Abstract
In order to gain insight into the effects of human breast milk on the development of the intestinal bifidobacteria and associated health effects, the transcriptome of Bifidobacterium longum LMG 13197 grown in breast milk and formula milk containing galactooligosaccharides (GOS) and long-chain fructooligosaccharides was compared to that obtained in a semisynthetic medium with glucose. Total RNA was isolated from exponentially growing cells and hybridized to a clone library-based microarray. Inserts of clones with significant hybridization signals were sequenced and identified. The B. longum transcriptomes obtained during growth on human and formula milk were more similar to each other than to that obtained from growth in semisynthetic medium with glucose. Remarkably, there were only a few genes implicated in carbohydrate metabolism that were similarly upregulated during growth in both human and formula milk although oligosaccharides were added to the formula. Common highly upregulated genes notably included putative genes for cell surface type 2 glycoprotein-binding fimbriae that are implicated in attachment and colonization in the intestine. Genes involved in carbohydrate metabolism formed the dominant group specifically upregulated in breast milk and included putative genes for N-acetylglucosamine degradation and for metabolism of mucin and human milk oligosaccharides via the galactose/lacto-N-biose gene cluster. This supports the notion that the bifidogenic effect of human milk is to a great extent based on its oligosaccharides. The transcriptional effect of semisynthetic medium containing GOS, which, like human milk, contains a large amount of lactose and galactose, on the B. longum transcriptome was also studied and revealed substantial similarity with carbohydrate-utilization genes upregulated during growth in human milk. This knowledge provides leads to optimizing formula milk to better simulate the observed bifidogenic effects of human breast milk.
The developing gut microbiota is profoundly influenced by the feeding regimen of babies, and typically within a few days of life, human milk stimulates bifidobacteria to become quite dominant (1, 28). In breast-fed infants bifidobacteria have been reported to reach up to 90% of the infant gut microbiota; in contrast, formula-fed infants have a more complex microbiota (14). The postnatal maturation of a balanced immune system requires constant microbial stimulation from the developing gut microbiota, and a high level of bifidobacteria has been associated with reduced intestinal infections (10, 18). Complex neutral oligosaccharides have been identified as the most likely so-called prebiotic factors in human milk that stimulate the growth of bifidobacteria in the infant gut (6, 11, 13). After lactose and lipids, the oligosaccharides are quantitatively the third components of human milk; they are only partially digested in the small intestine, so they reach the colon, where they selectively stimulate the development of microbiota dominated by bifidobacteria (11). Oligosaccharides with structures identical to human milk are not yet available as dietary ingredients because of their complexity (6). The core molecule of human milk oligosaccharides is characterized by repetitive attachment of monosaccharide units (galactose, fucose, N-acetyl-glucosamine, and sialic acid) to the basic molecule of lactose, thus forming compounds with both linear and branched structures (11). Proteolytic fragments of major human milk proteins were also shown to be effective as a growth factor for bifidobacteria (25). Prebiotics are defined as nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon and thus improve host health (15). Like human milk oligosaccharides, the supplementation of infant formula with certain prebiotics stimulates the number of fecal bifidobacteria (2, 8, 11, 16, 36). Feeding infants formula supplemented with galactooligosaccharides and long-chain fructooligosaccharides (GOS-lcFOS, 1:9) significantly increased the number of bifidobacteria and was accompanied by a reduction of pathogens in both preterm infants and term infants (7, 22, 23). Moreover, the GOS-lcFOS supplement caused the microbial diversity to closely resemble the microbiota of breast-fed infants, also at the level of the different Bifidobacterium species (2, 16). GOS are manufactured from lactose by using the enzyme β-galactosidase to attach glycosyl residues, which results in complex mixtures with various glycosidic linkages (12). Note that the core molecule in human milk is characterized by repetitive attachment of galactose and N-acetylglucosamine in β-glycosidic linkage to lactose (8). GOS alone have previously been demonstrated to be metabolized by bifidobacteria in vitro (42) and to increase fecal bifidobacterial numbers in vivo (27).
The molecular mechanisms that underlie the influence of breast-feeding on the development of the intestinal bifidobacterial community are not yet completely understood. The publication of the genome sequence from Bifidobacterium longum NCC2705 revealed that the chromosome encodes numerous genes for carbohydrate utilization collectively termed the glycobiome (38). Sequence data indicated a preference for di-, tri-, and oligosaccharides, pointing toward a bias for complex oligosaccharides (33, 34) complemented with transporters for a variety of disaccharides and oligosaccharides (33). This suggests adaptation to a special colonic niche and utilization of specific dietary components (38). Recently, a bifidobacterium-specific microarray platform comprising clone library-containing inserts of seven bifidobacterial species was constructed (9). In the present study the effects of human milk and formula milk on the global gene expression of B. longum were investigated to gain some insight into the impact of the milks on the development and colonization of intestinal bifidobacteria within the infant gut. Transcriptomics was performed on B. longum LMG 13197 during growth in human and formula milk with GOS-lcFOS and in comparison to semisynthetic medium with glucose. This was complemented with a study of the metabolic effect of semisynthetic medium with GOS on the B. longum transcriptome.
MATERIALS AND METHODS
Bacterial strains and cultivation conditions.
B. longum LMG 13197 was obtained from the Laboratorium voor Microbiologie (LMG) collection (Ghent, Belgium) and has been isolated from human infant feces. B. longum LMG 13197 was maintained in deMan-Rogosa-Sharpe (MRS) medium and glycerol (1:1, vol/vol) at −80°C. An overnight culture (no more than 12 h old) grown in Trypticase-peptone-yeast extract-glucose medium, pH 7 (5), was used as an inoculum (5%, vol/vol) after two washing steps to remove residual medium.
Growth in milk and semisynthetic medium with prebiotics.
