Abstract
Aspergillus fumigatus is an opportunistic human pathogenic fungus causing severe infections in immunocompromised patients. Cyclic AMP (cAMP) signal transduction plays an important role in virulence. A central component of this signaling cascade is protein kinase A (PKA), which regulates cellular processes by phosphorylation of specific target proteins. Here we describe the generation and analysis of A. fumigatus mutants expressing the gene encoding the catalytic subunit of PKA, pkaC1, under control of an inducible promoter. Strains overexpressing pkaC1 showed high PKA activity, reduced growth, sporulation deficiency, and formation of a dark pigment in the mycelium. These data indicate that cAMP-PKA signaling is involved in the regulation of important processes, such as growth, asexual reproduction, and biosynthesis of secondary metabolites. Furthermore, elevated PKA activity led to increased expression of the pksP gene. The polyketide synthase PksP is an essential enzyme for production of dihydroxynaphthalene-melanin in A. fumigatus and contributes to virulence. Our results suggest that increased pksP expression is responsible for pigment formation in the mycelium. Comparative proteome analysis of the pkaC1-overexpressing strain and the wild-type strain led to the identification of proteins regulated by the cAMP-PKA signal transduction pathway. We showed that elevated PKA activity resulted in activation of stress-associated proteins and of enzymes involved in protein biosynthesis and glucose catabolism. In contrast, proteins which were involved in nucleotide and amino acid biosynthesis were downregulated, as were enzymes involved in catabolism of carbon sources other than glucose.
The importance of Aspergillus fumigatus as a human pathogenic fungus has increased notably over the last years. Today, A. fumigatus is the most important airborne fungal pathogen causing invasive mycoses (for an overview, see references 4 and 28). Sensing of environmental stimuli and transduction of the corresponding signal via the cyclic AMP (cAMP) signaling cascade play an essential role in the virulence of a variety of human and plant pathogenic fungi, including Cryptococcus neoformans, Magnaporthe grisea, and A. fumigatus (1, 9, 19). They enable the fungus to adapt to changing environmental conditions, e.g., after invasion of the host tissue, by activation of factors which protect the pathogen against defense mechanisms of the host immune system. In eukaryotes, exogenous signals are sensed by defined transmembrane receptors on the surface of the cell, resulting in activation of receptor-bound heterotrimeric G proteins. In their inactive state, these G proteins consist of three subunits, designated Gα, Gβ, and Gγ. The Gα subunit binds GDP. After binding of a signal molecule to the receptor, GDP is exchanged with GTP. Subsequently, the G protein dissociates from the receptor and the Gα subunit is released from the βγ heterodimer. The Gα-GTP monomer formed by GpaB activates the adenylate cyclase (ACYA) that generates cAMP from ATP.
A central component of the cAMP signaling cascade is protein kinase A (PKA). PKA is a serine/threonine kinase which is conserved in eukaryotes. In the inactive state, PKA forms a heterotetrameric complex, consisting of two PKA catalytic (PKAC) subunits that are bound by two regulatory (PKAR) subunits. Each PKAR subunit has an autophosphorylation site for the PKAC subunit as well as two tandem copies of a cAMP binding site. After binding of two molecules of cAMP to these binding motifs, the catalytic and regulatory subunits dissociate as a result of a conformational change of the heterotetramer. The activated catalytic subunits are now able to phosphorylate target proteins, such as transcription factors.
As a counterpart of ACYA, phosphodiesterases hydrolyze intracellular cAMP to AMP to prevent constitutive activation of PKA and to reset the signaling cascade for the response to new environmental signals. For A. fumigatus, this model of cAMP signaling is based on results obtained by analysis of mutants of the cAMP cascade. Genes encoding the Gα subunit GpaB, the adenylate cyclase ACYA, the PKA catalytic subunit PKAC1, and PKAR were deleted, and the corresponding mutants were analyzed (18, 19, 34). The ΔpkaC1, ΔpkaR, and ΔacyA mutants were severely delayed in growth and sporulation, whereas the ΔgpaB mutant showed only a slight decrease in growth rate and spore formation. In contrast to its nearly unaffected growth, the ΔgpaB mutant showed a significant attenuation in virulence (19), underlining the importance of the cAMP-PKA signaling cascade for the virulence of this fungus.
In the A. fumigatus genome, two different genes for PKA catalytic subunits were identified, namely, pkaC1 and pkaC2 (19). Somehow PKAC2 is not active, because its nucleotide binding site does not contain the consensus sequence necessary for binding of ATP. Furthermore, deletion of pkaC1 resulted in a complete loss of PKA activity (19). This led to the assumption that PKAC1 is the single active PKAC subunit in A. fumigatus.
The aim of this study was to identify proteins of A. fumigatus that were regulated by PKA. Ectopic integration of pkaC1 under control of the inducible promoter of the isocitrate lyase gene (acuDp) (2) resulted in transgenic mutants whose level of active catalytic subunits could be drastically increased. The mutants were analyzed phenotypically and by comparative two-dimensional (2D) gel electrophoresis to identify putative target proteins/genes of PKA.
MATERIALS AND METHODS
Fungal and bacterial strains, media, and growth conditions.
The A. fumigatus ATCC 46645 wild-type strain was used for DNA isolation and to generate pkaC1Oex mutants. An A. fumigatus pksP strain was used for generation of pksP-pkaC1Oex mutants. Due to a mutation in the pksP gene, this strain is impaired in dihydroxynaphthalene (DHN)-melanin biosynthesis, producing white conidia and showing strong attenuation in virulence (15). A pksPp-lacZ strain (18) was used for the generation of pksPp-lacZ-ΔpkaR and pksPp-lacZ-pkaC1Oex mutants. The pksPp-lacZ strain contains the pksP promoter fused with the lacZ reporter gene for the quantification of pksP expression. A. fumigatus was cultivated at 37°C in Aspergillus minimal medium (AMM) as described previously (32). For solid medium, AMM containing 1.5% (wt/vol) agar was used. For transformation of Escherichia coli, strain TOP10F′ (Invitrogen, Germany) was used. E. coli strains were grown at 37°C in LB medium supplemented with 100 μg ml−1 of ampicillin.
Standard DNA techniques.
Standard techniques for manipulation of DNA were carried out as described previously (22). Chromosomal DNA of A. fumigatus was prepared using a Master Pure yeast DNA purification kit (Epicentre). For Southern blot analysis, chromosomal DNA of A. fumigatus was digested with SacI. DNA fragments were separated in an agarose gel and blotted onto Hybond N+ nylon membranes (GE Healthcare Bio-Sciences, Germany). Labeling of DNA probes, hybridization, and detection of DNA-DNA hybrids were performed using DIG labeling mix, DIG Easy Hyb, and a CDP-Star ready-to-use kit (Roche Applied Science, Germany), respectively, according to the manufacturer's recommendations.
Generation of recombinant plasmids and transformation of A. fumigatus.
