Abstract
We demonstrate that transcription of the gene swrAA, required for swarming migration in Bacillus subtilis, is driven by two promoters: a sigD-dependent promoter and a putative sigA-dependent promoter, which is inactive during growth in liquid Luria-Bertani medium and becomes active in the presence of the phosphorylated form of the response regulator DegU or on semisolid surfaces. Since sigD transcription is enhanced by SwrAA, this finding reveals that swrA expression is controlled by a positive feedback loop. We also demonstrate that the positive action of SwrAA in swimming and swarming motility is prevented in strains carrying a deletion of the two-component system degS-degU and that this effect is independent of swrAA transcription. Therefore, both DegU and SwrAA must be present to achieve full motility in B. subtilis.
A wild-type copy of the swrAA gene is necessary for swarming motility in both undomesticated and laboratory strains of Bacillus subtilis (3, 12). Laboratory strains (e.g., 168) that carry an sfp° allele and a frameshift mutation in the swrAA gene have a nonswarmer (Swr−) phenotype (3, 12, 13, 14, 31). The role played by swrAA in swarming is to enhance transcription of the operon fla/che, which contains sigD, the gene coding for the alternative sigma factor σD, as well as genes necessary for flagellum biosynthesis and chemotaxis (14). This notwithstanding, SwrAA does not resemble a DNA binding protein and does not show any particular feature by in silico analysis, nor does it display any similarity to characterized entries in protein databases, hampering the elucidation of its mechanism of action.
In order to gain insights into the biological role that it plays in the activation of the swarming behavior, our efforts were concentrated on the expression profile of the swrA dicistronic operon which contains swrAA (3).
The pleiotropic effects on the synthesis of degradative enzymes, competence, sporulation, and motility of mutations in the two-component system DegS-DegU have been extensively described previously (16, 23). In particular, motility is negatively affected by degS(Hy) and degU(Hy) mutations. These mutations increase the half-life of the phosphorylated form of DegU (DegU∼P) (1, 6, 23). In contrast, the necessity of a low level of DegU∼P, for motility in general (36) and for swarming in particular (15, 37), has been pointed out for undomesticated and laboratory strains, but its role has yet to be established. Albeit in previous reports swrAA was never identified as a DegU-regulated gene (18, 26), it has been recently shown that swrAA transcription is negatively affected by deletions of degU or degS (15).
Here we demonstrate that swrAA has two promoters: a σD-dependent promoter, active in planktonic growth, and a putative σA-dependent promoter triggered by DegU∼P, in concordance with published results (15). Furthermore, we show that in the absence of the functional alleles of either DegU or SwrAA B. subtilis is unable to fully swim as well as to swarm (12, 37). Although DegU activates swrAA transcription, SwrAA overexpression per se does not compensate for the loss of DegU, suggesting that DegU cooperates with SwrAA to achieve complete motility in B. subtilis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacillus subtilis strains used in this study are listed in Table 1. All strains were grown at 37°C in Luria-Bertani broth (LB broth; tryptone, 10 g; yeast extract, 5 g; NaCl, 10 g per liter) (30). Media were routinely solidified with 1.5% agar, unless otherwise indicated. The Escherichia coli strains DH5α (supE44 lacU169 [Δ80lacZΔM15] hsdR17[rK− mK+] recA1 endA1 gyrA96 thi-1 relA1), used for molecular cloning, and BL21(DE3) (F− ompT hsdSB[rB− mB−] gal dcm [DE3]), used for protein purification, were grown at 37°C in LB broth. When required, media were supplemented with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (100 μg/ml), ampicillin (100 μg/ml), kanamycin (2 μg/ml), chloramphenicol (5 μg/ml), phleomycin (5 μg/ml), spectinomycin (60 μg/ml), and erythromycin (1 and 50 μg/ml). When appropriate, 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the media.
TABLE 1.