Human milk was aseptically collected from two volunteers with a sterile pump and immediately frozen at −20°C. Written consent was received from all subjects who provided human breast milk. Commercial formula milk (Nutrilon 1 with GOS and polyfructose; Nutricia, Zoetermeer, The Netherlands) was dissolved to 10% in demineralized water. Both milks were centrifuged for 5 min at 5,000 rpm (4°C) and filtered to remove fat. Filtered-sterilized cysteine was added (0.5 g/liter), and the pH was adjusted to 6.5. Fifty milliliters of milk was added to sterile flasks with stoppers; flasks were sealed with aluminum rings and placed in a water bath for 20 min at 80°C. After flasks were cooled to room temperature, they were flushed with CO2/N2 using sterile filters. They were incubated a second time in a water bath for 20 min at 80°C and cooled to room temperature overnight. A semisynthetic medium was prepared with a minimal carbon source (6.7 g/liter yeast nitrogen base [Difco]), 0.5 g/liter cysteine, 1 ml of Tween 80, and a 40% [vol/vol] salt solution consisting of 0.2 g/liter CaCl2, 1 g/liter K2HPO4, 1 g/liter KH2PO4, 10 g/liter NaHCO3, 2 g/liter NaCl, 0.5 g/liter casein enzymatic hydrolysate, 0.05 g/liter sodium thioglycolate) such that it could be supplemented with the different carbon sources to be monitored (19). Transcriptomics of B. longum in human or formula milks were compared to the semisynthetic medium containing 5 g/liter glucose as a sole carbon source (hereafter, glucose medium). The number of CFU was determined by plating on MRS agar medium (Difco, France) with cysteine (0.5 g/liter), followed by incubation at 37°C anaerobically. The semisynthetic medium supplemented with GOS (composed of 33% disaccharides, 39% trisaccharides, 18% tetrasaccharides, 7% pentasaccharides, and 3% hexa-, hepta-, and octasaccharides; Friesland Foods, The Netherlands) (hereafter, GOS medium) as a sole carbon source was used to study the transcriptomics of B. longum in prebiotics. For comparative purposes B. longum was grown in semisynthetic medium with glucose or no carbon source. Carbon solutions were sterilized separately and added prior to fermentation at a final concentration of 5 g/liter. All media and carbon solutions were flushed with CO2/N2; flasks were closed with rubber stoppers, sealed with aluminum rings, and sterilized at 120°C for 15 min. Growth was followed by measuring the optical density at 600 nm (OD600). All tests were performed in duplicate at 37°C with agitation (200 rpm) for 10 h, and samples were taken every 2 h for pH measurements and determination of the number of CFU by plating on MRS medium with cysteine (0.5 g/liter), as described above.
RNA isolation and purification.
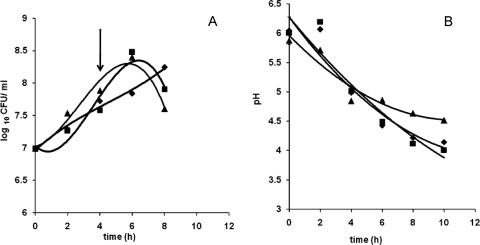
Cells for RNA isolation (50 ml) were harvested at mid-exponential phase (pH 5; OD600 of 0.5) and concentrated immediately by centrifugation at 12,000 rpm for 10 min at 4°C. After centrifugation, the supernatant was discarded, and the cell pellet was snap frozen in liquid nitrogen and stored at −80°C. Samples for RNA isolation were taken from milk and glucose medium cultures after 4 h and from GOS cultures after 6 h (Fig. 1 and 2). RNA isolation was performed using the Macaloid clay method as previously described (35). Briefly, cell pellets were resuspended in 0.5 ml of Tris-EDTA buffer (10 mM; pH 7.5) added to tubes containing 0.8 g of 0.1-mm zirconia beads (Biospec Products, Bartlesville, OK), 0.18 g of Macaloid clay in Tris-EDTA buffer (Kronos Titan GmbH, Leverkusen, Germany), 50 μl of 10% sodium dodecyl sulfate (SDS), and 0.5 ml of acidic phenol (pH 3.75). Cells were disrupted using three treatments of 45 s in a Fastprep (Qbiogene) interspersed by a 90-s incubation on ice. After disruption the samples were centrifuged at 4°C for 10 min at 12,000 rpm. The aqueous phase was used for RNA isolation by extraction with chloroform-phenol-isoamylalcohol (1:1:1) twice and with chloroform-isoamylalcohol (1:24) once. This was followed by a cleaning step using RNeasy columns (Qiagen Sciences, MD), according to the RNeasy cleaning protocol, and treatment with DNase (Roche Diagnostics, Mannheim, Germany) on the column. After elution from the column, RNA concentrations were measured using the Nanodrop ND-1000 (Nanodrop Technologies, Wilmington, DE). Additionally, quality and concentration of the RNA were measured using an Experion Automated Electrophoresis System (Bio-Rad) with RNA StdSens microfluidic chips. Total RNA samples with a 16S/23S rRNA ratio of at least 2.0 were used for microarray experiments.
FIG. 1.
Growth (log10 CFU/ml) (A) and pH curves (B) for B. longum in glucose medium (⧫), human breast milk (▪), and formula milk (▴). An arrow indicates the harvest point for RNA isolation.
FIG. 2.
Growth (OD600) (A) and pH (B) of B. longum on glucose (⧫), GOS (▪), and no carbon source (×). An arrow indicates the harvest point for RNA isolation. Error bars indicate standard deviations.
Reverse transcription of RNA.
Five micrograms of RNA was mixed with 2.5 μl of random p6 primer (2 μg/μl; Roche Diagnostics, Mannheim, Germany) and 2 U of RNasin (Promega, Madison, WI). This was incubated on ice for 10 min following an incubation of 5 min at 70°C and cooled to room temperature. On ice, 5 U of RNasin, 0.1 μM dithiothreitol, aminoallyl-deoxynucleoside triphosphate, and 200 U of Superscript II RT enzyme (Life Technologies S.A., Merelbeke, Belgium) were added and incubated for 3 h at 42°C. RNA was hydrolyzed by adding 6.25 μmol of NaOH and incubated at 37°C for 30 min. To neutralize pH, 6.25 μmol of acetate was added. Unincorporated aminoallyl-dUTP and free amines were removed using QIAquick columns (Qiagen Sciences) according to the supplier's protocol.
Fluorescent labeling and hybridization to bifidobacterial microarrays.