Plasmid pacuDpkaC1 was used for inducible overexpression of the pkaC1 gene in A. fumigatus. For construction of pacuDpkaC1, a 1-kb fragment of the acuD promoter was isolated via BamHI restriction from plasmid pDsRed_iclp (2) and inserted into the BamHI-digested plasmid pUC18 (Fermentas, Germany), resulting in plasmid pUCacuDp. Using primers PKAC1-F-Xba and PKAC1-R-Xba (Table 1) and A. fumigatus wild-type chromosomal DNA as a template, the pkaC1 gene was amplified by PCR, employing Bio-X-Act Short DNA polymerase (Bioline, Germany). The resulting 2-kb PCR product was inserted into plasmid pCR2.1TOPO (Invitrogen, Germany) by TOPO-TA cloning and verified by sequencing. After digestion with XbaI, the pkaC1-carrying DNA fragment was inserted into the XbaI restriction site of pUCacuDp, resulting in plasmid pUCacuDpkaC1. This plasmid was cut with SfoI and SmaI, and the 3.2-kb blunt-ended fragment comprising the acuD promoter in frame with the pkaC1 sequence was inserted into the SfoI site of plasmid pAN8-1 (GenBank accession number Z32751), containing the phleomycin resistance cassette. The resulting plasmid was designated pacuDpkaC1.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′) |
|---|---|
| PKAC1-F-Xba | TCTAGAAATGCCGACTTTAGGAGGTCTC |
| PKAC1-R-Xba | TCTAGAGTATCTCTCAGATGCGTC |
| PKAC1-int-F | AGA CGC CGT CGA CTT GCT C |
| acuD-F | CGGATCCGAAGGACAGGAAC |
| PKAR-for | CTTGTCATCACGTCTGTCCTC |
| PKAR-rev | GACGATGACTCGAATGTGGTTG |
For deletion of the pkaR gene in A. fumigatus, using pyrithiamine resistance as a selection marker, plasmid pCR2.1ΔpkaR-ptrA was generated as follows. A PCR product carrying the pkaR gene, including 1-kb flanking regions, was obtained by using primers PKAR-for and PKAR-rev and genomic A. fumigatus DNA as a template. The DNA fragment was ligated into vector pCR2.1TOPO (Invitrogen, Germany), resulting in plasmid pCR2.1pkaR. This plasmid was digested with NheI. Blunt ends were then generated by applying the DNA polymerase I large (Klenow) fragment (New England Biolabs, Germany). The product was digested with XmaI. The pyrithiamine resistance gene was inserted as a 2-kb DraI/XmaI fragment obtained from plasmid pCR2.1mpkA-ptrA (a gift from V. Valiante). For transformation, the pkaR deletion construct was amplified by PCR, using primers PKAR-for and PKAR-rev and plasmid pCR2.1ΔpkaR-ptrA as a template. Transformation of A. fumigatus was carried out using protoplasts as described previously (32). When selection for phleomycin resistance was used, phleomycin (Invivogen) was added to the medium to a final concentration of 80 μg ml−1. When selection for pyrithiamine resistance was used, pyrithiamine (Sigma-Aldrich, Germany) was added to the medium to a final concentration of 0.1 μg ml−1.
Quantification of conidial production.
To investigate the effect of pkaC1 overexpression on production of conidia in A. fumigatus, 105 conidia were plated on AMM agar plates containing glucose (50 mM) and glucose (50 mM)-acetate (100 mM) as carbon sources. After 3 days, conidia were harvested in 10 ml 0.9% (wt/vol) NaCl-0.1% (vol/vol) Tween 80, filtered using a cell strainer (Becton Dickinson, Germany), and counted using a Thoma chamber.
Protein extraction from A. fumigatus.
For protein extraction, 108 conidia of the A. fumigatus wild type and the pkaC1Oex strain were inoculated in 100 ml AMM containing 50 mM glucose and cultivated for 16 h on a rotary shaker. After this precultivation step, the mycelium was harvested by filtration using Miracloth, washed, transferred to AMM containing 100 mM acetate as the sole carbon source (inducing conditions), and incubated for a further 8 hours. Mycelium was harvested and immediately frozen in liquid nitrogen. After grinding of the mycelium to a fine powder, using a mortar and pestle, proteins were extracted for proteome analysis as described previously (17). For determination of PKA activity, 100 mg of the ground mycelium was resuspended in 500 μl ice-cold extraction buffer (25 mM Tris-HCl, pH 7.4, 1 mM dithiothreitol, 1 mM EDTA), and a PKA assay (Promega, Germany) was applied. Protein extracts were centrifuged for 10 min at 13,000 rpm at 4°C, and the supernatant was used for further experiments.
Proteome analysis.
Proteome analysis of A. fumigatus was carried out essentially as described previously (14, 17). In brief, protein extracts for 2D gel electrophoresis were purified by phenol extraction (13). The dried protein pellets were resuspended in lysis buffer. The absolute amount of 300 μg protein was applied via anodic cup loading to rehydrated IPG strips with a nonlinear pH gradient from 3 to 11 (GE Healthcare Bio-Sciences, Germany). The second dimension of electrophoresis was performed on an Ettan DALTsix system (GE Healthcare Bio-Sciences, Germany). Gels were stained with colloidal Coomassie blue (20). For each strain analyzed, three replicas of three gels were performed. Images were analyzed with Delta 2D software (version 3.4; Decodon, Germany). After background subtraction and normalization, spots were quantified using % spot volumes. Only spots with a ratio of >2 and a P value of <0.05 using Student's t test were regarded as significantly regulated. Protein spots were excised manually and digested with trypsin according to the protocol of Bruker Daltonics (adapted from the method described in reference 24). The samples were analyzed by matrix-assisted laser desorption ionization-tandem time of flight (Ultraflex 1; Bruker Daltonics, Germany) and subsequently identified by searching the NCBI database, using the MASCOT interface (MASCOT 2.1.03; Matrix Science, United Kingdom) with the following parameters: Cys as an S-carbamidomethyl derivative, Met in oxidized form (variable), one missed cleavage site, and a peptide mass tolerance of 200 ppm. Hits were considered significant according to the MASCOT score (P < 0.05). Database analysis was refined by using Protein Scape 1.3 software (Protagen, Germany).
β-Gal activity assays.
β-Galactosidase (β-Gal) activity was determined with o-nitrophenyl-β-d-galactoside (ONPG) as the substrate. Extinction of the sample was measured at 420 nm. Specific activities were calculated as previously described for Aspergillus nidulans (5).
Determination of cAMP-dependent PKA activity.
For determination of PKA activity, the PepTag assay for nonradioactive detection of cAMP-dependent PKA (Promega, Germany) was applied. This assay uses a positively charged kemptide coupled to a fluorescent dye as a substrate specific for PKA. Visualization of the kemptide was done by using UV light. The PKA activity assay was applied as described previously (19). Protein extracts were adjusted to identical concentrations. PKA holoenzymes in the sample were activated by incubation with 1 μM cAMP. The quantity of free catalytic PKA subunits was determined by incubation of the samples without the addition of cAMP.
RESULTS
Generation of pkaC1-overexpressing mutants.
