Abstract
The metabolism of isoflavones by gut bacteria plays a key role in the availability and bioactivation of these compounds in the intestine. Daidzein and genistein are the most common dietary soy isoflavones. While daidzein conversion yielding equol has been known for some time, the corresponding formation of 5-hydroxy-equol from genistein has not been reported previously. We isolated a strictly anaerobic bacterium (Mt1B8) from the mouse intestine which converted daidzein via dihydrodaidzein to equol as well as genistein via dihydrogenistein to 5-hydroxy-equol. Strain Mt1B8 was a gram-positive, rod-shaped bacterium identified as a member of the Coriobacteriaceae. Strain Mt1B8 also transformed dihydrodaidzein and dihydrogenistein to equol and 5-hydroxy-equol, respectively. The conversion of daidzein, genistein, dihydrodaidzein, and dihydrogenistein in the stationary growth phase depended on preincubation with the corresponding isoflavonoid, indicating enzyme induction. Moreover, dihydrogenistein was transformed even more rapidly in the stationary phase when strain Mt1B8 was grown on either genistein or daidzein. Growing the cells on daidzein also enabled conversion of genistein. This suggests that the same enzymes are involved in the conversion of the two isoflavones.
Isoflavones have been implicated in the prevention of hormone-dependent and age-related diseases, including cancer, osteoporosis, menopausal symptoms, and cardiovascular diseases (6, 21, 38, 46). Moreover, owing to their ability to induce hormonal and metabolic changes, isoflavones might favorably influence obesity and type 2 diabetes (43). Based on their structural similarity to endogenous estrogens, isoflavones may bind to estrogen receptors and display agonistic or antagonistic effects (24). Besides hormone-dependent effects, isoflavones show hormone-independent activities, including antioxidative and antiproliferative properties and enzyme inhibition (18, 25, 41). A rich dietary source of isoflavones is soy products containing predominantly daidzin and genistin or the corresponding aglycones, daidzein and genistein (20).
Biotransformation is an important factor in regulating the biological activity of dietary isoflavones. The metabolites formed may have effects that differ from those of the parent compound. Gut bacteria play a crucial role in the metabolism of isoflavones, as has been demonstrated previously for daidzein. Metabolites of bacterial daidzein transformation are dihydrodaidzein, O-desmethylangolensin, and equol (10, 35). In addition, tetrahydrodaidzein has been detected in human urine (26). In vitro studies suggest that O-desmethylangolensin and equol are more biologically active than their precursor, daidzein (e.g., they bind to estrogen receptors with greater affinity) (3). In humans, there are substantial interindividual variations in the metabolism of daidzein. Approximately 30 to 50% of humans are capable of producing considerable amounts of equol from ingested daidzein, which has been explained by differences in the composition of the gut microbiota (8, 12, 34, 36). The interindividual variability in equol formation appears to be unique to humans. The microbiotas of animals, including rats and mice, uniformly produce equol from daidzein (1, 5, 7, 27, 53). So far, only a few bacterial strains involved in equol formation have been described, and some of these strains catalyze only single reaction steps (23, 29, 31, 45, 49, 52, 54).
Gut microbiota and isolated bacterial strains transform genistein to dihydrogenistein, 6′-hydroxy-O-desmethylangolensin, and 2-(4-hydroxyphenyl)propionic acid (10, 15, 23, 39, 51, 52). Although dihydrogenistein formation from genistein corresponds to dihydrodaidzein formation from daidzein, further conversion yielding 5-hydroxy-equol, the metabolite analogous to equol, has not been observed previously.
Here, we demonstrate the conversion of daidzein and genistein to equol and 5-hydroxy-equol, respectively, by a strictly anaerobic bacterium newly isolated from the mouse intestine.
MATERIALS AND METHODS
Chemicals.
Daidzein and 2-(4-hydroxyphenyl)propionic acid were purchased from Acros Organics (Geel, Belgium). Dihydrodaidzein was obtained from Toronto Research Chemicals (Toronto, Canada), and equol was obtained from Fluka (Deisenhofen, Germany). Genistein was purchased from Roth (Karlsruhe, Germany). Dihydrogenistein, 6′-hydroxy-O-desmethylangolensin, and O-desmethylangolensin were prepared by using previously described methods (47, 48).
Isolation procedure.