The microarray slides were provided by TNO Nutrition and Food Research, The Netherlands (9). The array contained 2,000 spots of a B. longum LMG 13197 clone library containing an average insert size of approximately 1.5 kb covering 85% of the genome. Slides were incubated in preheated prehybridization solution (1% bovine serum albumin, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% SDS; filtered by 0.45-μm-pore-size filters) at 42°C for 45 min under rotation, washed, and dried under N2 flow. cDNA was dissolved in 4.5 μl of 0.1 M sodium carbonate buffer, pH 9.0, for 10 min at room temperature. An aliquot of 4.5 μl of the appropriate N-hydroxysuccinimide ester Cy dye was added and incubated in the dark at room temperature for 1 h. After incubation, unincorporated dyes were removed using Autoseq G50 columns (AmershamBiosciences, Freiburg, Germany). The Cy5- and Cy3-labeled probes were combined and dried using vacuum centrifugation. The samples were dissolved in 45 μl of Easyhyb buffer at 42°C for 15 min. The preparations were mixed well and spun down briefly. The subsequent steps were performed at 42°C. The prehybridized microarray slides were placed on hybridization chambers (Corning) moistened with MilliQ water. Hybridization mixtures were pipetted onto the slides and covered with glass lids. The chambers were immediately sealed, and the hybridization was then allowed to take place overnight at 42°C. Afterwards, the slides were washed by placing them in 50-ml tubes containing the following wash solutions at 37°C and agitated for 10 s: 1× SSC-0.2% SDS, 0.5× SSC, 0.2× SSC; finally the slides were washed twice in 0.2× SSC with agitation on a rotation plateau for 10 min. The slides were dried under N2 flow and scanned using ScanArray (Perkin Elmer) using two wavelengths corresponding to the excitation wavelengths of the Cy3 and Cy5 dyes. Scanned images of the slides were further processed with ImaGene, version 5.6, software.
Analysis of microarray data.
The microarray experimental design for the various samples is presented in Fig. 3. Samples for each condition were hybridized in duplicate. Human milk samples were compared to the formula milk and to samples of semisynthetic medium with glucose, which were also compared to GOS medium samples. For data analysis, flagged spots were eliminated, followed by background subtraction, log transformation, and Lowess fit normalization, using the Arraynorm program (http://genome.tugraz.at). Positive signals in biological replicates were analyzed for significant differential expression, using significance analysis of microarray with the Tigr Multiarray viewer program (37). Raw microarray data are available in the supplemental material.
FIG. 3.
Hybridization scheme for the different growth media tested. The arrowheads correspond to the Cy5-labeled samples, and the dots at the ends of the arrows correspond to the Cy3-labeled samples.
Nucleotide sequencing and analysis.
A selection of inserts was sequenced by GATC Biotech (Germany). The Smith and Waterman algorithm (40) was applied to search the obtained sequences against the complete protein and nucleotide sequences of all completely sequenced genomes present in the NCBI repository on 18 March 2007. In addition, extra searches were performed using only the complete genomes of B. longum NCC2705 and Bifidobacterium adolescentis ATCC 15703 (Gifu University, Life Science Research Center, Japan). For the nucleotide searches, all matches with the genome sequences were analyzed for annotated features using the protein and RNA annotation files of the genomes.
RESULTS AND DISCUSSION
Growth and gene expression of B. longum in human and formula milks and glucose medium.
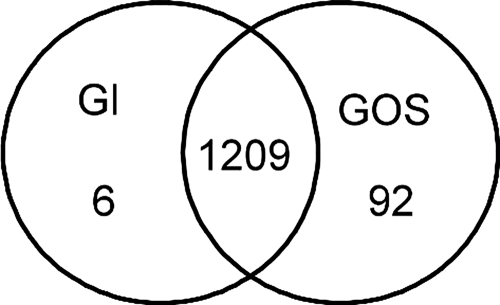
To identify the different effects of human and formula milk on the metabolism of B. longum, the transcriptomes obtained after growth in the milks were compared to the transcriptome obtained during growth in glucose medium. B. longum displayed a lower growth rate in the human milk than in the formula milk and glucose medium (Fig. 1), possibly due to the presence of antimicrobial substances (39). The growth rate appeared highest in the formula milk, and there was a corresponding rapid drop in the pH. The pH profiles for human milk and glucose medium were comparable. Mid-exponential phase for all media was reached after approximately 4 h, and at this time samples were taken for RNA isolation and subsequently hybridized to clone-based B. longum microarrays. In order to identify genes that were specifically induced upon growth under the different conditions, the clones with significant hybridization signals due to the different conditions were grouped, and the numbers of each group are presented as Venn diagrams (Fig. 4). Growth in human or formula milks resulted in the expression of B. longum RNA that shared significant hybridization signals with 409 clones. In the sections below, the genes expressed under the various conditions are described and compared.
FIG. 4.
Venn diagram showing the number of differential and commonly hybridized clones in human breast milk (BM) compared to formula milk (FM) and human milk compared to glucose medium (Gl).
Analysis of the microarrays identified 92 clones that showed significant hybridization signals with cDNA from RNA samples isolated from human milk compared to formula and glucose medium; 35 of these clones were sequenced, and the genes were tentatively identified (Table 1). Of these clones, 21 contained genes predicted to be involved in carbohydrate metabolism and sugar membrane transport, and 6 could code for general cellular processes; the function of 8 other clones was unknown.
TABLE 1.