To identify target proteins and metabolic pathways regulated by cAMP-PKA signal transduction, an A. fumigatus strain with enhanced PKA activity was generated. This enhanced activity was achieved by overexpressing the pkaC1 gene, encoding the catalytic subunit of PKA, which leads to an excess of free PKAC subunits. To exclude the possibility that constitutive overexpression results in a drastic growth defect, sporulation deficiency, or even lethality, the expression of pkaC1 was placed under the control of the A. fumigatus acuD promoter, which is activated by C2 carbon sources (2). Therefore, overexpression of pkaC1 was controllable by using different carbon sources, e.g., acetate for induction and glucose for repression. A schematic map of plasmid pacuDp-pkaC1, used for transformation of the A. fumigatus wild type, is shown in Fig. 1A. As a resistance marker, the phleomycin resistance gene ble under control of the gpdA promoter from A. nidulans was employed.
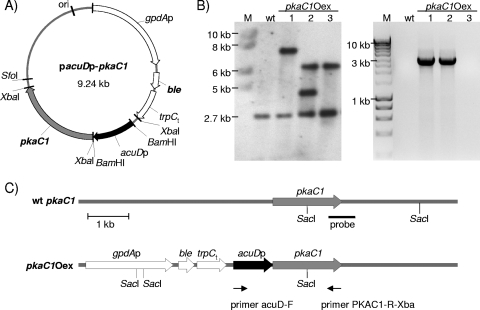
FIG. 1.
Generation of inducible pkaC1-overexpressing A. fumigatus strains. (A) Map of plasmid pacuDp-pkaC1 used for transformation of A. fumigatus wild type. pkaC1, pkaC1 gene amplified from A. fumigatus genomic DNA, including 3′-untranslated region; acuDp, promoter of the A. fumigatus acuD gene; gpdAp, promoter of the gpdA gene from A. nidulans; ble, phleomycin resistance gene; trpCt, terminator sequence. (B) Southern blot analysis (left) and PCR analysis (right) of the wild type (wt) and pkaC1Oex transformant strains. Lanes M, DNA marker. (C) Schematic drawings of the A. fumigatus wild-type pkaC1 genomic locus and the pkaC1Oex construct.
Southern blot analysis was performed to identify pkaC1-overexpressing mutants. Genomic DNAs of selected transformants and the wild-type strain were digested with SacI. For hybridization, a 640-bp probe specific for detection of the 3′ region of the pkaC1 gene was used, as presented schematically in Fig. 1C. As shown in Fig. 1B (left), a band with a size of 2.7 kb was obtained for all tested strains, indicating the presence of the endogenous pkaC1 gene. Additional bands with different sizes were obtained for transformants 1, 2, and 3, indicating successful integration of an additional pkaC1 sequence. To verify the integration of the complete acuDp-pkaC1 sequence, PCR analysis of the transformants was carried out. By using the specific primers acuD-F and PKAC1-R-Xba, a DNA fragment with a size of 2.8 kb was obtained only when the complete sequence, starting from the acuD promoter and ending at the 3′-untranslated region of the pkaC1 gene, was present in the genomes of the transformants. As shown in Fig. 1B (right panel), transformants 1 and 2, but not 3, gave the expected signal. Transformant strain 1, designated pkaC1Oex, was used for further studies, including proteome analysis.
Phenotypic analysis of the pkaC1-overexpressing mutant.
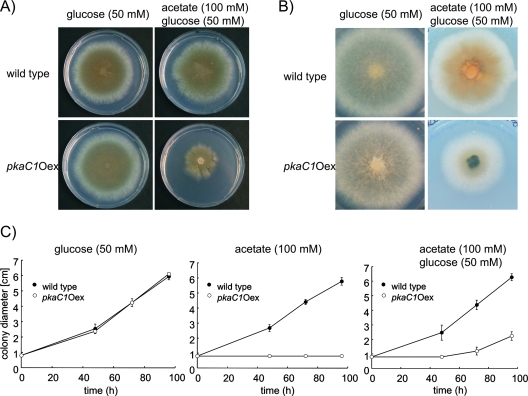
To compare the phenotypes of the pkaC1Oex strain and the wild type, conidia of these strains were point inoculated on agar plates containing different carbon sources. After incubation for 72 h at 37°C on AMM agar plates with glucose as the sole carbon source, the wild-type and pkaC1Oex strains showed no differences (Fig. 2A and B). To induce pkaC1 expression via the acuD promoter, conidia were inoculated on AMM agar plates containing 100 mM acetate as the sole carbon source. In contrast to growth on glucose, the pkaC1Oex mutant was not able to germinate (Fig. 2C). Microscopic analysis showed that pkaC1Oex conidia were swollen but were not able to germinate (data not shown). To test whether the conidia were still viable at this stage (at 4 days postinoculation), a drop of glucose solution was added to the conidia on the agar plate. The conidia started to germinate (data not shown). In contrast, conidia of the wild type were able to germinate and to grow normally on acetate as the sole carbon source. To enable the pkaC1Oex strain to germinate under inducing conditions, conidia were inoculated on AMM agar plates containing 100 mM acetate and 50 mM glucose. As shown in Fig. 2C, the addition of glucose to acetate-containing medium enabled the conidia to germinate. However, in comparison to the wild-type strain, which grew normally on glucose-acetate medium, the pkaC1Oex strain exhibited a drastically altered phenotype. Radial growth of the mutant was strongly reduced (Fig. 2A and C), and the mycelium produced a dark pigment visible on the back side of the agar plates (Fig. 2B).
FIG. 2.
Phenotypic analysis of the pkaC1Oex mutant under noninducing (glucose) and inducing (acetate-glucose) conditions. Front views (A) and back views (B) of 5-day-old colonies grown on AMM agar plates with glucose and acetate-glucose are shown. (C) Determination of radial growth of wild-type and pkaC1Oex strains cultivated on medium containing different concentrations of glucose and/or acetate as the carbon source. Spores (103) were point inoculated, and the plates were incubated at 37°C for the indicated times.
PKA enzyme activity was increased in the pkaC1-overexpressing strain.
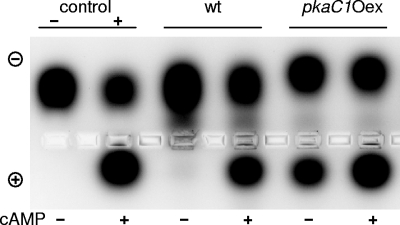
To determine whether induced overexpression of pkaC1 resulted in active PKAC subunits, PKA activity was measured in protein extracts of the pkaC1Oex mutant and compared to that of the wild-type strain. For this purpose, PKA activity was assayed using a colored peptide (kemptide) specifically recognized and phosphorylated by PKA. For generation of protein extracts, the A. fumigatus strains were preincubated in AMM-glucose and then shifted to AMM-acetate for induction of pkaC1 overexpression. As shown in Fig. 3, the nonphosphorylated kemptide migrated to the cathode, whereas the phosphorylated and therefore negatively charged kemptide migrated to the anode. The stronger the signal, the larger was the amount of phosphorylated kemptide in the protein extract, indicating increased PKA activity. This assay clearly showed that in the wild type incubated without cAMP, hardly any PKA activity was detectable. This was due to the regulatory subunits, which bind most of the catalytic subunits and keep them inactive. In contrast, a strong signal due to phosphorylated kemptide was detectable after addition of cAMP to the wild-type extract. This obviously was the result of cAMP molecules binding to PKAR subunits, followed by the release of active PKAC1 subunits.