Strain Mt1B8 was isolated from the ileum of a 12-week-old female TNFΔARE C57BL/6 mouse (28) in the course of experiments aiming at identification of bacteria associated with inflamed mucosa. Animal use was approved by the Bavarian Animal Care and Use Committee (approval no. 55.2-1-54-2531-74-06). The ileal sample was prepared as described previously (17). Strain Mt1B8 was isolated on Mt1 agar after incubation of an undiluted ileal cell suspension (100 μl) at 37°C for 9 days under anaerobic conditions in sealed jars using AnaeroGen sachets (Oxoid). The composition of Mt1 agar (pH 7.7) was 5 g/liter mucin (catalog no. M1778; Sigma), 500 mg/liter cysteine, 1 mg/liter yeast extract, 20 μg/liter folic acid, 20 μg/liter vitamin B12, 50 mM NaHCO3, 10 mM CH3COONa, 5 mM Na2HPO4, 5 mM NaCl, 3 mM KH2PO4, 1 mM CaCl2, 1 mM MgCl2, 10 μM FeCl3, and 1% (wt/vol) agar. Strain purity was ensured as described previously (14). Strain Mt1B8 was a strictly anaerobic, gram-positive, rod-shaped bacterium that grew as single cells, as determined by microscopic observation after Gram staining and by the KOH test (22). An analysis of a partial sequence (1,338 bp) of the 16S rRNA gene of strain Mt1B8 was performed as described previously (13), and the results showed that strain Mt1B8 is a member of the family Coriobacteriaceae. Since three equol-forming bacteria isolated from rat and human intestines (29, 30, 49) also belong to the Coriobacteriaceae, we focused on the conversion of isoflavones by strain Mt1B8.
Bacterial growth.
Strain Mt1B8 was routinely kept and grown under strictly anoxic conditions in brain heart infusion (BHI) broth (Roth, Karlsruhe, Germany) in Hungate tubes with butyl rubber stoppers and screw caps. The BHI broth was supplemented with 0.5 g/liter cysteine hydrochloride (Merck, Darmstadt, Germany). The 16-ml tubes containing 10 ml medium and an H2-CO2 (80:20, vol/vol) gas phase were inoculated with 100 μl of an overnight culture and incubated at 37°C. Bacterial growth was monitored turbidometrically by determining the optical density at 600 nm (OD600). The anoxic techniques used have been described elsewhere (9).
Conversion experiments.
For the conversion experiments, the isoflavonoids were dissolved in dimethyl sulfoxide (20 mM stock solutions) and sterile filtered (Millex-GV filter; Millipore, Billerica, MA). To tubes containing 10 ml BHI broth, 50 μl (daidzein, genistein, dihydrogenistein) or 32 μl (dihydrodaidzein) of a stock solution was added by using a syringe. The tubes were inoculated with 200 μl of an overnight culture (ca. 2.8 × 106 cells) of strain Mt1B8 and incubated at 37°C. As controls, isoflavonoids and bacteria were incubated separately in medium. Samples were taken at different times with a syringe and centrifuged at 14,000 × g for 5 min. The supernatants (20 μl) were directly used for high-performance liquid chromatography (HPLC) analysis.
For the induction experiments, strain Mt1B8 was grown in BHI broth supplemented with daidzein, genistein, dihydrodaidzein, or dihydrogenistein at a final concentration of 100 μM. In parallel, cultures were grown in the absence of isoflavonoids. Following incubation for 14 h, the same isoflavonoid or another isoflavonoid was added to the same cultures at a final concentration of 100 μM. The tubes were incubated at 37°C for another 26 h. Samples were taken every 2 h for use in HPLC analysis, determination of the OD600, and protein measurement. Following disruption of cells by heating them in 0.44 M NaOH, the protein concentration was determined by the bicinchoninic acid method (BCA-1 kit; Sigma, Deisenhofen, Germany) with bovine serum albumin as the standard.
HPLC analysis.
Isoflavones and their aromatic metabolites were separated using an HPLC system (Gynkotek, Munich, Germany) equipped with a model 480 pump, an ERC-5515 degasser, a GINA 50 autosampler, an STH 585 column oven, a UVD 320S diode array detector, and a reversed-phase C18 column (LiChrospher 100 RP-18; 5 μm; 250 by 4 mm; Merck, Darmstadt, Germany). The column temperature was kept at 37°C. The mobile phase was a gradient of water-acetic acid (98/2, vol/vol) (solvent A) and methanol (solvent B) (5 to 55% solvent B in 15 min, 55% solvent B for 10 min, 55 to 100% solvent B in 3 min, and 100% solvent B for 4 min) at a flow rate of 0.8 ml/min. Detection was at 280 nm. The compounds were identified on the basis of their retention times and UV spectra (200 to 355 nm) in comparison with those of standard reference compounds. Calibration curves were used for quantification. For control of the HPLC system and data processing, the Chromeleon software (version 6.40; Dionex, Sunnyvale, CA) was used.
UPLC-ESI-MS analysis.
For further characterization by ultraperformance liquid chromatography (UPLC)-coupled mass spectrometry (MS), the final product of genistein metabolism by strain Mt1B8 was isolated by HPLC from the fermentation supernatant. Fractions containing the genistein product were collected manually and used for UPLC-MS analysis. The UPLC system (Acquity Ultra Performance LC; Waters, Milford, MA) consisted of a solvent manager, a sample manager, and a diode array detector and was connected to a triple quadrupole mass spectrometer with a Z-spray API electrospray ionization (ESI) source (Quattro Premier XE; Waters, Milford, MA). The column was a UPLC BEH C18 column (1.7 μm; 50 by 2.1 mm; Waters, Milford, MA). The column temperature was maintained at 25°C. The mobile phase was a gradient of water-formic acid (95/5, vol/vol; pH 2.0) (solvent A) and methanol (solvent B) (0 to 40% solvent B in 3.10 min, 40% solvent B for 0.40 min, and 40 to 100% solvent B in 1.50 min) at a flow rate of 0.35 ml/min. A 4-μl aliquot of a sample was injected. MS-MS analyses were carried out in positive ionization mode using a capillary voltage of 0.7 kV, a source block temperature of 100°C, and a desolvation temperature of 450°C. The collision gas was argon at a pressure of 3.1 × 10−1 Pa. The cone voltage was 25 V, and the collision energy was 13 eV. Data were analyzed using the MassLynx software (version 4.1; Waters, Milford, MA).
