effects of SGF (pH 2.0 or 2.4) on free phage were not assayed.
FIG. 4.
(A) Survival of microencapsulated phage Felix O1 after exposure to SGF with 3.2 mg ml−1 pepsin at pH 2.0 (○) and pH 2.4 (•) and SM buffer of pH 7.5 (▾) at 37°C for 0, 5, 15, 30, 60, 90, and 120 min. Treatments yielding undetectable phage (<103 PFU g−1 of microsphere for microencapsulated phage) were considered to be zero. (B) Release of microencapsulated phage Felix O1 in SIF of pH 6.8 at 37°C. (C) Stability of microencapsulated phage Felix O1 throughout 6 weeks of storage. Values are shown for wet microspheres loaded with phage stored at 4°C (▾) and dried microencapsulated phage stored at 4°C (▪) and 22°C (□). Each value in the figure represents the means ± SD (n = 3).
Stability of free and microencapsulated bacteriophage Felix O1 in bile salts.
The results presented in Table 1 illustrate the survival of free and microencapsulated bacteriophage Felix O1 in the presence of bile. Significant protection of bacteriophage Felix O1 in high bile concentrations by microencapsulation was observed. The titers of free phage in 1% and 2% bile salts after 3 h of incubation decreased by 1.29 and 1.67 log units, respectively, while after 1 h of incubation at these concentrations, titers decreased by 0.09 log and 0.58 log units, respectively. Conversely, the viability of microencapsulated phage was fully maintained after 1-h and 3-h incubations in both bile concentrations.
TABLE 1.
Survival of free and encapsulated bacteriophage Felix O1 after treatments with bilea
| Phage type (treatment period) | Survival (log10 PFU ml−1 or g−1 of microspheres) at the indicated bile concnb
|
||
|---|---|---|---|
| 0% | 1% | 2% | |
| Free phage (1 h) | 7.33 ± 0.05 AP | 7.24 ± 0.08 AP | 6.75 ± 0.35 AQ |
| Encapsulated phage (1 h) | 7.35 ± 0.14 AP | 7.31 ± 0.17 AP | 7.38 ± 0.06 BP |
| Free phage (3 h) | 7.36 ± 0.09 AP | 6.07 ± 0.12 BQ | 5.69 ± 0.03 CR |
| Encapsulated phage (3 h) | 7.39 ± 0.08 AP | 7.34 ± 0.05 AP | 7.27 ± 0.02 BP |
Bacteriophage Felix O1 was treated with simulated bile at concentrations of 1% and 2% at 37°C for 1 and 3 h.
Values shown are means ± standard deviations (n = 3). Within columns, the same letter (A to C) is placed next to combinations whose means were not significantly different (P > 0.05). Within rows, the same letter (P to R) is placed next to combinations whose means were not significantly different (P > 0.05).
In vitro release of phage from microspheres in SIF.
Microspheres should not only effectively protect phage from adverse gastric conditions but also permit the release of phage at the desired site (e.g., small intestine) in a viable form. The release profile of viable phage from chitosan-alginate microspheres in SIF is shown in Fig. 4B. When encapsulated phage Felix O1 was placed into SIF of pH 6.8, the microspheres began to swell and disintegrate and had a sustained release following a burst effect. The phage numbers gradually increased from 1.5 × 105 PFU at 30 min to almost a complete release (1.6 ×107 PFU) after a 5-h incubation in SIF.
Stability of microencapsulated phage Felix O1 during storage at 4°C and 22°C.
Preparation in a dry form is desirable for both prolonged storage and application of microencapsulated phage. Since the phage particles were sensitive to freezing and lyophilization (7), air drying at room temperature (22°C) was chosen as the drying method for fresh microspheres in this study. The process of drying without protective agents resulted in almost complete inactivation of phage (data not shown). Trehalose is a well-known stabilizing agent for viruses (4) and was used as a stabilizing agent in this study for phage Felix O1 during an air-drying process and subsequent storage. Viable phage was assayed during the drying process, and a mean phage loss of 1.20 log units was observed. Figure 4C shows the stability of dried microencapsulated phage during 6 weeks of storage at 4°C and 22°C. The survival of microencapsulated phage in dried form decreased from 5.0 × 107 to 6.3 × 106 PFU g−1 microspheres for 6 weeks of storage at 4°C (12.6% survival). However, upon storage at 22°C, the phage numbers decreased from 3.6 × 107 to 2.3 × 106 PFU g−1 microspheres (6.4% survival). The results demonstrate that dried encapsulated phage stored at 4°C shows better stability than phage kept at room temperature. For wet microspheres, it was found that no decrease in viability occurred following 6 weeks of storage at 4°C.