Bacillus subtilis strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| MH5636 | trpC2 pheA1 cat His6-rpoC; Cm | 28 |
| PB5094 | trpC2 swrAA mutant Δ(degS degU); Km | QB4238 (23) |
| PB5249 | trpC2 pheA1 swrAA+ | 31 |
| PB5342 | trpC2 pheA1 swrAA mutant Δ(degS degU); Km | PB5094 → PB5370 |
| PB5343 | trpC2 pheA1 swrAA+ Δ(degS degU); Km | PB5094 → PB5249 |
| PB5370 | trpC2 pheA1 swrAA mutant | This study |
| PB5383 | trpC2 pheA1 swrAA+degU32(Hy); Sp | Amati et al., unpublished |
| PB5392 | trpC2 pheA1 PswrAWT-swrAA+; Km | pCCPswrAWT → PB5249 |
| PB5393 | trpC2 pheA1 PswrAA−-swrAA+; Km | pCCPswrAA− → PB5249 |
| PB5394 | trpC2 pheA1 PswrAD−-swrAA+; Km | pCCPswrAD− → PB5249 |
| PB5395 | trpC2 pheA1 PswrADA−-swrAA+; Km | pCCPswrADA− → PB5249 |
| PB5396 | trpC2 pheA1 PswrAWT-swrAA mutant; Km | pCCPswrAWT → PB5370 |
| PB5397 | trpC2 pheA1 PswrAA−-swrAA mutant; Km | pCCPswrAA− → PB5370 |
| PB5398 | trpC2 pheA1 PswrAD−-swrAA mutant; Km | pCCPswrAD− → PB5370 |
| PB5399 | trpC2 pheA1 PswrADA−-swrAA mutant; Km | pCCPswrADA− → PB5370 |
| PB5400 | trpC2 pheA1 swrAA+pks::PswrAWT-lacZ; Cm | pJM783PswrAWT → PB5249 |
| PB5401 | trpC2 pheA1 swrAA+pks::PswrAA−-lacZ; Cm | pJM783PswrAA− → PB5249 |
| PB5402 | trpC2 pheA1 swrAA+pks::PswrAD−-lacZ; Cm | pJM783PswrAD− → PB5249 |
| PB5403 | trpC2 pheA1 swrAA+pks::PswrADA−-lacZ; Cm | pJM783PswrADA− → PB5249 |
| PB5404 | trpC2 pheA1 swrAA mutant pks::PswrAWT-lacZ; Cm | pJM783PswrAWT → PB5370 |
| PB5405 | trpC2 pheA1 swrAA mutant pks::PswrAA−-lacZ; Cm | pJM783PswrAA− → PB5370 |
| PB5406 | trpC2 pheA1 swrAA mutant pks::PswrAD−-lacZ; Cm | pJM783PswrAD− → PB5370 |
| PB5407 | trpC2 pheA1 swrAA mutant pks::PswrADA−-lacZ; Cm | pJM783PswrADA− → PB5370 |
| PB5408 | trpC2 pheA1 swrAA+degU32(Hy) pks::PswrAWT-lacZ; Cm Sp | PB5383 → PB5400 |
| PB5409 | trpC2 pheA1 swrAA+degU32(Hy) pks::PswrAA−-lacZ; Cm Sp | PB5383 → PB5401 |
| PB5410 | trpC2 pheA1 swrAA+degU32(Hy) pks::PswrAD−-lacZ; Cm Sp | PB5383 → PB5402 |
| PB5411 | trpC2 pheA1 swrAA+degU32(Hy) pks::PswrADA−-lacZ; Cm Sp | PB5383 → PB5403 |
| PB5412 | trpC2 pheA1 swrAA mutant degU32(Hy) pks::PswrAWT-lacZ; Cm Sp | PB5383 → PB5404 |
| PB5413 | trpC2 pheA1 swrAA mutant degU32(Hy) pks::PswrAA−-lacZ; Cm Sp | PB5383 → PB5405 |
| PB5414 | trpC2 pheA1 swrAA mutant degU32(Hy) pks::PswrAD−-lacZ; Cm Sp | PB5383 → PB5406 |
| PB5415 | trpC2 pheA1 swrAA mutant degU32(Hy) pks::PswrADA−-lacZ; Cm Sp | PB5383 → PB5407 |
| PB5426 | trpC2 pheA1 swrAA mutant pks::PswrAWT-lacZ; ΔsigD; Cm Em | pLD11 → PB5404 |
| PB5427 | trpC2 pheA1 swrAA+pks::PswrAWT-lacZ; ΔsigD; Cm Em | pLD11 → PB5400 |
| PB5428 | trpC2 pheA1 swrAA mutant degU32(Hy) pks::PswrAWT-lacZ; ΔsigD; Cm Em Sp | PB5383 → PB5426 |
| PB5429 | trpC2 pheA1 swrAA+degU32(Hy) pks::PswrAWT-lacZ; ΔsigD; Cm Em Sp | PB5383 → PB5425 |
Genetic techniques.