B. longum genes upregulated in human milk compared to formula milk and glucose medium
| Locus tag(s)a | Function | KEGG function codeb | Fold change (log 2 ratio)c
|
|
|---|---|---|---|---|
| Glucose | Formula | |||
| BL1638 | Solute binding protein of ABC transporter for sugars | MT | 5.54 | 0 |
| DVU1515 | Type II DNA modification methyltransferase, putative (Desulfovibrio vulgaris subsp. vulgaris strain Hildenborough) | FU | 5.07 | 6.46 |
| BL1639 | Permease of ABC transporter for sugars | MT | 4.52 | 4.39 |
| BL1642 | Putative desulfatase, possibly for mucin | FU | 4.47 | 4.56 |
| BL1673-BL1674 | Possible lactaldehyde reductase; probable glycosyltransferase | C, FU | 4.41 | 4.09 |
| BL0978 | β-Galactosidase (lacZ) | C | 4.29 | 2.40 |
| EF2229 | Hypothetical protein (Enterococcus faecalis V583) | FU | 3.85 | − |
| BL1344 | N-Acetylglucosamine-6-phosphate deacetylase | C | 3.26 | 3.62 |
| BL0919-BL0920 | Possible efflux transporter protein | FU | 3.22 | 3.53 |
| BL1343 | Glucosamine-6-phosphate deaminase | C | 3.13 | 4.0 |
| BL1673 | Possible lactaldehyde reductase | C | 2.98 | − |
| BL0454-BL0455 | Narrowly conserved hypothetical protein; widely conserved hypothetical membrane protein | FU | 2.93 | 5.06 |
| BL1674 | Probable glycosyltransferase | C | 2.61 | − |
| BL1211 | Galactose-1-phosphate uridylyltransferase | C | 2.11 | − |
| BL0992 | 30S ribosomal protein S1 | TR | 2.09 | − |
| BL1411 | Cell division protein FtsK | PS | 1.99 | 2.04 |
| BL0951-BL0953 | Formate acetyltransferase; hypothetical protein; glutamine-dependent NAD(+) synthetase | C, FU, NM | 1.90 | 2.27 |
| BL0675 | Possible cell surface protein similar to FimA fimbrial subunit of A. naeslundii | FU | 1.73 | −1.78 |
| BL0676 | Sortase-like protein similar to fimbria-associated protein of A. naeslundii | FU | 1.69 | −1.71 |
| BL1164 | Probable solute binding protein of ABC transporter system for sugars | MT | 1.44 | 0 |
| BL0949-BL0950 | Narrowly conserved hypothetical protein; pyruvate formate-lyase 1 activating enzyme | FU, EM | 1.40 | − |
| BL1402-BL1403 | Response regulator of two-component system; atypical histidine kinase sensor of two-component system | EP | 1.24 | − |
| BL1589 | 30S ribosomal protein S17 | TR | 1.22 | − |
| BL0197-BL0198 | Possible ATP binding protein of ABC transporter; hypothetical membrane protein with unknown function | FU | 1.17 | − |
| BL1164-BL1165 | Probable solute binding protein of ABC transporter system for sugars; probable solute binding protein of ABC transporter system for sugars | MT | 1.11 | 0 |
| BL0716 | Transketolase | C | 0.99 | − |
| BL0715 | Transaldolase | C | 0.81 | − |
| BL0157 | Narrowly conserved hypothetical protein | FU | 0.56 | − |
| BL0671-BL0672 | Ribonucleotide-diphosphate reductase beta subunit; probable glycosyltransferase | N, FU | 0.47 | −1.73 |
| BL0433-BL0434 | Protein PII-uridylyltransferase; nitrogen regulatory protein N-II | EP, NM, EM | 0.36 | −2.34 |
| BL1545-BL1546 | 30S ribosomal protein S15; a polyribonucleotide nucleotidyl-transferase | TR, N | 0.22 | − |
| BL0976 | Galactoside symporter (lacS) | MT | 0.20 | − |
| BL0742 | Probable helicase | RR | 0.16 | − |
| BL0284 | Fem AB-like protein for formation of peptidoglycan | CP | − | 0 |
| BL0529 | Probable α-1,4-glucosidase | C | − | 0 |
| BL0544 | α-l-Arabinosidase | C | − | 0 |
| BL0872 | Possible ABC transporter component | MT | − | 0 |
| BL0966 | Phosphoglycerate mutase | C | − | 0 |
| BL0993-BL0994 | Methylenetetrahydrofolate dehydrogenase (NADP+), solute binding protein (zinc/manganese) of ABC transporter system | CV, MT | − | 0 |
| BL1169-BL1170 | Probable permease of ABC transporter system for sugars | MT | − | 0 |
| BL1731-BL1732 | Hypothetical protein; methionine aminopeptidase | NM | − | 0 |
Genes that were significantly upregulated in human milk compared to both glucose medium and formula milk are in boldface.
KEGG, Kyoto Encyclopedia of Genes and Genomes. Function codes are as follows: C, carbohydrate metabolism; CV, metabolism of cofactors and vitamins; EM, energy metabolism; EP, environmental information processing; FU, function unknown; MT, membrane transport; N, nucleotide metabolism; NM, nitrogen metabolism; PS, cellular processes and signaling; RR, replication and repair; TR, translation.
0, expressed but not upregulated; −, no significant signal detected.
Genes expressed during growth in both human and formula milks versus semisynthetic medium containing glucose.
Hybridization patterns of both human and formula milk were compared to glucose medium to identify specific milk-induced gene expression of B. longum.
(i) Carbohydrate metabolism.
Despite the addition of oligosaccharides to the formula milk, there were only a few genes implicated in carbohydrate metabolism that were similarly upregulated during growth in both formula and human milk. These included two putative glycosyl hydrolases (BL0529 and BL0544) (Tables 1 and 2) and an ATP-binding cassette (ABC)-type transporter (BL1164-BL1165), which were reported to be induced by FOS and lactose in semisynthetic medium (33), and a solute-binding protein of ABC transporter for sugars (BL1638) (Table 1).
TABLE 2.
B. longum genes highly upregulated in formula compared to human milk
| Locus tag(s)a | Function | KEGG function codeb | Fold change (log 2 ratio) |
|---|---|---|---|
| BL0012-BL0013 | Hypothetical protein weakly similar to putative transcriptional regulator from Streptomyces; proline/betaine transporter | FU | 4.30 |
| BL1647 | Hypothetical protein | FU | 4.23 |
| BL1534 | Putative biotin biosynthesis protein BioY | FU | 3.45 |
| BL0841-BL0842 | Hypothetical protein; widely conserved hypothetical transmembrane protein with duf013 | FU | 3.01 |
| BL1537 | Fatty acid synthetase (Fas) | LM | 2.86 |
| BL1775 | Fragment of β-galactosidase | C | 2.83 |
| BL1148 | Deoxyguanosinetriphosphate triphosphohydrolase-like protein | N | 2.63 |
| BL1114-BL1115 | Hypothetical protein in ImpB/MucB/SamB family of UV repair proteins; S-adenosyl-l-homocysteine hydrolase | RR, A | 2.45 |
| BL0841-BL0842 | Hypothetical protein; widely conserved hypothetical transmembrane protein with duf013 | FU | 2.45 |
| BL0433-BL0435 | Protein PII-uridylyltransferase; nitrogen regulatory protein N-II; possible ammonium ion transporter | NM, MT | 2.34 |
| BL0613 | Probable integral membrane transporter | MT | 1.96 |
| BL0675 | Possible cell surface protein similar to FimA fimbrial subunit of A. naeslundii | FU | 1.78 |
| BL0671-BL0672 | Ribonucleotide-diphosphate reductase β subunit; probable glycosyltransferase | N, FU | 1.73 |
| BL0676 | Sortase-like protein similar to fimbria-associated protein of A. naeslundii | FU | 1.71 |
| BL0433-BL0434 | Protein PII-uridylyltransferase; nitrogen regulatory protein N-II | NM | 1.63 |
| BL1647-BL1648 | Hypothetical protein | FU | 1.60 |
| BL0604 | Phosphoenolpyruvate carboxylase | C, EM | 0.69 |
Genes that were also induced in human milk compared to glucose are shown in boldface.