DISCUSSION
It is well known that viruses are typically damaged irreversibly by exposure to low pH, organic solvents, dehydration, and heat (32, 33, 45). The current experiment showed that the viability of free phage Felix O1 was rapidly lost upon exposure to a simulated acidic gastric environment and, to a lesser extent, upon exposure to bile salts. Therefore, ensuring phage stability is the key consideration in the design of effective microencapsulation methods. Not only should microencapsulation processes employ physically mild conditions, but also the selected materials should be compatible with the phage and not compromise its biological activity. Moreover, when the objective is to deliver viable phage to the gut, the encapsulation materials should protect phage from acid and enzymes present in gastric fluid and dissolve or swell easily in a weakly alkaline intestinal medium. The current encapsulation process was performed in a mild aqueous-based environment, and our results show that these encapsulation and coating processes had no detrimental effects on phage viability. Similar to previous reports on encapsulation of probiotic bacteria in chitosan-alginate microspheres (24), a high phage loading efficiency was also found in this study (93.3%). When the sodium alginate solution containing phage was dropped into a gelation medium of calcium chloride, the droplets formed gel microspheres instantaneously, entrapping the phage in a three-dimensional network of ionically cross-linked alginate. Additionally, the encapsulated phage showed excellent stability during storage under wet conditions. Spermine-alginate and poly(dl-lactide-co-glycolide) have been previously used for rotavirus microencapsulation, but only approximately 14% and 30%, respectively, of the initial quantity of virus were entrapped within microspheres (29, 39). Therefore, it appears that Ca-alginate matrices have a good compatibility with encapsulated phage Felix O1.
Gastric juice survival is a prerequisite when phage is administered orally. The nonencapsulated phage Felix O1 was extremely sensitive to low-pH conditions, which is similar to the pig stomach pH. This is in agreement with a previous report in which none of three Vibrio vulnificus phage strains was recovered from SGF within 2 min at pH 2.5 to 2.7 (23). However, phage λ appears to be more acid resistant and was stable in SM buffer of pH 3.0 during storage at ambient temperature for 24 h (21). Clearly, there was a substantial strain variation in response to acid, but variations in assay buffer and incubation conditions might also contribute to the differences in the acid tolerance of phage. The alginate gel network has the properties of shrinking in low pH and dissolving in higher pH (15), which permits both the protection of phage from adverse gastric conditions and the release of phage at desired sites (small intestine) in a viable form. In this study, the survival of phage encapsulated in chitosan-alginate microspheres was much higher than that of free phage in SGF, even in the lower-pH solution, although partial loss of viability was still evident. A previous study also showed that survival of calcium-alginate-immobilized bifidobacteria was increased compared to nonencapsulated bacteria upon exposure to simulated gastrointestinal conditions (26). The protection mechanism of phage by encapsulation is likely achieved by limiting the direct contact of phage with an acidic medium. Immediate exposure of naked free phage to a very low pH SGF decreases viability. Presumably, encapsulation in a gel network protects the phage by reducing the diffusion rate of the protons into the bead matrix. Tang et al. have shown that the diffusion rate of acids (0.1 M hydrochloric acid) is significantly lower in an alginate network than in water (42). In addition, the drying and chitosan coating of alginate microspheres resulted in a very compact structure with a significantly reduced pore size, as demonstrated by DeGroot and Neufeld (11) and Gåserød (14), retarding proton diffusion into alginate matrix. It is therefore possible that phage located in the core of the microsphere is subjected to a more moderate pH during the initial exposure. However, the partial loss of phage indicates that the microspheres cannot completely prevent the acids from diffusing into the microspheres. The fact that phage viability decreased with increasing exposure time to SGF supports this hypothesis. A previous study has shown that calcium alginate microspheres with a diameter below 100 μm were not effective in protecting bifidobacteria subjected to simulated gastric acid (19). Therefore, it is reasonable to expect that the protection effects could be further improved by increasing the size of the microspheres.