B. subtilis strains were transformed with chromosomal or plasmid DNA by the procedure previously described (17). E. coli transformation was performed according to standard protocols (30).
Strain construction.
All primers used are listed in Table 2. To construct a strain carrying the swrAA mutant gene (PB5370), a PCR fragment from PB1831 (31), amplified using primers yvjB-F and yvjD2661, was cotransformed with plasmid pDG148 (35) in PB5249 (swrAA+) and selected for phleomycin resistance. Colonies were screened according to the swrAA mutant phenotype (loss of swarming motility): the Swr mutant clones obtained were kept on antibiotic-free medium to facilitate plasmid loss, and the swrA locus was sequenced.
TABLE 2.
Primers used in this study
| Primer | Sequence (restriction sites underlined) |
|---|---|
| A-rev | 5′-ACCGCTCGAGTTGTGAACCCCCATTTTCTTTATACAGATAAGCAC-3′ |
| DPSigA F | 5′-GTTGCCTATCTTTGTTTACTTCAAAATATAAGAAG-3′ |
| DPSigA R | 5′-CTTCTTATATTTTGAAGTAAACAAAGATAGGCAAC-3′ |
| DPSigD F | 5′-GGACTGTTATTACCCATCAATATATGAGAGAGAC-3′ |
| DPSigD R | 5′-GTCTCTCTCATATATTGATGGGTAATAACAGTCC-3′ |
| FlgB2 | 5′-GCTTAATATCCGCTCTGCTCAAGGCA-3′ |
| mk120 | 5′-GCGCTGCAATATTGTGGTTAATTCTC-3′ |
| mk170 | 5′-GTTCTTTTGGCTCGCACTGTTGTTTG-3′ |
| mk237 | 5′-AGATCGCAAGACCTGCTGCGTC-3′ |
| Pflg | 5′-TGAAGCTTGGAATTGACGCCCC-3′ |
| PKS-for | 5′-GGGAATTCGCTTACACCCTGCAGGTC-3′ |
| PKS-rev | 5′-CCCGAATTCAACCAGTCTTTG-3′ |
| upFla/Che | 5′-TCTCGGGTTGAAAGTCTTTCTATG-3′ |
| Up-PromA | 5′-CCGAATTCTTTGTGCTTAAAGAGATTATGGATC-3′ |
| yvjD3220 | 5′-ACGGAATTCTTATTACAAAGCGGTACAGACCGC-3′ |
| yvjD2661 | 5′-CGCGAATCCGCAATGAAACCGAGAGGAATC-3′ |
| yvjB.rev.X | 5′-CGCCGCTCGAGCACAAAGAAACAGGAGATG-3′ |
| yvjB-F | 5′-GGGGTACCAATGGGGGACGGCAGCAAC-3′ |
| yvzD1 | 5′-CTGGAATCCTTGAAGAGGGCAAGTATTGTG-3′ |
| yvzD-R | 5′-GCGGATCCGCGCTTTTTAACAGTTCAATTCC-3′ |
| 301 | 5′-CGGGATCCCAAGATCGCCCGCTTCCCGGAT-3′ |
| 302 | 5′-AAAACTGCAGCATTGTTATCCCCCTAATACCT-3′ |
| 303 | 5′-CCATCGATGAATCTGCTGGAAAAAGTGATACA-3′ |
| 304 | 5′-GGGGTACCCGCTTTATTTTCTATGAGTTTCTCAT-3′ |
For the construction of strains with the in-locus swrA promoter mutations, a DNA segment corresponding to the 3′ region of ctpB and part of the intergenic region between ctpB and swrAA was amplified from PB5249 DNA using primers yvjB-F (KpnI site) and yvjB.rev.X (XhoI site). The fragment was inserted between the KpnI/XhoI sites of pJM114 (27), downstream of the kanamycin resistance gene, thus generating pCC0. The region upstream of the swrAA open reading frame