SPE.
For nuclear magnetic resonance (NMR) analysis, the final product of genistein metabolism by strain Mt1B8 was isolated from approximately 50 ml of the fermentation supernatant (initial concentration of genistein, 100 μM) by solid-phase extraction (SPE). An octadecyl (C18) column (3 ml; 500 mg; Bakerbond, Phillipsburg, NJ) was conditioned three times with 2 ml of methanol and three times with 2 ml of water. After this, 2 ml of the fermentation supernatant was loaded onto the column, and this was followed by two washes with 2 ml of 3.7 mM aqueous HCl and one wash with 2 ml of 40% (vol/vol) aqueous methanol. The column was dried at room temperature for 10 min. The genistein metabolite was eluted with 2 ml of 60% (vol/vol) aqueous methanol. The eluates were pooled, dried by vacuum centrifugation (RC 10.22; Jouan, Saint-Nazaire, France), and dissolved in water.
NMR analysis.
The final product of genistein conversion by strain Mt1B8 was isolated from the fermentation supernatant by SPE as described above. For further purification, 100-μl samples were separated using the HPLC system described above. The fractions containing the genistein metabolite were manually collected, pooled, and dried by vacuum centrifugation. 1H NMR spectra (500 MHz) and 13C NMR spectra (125 MHz) were recorded in dimethyl sulfoxide-d6 using a Bruker Avance 500 instrument. For 1H NMR of 5-hydroxy-equol: δ = 2.69-2.74 (m, 1H, 4-H), 4.06-4.09 (m, 1H, 2-H), 4.32-4.35 (m, 1H, 2-H), 5.69, 5.88 (each d, J = 2.2 Hz, 2H, 6-H, 8-H), 6.70 (d, J = 8.5 Hz, 2H, 3′-H, 5′-H), 7.08 (d, J = 8.5 Hz, 2H, 2′-H, 6′-H); signals for two aliphatic protons (4-H, 3-H) were not assigned. For 13C NMR of 5-hydroxy-equol: δ = 70.10 (C-2), 94.17, 95.18 (C-6, C-8), 115.35 (C-3′, C-5′), 128.37 (C-2′, C-6′), 155.44, 156.19, 156.24, 156.44 (C-4′, C-5, C-7, C-8a); signals for four carbons (C-3, C-4, C-4a, C-1′) were not assigned.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain Mt1B8 has been deposited in the EMBL nucleotide sequence database under accession number AM747811.
RESULTS AND DISCUSSION
Daidzein conversion by strain Mt1B8.
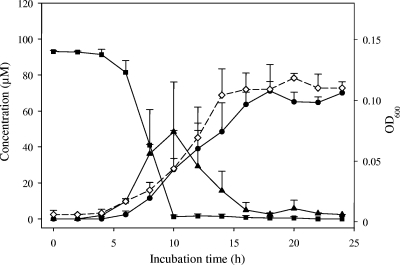
Growing cells of strain Mt1B8 completely transformed ca. 100 μM daidzein in 10 h (Fig. 1). The conversion of daidzein started concurrently with growth of the culture, and the maximal rate was reached after 6 to 10 h during the exponential growth phase. From daidzein, a nearly equimolar concentration of equol (70 μM) was obtained as the final product within 18 h. In the course of daidzein conversion, one intermediate was observed, which was identified as dihydrodaidzein. The maximal concentration of dihydrodaidzein (48 μM) was detected after 10 h of incubation (Fig. 1). When directly used as a substrate, dihydrodaidzein (ca. 65 μM) was also transformed by growing cells of strain Mt1B8 to equol (50 μM) within 12 h (data not shown).
FIG. 1.
Time course of conversion of daidzein (▪) via dihydrodaidzein (▴) to equol (•) by strain Mt1B8. Cell growth is indicated by the OD600 (⋄) (y axis on the right). The symbols indicate the means of triplicate experiments. The error bars indicate standard deviations.