Gastric emptying rates are an important factor that should be considered for survival of phage Felix O1 passing through the stomach. Transit time through the stomach is highly variable and is affected by the dosage form and the particle size in the case of administration of a solid meal (9). Previous studies indicate that solutions and small pellets of less than 2 mm empty from the stomach rapidly (9). The rate of gastric emptying in pigs is somewhat slower than that found in humans, with the mean times for 50% gastric emptying for liquid and pellet systems of 1.4 to 2.2 h (10), while typical values for the emptying of such dosage forms in humans are 0.5 to 1.5 h (9). The size of the microspheres produced in this study and the fact that the encapsulated phage appears to be viable over a 1.5-h period at pH 2.4 suggest that a sufficient amount of phage should remain viable after passage through the stomach when administered along with feed. This is because the stomach pH is likely to be much higher than pH 2.4 after a meal due to the buffering effect of ingested food. For example, during a meal, gastric pH increases to an average value of 5.0 in humans (13). In addition, the ingested food constituents may provide protection for phage against extreme pH values, which has been demonstrated in a study involving bacteria (49). O'Flynn et al. (34) have previously reported that phage Felix O1 can survive exposure to gastric contents directly collected from porcine stomachs at pH 2.5 for up to 2 h; this disagreement is presumably due to the difference in assay medium used. It is expected that as soon as the phage leaves the stomach, the low pH surrounding the microspheres will be neutralized by intestinal juice, and the microspheres will be gradually disintegrated at this higher pH, causing release of phage from the microspheres. In this study, complete release of phage from the microspheres was observed within 6 h of incubation in SGF. The released phage would be distributed throughout the small intestine during transit and should be available to bind and infect the target pathogens.
Bile acid formed from cholesterol in the liver is one of the anionic surfactants secreted in the gastrointestinal tract. Although there are few reports on bile resistance of bacteriophage, phage Felix O1 appears to be less bile tolerant than the staphylococcal phage K (unpublished data) and three previously reported phage of V. vulnificus (23), which were found to be resistant to 1% and 2% bile without loss of viability for up to 3 h at 37°C. The difference between the reduction in titer of free phage and that of encapsulated phage was statistically significant (P < 0.05). When microencapsulated phage was exposed to bile salts, the viability of phage was fully maintained. Chitosan is a polysaccharide with polycationic properties; its salts are able to interact with anionic compounds in solution. Chitosan could adsorb bile acids through the ion-exchange action that occurs when chitosan salts and bile acids form an insoluble complex on the chitosan-alginate membrane (24, 30). This will hinder the diffusion of bile salts into the microsphere core and protect the encapsulated phage from interacting with the bile salts. In a previous study, Krasaekoopt et al. also reported that the chitosan-coated alginate beads provided better protection for probiotic bacteria in bile salt solution than either poly-l-lysine or alginate (24). Although exposure to bile resulted in some loss in viability of phage Felix O1 compared to gastric acid, bile does not appear to be a major concern for oral delivery of phage Felix O1.
In conclusion, microencapsulation of bacteriophage Felix O1 in alginate-chitosan microspheres significantly improves the survival of this phage under laboratory conditions designed to simulate the pig gastrointestinal tract. The current encapsulation approach could provide a possible delivery technology for improving the efficacy of bacteriophage in oral therapeutic applications. Future work is required to explore other surface-coating materials with characteristics that will allow for long-term storage of encapsulated phage and targeted delivery of phage to sites in the gastrointestinal tract and that will enhance the protection of bacteriophage under harsh acidic conditions. The enteric polymers would be an attractive choice for use since they have been demonstrated to provide effective protection for bacteria against in vitro simulated gastric conditions (5, 35).
Acknowledgments
This work was supported by Agriculture and Agri-Food Canada research grant 169. Y. Ma received a graduate scholarship from the China Scholarship Council.
Footnotes
Published ahead of print on 30 May 2008.