and the swrAA gene itself were amplified using primers Up-PromA (EcoRI site) and yvjD3220 (EcoRI site). The amplified product was cloned upstream of the kanamycin resistance gene of pCC0, thus producing pCCPswrAWT. Mutations were introduced in pCCPswrAWT by site-directed mutagenesis PCR, using primers DPSigD F and DPSigD R, deleting 3 nucleotides (nt) from the σD consensus sequence, and/or primers DPSigA F and DPSigA R, deleting 3 nt from the σA consensus sequence. The plasmids obtained are listed in Table 3. swrAA+ strains containing the promoter mutations, PB5392 to PB5395, were produced by transformation of PB5249 with these plasmids linearized with XmnI and by selection for kanamycin resistance. Strains were checked by phenotypic assays and verified by diagnostic restriction digestion with EcoRV (for D− mutations) or BfaI (for A− mutations). To introduce the mutations into PB5370 (swrAA mutant), generating strains PB5396 to PB5399, the same procedure was followed, but plasmids were digested with ScaI, allowing recombination upstream of the nine-adenine stretch in the swrAA gene. The integrative vector pJM783 (27), which generates transcriptional fusions to lacZ, was used for studying swrAA promoter activity. Because of the deficiency of unique restriction sites in pJM783, pCAPs (Roche) vector was used as an intermediate step. A DNA segment corresponding to the swrAA promoter was amplified from PB5249 chromosomal DNA using primers Up-PromA and A-rev and was inserted in the MluNI restriction site in the catabolite gene activator protein gene. This plasmid was first digested with SphI, filled by T4 DNA polymerase, and digested with EcoRI, to extract a fragment containing the PswrA promoter that was cloned into the EcoRI and SmaI site of pJM783, creating pJM783A. A 700-bp fragment from the pksA gene was amplified from PB5249 chromosomal DNA using primers PKS-for (EcoRI site) and PKS-rev (EcoRI site) and inserted into the EcoRI site in pJM783A. One clone carrying the pksA fragment, in the orientation opposite PswrA, was selected, creating the plasmid pJM783PswrAWT. Mutations of PswrA were introduced in pJM783PswrAWT by site-directed mutagenesis PCR using primers DPSigD F and DPSigD R and/or DPSigA F and DPSigA R. These plasmids were used to transform strains PB5249 and PB5370, obtaining strains PB5400 to PB5407. The PfliDST-lacZ reporter fusion inserted in pks was derived by transformation with DNA from a strain carrying PfliD-224 (9).