So far, only a few bacteria have been reported to catalyze the complete conversion of daidzein to equol. Asaccharobacter celatus and Adlercreutzia equolifaciens, which are phylogenetically related to strain Mt1B8, also form dihydrodaidzein as an intermediate and were isolated from rat cecal contents and human feces, respectively (29, 30). Two other strains, Eubacterium sp. strains D1 and D2, were isolated from feces of pigs (54). All other species isolated so far apparently catalyze only certain steps in the conversion of daidzein to equol. Clostridium sp. strain HGH6 and Clostridium-like strain TM-40 from human feces and Lactobacillus sp. strain Niu-O16 from the bovine rumen convert daidzein to dihydrodaidzein (23, 45, 52). Eggerthella sp. strain Julong 732, which was isolated from human feces, transforms dihydrodaidzein to equol (49). By combining Lactobacillus sp. strain Niu-O16 and Eggerthella sp. strain Julong 732, formation of equol from daidzein has been demonstrated (50). For humans, complete conversion of daidzein to equol was observed when a partially defined mixed culture isolated from feces (19) or complex fecal microbiota (35, 42, 44) was used. Alternatively, daidzein may be transformed to O-desmethylangolensin by cleavage of the C ring, as catalyzed by the human intestinal species Eubacterium ramulus (39, 51).
Genistein conversion by strain Mt1B8.
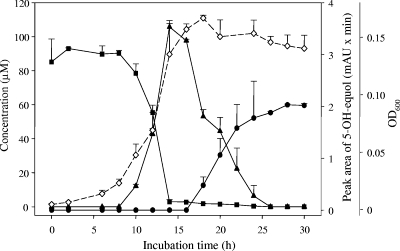
Genistein (ca. 100 μM) was completely transformed to dihydrogenistein by growing cells of strain Mt1B8 within 14 h (Fig. 2). The conversion of genistein started with a delay after growth of the culture, and the maximal rate was reached between 10 and 14 h during the exponential growth phase. Dihydrogenistein was converted further within 25 h, and one final product was formed. Although the cell densities were higher in the fermentation experiment with genistein, the transformation of this isoflavone started with a delay (Fig. 2) compared to the transformation of daidzein by strain Mt1B8 (Fig. 1). Moreover, the end product of genistein conversion appeared late, leading to accumulation of the intermediate dihydrogenistein (Fig. 2). In contrast, equol was observed immediately after daidzein conversion started, and the amount of dihydrodaidzein formed was smaller than the amount of dihydrogenistein (Fig. 1). Remarkably, incubation of dihydrogenistein (ca. 100 μM) with growing cells of strain Mt1B8 led to only a slight decrease in the dihydrogenistein concentration (18 μM within 40 h) (data not shown). However, the same metabolite which was observed as the end product of genistein transformation was also formed from dihydrogenistein, but at a much lower level (0.008 milli-absorption units × min).
FIG. 2.
Time course of conversion of genistein (▪) via dihydrogenistein (▴) to the metabolite that was identified as 5-hydroxy-equol (•) by strain Mt1B8. Since a standard 5-hydroxy-equol reference compound was not available, values are expressed as peak areas (first y axis on the right). Cell growth is indicated by the OD600 (⋄) (second y axis on the right). The symbols indicate the means of duplicate experiments. The error bars indicate standard deviations. mAU, milli-absorption units.
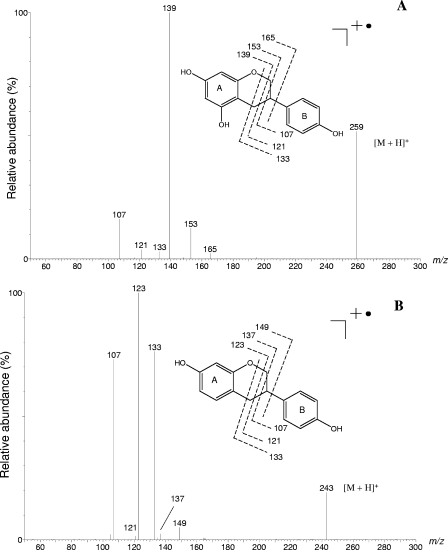
The elution behavior during HPLC analysis and the UV spectrum of the genistein product (absorption maxima at 232 and 280 nm) did not correspond to the elution behavior and UV spectrum of the previously reported microbial genistein metabolites, 6′-hydroxy-O-desmethylangolensin, 2-(4-hydroxyphenyl)propionic acid, 4-ethylphenol, and 1,3,5-trihydroxybenzene (4, 10, 15, 16, 23, 39, 40, 51, 52). Further characterization of the metabolite by UPLC-ESI-MS analysis resulted in a protonated molecule at m/z 259 [M+H]+, indicating a hydroxylated form of equol (Fig. 3A). The product ion spectrum for m/z 259 revealed a major fragment at m/z 139 and additional peaks at m/z 165, 153, 133, 121, and 107 (Fig. 3A). For comparison, the daidzein metabolite, equol, was analyzed in parallel by MS. From the molecule ion peak at m/z 243, fragments at m/z 149, 137, 133, 123, 121, and 107 were formed (Fig. 3B). As previously observed for the molecular peaks of the genistein metabolite and equol, a mass difference of 16 was also evident for the peaks at m/z 165 (equol, m/z 149), m/z 153 (equol, m/z 137), and m/z 139 (equol, m/z 123). Thus, these fragments most likely represent A-ring derivatives, whereas m/z 133, 121, and 107, which are present in both spectra, originate from the B ring (Fig. 3A and B). Standard reference compounds of hydroxylated equol derivatives were not available to confirm the proposed structure of 5-hydroxy-equol. Therefore, the metabolite was isolated from the supernatant of genistein fermentation by strain Mt1B8 using SPE, purified by analytical HPLC, and subjected to 1H and 13C NMR analysis. Although only a small quantity of the compound could be obtained, the NMR data confirmed that it was 5-hydroxy-equol (see Materials and Methods).