REFERENCES
- 1.Anal, A. K., and W. F. Stevens. 2005. Chitosan-alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 290:45-54. [DOI] [PubMed] [Google Scholar]
- 2.Anal, A. K., and H. Singh. 2007. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 18:240-251. [Google Scholar]
- 3.Atterbury, R. J., M. A. P. Van Bergen, F. Ortiz, M. A. Lovell, J. A. Harris, A. De Boer, J. A. Wagenaar, V. M. Allen, and P. A. Barrow. 2007. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 73:4543-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieganski, R. M., A. Fowler, J. R. Morgan, and M. Toner. 1998. Stabilization of active recombinant retroviruses in an amorphous dry state with trehalose. Biotechnol. Prog. 14:615-620. [DOI] [PubMed] [Google Scholar]
- 5.Chan, E. S., and Z. Zhang. 2002. Encapsulation of probiotic bacteria Lactobacillus acidophilus by direct compression. Food Bioprod. Process. 80:78-82. [Google Scholar]
- 6.Chibani-Chennoufi, S., J. Sidoti, A. Bruttin, E. Kutter, S. Sarker, and H. Brüssow. 2004. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob. Agents Chemother. 48:2558-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, W. A. 1962. Comparison of several methods for preserving bacteriophages. Appl. Environ. Microbiol. 10:466-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cormican, M., N. DeLappe, C. O'Hare, G. Doran, D. Morris, G. Corbett-Feeney, S. Fanning, M. Daly, M. Fitzgerald, and J. Moore. 2002. Salmonella enterica serotype Bredeney: antimicrobial susceptibility and molecular diversity of isolates from Ireland and Northern Ireland. Appl. Environ. Microbiol. 68:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, S. S., J. G. Hardy, and J. W. Fara. 1986. Transit of pharmaceutical dosage forms through the small intestine. Gut 27:886-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, S. S., L. Illum, and M. Hinchcliffe. 2001. Gastrointestinal transit of dosage forms in the pig. J. Pharm. Pharmacol. 53:33-39. [DOI] [PubMed] [Google Scholar]
- 11.DeGroot, A. R., and R. J. Neufeld. 2001. Encapsulation of urease in alginate beads and protection from α-chymotrypsin with chitosan membranes. Enzyme Microb. Technol. 29:321-327. [Google Scholar]
- 12.De Muth, J. E. 1999. Basic statistics and pharmaceutical statistical applications. Marcel Dekker, New York, NY.
- 13.Dressman, J. B., R. R. Berardi, L. C. Dermentzoglou, T. L. Russell, S. P. Schmaltz, J. L. Barnett, and K. M. Jarvenpaa. 1990. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 7:756-761. [DOI] [PubMed] [Google Scholar]
- 14.Gåserød, O., O. Smidsrød, and G. Skjåk-Bræk. 1998. Microcapsules of alginate-chitosan II. A quantitative study of the interaction between alginate and chitosan. Biomaterials 19:1815-1825. [DOI] [PubMed] [Google Scholar]
- 15.George, M., and T. E. Abraham. 2006. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J. Control. Release 114:1-14. [DOI] [PubMed] [Google Scholar]
- 16.Gill, J. J., J. C. Pacan, M. E. Carson, K. E. Leslie, M. W. Griffiths, and P. M. Sabour. 2006. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 50:2912-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill, J. J., T. Hollyer, and P. M. Sabour. 2007. Bacteriophages and phage-derived products as antibacterial therapeutics. Expert Opin. Ther. Pat. 17:1341-1350. [Google Scholar]
- 18.Goode, D., V. M. Allen, and P. A. Barrow. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69:5032-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen, L. T., P. M. Allan-Wojtas, Y. L. Jin, and A. T. Paulson. 2002. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 19:35-45. [Google Scholar]
- 20.Higgins, J. P., S. E. Higgins, K. L. Guenther, W. Huff, A. M. Donoghue, D. J. Donoghue, and B. M. Hargis. 2005. Use of a specific bacteriophage treatment to reduce Salmonella in poultry products. Poult. Sci. 84:1141-1145. [DOI] [PubMed] [Google Scholar]
- 21.Jepson, C. D., and J. B. March. 2004. Bacteriophage lambda is a highly stable DNA vaccine delivery vehicle. Vaccine 22:2413-2419. [DOI] [PubMed] [Google Scholar]
- 22.Joerger, R. D. 2003. Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 82:640-647. [DOI] [PubMed] [Google Scholar]
- 23.Koo, J., A. Depaola, and D. L. Marshall. 2000. Effect of simulated gastric fluid and bile on survival of Vibrio vulnificus and Vibrio vulnificus phage. J. Food Prot. 63:1665-1669. [DOI] [PubMed] [Google Scholar]
- 24.Krasaekoopt, W., B. Bhandari, and H. Deeth. 2004. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 14:737-743. [Google Scholar]
- 25.Lameiro, M. H., R. Malpique, A. C. Silva, P. M. Alves, and E. Melo. 2006. Encapsulation of adenoviral vectors into chitosan-bile salt microparticles for mucosal vaccination. J. Biotechnol. 126:152-162. [DOI] [PubMed] [Google Scholar]
- 26.Lee, K. Y., and T. R. Heo. 2000. Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salt solution. Appl. Environ. Microbiol. 66:869-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, X. D., W. Y. Yu, Y. Zhang, W. M. Xue, W. T. Yu, Y. Xiong, X. J. Ma, Y. Chen, and Q. Yuan. 2002. Characterization of structure and diffusion behaviour of Ca-alginate beads prepared with external or internal calcium sources. J. Microencapsul. 19:775-782. [DOI] [PubMed] [Google Scholar]
- 28.Loc Carrillo, C., R. J. Atterbury, A. el-Shibiny, P. L. Connerton, E. Dillon, A. Scott, and I. F. Connerton. 2005. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71:6554-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser, C. A., T. J. Speaker, and P. A. Offit. 1998. Effect of water-based microencapsulation on protection against EDIM rotovirus challenge in mice. J. Virol. 72:3859-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata, Y., S. Toniwa, E. Miyamoto, and S. Kawashima. 1999. Preparation of alginate gel beads containing chitosan salt and their function. Int. J. Pharm. 176:265-268. [DOI] [PubMed] [Google Scholar]
- 31.Murthy, K., and R. Engelhardt. 2006. Encapsualted bacteriophage formulation. International patent WO 2006047871.