A, amino acid metabolism; LM, lipid metabolism. For other abbreviations, see Table 1, footnote b.
(ii) Nitrogen metabolism.
Genes predicted to encode protein PII-uridylyltransferase (glnD) and a nitrogen regulatory protein N-II (glnB) (BL0433 and BL0434, respectively) were upregulated during growth of B. longum in human milk compared to glucose medium and to an even greater degree in formula milk. These genes were shown to be involved in regulation of glutamine synthetase activity in Escherichia coli that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine (32). The B. longum genome contains all homologs needed for biosynthesis of pyrimidine and purine nucleotides from glutamine (38). Glutamate synthetase is regulated through a complex series of events in E. coli and many bacteria in response to a low ammonia concentration in the environment (31). Interestingly, ammonia may be liberated by glucosamine-6-P isomerase (BL1343), and the predicted gene for this enzyme (see below) was significantly upregulated in human milk. A clone encoding a hypothetical protein and a putative methionine aminopeptidase (BL1731 and BL1732, respectively) could indicate removal of the N-terminal methionine from nascent proteins; this clone was upregulated in both milks. Two of the other putative specific transport systems for peptides in the B. longum DJ010A (26) were not detected, either due to low signals or possibly due to the absence of these genes in the clone library. The nitrogen content in formula milk originates mainly from proteins and oligopeptides. The nitrogen regulatory proteins as well as genes involved in amino acid metabolism indicate assimilation of nitrogen by B. longum via peptide hydrolysis. Another upregulated gene shared by both milks was a putative ABC transporter for zinc and manganese (BL0993 and BL0994).
(iii) Colonization factors.
Two genes for cell surface proteins with high similarity to the type 2 glycoprotein-binding FimA fimbrial subunit and sortase-like fimbria-associated protein of the oral inhabitant Actinomyces naeslundii, in clones BL0675 and BL0676, respectively (Tables 1 and 2), were highly upregulated during growth of B. longum in human milk and even more in formula milk (Table 2). These proteins are involved in the adhesion of A. naeslundii to microbial oral biofilms and interaction with epithelial surfaces (29, 32). The putative FimA fimbrial subunit clone BL0675 contained other motifs: a putative immunoglobulin domain found in other cell-surface proteins and a Cna protein B-type domain found in the Staphylococcus aureus collagen-binding surface protein, which forms a repetitive β-sandwich structure that presents the ligand-binding domain away from the bacterial cell surface. The putative sortase-like fimbria-associated surface protein BL0676 contained the sortase LPXTG motif, which can function as an anchor on the cell wall. There is some tentative evidence that bifidobacteria have fimbria-like structures (38); however, further characterization is required to determine if they contribute to attachment or retention in the human intestine.
Genes upregulated specifically during growth in human milk.
Complex neutral oligosaccharides have been identified as the most likely prebiotic factors in human milk that stimulate the growth of bifidobacteria in the infant gut. This was supported by the high percentage of upregulated genes with predicted functions in the transport and metabolism of oligosaccharides in human compared to formula milk (Table 1). Significantly upregulated genes included those for putative N-acetylglucosamine-6-P deacetylase (BL1344) that catalyzes the deacetylation of N-acetyl-d-glucosamine 6-P and glucosamine-6-phosphate isomerase 1 (BL1343), which converts d-glucosamine-6-P to d-fructose-6-P and ammonia. N-Acetylglucosamine-containing oligosaccharides are regarded as the most bifidogenic oligosaccharides in human milk (24). Consequently B. longum would be able to use the N-acetylglucosamine-6-P in the human milk as a source of carbon and nitrogen. Other significant genes involved in carbohydrate metabolism upregulated specifically in human milk included a putative desulfatase (BL1642) and sugar permease subunit (BL1639) (Table 1). The latter forms part of a novel putative operon for metabolism of mucin sugars and human milk oligosaccharides in B. longum JCM1217. The complete operon encodes an ABC-type sugar transporter, lactose-N-biose phosphorylase, mucin desulfatase, galactose-1-phosphate uridylyltransferase, and UDP-glucose 4-epimerase. It has been speculated that this operon plays a critical role in the bifidogenic effect of human milk as the lacto-N-biose structure appears to be unique to human milk oligosaccharides (20); moreover, intestinal colonization by bifidobacteria would be enhanced by metabolism of mucin sugars. The putative (galacto)oligosaccharide transporter genes (BL1638 and BL1639) were previously found to be induced in vitro by FOS and expressed but not induced by lactose, raffinose, and maltose (33). Another gene involved in galactose metabolism, a galactose-1-phosphate uridylyltransferase gene (BL1211), was also specifically upregulated in breast milk.
As expected, since lactose is the predominant sugar in milk, a putative galactoside transporter gene for lactose transport, lacS (in clone BL0976), was upregulated in human milk although rather surprisingly not in formula milk. In fact, the amount of lactose reaching the newborn colon from breast as well as formula milk is low since it is absorbed in the ileum (11). However, the expression of lacS in B. longum NCC2705 was shown to be induced by several carbohydrates besides lactose including raffinose, maltose, and FOS (33, 34), so perhaps alternative sugar sources such as oligosaccharides specific to human milk promoted the upregulation. Significant upregulation of a putative β-galactosidase gene (lacZ, or BL0978) was also detected during growth in human milk and again not in formula milk; lacZ may be involved in hydrolysis of lactose to glucose and galactose, and/or alternatively in hydrolysis of terminal nonreducing β-d-galactose residues in milk oligosaccharides (8). A tentative lactaldehyde reductase (BL1673) gene that anaerobically reduces l-lactaldehyde (a product of both the l-fucose and l-rhamnose catabolic pathways to l-1,2-propanediol) was highly upregulated (30) only in human milk. It is noteworthy that l-fucose can be derived from human milk oligosaccharides (24) or mucin sugars. Putative genes for transketolase and transaldolase, involved in the pentose phosphate pathway (BL0715, BL0716, and BL0966), were upregulated to a great extent in the human milk compared to glucose medium. The pentose phosphate pathway, besides energy metabolism, is used for reducing agents, nucleotide formation, and reducing equivalents for synthetic purposes (41), suggesting an enhanced requirement for the latter in human milk. The bifidobacterial transaldolase protein was previously identified in vivo in fresh infant feces as well as in batch cultures in MRS medium via a proteomics approach (21). This supports the assumption that the genes expressed in these in vitro studies in milk can also be expected in vivo.