FIG. 3.
ESI product-ion mass spectrum of the protonated molecule (m/z 259) of 5-hydroxy-equol (A). Subsequently, the identity of this metabolite was confirmed by NMR analysis. For comparison, the product-ion spectrum of the protonated molecule of equol (m/z 243) was recorded (B).
Whereas dihydrogenistein is the end product of genistein conversion by Lactobacillus sp. strain Niu-O16 (52), the C-ring cleavage yielding 6′-hydroxy-O-desmethylangolensin and subsequently 2-(4-hydroxyphenyl)propionic acid is catalyzed by Eubacterium ramulus (39, 51). Although proposed to be a metabolite of microbial genistein conversion (2, 11), so far, 5-hydroxy-equol has been detected neither in vitro nor in vivo as a product of the human or rat intestinal microbiota. This suggests that strain Mt1B8 is not common in humans and rats but is specific to mice. However, the metabolism of genistein has not been studied in mice to date. On the other hand, 5-hydroxy-equol may have escaped detection because a standard reference compound is not commercially available. Different hydroxyl-substituted equol derivatives are products of phase I metabolism of equol by host enzymes (37). Interestingly, 5-hydroxy-equol was reported to show an antioxidant activity superior to that of genistein (2). As shown for other isoflavonoids, including daidzein, genistein, and equol, 5-hydroxy-equol is also expected to bind to estrogen receptors, preferably to estrogen receptor β (32, 33, 42).
Induction of isoflavonoid conversion.
During growth of strain Mt1B8, daidzein, genistein, dihydrodaidzein, and dihydrogenistein were converted to the end products equol and 5-hydroxy-equol. When the isoflavonoids were added after 14 h of growth (i.e., at the beginning of the stationary phase), the ability of strain Mt1B8 to transform these compounds was greatly reduced (Table 1). During 25 h of incubation with daidzein and genistein only very small amounts of dihydrodaidzein and dihydrogenistein, respectively, were formed. No metabolites were observed when dihydrodaidzein or dihydrogenistein was incubated with strain Mt1B8 under these conditions. When cells were grown in the presence of daidzein, genistein, dihydrodaidzein, and dihydrogenistein, the second dose of these compounds added after 14 h of incubation was rapidly converted to the same metabolites that were observed with growing cells. The conversion rates for cultures grown in the presence of the isoflavonoids were up to 54-fold higher than those for cultures not preincubated with these compounds (Table 1). Thus, the expression of encoding genes does not appear to be constitutive but is inducible by the substrates of the enzymes involved. To date, there have been no other reports of the induction of enzymes responsible for the transformation of (iso)flavonoids by gut bacteria. The isoflavonoids did not appear to affect cell growth, since the cell densities were similar whether isoflavonoids were present or absent (data not shown). The time courses of daidzein and genistein transformation and metabolite generation in the stationary phase were similar for cells preincubated with these isoflavones. The delay in the conversion of genistein and the formation of 5-hydroxy-equol observed with growing cells were not observed. However, as observed for growing cultures, the rate of conversion of dihydrogenistein in the stationary phase for cells grown in the presence of this isoflavonoid was lower than the rates of conversion of the other isoflavonoids (Table 1).
TABLE 1.
Initial conversion rates (0 to 2 h) for isoflavonoid substrates added to cultures of strain Mt1B8 during the stationary growth phasea
| Substrate | Conversion rate (μmol h−1 mg protein−1)
|
|
|---|---|---|
| Without isoflavonoid | With isoflavonoidb | |
| Daidzein | 0.03 | 1.61 (daidzein) |
| Dihydrodaidzein | 0.04 | 1.80 (dihydrodaidzein) |
| Genistein | 0.05 | 1.28 (genistein) |
| 2.31 (daidzein) | ||
| Dihydrogenistein | 0.02 | 0.64 (dihydrogenistein) |
| 1.26 (genistein) | ||
| 1.12 (daidzein) | ||
The cultures were initially grown for 14 h in the absence or presence of the same or another isoflavonoid. The levels of growth (OD600) were similar with and without isoflavonoids. The values are the means of triplicate experiments.
The isoflavonoids used for preincubation are indicated in parentheses.
In addition, the effect of preincubation with isoflavonoids other than those added in the stationary phase was tested. The presence of daidzein during cell growth enabled conversion not only of daidzein but also of genistein during the stationary phase at similar rates (Table 1). This suggests that identical enzymes catalyze the conversion of the two isoflavones. Furthermore, the transformation of dihydrogenistein was also induced by preincubation with either genistein or daidzein. Remarkably, the resulting conversion rates were even higher than those observed following induction by dihydrogenistein (Table 1). This suggests that dihydrogenistein conversion is more efficiently induced when this compound (or dihydrodaidzein) is formed inside the cell.