- 32.Nechaeva, E. 2002. Development of oral microencapsulated forms for delivering viral vaccines. Expert. Rev. Vaccines 1:385-397. [DOI] [PubMed] [Google Scholar]
- 33.Offit, P. A., C. A. Khoury, C. A. Moser, H. F. Clark, J. E. Kim, and T. J. Speaker. 1994. Enhancement of rotavirus immunogenicity by microencapsulation. Virology 203:134-143. [DOI] [PubMed] [Google Scholar]
- 34.O'Flynn, G., A. Coffey, G. F. Fitzgerald, and R. P. Ross. 2006. The newly isolated lytic bacteriophages st104a and st104b are highly virulent against Salmonella enterica. J. Appl. Microbiol. 10:251-259. [DOI] [PubMed] [Google Scholar]
- 35.Rao, A. V., N. Shiwnarain, and J. Maharaj. 1989. Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Can. Inst. Food. Sci. Technol. J. 22:345-349. [Google Scholar]
- 36.Reece, K., S. Frye, W. Dutch, M. Lising, T. Bittner, S. Raza, J. Manry, R. Nieto, D. Walton, and M. Healy. 2005. Serotyping of Salmonella isolates using the DiversiLab System and associated Salmonella database, abstr. Z-051. Abstr. 105th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Smith, H. W., and M. B. Huggins. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 129:2659-2675. [DOI] [PubMed] [Google Scholar]
- 39.Sturesson, C., and L. Wikingsson. 2000. Comparison of poly(acryl starch) and poly(lactide-co-glycolide) microspheres as drug delivery system for a rotavirus vaccine. J. Control. Release 68:441-450. [DOI] [PubMed] [Google Scholar]
- 40.Sulakvelidze, A., Z. Alavidze, and J. G. Morris, Jr. 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sulakvelidze, A., and P. Barrow. 2005. Phage therapy in animals and agribusiness, p. 335-380. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages, biology and applications. CRC Press, Boca Raton, FL.
- 42.Tang, M., P. Dettmar, and H. Batchelor. 2005. Bioadhesive oesophageal bandages: protection against acid and pepsin injury. Int. J. Pharm. 292:169-177. [DOI] [PubMed] [Google Scholar]
- 43.Uludag, H., P. DeVos, and P. A. Tresco. 2000. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 42:29-64. [DOI] [PubMed] [Google Scholar]
- 44.U.S. Pharmacopeial Convention. 2004. The United States pharmacopeia, 27th ed., p. 2728. U.S. Pharmacopeial Convention, Rockville, MD.
- 45.Waddell, T. E., R. Johnson, and A. Mazzocco. 2004. Methods and compositions for controlled release of bioactive compounds. Canadian patent CA2463827.
- 46.Wan, J., J. B. Gordon, K. Muirhead, M. W. Hickey, and M. Coventry. 1997. Incorporation of nisin in micro-particles of calcium alginate. Lett. Appl. Microbiol. 24:153-158. [DOI] [PubMed] [Google Scholar]
- 47.Whichard, J. M., N. Sriranganathan, and F. W. Pierson. 2003. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquid culture and on chicken frankfurters. J. Food Prot. 66:220-225. [DOI] [PubMed] [Google Scholar]
- 48.Xue, W. M., W. T. Yu, X. D. Liu, X. He, W. Wang, and X. J. Ma. 2004. Chemical method of breaking the cell-loaded sodium alginate-chitosan microcapsules. Chem. J. Chin. Univ. 25:1342-1346. [Google Scholar]
- 49.Zhu, H., C. A. Hart, D. Sales, and N. B. Roberts. 2006. Bacterial killing in gastric juice—effect of pH and pepsin on Escherichia coli and Helicobacter pylori. J. Med. Microbiol. 55:1265-1270. [DOI] [PubMed] [Google Scholar]