Highly upregulated genes in formula milk versus human milk.
There were 54 genes significantly upregulated in formula milk compared to human milk (and glucose medium), and the sequences of 17 clones have been determined (Table 2). Among the genes tentatively identified were those annotated for carbohydrate utilization, including a fragment of lacZ and a glucosyltransferase (BL1775 and BL0672). Overall, the number of differentially expressed genes involved in carbohydrate metabolism in formula milk was much lower than the number upregulated in human milk. One could speculate that this is due to a lower carbohydrate metabolism by B. longum in formula milk with GOS-lcFOS than in human milk, as the complexity of the carbohydrates in breast milk is much higher and is likely to induce a more complex carbohydrate metabolism than the formula milk. However, an insufficient number of clones were sequenced and identified to confirm this.
A few of the genes were also upregulated during growth of B. longum in human milk but more strongly in formula milk (Table 2). Specifically, the putative genes for regulation of glutamine synthase activity (BL0433 and BL0434) were more highly upregulated in formula milk than in human milk. The same was observed for the potential fimbria genes discussed above. Other formula-specific upregulated genes were predicted to be involved in transport such as a possible oxalate/formate transporter (BL0613), lipid metabolism (BL1537), and several genes of unknown function. A nucleotide hydrolyzing gene (BL1148) was found to be upregulated, as well as a ribonucleotide-2P reductase gene (BL0671), possibly due to the influence of the high level of nucleotides in formula compared to human milk. A putative bioY gene (BL1534) encoding part of a group of BioMNY proteins, which are considered to constitute tripartite biotin ABC-transporters in prokaryotes, was significantly upregulated in formula milk (17). This might be due to the presence of a high concentration of biotin in the formula milk (17 μg/liter) compared to the human milk used in this study; the level in the latter can vary from 5 to 11 μg/liter (4). Enzymes needed for synthesis of biotin appeared to be missing from the B. longum genome sequence (38).
Growth and transcriptomics of B. longum in GOS medium.
The transcriptome of B. longum growing on GOS medium compared with glucose medium was studied at mid-exponential growth (Fig. 2).
B. longum showed a relatively lower specific growth rate on GOS (μ = 0.24 ± 0.008 h−1) than on glucose (μ = 0.27 ± 0.044 h−1) with a concomitant slower production of acids. No growth was observed when the bacteria were grown on the semisynthetic medium without a carbon source (Fig. 2). Comparison of the transcriptome of B. longum grown in GOS medium with glucose showed 91 clones with significantly induced signals (Table 3). Sequencing of 36 clones revealed 25 involved in carbohydrate metabolism and transport, 1 in amino acid biosynthesis, 1 in ribosomal genes, 1 in genetic information, 1 in cellular processes, and 17 with unknown functions. The Venn diagram (Fig. 5) indicates that many more genes are upregulated with growth in GOS than with growth in glucose, probably due to the complex nature of GOS. The degradation of GOS requires a wide range of enzymes and transporters for uptake into the cell, and these are discussed below.
TABLE 3.
B. longum induced genes by growth on GOS compared to glucose medium
| Locus tag(s)a | Function | KEGG function coded | Fold change (log 2 ratio) |
|---|---|---|---|
| BL0978 | β-Galactosidase (lacZ) | C | 3.9 |
| BL1673-BL1674 | Possible lactaldehyde reductase; probable glycosyltransferase | C, FU | 3.83 |
| BL1164b | Probable solute binding protein of ABC transporter system for sugars | MT | 3.75 |
| BL1164-BL1165b | Probable solute binding protein of ABC transporter system for sugars; probable solute binding protein of ABC transporter system for sugars | MT | 3.43 |
| BL1673 | Possible lactaldehyde reductase | C | 3.38 |
| BL1402-BL1403 | Response regulator of two-component system; atypical histidine kinase sensor of two-component system | FU | 3.12 |
| BL0157 | Narrowly conserved hypothetical protein | FU | 3.09 |
| BL0157-BL0158 | Narrowly conserved hypothetical protein; very narrowly conserved hypothetical protein | FU | 2.96 |
| BL0919 | Possible efflux transporter protein | FU | 2.78 |
| BL0976 | Galactoside symporter (lacS) | C | 2.66 |
| BL0949-BL0950 | Narrowly conserved hypothetical protein; pflA pyruvate formate-lyase 1 activating enzyme | FU, PyM | 2.46 |
| BL0260-BL0261 | Sugar transport system permease protein; sugar transport system permease protein | MT | 2.42 |
| BL0950-BL0951 | Pyruvate formate-lyase 1 activating enzyme, formate acetyltransferase | PyM | 2.24 |
| BL0259 | β-Galactosidase I | C | 2.20 |
| BL0951-BL0953 | Formate acetyltransferase (pfl); hypothetical protein; glutamine-dependent NAD(+) synthetase (nadE) | PyM, FU, CV | 2.17 |
| BL1638 | Solute binding protein of ABC transporter for sugars | MT | 2.13 |
| BL1523 | Sugar permease of ABC transporter system | MT | 2.01 |
| DVU1515 | Putative type II DNA modification methyltransferase (Desulfovibrio vulgaris subsp. vulgaris strain Hildenborough) | FU | 1.77 |
| BL0157 | Narrowly conserved HP | FU | 1.58 |
| BL1639 | Permease of ABC transporter for sugars | MT | 1.57 |
| BL1674 | Probable glycosyltransferase | FU | 1.49 |
| BL0978 b | β-Galactosidase (lacZ) | C | 1.45 |
| BL0699 | Hypothetical myosin-like protein with unknown function | FU | 1.39 |
| BL0454-BL0455 | Narrowly conserved hypothetical protein; widely conserved hypothetical membrane protein | FU | 1.30 |
| BL1211 | Galactose-1-phosphate uridylyltransferase | C | 1.24 |
| BL1411 | Cell division protein FtsK | PS | 1.17 |
| BL1571-BL1573 | 50S ribosomal protein L13; rpsI 30S ribosomal protein S9; probable glycogen operon protein GlgX | R, C | 1.05 |
| BL1098 | Elongation factor G | GI | 1.04 |
| BL1646-BL1647 | Possible histidine kinase sensor of two component system; hypothetical protein | FU | 0.92 |
| BL1647-BL1648b | Hypothetical protein | FU | 0.89 |
| BL1396-BL1397 | Probable cation-transporting ATPase; aconitate hydratase | FU, C | 0.69 |
| BL1731-BL1732c | Hypothetical protein; methionine aminopeptidase (map) | PM | 0.57 |
Genes in boldface are also induced in human milk compared to glucose; underlined genes are induced in human milk compared to formula milk.