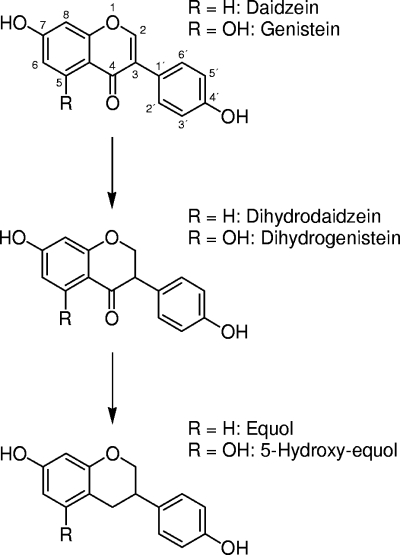
In summary, strain Mt1B8 converts daidzein and genistein to the analogous metabolites, equol and 5-hydroxy-equol, via the same pathway (Fig. 4). Thus, besides the intensively studied equol formation, intestinal bacteria might also contribute to bioactivation of genistein by converting it to 5-hydroxy-equol.
FIG. 4.
Anaerobic conversion of daidzein and genistein by strain Mt1B8 isolated from the mouse intestine.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant BR 2269/3-1).
We are indebted to Jeanett Haeger for technical assistance. We thank Frank Lehmann (Pharmaceutical Institute, University of Bonn, Bonn, Germany) for synthesis of standard reference compounds and George Kollias (Institute of Immunology, Biomedical Sciences Research Center Alexander Flemming, Vari, Greece) for providing the TNFΔARE C57BL/6 mice.
Footnotes
Published ahead of print on 6 June 2008.
REFERENCES
- 1.Adlercreutz, H., P. I. Musey, T. Fotsis, C. Bannwart, K. Wahala, T. Makela, G. Brunow, and T. Hase. 1986. Identification of lignans and phytoestrogens in urine of chimpanzees. Clin. Chim. Acta 158:147-154. [DOI] [PubMed] [Google Scholar]
- 2.Arora, A., M. G. Nair, and G. M. Strasburg. 1998. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 356:133-141. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson, C., C. L. Frankenfeld, and J. W. Lampe. 2005. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp. Biol. Med. 230:155-170. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, S., L. Coward, M. Kirk, and J. Sfakianos. 1998. HPLC-mass spectrometry analysis of isoflavones. Proc. Soc. Exp. Biol. Med. 217:254-262. [DOI] [PubMed] [Google Scholar]
- 5.Bayer, T., T. Colnot, and W. Dekant. 2001. Disposition and biotransformation of the estrogenic isoflavone daidzein in rats. Toxicol. Sci. 62:205-211. [DOI] [PubMed] [Google Scholar]
- 6.Birt, D. F., S. Hendrich, and W. Wang. 2001. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol. Ther. 90:157-177. [DOI] [PubMed] [Google Scholar]
- 7.Blair, R. M., S. E. Appt, A. A. Franke, and T. B. Clarkson. 2003. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis). J. Nutr. 133:2262-2267. [DOI] [PubMed] [Google Scholar]
- 8.Bolca, S., S. Possemiers, A. Herregat, I. Huybrechts, A. Heyerick, S. De Vriese, M. Verbruggen, H. Depypere, D. De Keukeleire, M. Bracke, S. De Henauw, W. Verstraete, and T. Van de Wiele. 2007. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J. Nutr. 137:2242-2246. [DOI] [PubMed] [Google Scholar]
- 9.Braune, A., M. Gütschow, W. Engst, and M. Blaut. 2001. Degradation of quercetin and luteolin by Eubacterium ramulus. Appl. Environ. Microbiol. 67:5558-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y. C., and M. G. Nair. 1995. Metabolism of daidzein and genistein by intestinal bacteria. J. Nat. Prod. 58:1892-1896. [DOI] [PubMed] [Google Scholar]
- 11.Chang, Y. C., M. G. Nair, and J. L. Nitiss. 1995. Metabolites of daidzein and genistein and their biological activities. J. Nat. Prod. 58:1901-1905. [DOI] [PubMed] [Google Scholar]
- 12.Clavel, T., M. Fallani, P. Lepage, F. Levenez, J. Mathey, V. Rochet, M. Serezat, M. Sutren, G. Henderson, C. Bennetau-Pelissero, F. Tondu, M. Blaut, J. Dore, and V. Coxam. 2005. Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. J. Nutr. 135:2786-2792. [DOI] [PubMed] [Google Scholar]
- 13.Clavel, T., G. Henderson, C. A. Alpert, C. Philippe, L. Rigottier-Gois, J. Dore, and M. Blaut. 2005. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microbiol. 71:6077-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavel, T., R. Lippman, F. Gavini, J. Dore, and M. Blaut. 2007. Clostridium saccharogumia sp. nov. and Lactonifactor longoviformis gen. nov., sp. nov., two novel human faecal bacteria involved in the conversion of the dietary phytoestrogen secoisolariciresinol diglucoside. Syst. Appl. Microbiol. 30: 16-26. [DOI] [PubMed] [Google Scholar]