TABLE 3.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pJM114 | Integrative vector; Km | 27 |
| pCCPswrAWT | pJM114 PswrAWT-swrAA+; Km | This study |
| pCCPswrAA− | pJM114 PswrAA−-swrAA+; Km | This study |
| pCCPswrAD− | pJM114 PswrAD−-swrAA+; Km | This study |
| pCCPswrADA− | pJM114 PswrADA−-swrAA+; Km | This study |
| pJM783 | Integrative vector, promoterless lacZ preceded by rbsspoVG; Cm | 27 |
| pJM783PswrAWT | pJM783 PswrAWT-lacZ; Cm | This study |
| pJM783PswrAA− | pJM783 PswrAA−-lacZ; Cm | This study |
| pJM783PswrAD− | pJM783 PswrAD−-lacZ; Cm | This study |
| pJM783PswrADA− | pJM783 PswrADA−-lacZ; Cm | This study |
| pHCMC05 | Replicative vector; Pspac promoter; Cm | 24 |
| pSwrAA | pHCMC05 Pspac-swrAA; Cm | This study |
| pDG148 | Replicative vector; Pspac promoter; Km Bm | 35 |
| pBG9 | FlgM-overexpressing plasmid | 2 |
| pYFC-11 | SigD-overexpressing plasmid | 5 |
| pJM109B | Integrative vector; Em | 27 |
| pLD11 | sigD::Em in pJM109B | This study |
To insert the degU32(Hy) mutation, strains from PB5400 to PB5407 were transformed with chromosomal DNA of PB5383, in which the degU32(Hy) mutation is associated with a spectinomycin resistance gene, obtaining strains PB5408 to PB5415. Construction of PB5383 will be described in detail elsewhere (G. Amati et al., unpublished data).
To place the swrAA coding sequence under control of the IPTG-inducible Pspac promoter on a replicative vector, chromosomal DNA of PB5249 was amplified with primers yvzD1 (BamHI) and yvzD-R (BamHI). After BamHI digestion, the fragment was ligated to BamHI-restricted pHCMC05 (24), thus producing pSwrAA. This plasmid and the empty pHCMC05 were used to transform PB5249 and PB5370, alone or in combination with chromosomal DNA from PB5342 or PB5343, selecting for chloramphenicol and kanamycin resistance when required. Several different clones were tested in motility assays.
To inactivate sigD, its flanking regions were cloned into the corresponding restriction sites of plasmid pJM109B (27), using primers 301 (BamHI) and 302 (PstI) and primers 303 (ClaI) and 304 (KpnI), respectively, giving pLD11 (constructed by L. De Riso in our laboratory). PB5404 and PB5400 were transformed with pLD11 and selected with erythromycin, giving strains PB5426 and PB5427, respectively. Both strains were subsequently transformed with chromosomal DNA of PB5383, obtaining strains PB5428 and PB5429, respectively.
Plasmids, listed in Table 3, were verified by sequencing.
Motility assays.
Cells, previously grown on LB broth-1.5% agar plates with appropriate antibiotic, were seeded onto the center of an 8.5-cm plate containing freshly prepared LB broth plus 0.2% agar to evaluate swimming motility. Swarming was evaluated on 8.5-cm plates containing freshly prepared LB broth with 0.7% agar. On the surface of swarming plates, 10 μl of 2 mM surfactin was spread, and plates were dried for 10 min in a 40°C incubator before cells were spotted at the center of the plate. Swimming and swarming plates were incubated at 30°C; diameters of halos due to bacterial migration were recorded 13 or 16 h postinoculation, as indicated. For complementation experiments with pSwrAA, swimming and swarming plates contained 5 μg/ml chloramphenicol; when appropriate, 1 mM IPTG was added.
β-Galactosidase activity assay.
To assay β-galactosidase activity, overnight cultures, grown in peptone-yeast extract and 0.5% glucose, were washed with physiological solution and diluted, in fresh LB medium supplemented with 100 μM of FeCl3, to an optical density at 600 nm (OD600) of 0.2. Samples were taken at 30-min intervals for OD600 readings, and β-galactosidase activity was determined. The β-galactosidase activity, based on OD600 readings, was calculated according to the formula (OD420 × 1.5)/(OD600 × sample volume in ml × reaction time in min × 0.00486) and expressed as modified Miller units (MU) (19). Results shown are the means of at least three different experiments, done in duplicate.
Sporulation efficiency was calculated by plating serial dilutions of samples taken from the growth curves directly or after a 10-min incubation at 80°C as published previously (25).
Protein purification.