FIG. 3.
Analysis of PKA activity of wild-type (wt) and pkaC1Oex transformant strains. Depending on the PKA activity, the net charge of the substrate kemptide was altered with respect to its phosphorylation status. As a positive control (+), the purified PKAC subunit was used, and as a negative control (−), protein extraction buffer without enzyme was used.
The PKA activity pattern of the pkaC1Oex mutant was different from that of the wild type, as strong PKA activity was detectable even without the addition of cAMP (Fig. 3). This result indicated that PKA activity in the pkaC1Oex mutant was significantly increased after induction of pkaC1 expression. The presence of free catalytic subunits in the cytoplasm thus resulted in high PKA activity, irrespective of the availability of cAMP.
Overexpression of pkaC1 and deletion of pkaR resulted in increased β-Gal activity in a pksPp-lacZ reporter strain.
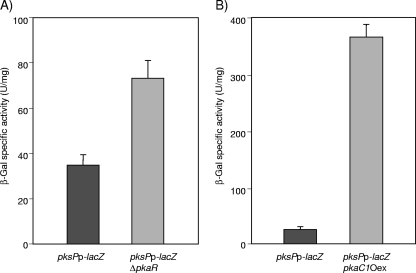
A. fumigatus mutants with enhanced PKA activity, either due to overexpression of pkaC1 or as a result of deletion of pkaR (34), develop a darkly pigmented mycelium. Previously, the pksP gene, encoding a polyketide synthase, was identified to be essential for DHN-melanin biosynthesis in A. fumigatus (15, 30). To investigate a possible influence of PKA activity on expression of the pksP gene, two different mutants, the ΔpkaR and pkaC1Oex strains, were generated using the pksPp-lacZ strain, containing the pksP promoter fused with the lacZ reporter gene. Expression of the pksPp-lacZ gene fusion in A. fumigatus was determined by measurement of the β-Gal activity in protein extracts of the pksPp-lacZ ΔpkaR mutant, the pksPp-lacZ-pkaC1Oex mutant, and the parental strain. Figure 4 shows that specific β-Gal activity was increased 2-fold in the pksPp-lacZ ΔpkaR strain (Fig. 4A) and 10-fold in the pksPp-lacZ-pkaC1Oex strain (Fig. 4B), indicating that in A. fumigatus expression of the pksP gene is positively regulated by enhanced PKA activity.
FIG. 4.
Determination of specific β-Gal activity for quantification of pksPp-lacZ expression. (A) The pksPp-lacZ strain, bearing a pksP promoter-lacZ gene fusion, and the pksPp-lacZ ΔpkaR mutant, with a deletion of the pkaR gene, were cultivated for 28 h in AMM. Protein extracts were analyzed for β-Gal activity. (B) The pksPp-lacZ and pksPp-lacZ-pkaC1Oex strains were precultivated for 16 h in AMM. The mycelia were then shifted to acetate-containing medium and further incubated for 8 h for induction of pkaC1 overexpression. Protein extracts were analyzed for β-Gal activity.
Overexpression of pkaC1 led to reduced conidiation.
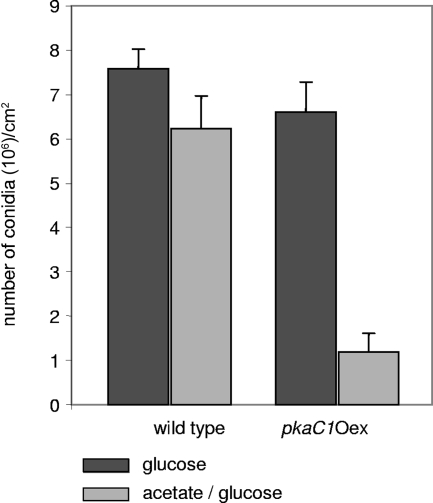
In previous experiments, it was shown that cAMP signal transduction regulates conidiation (19). To investigate the effect of pkaC1 overexpression on production of conidia, wild-type and pkaC1Oex strains were grown on AMM agar plates containing glucose and glucose-acetate as carbon sources. After 3 days, the conidia were harvested and counted (Fig. 5). On glucose (repressing conditions), the number of conidia derived from the pkaC1Oex mutant was similar to that calculated for the wild type. In contrast, under pkaC1 overexpression conditions (acetate-containing medium; inducing conditions), the number of conidia produced by the pkaC1Oex mutant reached only 18% of the number of conidia produced by the wild type on the same medium. This indicates that pkaC1 overexpression severely reduced the production of conidia.
FIG. 5.
Quantification of sporulation of wild-type and pkaC1Oex strains. AMM agar plates containing glucose or acetate-glucose were inoculated with 105 spores. After incubation for 3 days at 37°C, the number of conidia was determined.
Identification of putative PKA target proteins by proteome analysis.
A comparative proteome analysis was performed to identify putative target proteins of PKA. For this purpose, the pkaC1Oex strain was preincubated in glucose-containing medium to allow germination of the conidia. pkaC1 overexpression was then induced by shifting the mycelia to acetate-containing medium. This experiment led to the identification of proteins that were differentially synthesized compared with the wild-type strain (Fig. 6). Overall, 98 protein spots on the 2D gel containing the wild-type proteins showed an increase of the spot volume of larger than twofold (P value of <0.05). In contrast, the 2D gel for the pkaC1Oex strain showed only 44 upregulated protein spots. Hence, 69% of the differentially synthesized proteins were downregulated in the pkaC1Oex strain, and 31% were upregulated compared with the wild type. By mass spectrometry, 53 of the 98 downregulated and 28 of the 44 upregulated protein spots were identified. The results are summarized in detail in Tables 2 and 3. Among the upregulated spots, many proteins were identified that are involved in protein biosynthesis, e.g., ribosomal proteins and translation elongation factors. Some of the downregulated proteins identified are involved in the biosynthesis of nucleotide bases and amino acids. For example, the carbamoyl phosphate synthetase large subunit is a key enzyme in pyrimidine and arginine biosynthesis (12). Ade1p is involved in purine biosynthesis (11).
FIG. 6.
2D gel electrophoresis of protein extracts of the wild type and the pkaC1Oex strain. To identify proteins that were synthesized due to elevated PKA activity, conidia of both strains were preincubated in AMM containing glucose (noninducing conditions). The mycelia were then transferred to AMM containing 100 mM acetate as the sole carbon source and further incubated for 8 hours. Under these conditions, pkaC1 expression was strongly induced in the pkaC1Oex strain, in contrast to the wild-type strain. The figure shows a dual-channel image produced by Delta2D 3.5 software after alignment of all images, using an implemented warping strategy. The blue areas represent the control gel for the wild type, and the orange areas represent the gel for the pkaC1Oex strain (black areas show overlap of both strains). The orientation of isoelectric focusing is indicated. The numbers (spot identities) refer to proteins whose levels changed significantly in the pkaC1Oex strain in comparison to those in the wild-type strain (Tables 2 and 3).