Induced in formula compared to human milk.
Expressed but not induced under all other conditions.
GI, genetic information processing; PM, protein metabolism, PyM, pyruvate metabolism. For other abbreviations, see Table 1, footnote b.
FIG. 5.
Venn diagram showing the number of differential and commonly hybridized clones of GOS compared to glucose (Gl).
Genes specifically upregulated in GOS medium.
In comparison to glucose medium and the human and formula milks, putative genes unique to GOS included β-galactosidase I (BL0259) and genes coding for an ABC sugar permease transporter (BL0260 and BL0261) within the same gene cluster. These proteins were reported to be similar to the predicted lactose ABC permease of Streptomyces coelicolor and were shown to be expressed by growth on lactose, raffinose, maltose, and FOS by B. longum NCC2705 (33). Another sugar permease transport system (BL1523) was also specifically induced by GOS; the latter was previously shown to be induced by raffinose in vitro (33) and also in this study (data not shown).
Shared upregulated genes in GOS medium with human or formula milks.
There was substantial overlap in the upregulated carbohydrate utilization genes between the GOS medium and human milk. Other putative genes for β-galactosidase (BL0978), galactoside transport (BL0976), galactose-1-phosphate uridylyltransferase (BL1211), glycosyltransferase (BL1674), and possible lactaldehyde reductase (BL1673) were detected and were also upregulated in human milk (Tables 1 and 3), indicating specifically induced carbohydrate metabolism. Genes BL1638 and BL1639, which form part of a predicted transporter in a novel gene cluster for metabolism of mucin sugars and human milk oligosaccharides as described above (Table 3) (20), and other genes for transport of sugars (BL1164-BL1165) were significantly upregulated in GOS medium and human and formula milks compared to glucose medium.
Conclusions.
The gene expression of B. longum, a common intestinal bacterium, was studied to gain insight into the effects of human breast milk versus formula milk on its functionality and associated health effects within the human intestine. Transcriptomes for human milk and GOS medium showed substantial overlap in the upregulation of carbohydrate utilization genes, presumably due to the large amount of GOS and galactose in these substrates. In contrast, there were fewer genes for sugar utilization shared between the human and formula milks among the clones sequenced despite the GOS-lcFOS (1:9) oligosaccharides present in the formula milk. Moreover, genes involved in carbohydrate metabolism formed the most dominant group specifically upregulated in breast milk. The latter included putative genes for N-acetylglucosamine degradation (BL1642) and metabolism of mucin and human milk oligosaccharides via the novel galactose/lacto-N-biose operon with associated sugar transporters (BL1638 and BL1639), of which only the transporters were also induced by GOS and formula milk with GOS-lcFOS. This supports the reported findings that the bifidogenic effect of human milk is to a great extent based on its unique and complex oligosaccharides. It was interesting that the putative genes for cell surface type 2 glycoprotein-binding fimbriae that are implicated in attachment and colonization in the intestine appeared to be upregulated only in the milks. Importantly, the present study generates leads for further investigation of genes involved in the metabolism and colonization of B. longum in the infant human intestine.
Supplementary Material
Acknowledgments
This work was supported by the Dutch Ministry of Economic Affairs through the Innovation Oriental Research Program on Genomics (IOP Genomics: IGE01016).
We are very grateful to volunteers for providing milk. We thank Michiel Wells for assistance with database searching and are grateful to Friesland Foods Corporate Research (Deventer, The Netherlands) for their kind gift of purified GOS.
Footnotes
Published ahead of print on 6 June 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adlerberth, I., D. P. Strachan, P. M. Matricardi, S. Ahrne, L. Orfei, N. Aberg, M. R. Perkin, S. Tripodi, B. Hesselmar, R. Saalman, A. R. Coates, C. L. Bonanno, V. Panetta, and A. E. Wold. 2007. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J. Allergy Clin. Immunol. 120:343-350. [DOI] [PubMed] [Google Scholar]
- 2.Bakker-Zierikzee, A. M., M. S. Alles, J. Knol, F. J. Kok, J. J. M. Tolboom, and J. G. Bindels. 2005. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br. J. Nutr. 94:783-790. [DOI] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Bates, C. J., and A. Prentice. 1994. Breast milk as a source of vitamins, essential minerals and trace elements. Pharmacol. Ther. 62:193-220. [DOI] [PubMed] [Google Scholar]
- 5.Biavati, B., and P. Mattarelli. 1991. Bifidobacterium ruminantium sp. nov. and Bifidobacterium merycicum sp. nov. from the rumens of cattle. Int. J. Syst. Bacteriol. 41:163-168. [DOI] [PubMed] [Google Scholar]
- 6.Boehm, G., S. Fanaro, G. Moro, J. Knol, S. Arslanoglu, F. Mosca, and B. Stahl. 2004. Prebiotic oligosaccharides in infant nutrition: effects on intestinal flora. Agro Food Ind. Hi-Technol. 15:14-16. [Google Scholar]
- 7.Boehm, G., M. Lidestri, P. Casetta, J. Jelinek, F. Negretti, B. Stahl, and A. Marini. 2002. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch. Dis. Child. 86:178-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehm, G., and B. Stahl. 2007. Oligosaccharides from milk. J. Nutr. 137:847S-849S. [DOI] [PubMed] [Google Scholar]
- 9.Boesten, R. J., F. Schuren, and W. M. de Vos. 2005. Genotyping of bifidobacteria using a mixed-species microarray. Abstr. 8th Symp. Lactic Acid Bacteria, Egmond aan Zee, The Netherlands, 28 August to 1 September 2005.