Bacillus subtilis RNA polymerase core enzyme was purified from MH5636 essentially as described previously (28). The RNA polymerase fraction was dialyzed against DB (50 mM sodium phosphate, pH 8.0, 100 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 50% glycerol), and aliquots were stored at −70°C. The protein concentration, determined by the Bradford assay, was 2.8 mg/ml. Recombinant FlgM was overexpressed from pBG9 and purified as described previously (2), omitting the size-exclusion chromatographic step. In fractions containing FlgM, glycerol was added to a final concentration of 20%. The FlgM concentration was 0.3 mg/ml. Recombinant SigD was overexpressed from pYFC-11 (5) in E. coli BL21(DE3) and purified as described previously (11) with the following modifications: cells were grown to mid-logarithmic phase at 37°C in 1 liter of LB medium with 100 mg ampicillin. IPTG was added to 0.5 mM, and cells were harvested after 3 h of further incubation. The cell pellet was resuspended in 20 ml TNEG (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 5% glycerol, 0.1 mM EDTA, 0.1 mM dithiothreitol) and lysed by sonication. Triton X-100 was added to an 0.5% (vol/vol) final concentration, and the inclusion bodies were recovered by centrifugation. The pellet was washed twice with 20 ml of TNEG containing 0.5% Triton X-100. The pellet was finally resuspended in 10 ml of TNEG containing 0.4% Sarkosyl and incubated for 30 min at 20°C before centrifugation. The supernatant was slowly diluted 10 times with TNEG at 4°C and dialyzed for 16 h against 1 liter TNEG. After centrifugation, the supernatant was applied on a Q-Sepharose column (GE Healthcare) equilibrated with TNEG, and after washing, SigD was eluted with a linear salt gradient from buffer TNEG to TNEG containing 1 M NaCl. Peak fractions were pooled, dialyzed against 1 liter of TNEG containing 50% glycerol, and stored at −80°C in aliquots. The concentration of SigD was 2 mg/ml. Purified recombinant SigA was a generous gift from A. Albertini.
Runoff transcription assay.
Runoff transcription assays were performed with promoter fragments PCR amplified from the different pCCPswrA plasmids or from PB5249 chromosomal DNA (for Pfla/che and Phag). For PswrA Up-PromA and A-rev primers were used; primers upFla/Che and FlgB2 amplified Pfla/che, and primers Pflg and mk120, mk170, or mk237 were used to produce Phag. Reactions (25-μl mixtures) were performed as follows: 3.4 pmol of B. subtilis RNA polymerase was preincubated for 15 min on ice with 34 pmol of σD or 50 pmol of σA and 60 pmol of FlgM when indicated. The protein complexes were then transferred in tubes containing 0.25 pmol template DNA, 1 μl RNasin (Promega), 10 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 150 mM KCl, and 1 mM dithiothreitol and incubated for 8 min at 37°C. ATP, CTP, and GTP (1 mM each, final concentration); UTP (0.12 mM); and 0.2 μl of [α-32P]UTP were added. Incubation was stopped after 8 min by addition of 100 μl of stop solution (2.5 M NH4OAc, 10 mM EDTA, 0.2 mg/ml glycogen). Following phenol-chloroform extraction and ethanol precipitation, samples were analyzed by electrophoresis on 6% acrylamide-8 M urea-polyacrylamide gels. Dried gels were exposed on film.
RESULTS
Identification of the swrAA promoter in vitro.
Inspection of the region upstream of the swrAA open reading frame allowed for the identification of sequences that have high homology to the consensus sequences of σA (SigA)- and σD (SigD)-dependent promoters (Fig. 1).
FIG. 1.
The promoter of the swrA operon. DNA sequence of the 361-nt region upstream of the swrAA start codon (TTG). Sequences corresponding to the putative σD- and σA-dependent promoters are underlined, and the promoter's identity is indicated above (33). The consensus sequence for σD-dependent promoters is TAAA-(N)12-16-GCCGATAT, whereas for σA promoters it is TTGACA-(N)13-22-TATAAT. An arrow above the DNA sequence specifies the estimated σD-directed transcription start site (position −235/−236). Nucleotides deleted to obtain the mutant promoters are in bold.