TABLE 2.
Downregulated proteins of A. fumigatus pkaC1Oex strain in comparison to the wild type after induction of pkaC1 expression
| Putative function and spot no.a | Protein name | Locus tag | % Sequence coverage | Regulation (fold) |
|---|---|---|---|---|
| Protein biosynthesis | ||||
| 403 | Translation elongation factor EF-2 subunit, putative | AFUA_2G13530 | 13.5 | 3.7 |
| 441 | Translation elongation factor EF-2 subunit, putative | AFUA_2G13530 | 19.4 | 5.6 |
| 418 | Polyadenylate-binding protein | AFUA_1G04190 | 50.5 | 2.4 |
| 852 | 40S ribosomal protein S0 | AFUA_3G13320 | 50.5 | 8.1 |
| Purine biosynthesis | ||||
| 426 | Ade1p protein | AFUA_6G04730 | 27.5 | 2.3 |
| Pyrimidine/arginine biosynthesis | ||||
| 275 | Carbamoyl-phosphate synthetase, large subunit | AFUA_2G10070 | 18.2 | 3.2 |
| Tricarboxylic acid cycle | ||||
| 1007 | Malate dehydrogenase, NAD dependent | AFUA_6G05210 | 71.5 | 3.0 |
| Glyoxylate cycle | ||||
| 565 | Malate synthase | AFUA_6G03540 | 32.4 | 2.4 |
| 628 | Malate synthase | AFUA_6G03540 | 25.4 | 2.7 |
| 634 | Malate synthase | AFUA_6G03540 | 37.9 | 2.7 |
| 2100 | Isocitrate lyase (AcuD) | AFUA_6G02860 | 17.4 | 2.4 |
| Gluconeogenesis | ||||
| 619 | Phosphoenolpyruvate carboxykinase (AcuF) | AFUA_6G07720 | 28.3 | 2.8 |
| 2203 | Phosphoenolpyruvate carboxykinase (AcuF) | AFUA_6G07720 | 34.6 | 2.5 |
| Pentose phosphate pathway | ||||
| 1039 | Ribose 5-phosphate isomerase A | AFUA_6G10610 | 18.4 | 2.1 |
| Acetate degradation | ||||
| 501 | Acetyl-CoA synthetase (FacA) | AFUA_4G11080 | 16.0 | 3.5 |
| 510 | Acetyl-CoA synthetase (FacA) | AFUA_4G11080 | 18.2 | 11.0 |
| 518 | Acetyl-CoA synthetase (FacA) | AFUA_4G11080 | 26.7 | 2.8 |
| 519 | Acetyl-CoA synthetase (FacA) | AFUA_4G11080 | 29.7 | 4.8 |
| 2266 | Acetyl-CoA synthetase (FacA) | AFUA_4G11080 | 30.4 | 7.6 |
| Alcohol degradation | ||||
| 891 | Alcohol dehydrogenase (ADH1) | AFUA_7G01010 | 37.7 | 2.8 |
| 914 | Alcohol dehydrogenase (ADH1) | AFUA_7G01010 | 33.7 | 2.6 |
| Fatty acid degradation | ||||
| 791 | 3-Ketoacyl-CoA ketothiolase (Kat1), putative | AFUA_1G12650 | 45.5 | 3.0 |
| Propionate degradation | ||||
| 771 | Methylcitrate synthase | AFUA_6G03590 | 40.0 | 2.4 |
| Electron transport and reduction | ||||
| 835 | Flavohemoprotein | AFUA_8G06080 | 22.9 | 2.2 |
| 2179 | Assimilatory sulfite reductase | AFUA_6G08920 | 17.0 | 2.2 |
| Cytoskeleton assembly | ||||
| 701 | Tubulin beta-2 subunit | AFUA_7G00250 | 22.6 | 3.2 |
| 710 | Tubulin beta-2 subunit | AFUA_7G00250 | 23.3 | 2.0 |
| 780 | Septin | AFUA_5G03080 | 21.6 | 2.7 |
| Protection against oxidative stress | ||||
| 464 | Mycelial catalase Cat1 | AFUA_3G02270 | 34.6 | 2.1 |
| 466 | Mycelial catalase Cat1 | AFUA_3G02270 | 37.8 | 5.8 |
| Siderophore biosynthesis | ||||
| 656 | l-Ornithine N-5-oxygenase | AFUA_2G07680 | 45.5 | 2.7 |
| Hemolysis | ||||
| 1457 | Asp hemolysin | AFUA_3G00590 | 50.4 | 2.4 |
| Others and proteins of unknown function | ||||
| 597 | GMC oxidoreductase | AFUA_3G01580 | 49.1 | 2.4 |
| 603 | GMC oxidoreductase | AFUA_3G01580 | 25.9 | 8.0 |
| 610 | GMC oxidoreductase | AFUA_3G01580 | 41.3 | 2.8 |
| 545 | AMP-binding domain protein, putative | AFUA_1G05980 | 38.3 | 3.6 |
| 593 | Pyruvate decarboxylase PdcA, putative | AFUA_3G11070 | 37.1 | 2.1 |
| 611 | Pyridine nucleotide-disulfide oxidoreductase (Nfrl), putative | AFUA_7G02070 | 37.1 | 3.2 |
| 719 | Homocitrate synthase | AFUA_4G10460 | 22.2 | 2.1 |
| 739 | Gamma-butyrobetaine hydroxylase subfamily, putative | AFUA_2G14970 | 43.3 | 2.6 |
| 776 | Acyl-CoA dehydrogenase family protein | AFUA_5G06500 | 24.6 | 2.2 |
| 834 | 2-Amino-3-carboxymuconate-6-semialdehyde decarboxylase, putative | AFUA_5G12460 | 27.9 | 2.0 |
| 903 | Ran/Spi1 binding protein | AFUA_5G12180 | 45.7 | 2.5 |
| 887 | Unknown function | AFUA_6G11850 | 57.4 | 2.4 |
| 1019 | Short chain dehydrogenase, putative | AFUA_4G08710 | 59.6 | 3.6 |
| 1021 | Unknown function | AFUA_8G00550 | 63.1 | 7.9 |
| 1103 | Outer mitochondrial membrane protein porin | AFUA_4G06910 | 33.0 | 2.4 |
| 1141 | DlpA domain protein | AFUA_4G10940 | 29.4 | 2.1 |
| 1165 | Unknown function | AFUA_5G14680 | 63.0 | 2.1 |
| 1195 | Rho-GDP dissociation inhibitor | AFUA_5G11380 | 29.4 | 2.2 |
| 1320 | HEX1 | AFUA_5G08830 | 30.3 | 2.3 |
| 2176 | PH domain protein | AFUA_4G12450 | 20.9 | 8.3 |
Some spots represent different isoforms of the same protein. Proteins were identified by peptide mass fingerprinting. Spot numbers are shown in Fig. 6.
TABLE 3.