- 15.Coldham, N. G., C. Darby, M. Hows, L. J. King, A. Q. Zhang, and M. J. Sauer. 2002. Comparative metabolism of genistin by human and rat gut microflora: detection and identification of the end-products of metabolism. Xenobiotica 32:45-62. [DOI] [PubMed] [Google Scholar]
- 16.Coldham, N. G., L. C. Howells, A. Santi, C. Montesissa, C. Langlais, L. J. King, D. D. Macpherson, and M. J. Sauer. 1999. Biotransformation of genistein in the rat: elucidation of metabolite structure by product ion mass fragmentology. J. Steroid Biochem. Mol. Biol. 70:169-184. [DOI] [PubMed] [Google Scholar]
- 17.Conte, M. P., S. Schippa, I. Zamboni, M. Penta, F. Chiarini, L. Seganti, J. Osborn, P. Falconieri, O. Borrelli, and S. Cucchiara. 2006. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 55:1760-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cos, P., T. De Bruyne, S. Apers, D. Vanden Berghe, L. Pieters, and A. J. Vlietinck. 2003. Phytoestrogens: recent developments. Planta Med. 69:589-599. [DOI] [PubMed] [Google Scholar]
- 19.Decroos, K., S. Vanhemmens, S. Cattoir, N. Boon, and W. Verstraete. 2005. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 183:45-55. [DOI] [PubMed] [Google Scholar]
- 20.Eldridge, A. C., and W. F. Kwolek. 1983. Soybean isoflavones: effect of environment and variety on composition. J. Agric. Food Chem. 31:394-396. [DOI] [PubMed] [Google Scholar]
- 21.Geller, S. E., and L. Studee. 2006. Soy and red clover for mid-life and aging. Climacteric 9:245-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregersen, T. 1978. Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur. J. Appl. Microbiol. Biotechnol. 5:123-127. [Google Scholar]
- 23.Hur, H. G., J. O. Lay, Jr., R. D. Beger, J. P. Freeman, and F. Rafii. 2000. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch. Microbiol. 174:422-428. [DOI] [PubMed] [Google Scholar]
- 24.Hwang, C. S., H. S. Kwak, H. J. Lim, S. H. Lee, Y. S. Kang, T. B. Choe, H. G. Hur, and K. O. Han. 2006. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 101:246-253. [DOI] [PubMed] [Google Scholar]
- 25.Jackman, K. A., O. L. Woodman, and C. G. Sobey. 2007. Isoflavones, equol and cardiovascular disease: pharmacological and therapeutic insights. Curr. Med. Chem. 14:2824-2830. [DOI] [PubMed] [Google Scholar]
- 26.Joannou, G. E., G. E. Kelly, A. Y. Reeder, M. Waring, and C. Nelson. 1995. A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J. Steroid Biochem. Mol. Biol. 54:167-184. [DOI] [PubMed] [Google Scholar]
- 27.Juniewicz, P. E., S. Pallante Morell, A. Moser, and L. L. Ewing. 1988. Identification of phytoestrogens in the urine of male dogs. J. Steroid Biochem. 31:987-994. [DOI] [PubMed] [Google Scholar]
- 28.Kontoyiannis, D., M. Pasparakis, T. T. Pizarro, F. Cominelli, and G. Kollias. 1999. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10:387-398. [DOI] [PubMed] [Google Scholar]
- 29.Maruo, T., M. Sakamoto, C. Ito, T. Toda, and Y. Benno. 2008. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 58:1221-1227. [DOI] [PubMed] [Google Scholar]
- 30.Minamida, K., K. Ota, M. Nishimukai, M. Tanaka, A. Abe, T. Sone, F. Tomita, H. Hara, and K. Asano. 2008. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int. J. Syst. Evol. Microbiol. 58:1238-1240. [DOI] [PubMed] [Google Scholar]
- 31.Minamida, K., M. Tanaka, A. Abe, T. Sone, F. Tomita, H. Hara, and K. Asano. 2006. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 102:247-250. [DOI] [PubMed] [Google Scholar]
- 32.Morito, K., T. Hirose, J. Kinjo, T. Hirakawa, M. Okawa, T. Nohara, S. Ogawa, S. Inoue, M. Muramatsu, and Y. Masamune. 2001. Interaction of phytoestrogens with estrogen receptors α and β. Biol. Pharm. Bull. 24:351-356. [DOI] [PubMed] [Google Scholar]
- 33.Muthyala, R. S., Y. H. Ju, S. Sheng, L. D. Williams, D. R. Doerge, B. S. Katzenellenbogen, W. G. Helferich, and J. A. Katzenellenbogen. 2004. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors α and β. Bioorg. Med. Chem. 12:1559-1567. [DOI] [PubMed] [Google Scholar]
- 34.Possemiers, S., S. Bolca, E. Eeckhaut, H. Depypere, and W. Verstraete. 2007. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: producer phenotyping and relation with intestinal community. FEMS Microbiol. Ecol. 61:372-383. [DOI] [PubMed] [Google Scholar]