- 10.Cebra, J. J. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69:1046S-1051S. [DOI] [PubMed] [Google Scholar]
- 11.Coppa, G. V., S. Bruni, L. Morelli, S. Soldi, and O. Gabrielli. 2004. The first prebiotics in humans: human milk oligosaccharides. J. Clin. Gastroenterol. 38:S80—S83. [DOI] [PubMed] [Google Scholar]
- 12.Crout, D. H., and G. Vic. 1998. Glycosidases and glycosyl transferases in glycoside and oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2:98-111. [DOI] [PubMed] [Google Scholar]
- 13.Fanaro, S., G. Boehm, J. Garssen, J. Knol, F. Mosca, B. Stahl, and V. Vigi. 2005. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatr. Suppl. 94:22-26. [DOI] [PubMed] [Google Scholar]
- 14.Favier, C. F., W. M. Vos de, and A. D. L. Akkermans. 2003. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe 9:219-229. [DOI] [PubMed] [Google Scholar]
- 15.Gibson, G. R., A. L. McCartney, and R. A. Rastall. 2005. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 93:S31-S34. [DOI] [PubMed] [Google Scholar]
- 16.Haarman, M., and J. Knol. 2005. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 71:2318-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebbeln, P., D. A. Rodionov, A. Alfandega, and T. Eitinger. 2007. Biotin uptake in prokaryotes by solute transporters with an optional ATP-binding cassette-containing module. Proc. Natl. Acad. Sci. USA 104:2909-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooper, L. V. 2004. Bacterial contributions to mammalian gut development. Trends Microbiol. 12:129-134. [DOI] [PubMed] [Google Scholar]
- 19.Kabel, M. A., L. Kortenoeven, H. A. Schols, and A. G. J. Voragen. 2002. In vitro fermentability of differently substituted xylo-oligosaccharides. J. Agric. Food Chem. 50:6205-6210. [DOI] [PubMed] [Google Scholar]
- 20.Kitaoka, M., J. Tian, and M. Nishimoto. 2005. Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl. Environ. Microbiol. 71:3158-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaassens, E. S., W. M. de Vos, and E. E. Vaughan. 2007. Metaproteomics approach to study the functionality of the microbiota in the human infant gastrointestinal tract. Appl. Environ. Microbiol. 73:1388-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knol, J., P. Scholtens, C. Kafka, J. Steenbakkers, S. Gro, K. Helm, M. Klarczyk, H. Schopfer, H. M. Bockler, and J. Wells. 2005. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 40:36-42. [DOI] [PubMed] [Google Scholar]
- 23.Kolida, S., K. Tuohy, and G. R. Gibson. 2002. Prebiotic effects of inulin and oligofructose. Br. J. Nutr. 87:S193-S197. [DOI] [PubMed] [Google Scholar]
- 24.Kunz, C., S. Rudloff, W. Baier, N. Klein, and S. Strobel. 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu. Rev. Nutr. 20:699-722. [DOI] [PubMed] [Google Scholar]
- 25.Liepke, C., K. Adermann, M. Raida, H. Magert, W. Forssmann, and H. Zucht. 2002. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur. J. Biochem. 269:712-718. [DOI] [PubMed] [Google Scholar]
- 26.Lorca, G. L., R. D. Barabote, V. Zlotopolski, C. Tran, B. Winnen, R. N. Hvorup, A. J. Stonestrom, E. Nguyen, L. W. Huang, D. S. Kim, and M. H. Saier, Jr. 2007. Transport capabilities of eleven gram-positive bacteria: comparative genomic analyses. Biochim. Biophys. Acta 1768:1342-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macfarlane, S., G. T. Macfarlane, and J. H. Cummings. 2006. Review article: prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 24:701-714. [DOI] [PubMed] [Google Scholar]
- 28.Magne, F., W. Hachelaf, A. Suau, G. Boudraa, I. Mangin, M. Touhami, K. Bouziane-Nedjadi, and P. Pochart. 2006. A longitudinal study of infant faecal microbiota during weaning. FEMS Microbiol. Ecol. 58:563-571. [DOI] [PubMed] [Google Scholar]
- 29.Mishra, A., A. Das, J. O. Cisar, and H. Ton-That. 2007. Sortase-catalyzed assembly of distinct heteromeric fimbriae in Actinomyces naeslundii. J. Bacteriol. 189:3156-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montella, C., L. Bellsolell, R. Perez-Luque, J. Badia, L. Baldoma, M. Coll, and J. Aguilar. 2005. Crystal structure of an iron-dependent group III dehydrogenase that interconverts l-lactaldehyde and l-1,2-propanediol in Escherichia coli. J. Bacteriol. 187:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson, D. L., and M. M. Cox. 2000. Lehninger principles of biochemistry, 3rd ed. Worth Publishers, New York, NY.
- 32.Palmer, R. J., Jr., S. M. Gordon, J. O. Cisar, and P. E. Kolenbrander. 2003. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J. Bacteriol. 185:3400-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parche, S., J. Amon, I. Jankovic, E. Rezzonico, M. Beleut, H. Barutcu, I. Schendel, M. P. Eddy, A. Burkovski, F. Arigoni, and F. Titgemeyer. 2007. Sugar transport systems of Bifidobacterium longum NCC2705. J. Mol. Microbiol. Biotechnol. 12:9-19. [DOI] [PubMed] [Google Scholar]
- 34.Parche, S., M. Beleut, E. Rezzonico, D. Jacobs, F. Arigoni, F. Titgemeyer, and I. Jankovic. 2006. Lactose-over-glucose preference in Bifidobacterium longum NCC2705: glcP, encoding a glucose transporter, is subject to lactose repression. J. Bacteriol. 188:1260-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pieterse, B., R. H. Jellema, and M. J. van der Werf. 2006. Quenching of microbial samples for increased reliability of microarray data. J. Microbiol. Methods 64:207-216. [DOI] [PubMed] [Google Scholar]
- 36.Roberfroid, M. 2007. Prebiotics: the concept revisited. J. Nutr. 137:830S-837S. [DOI] [PubMed] [Google Scholar]
- 37.Saeed, A. I., V. Sharov, J. White, J. Li, W. Liang, N. Bhagabati, J. Braisted, M. Klapa, T. Currier, M. Thiagarajan, A. Sturn, M. Snuffin, A. Rezantsev, D. Popov, A. Ryltsov, E. Kostukovich, I. Borisovsky, Z. Liu, A. Vinsavich, V. Trush, and J. Quackenbush. 2003. TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34:374-378. [DOI] [PubMed] [Google Scholar]
- 38.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M.-C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Severin, S., and X. Wenshui. 2005. Milk biologically active components as nutraceuticals: review. Crit. Rev. Food Sci. Nutr. 45:645-656. [DOI] [PubMed] [Google Scholar]
- 40.Smith, T. F., and M. S. Waterman. 1981. Identification of common molecular subsequences. J. Mol. Biol. 147:195-197. [DOI] [PubMed] [Google Scholar]
- 41.Stryer, L. 2001. Biochemistry, 5th ed. W. H. Freeman, New York, NY.
- 42.Vernazza, C. L., G. R. Gibson, and R. A. Rastall. 2006. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J. Appl. Microbiol. 100:846-853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.