Upregulated proteins of A. fumigatus pkaC1Oex strain in comparison to the wild type after induction of pkaC1 expression
| Putative function and spot no.a | Protein name | Locus tag | % Sequence coverage | Regulation (fold) |
|---|---|---|---|---|
| Protein biosynthesis | ||||
| 805 | Translation elongation factor EF-1 alpha subunit, putative | AFUA_1G06390 | 33.0 | 3.0 |
| 428 | Translation elongation factor EF-2 subunit, putative | AFUA_2G13530 | 31.3 | 2.1 |
| 315 | Elongation factor EF-3, putative | AFUA_7G05660 | 53.8 | 2.5 |
| 329 | Elongation factor EF-3, putative | AFUA_7G05660 | 39.1 | 5.6 |
| 337 | Elongation factor EF-3, putative | AFUA_7G05660 | 20.1 | 2.1 |
| 823 | Translation elongation factor EF-Tu, putative | AFUA_1G12170 | 68.0 | 2.1 |
| 968 | 40S ribosomal protein S0 | AFUA_3G13320 | 57.2 | 2.3 |
| 1026 | Cytosolic large ribosomal subunit protein L7A | AFUA_6G12990 | 53.1 | 3.3 |
| 1077 | Cytosolic small ribosomal subunit S4, putative | AFUA_3G06840 | 62.8 | 2.2 |
| 1118 | 60S ribosomal protein P0 | AFUA_1G05080 | 51.1 | 3.3 |
| C1 metabolism | ||||
| 397 | C1-THFS protein | AFUA_3G08650 | 29.0 | 3.4 |
| 879 | Methylenetetrahydrofolate dehydrogenase | AFUA_8G05330 | 34.9 | 2.6 |
| Pyridoxine biosynthesis | ||||
| 1012 | Pyridoxine biosynthesis protein | AFUA_5G08090 | 31.8 | 2.3 |
| Pyruvate metabolism | ||||
| 792 | Pyruvate dehydrogenase complex alpha subunit, putative | AFUA_1G06960 | 58.6 | 2.4 |
| 798 | Phosphatidyl synthase | AFUA_4G11720 | 24.3 | 4.6 |
| 799 | Phosphatidyl synthase | AFUA_4G11720 | 25.9 | 3.1 |
| 813 | Phosphatidyl synthase | AFUA_4G11720 | 24.1 | 2.9 |
| ATP synthesis | ||||
| 702 | Mitochondrial F1 ATPase subunit alpha, putative | AFUA_8G05320 | 50.5 | 2.7 |
| Pentose phosphate pathway | ||||
| 1016 | Ribose 5-phosphate isomerase A | AFUA_6G10610 | 48.9 | 2.4 |
| Purine degradation | ||||
| 807 | Allantoicase | AFUA_3G12560 | 44.9 | 4.9 |
| Protein-folding | ||||
| 862 | Hsp70 chaperone, putative | AFUA_1G07440 | 42.4 | 2.4 |
| Cell cycle and protein degradation | ||||
| 386 | Cell division control protein Cdc48 | AFUA_2G17110 | 47.5 | 4.3 |
| Others | ||||
| 633 | Choline oxidase (CodA), putative | AFUA_8G04090 | 54.6 | 2.1 |
| 1018 | HAD superfamily hydrolase, putative | AFUA_5G08270 | 52.0 | 2.8 |
| 1025 | Carbonyl reductase, putative | AFUA_5G09400 | 55.1 | 2.0 |
Some spots represent different isoforms of the same protein. Proteins were identified by peptide mass fingerprinting. Spot numbers are shown in Fig. 6.
Some important metabolic enzymes were also found to be regulated differentially. Among others, malate dehydrogenase, involved in the citric acid cycle, the phosphoenolpyruvate carboxy kinase of gluconeogenesis, and ribose 5-phosphate isomerase A of the pentose phosphate pathway were downregulated in the pkaC1Oex mutant. Furthermore, enzymes were identified that are involved in degradation or activation of acetate (acetyl-coenzyme A [acetyl-CoA] synthase), ethanol (alcohol dehydrogenase), propionate (methylcitrate synthase), and fatty acids (3-ketoacyl-CoA ketothiolase). Interestingly, two enzymes of the glyoxylate cycle were identified, namely, isocitrate lyase and malate synthase, whose synthesis was downregulated in the pkaC1Oex strain. The glyoxylate cycle allows the utilization of C2 carbon sources, such as ethanol or acetate, and is present only in bacteria, plants, and fungi.
Proteins involved in cytoskeleton assembly and cell cycle regulation were identified, i.e., β-tubulin and a septin family protein were downregulated and Cdc48 was found to be upregulated in the pkaC1Oex mutant. Two isoforms of the catalase Cat1 were significantly downregulated, as well as the l-ornithine-N-5-oxygenase, an essential enzyme of siderophore biosynthesis. For some proteins, no function was assigned in the database. These spots were designated proteins of unknown function.
DISCUSSION
Signal transduction via cAMP is a central signaling pathway in all eukaryotic cells to mediate cellular responses to environmental stimuli. In a large number of studies, elements of this cascade were identified, and their roles in different cellular processes, such as growth, development, and reproduction, and in the infection process were analyzed (for an overview, see references 18, 23, and 33). In this study, we further characterized the role of cAMP in cellular processes of the opportunistic human pathogenic fungus A. fumigatus. A central enzyme of cAMP signaling is PKA. Here we investigated the influence of enhanced PKA activity on A. fumigatus, and additionally, we aimed at the identification of PKA target proteins by a proteomic approach.
The generation of A. fumigatus strains with altered PKA activity was achieved by ectopic integration of the pkaC1 sequence under control of the acuD promoter. In such a strain, overexpression of pkaC1 was inducible, leading to an excess of free catalytic subunits. Consequently, the increase of PKA activity was independent of the intracellular cAMP level. This finding implies that in the pkaC1 overexpression strain, the intracellular level of PKAR subunits is not sufficient to bind and inactivate the excess of additionally generated PKAC1 subunits. A similar increase in PKA activity was observed by Zhao and coworkers (34) by deletion of the gene encoding the regulatory subunit of PKA, pkaR. In contrast to the case for the ΔpkaR mutant, the PKA activity in the pkaC1Oex strain could be increased further by the addition of cAMP. This is due to the cAMP-induced release of PKAC1 subunits bound by endogenous PKAR subunits.
Increased PKA activity in the pkaC1Oex mutant led to phenotypic changes similar to those of other fungal mutants with elevated PKA activity. For example, in Neurospora crassa, Aspergillus niger, and Colletotrichum lagenarium, deletion of pkaR resulted in reduced growth and conidiation (6, 26, 27). This agrees well with the observation that sporulation capacity was significantly reduced in the A. fumigatus pkaC1Oex mutant. Similar results were obtained for other A. fumigatus mutants affected in cAMP signaling, i.e., the ΔgpaB, ΔacyA, and ΔpkaC1 mutant strains (18, 19). In A. nidulans, overexpression of the pkaA gene, encoding one of the two PKAC subunits in this fungus, resulted in reduced sporulation but had no influence on growth (25), whereas deletion of pkaA resulted in increased sporulation and a severe growth defect. Furthermore, pkaR mutants of N. crassa and A. niger displayed apolar growth (6, 26). By deletion of ubc1, the PKAR subunit-encoding gene in Ustilago maydis, cytokinesis and budding were affected (10). In conclusion, in different fungi PKA regulates growth, cell proliferation, and sporulation.