- 35.Rafii, F., C. Davis, M. Park, T. M. Heinze, and R. D. Beger. 2003. Variations in metabolism of the soy isoflavonoid daidzein by human intestinal microfloras from different individuals. Arch. Microbiol. 180:11-16. [DOI] [PubMed] [Google Scholar]
- 36.Rowland, I. R., H. Wiseman, T. A. Sanders, H. Adlercreutz, and E. A. Bowey. 2000. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr. Cancer 36:27-32. [DOI] [PubMed] [Google Scholar]
- 37.Rufer, C. E., H. Glatt, and S. E. Kulling. 2006. Structural elucidation of hydroxylated metabolites of the isoflavan equol by gas chromatography-mass spectrometry and high-performance liquid chromatography-mass spectrometry. Drug Metab. Dispos. 34:51-60. [DOI] [PubMed] [Google Scholar]
- 38.Scalbert, A., C. Manach, C. Morand, C. Remesy, and L. Jimenez. 2005. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 45:287-306. [DOI] [PubMed] [Google Scholar]
- 39.Schoefer, L., R. Mohan, A. Braune, M. Birringer, and M. Blaut. 2002. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol. Lett. 208:197-202. [DOI] [PubMed] [Google Scholar]
- 40.Setchell, K. D. 1998. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 68:1333S-1346S. [DOI] [PubMed] [Google Scholar]
- 41.Setchell, K. D., and A. Cassidy. 1999. Dietary isoflavones: biological effects and relevance to human health. J. Nutr. 129:758S-767S. [DOI] [PubMed] [Google Scholar]
- 42.Setchell, K. D., C. Clerici, E. D. Lephart, S. J. Cole, C. Heenan, D. Castellani, B. E. Wolfe, L. Nechemias-Zimmer, N. M. Brown, T. D. Lund, R. J. Handa, and J. E. Heubi. 2005. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 81:1072-1079. [DOI] [PubMed] [Google Scholar]
- 43.Szkudelska, K., and L. Nogowski. 2007. Genistein—a dietary compound inducing hormonal and metabolic changes. J. Steroid Biochem. Mol. Biol. 105:37-45. [DOI] [PubMed] [Google Scholar]
- 44.Tamura, M. 2006. Comparison of the in vitro metabolism of isoflavones by fecal flora from human flora-associated mice and human. J. Sci. Food Agric. 86:1567-1570. [Google Scholar]
- 45.Tamura, M., T. Tsushida, and K. Shinohara. 2007. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 13:32-35. [DOI] [PubMed] [Google Scholar]
- 46.Usui, T. 2006. Pharmaceutical prospects of phytoestrogens. Endocr. J. 53: 7-20. [DOI] [PubMed] [Google Scholar]
- 47.Wahala, K., and T. Hase. 1989. Hydrogen transfer reduction of isoflavones. Heterocycles 28:183-186. [Google Scholar]
- 48.Wahala, K., A. Salakka, and H. Adlercreutz. 1998. Synthesis of novel mammalian metabolites of the isoflavonoid phytoestrogens daidzein and genistein. Proc. Soc. Exp. Biol. Med. 217:293-299. [DOI] [PubMed] [Google Scholar]
- 49.Wang, X. L., H. G. Hur, J. H. Lee, K. T. Kim, and S. I. Kim. 2005. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol. 71:214-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, X. L., H. J. Kim, S. I. Kang, S. I. Kim, and H. G. Hur. 2007. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Arch. Microbiol. 187:155-160. [DOI] [PubMed] [Google Scholar]
- 51.Wang, X. L., K. T. Kim, J. H. Lee, H. G. Hur, and S. I. Kim. 2004. C-ring cleavage of isoflavones daidzein and genistein by a newly-isolated human intestinal bacterium Eubacterium ramulus Julong 601. J. Microbiol. Biotechnol. 14:766-771. [Google Scholar]
- 52.Wang, X. L., K. H. Shin, H. G. Hur, and S. I. Kim. 2005. Enhanced biosynthesis of dihydrodaidzein and dihydrogenistein by a newly isolated bovine rumen anaerobic bacterium. J. Biotechnol. 115:261-269. [DOI] [PubMed] [Google Scholar]
- 53.Ward, W. E., S. Kim, D. Chan, and D. Fonseca. 2005. Serum equol, bone mineral density and biomechanical bone strength differ among four mouse strains. J. Nutr. Biochem. 16:743-749. [DOI] [PubMed] [Google Scholar]
- 54.Yu, Z. T., W. Yao, and W. Y. Zhu. 2008. Isolation and identification of equol-producing bacterial strains from cultures of pig faeces. FEMS Microbiol. Lett. 282:73-80. [DOI] [PubMed] [Google Scholar]