Interestingly, the pkaC1Oex mutant was unable to germinate on AMM-acetate agar plates. This is in contrast to the case for the ΔpkaR mutant, which is able to germinate when acetate is the sole carbon source (T. Heinekamp, unpublished data). The polarization defect of the pkaC1Oex strain was not due to a delayed death of the conidia. This was demonstrated by inoculating conidia on agar plates supplemented with acetate as the sole carbon source. After incubation for 4 days, glucose was added to the medium and the conidia started to germinate, indicating that the conidia were viable. A role of PKA in germination could also be shown for other aspergilli. In a pkaC-overexpressing A. niger mutant, germination was delayed (26). The same was observed for an A. nidulans PKA-deficient mutant. In contrast, conidia of a mutant overexpressing pkaB, encoding a putative second catalytic subunit of PKA of A. nidulans with minor importance, were not able to germinate (21). For a pkaR deletion mutant of A. fumigatus, retarded spore germination and a severe reduction of spore viability were reported (34). This indicates the importance of cAMP signaling for conidial germination. After induction of PKA activity, the mycelium of the pkaC1Oex strain changed its color due to the formation of a dark pigment. The same was observed in the pkaR mutant (34), implying that high PKA activity regulates the biosynthesis of this pigment. The polyketide synthase PksP is a key enzyme in the biosynthesis of the pigment DHN-melanin in A. fumigatus (15, 29). Mutants deficient for PksP produce white conidia instead of the gray-green conidia of the wild type and are strongly attenuated in virulence. Expression of the gene was found to be predominantly in the conidia and phialides but was also detected in hyphae during the infection process (16). Liebmann and coworkers suggested that regulation of pksP depends on cAMP signaling, based on the observation that in both ΔgpaB and ΔpkaC1 deletion mutants of A. fumigatus, pksP expression was drastically reduced (18, 19). These data make it very likely that DHN-melanin is the pigment produced due to high PKA activity in both the pkaC1Oex strain and the ΔpkaR mutant. Because overexpression of pkaC1 in a pksP mutant did not result in pigment formation (Heinekamp, unpublished data), these data indicate that the pigment produced in the pkaC1-overexpressing strain is in fact DHN-melanin. Consistently, as shown here, β-Gal activity was strongly increased in both a ΔpkaR strain and a pkaC1-overexpressing strain with an integrated reporter construct consisting of a fusion of the pksP promoter and the lacZ reporter gene. Previously, Zhao et al. (34) reported for a ΔpkaR mutant that the hyphal cell wall appeared thicker and more pigmented than that of the wild type. Therefore, increased pksP expression in A. fumigatus hyphae could result in DHN-melanin formation and pigmentation in hyphae of pkaC1Oex and ΔpkaR mutant strains.
Comparative proteomics of the wild type and a mutant strain with enhanced PKA activity was used for the identification of PKA target proteins and of the metabolism or biosynthesis pathways that are regulated by cAMP-PKA. In general, there are two straightforward approaches to generate mutants with enhanced PKA activity. On the one hand, deletion of the regulatory subunit results in increased PKA activity (34). On the other hand, as shown here, overexpression of the catalytic subunit also results in a strong increase of PKA activity. We could clearly show that both strategies led to mutants exhibiting similar phenotypes with regard to growth, pigmentation, and conidiation. However, for proteome analyses, it is important to compare strains with similar growth rates. Considering the severe germination and growth defects of the pkaR mutant, we chose the induced pkaC1 overexpression approach. By preincubating the pkaC1Oex mutant and the wild-type strain in glucose-containing medium (repressing conditions), normal germination and growth were achieved. The mycelia were then shifted to acetate-containing medium (inducing conditions). This experimental setup excluded possible side effects from germination defects and differences in growth rates.
The proteomic data imply that PKA regulates the utilization of C sources. This was inferred from the observation that a high PKA level induced the biosynthesis of enzymes necessary for growth on glucose. In contrast, low PKA activity increased the levels of enzymes for catabolism of C sources others than glucose, e.g., ethanol or acetate. Accordingly, enzymes essential for utilization of C2 carbon sources are less synthesized in the pkaC1Oex strain. This is consistent with the reduced growth rate of the pkaC1Oex strain on AMM agar plates with acetate as the carbon source. However, the finding that isocitrate lyase, a central enzyme of the glyoxylate cycle, was synthesized less (2.4-fold) in the pkaC1Oex strain also depicts the inherent complexity of the experimental setup of this study. The transcriptional regulation of isocitrate lyase is mediated by the acuD promoter, the same promoter that was used to induce pkaC1 overexpression. Therefore, interference of pkaC1 induction may be caused by elevated PKA activity.
PKA was found to regulate several important biosynthesis pathways, i.e., enzymes involved in nucleotide and amino acid biosynthesis. Based on uracil auxotrophic mutants bearing a mutation of the pyrG gene, encoding orotidine-5′-phosphate decarboxylase, it was shown that de novo pyrimidine biosynthesis is essential for germination and virulence of A. fumigatus (7). Interestingly, two protein spots, representing septin and β-tubulin, which are involved in cytoskeleton assembly, were downregulated by induced pkaC1 expression. Septins play an important role in morphogenesis and virulence in different fungi, e.g., Candida albicans (31) and U. maydis (3). In U. maydis, a septin essential for establishing normal cellular morphology for infection of plants was found to be regulated by the cAMP pathway (3). The proteome data also indicate that cell division and growth are reduced by high PKA activity. This correlates well with the growth defect observed for the ΔpkaR and pkaC1Oex strains. Therefore, PKA regulates fungal morphogenesis.
Some proteins, i.e., the translation elongation factor EF-2 (spots 403, 441, and 428) and the ribose 5-phosphate isomerase (spots 1039 and 1016), appeared as multiple spots (up- and downregulated) on the gels. This phenomenon might be caused by different posttranslational modifications in the wild-type and mutant strains resulting in altered electrophoretic properties of the respective proteins. For instance, the regulation of EF-2 by phosphorylation is well known from studies of Cryptococcus neoformans (8).
Taken together, the results of the proteome analysis demonstrate the diversity in cellular processes regulated by PKA in A. fumigatus. Signaling via cAMP-PKA plays an important role, especially in regulating C source sensing and metabolism. Further analysis of the PKA-mediated regulation of these cellular processes will help to determine the details of the multifactorial virulence process of A. fumigatus.
Acknowledgments
We thank Franziska Lessing and André Schmidt for help with proteomics. Robert Winkler is acknowledged for MS analyses, and Nancy Hannwacker and Silke Steinbach are acknowledged for excellent technical assistance.
This work was supported by the HKI and the International Leibniz Research School for Microbial and Biomolecular Interactions (ILRS) Jena.
Footnotes
Published ahead of print on 6 June 2008.
REFERENCES
- 1.Adachi, K., and J. E. Hamer. 1998. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 10:1361-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behnsen, J., P. Narang, M. Hasenberg, F. Gunzer, U. Bilitewski, N. Klippel, M. Rohde, M. Brock, A. A. Brakhage, and M. Gunzer. 2007. Environmental dimensionality controls the interaction of phagocytes with the pathogenic fungi Aspergillus fumigatus and Candida albicans. PLoS Pathog. 3:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce, K. J., H. Chang, C. A. D'Souza, and J. W. Kronstad. 2005. An Ustilago maydis septin is required for filamentous growth in culture and for full symptom development on maize. Eukaryot. Cell 4:2044-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brakhage, A. A. 2005. Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants. Curr. Drug Targets 6:875-886. [DOI] [PubMed] [Google Scholar]
- 5.Brakhage, A. A., and J. Van den Brulle. 1995. Use of reporter genes to identify recessive trans-acting mutations specifically involved in the regulation of Aspergillus nidulans penicillin biosynthesis genes. J. Bacteriol. 177:2781-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno, K. S., R. Aramayo, P. F. Minke, R. L. Metzenberg, and M. Plamann. 1996. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 15:5772-5782. [PMC free article] [PubMed] [Google Scholar]
- 7.d'Enfert, C., M. Diaquin, A. Delit, N. Wuscher, J. P. Debeaupuis, M. Huerre, and J. P. Latge. 1996. Attenuated virulence of uridine-uracil auxotrophs of Aspergillus fumigatus. Infect. Immun. 64:4401-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donovan, M. G., and J. W. Bodley. 1991. Saccharomyces cerevisiae elongation factor 2 is phosphorylated by an endogenous kinase. FEBS Lett. 291:303-306. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza, C. A., J. A. Alspaugh, C. Yue, T. Harashima, G. M. Cox, J. R. Perfect, and J. Heitman. 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21:3179-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold, S., G. Duncan, K. Barrett, and J. Kronstad. 1994. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8:2805-2816. [DOI] [PubMed] [Google Scholar]
- 11.Hartman, S. C., and J. M. Buchanan. 1958. Biosynthesis of the purines. XXII. 2-Amino-N-ribosylacetamide-5′-phosphate kinosynthase. J. Biol. Chem. 233:456-461. [PubMed] [Google Scholar]
- 12.Holden, H. M., J. B. Thoden, and F. M. Raushel. 1999. Carbamoyl phosphate synthetase: an amazing biochemical odyssey from substrate to product. Cell. Mol. Life Sci. 56:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacson, T., C. M. Damasceno, R. S. Saravanan, Y. He, C. Catala, M. Saladie, and J. K. Rose. 2006. Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat. Protoc. 1:769-774. [DOI] [PubMed] [Google Scholar]
- 14.Kniemeyer, O., F. Lessing, O. Scheibner, C. Hertweck, and A. A. Brakhage. 2006. Optimisation of a 2-D gel electrophoresis protocol for the human-pathogenic fungus Aspergillus fumigatus. Curr. Genet. 49:178-189. [DOI] [PubMed] [Google Scholar]
- 15.Langfelder, K., B. Jahn, H. Gehringer, A. Schmidt, G. Wanner, and A. A. Brakhage. 1998. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med. Microbiol. Immunol. (Berlin) 187:79-89. [DOI] [PubMed] [Google Scholar]
- 16.Langfelder, K., B. Philippe, B. Jahn, J. P. Latge, and A. A. Brakhage. 2001. Differential expression of the Aspergillus fumigatus pksP gene detected in vitro and in vivo with green fluorescent protein. Infect. Immun. 69:6411-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lessing, F., O. Kniemeyer, I. Wozniok, J. Loeffler, O. Kurzai, A. Haertl, and A. A. Brakhage. 2007. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryot. Cell 6:2290-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebmann, B., S. Gattung, B. Jahn, and A. A. Brakhage. 2003. cAMP signaling in Aspergillus fumigatus is involved in the regulation of the virulence gene pksP and in defense against killing by macrophages. Mol. Genet. Genomics 269:420-435. [DOI] [PubMed] [Google Scholar]
- 19.Liebmann, B., M. Muller, A. Braun, and A. A. Brakhage. 2004. The cyclic AMP-dependent protein kinase A network regulates development and virulence in Aspergillus fumigatus. Infect. Immun. 72:5193-5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuhoff, V., N. Arold, D. Taube, and W. Ehrhardt. 1988. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie brilliant blue G-250 and R-250. Electrophoresis 9:255-262. [DOI] [PubMed] [Google Scholar]
- 21.Ni, M., S. Rierson, J. A. Seo, and J. H. Yu. 2005. The pkaB gene encoding the secondary protein kinase A catalytic subunit has a synthetic lethal interaction with pkaA and plays overlapping and opposite roles in Aspergillus nidulans. Eukaryot. Cell 4:1465-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Santangelo, G. M. 2006. Glucose signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70:253-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu, K., and N. P. Keller. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staudohar, M., M. Bencina, P. J. van de Vondervoort, H. Panneman, M. Legisa, J. Visser, and G. J. Ruijter. 2002. Cyclic AMP-dependent protein kinase is involved in morphogenesis of Aspergillus niger. Microbiology 148:2635-2645. [DOI] [PubMed] [Google Scholar]
- 27.Takano, Y., K. Komeda, K. Kojima, and T. Okuno. 2001. Proper regulation of cyclic AMP-dependent protein kinase is required for growth, conidiation, and appressorium function in the anthracnose fungus Colletotrichum lagenarium. Mol. Plant-Microbe Interact. 14:1149-1157. [DOI] [PubMed] [Google Scholar]
- 28.Tekaia, F., and J. P. Latge. 2005. Aspergillus fumigatus: saprophyte or pathogen? Curr. Opin. Microbiol. 8:385-392. [DOI] [PubMed] [Google Scholar]
- 29.Tsai, H. F., Y. C. Chang, R. G. Washburn, M. H. Wheeler, and K. J. Kwon-Chung. 1998. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai, H. F., M. H. Wheeler, Y. C. Chang, and K. J. Kwon-Chung. 1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warenda, A. J., S. Kauffman, T. P. Sherrill, J. M. Becker, and J. B. Konopka. 2003. Candida albicans septin mutants are defective for invasive growth and virulence. Infect. Immun. 71:4045-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidner, G., C. d'Enfert, A. Koch, P. C. Mol, and A. A. Brakhage. 1998. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5′-monophosphate decarboxylase. Curr. Genet. 33:378-385. [DOI] [PubMed] [Google Scholar]
- 33.Yu, J. H., J. H. Mah, and J. A. Seo. 2006. Growth and developmental control in the model and pathogenic aspergilli. Eukaryot. Cell 5:1577-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao, W., J. C. Panepinto, J. R. Fortwendel, L. Fox, B. G. Oliver, D. S. Askew, and J. C. Rhodes. 2006. Deletion of the regulatory subunit of protein kinase A in Aspergillus fumigatus alters morphology, sensitivity to oxidative damage, and virulence. Infect. Immun. 74:4865-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]