In order to confirm the validity of our in silico analysis, we set up in vitro transcription assays for the promoter of the swrA operon (PswrA) using purified B. subtilis RNA polymerase. The entire sequence depicted in Fig. 1, starting from the ρ-independent terminator of the preceding ctpB gene (yvjB) up to the swrAA start codon (excluded), was used as a template (hereafter referred to as wt) with either σD or σA RNA polymerase (E) holoenzyme. Parallel reactions were run using the same template in which the putative consensus sequences for either σD or σA had been deleted (D− or A−, respectively), as specified in Fig. 1.
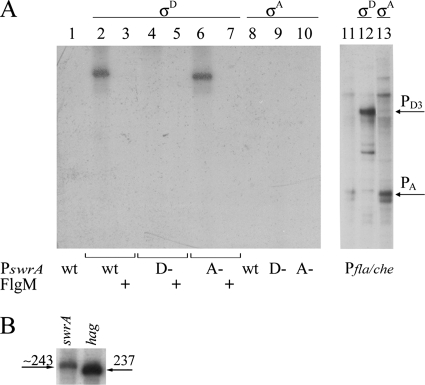
As shown in Fig. 2A, both the wt and A− templates can be efficiently transcribed by the B. subtilis EσD, and transcription is blocked by the addition of the σD-specific anti-sigma factor FlgM (10, 22) (lanes 3 and 7). As expected, when the D− template is used, transcription is completely abolished (lane 4), confirming that the swrA operon indeed possesses a σD-dependent promoter located between 267 and 243 nt upstream of the initiation codon.
FIG. 2.
In vitro swrA transcription is driven by the σD-dependent promoter. (A) The entire sequence shown in Fig. 1 and the promoter region of the fla/che operon were used as templates in runoff experiments with purified B. subtilis core RNA polymerase only (lanes 1 and 11) plus recombinant SigD (lanes 2 to 7 and lane 12) or SigA (lanes 8 to 10 and lane 13). The PswrA template sequence was in the wild-type form (wt) or contained a deletion of the σD promoter (D−) or of the σA promoter (A−). To confirm the identity of the sigma factor, the anti-σD factor FlgM was added where indicated (+). The E(σD) product obtained from the A− mutant swrA promoter (lane 6) migrates faster than the product from the wt promoter (lane 2) because of the deletion of 3 nt in the mutant template. (B) Transcription products obtained with E(σD) from PswrAWT (left) and Phag237 (right). The length of PswrA transcript was estimated from the migration of Phag237, Phag270, and Phag120 (not shown).
No σA-dependent transcription could be detected, while in the same conditions the fla/che promoter (8) could be transcribed by both sigma factors (Fig. 2A, lanes 12 and 13). Thus, although the putative σA-dependent PswrA is closer to the σA consensus than Pfla/che(A) (TAGACT—17 nt-TACAAT) (8), it is not recognized by EσA. From these data we concluded that the putative σA-dependent promoter identified in silico is not functional in vitro.
The site of swrA transcription initiation could not be determined by primer extension analysis despite experiments that were conducted under several conditions (with several enzymes, at different temperatures, and in different media) both in degUwt and in degU32(Hy) genetic backgrounds (see below). This could possibly be attributed to the presence of RNA secondary structures that can be predicted in the 5′ untranslated region of the swrA operon. To overcome this technical problem, the hag promoter was used as a ruler in runoff assays, since the σD-dependent transcription start site for hag, coding for flagellin in B. subtilis, is well characterized (20). Templates spanning different lengths but beginning from the same position in the hag promoter were transcribed and run in parallel with a PswrA transcript, whose initiation was approximately mapped at position −235/−236 (Fig. 2B), indicated by an arrow in Fig. 1.
Assessment of promoter activity by transcriptional fusions.
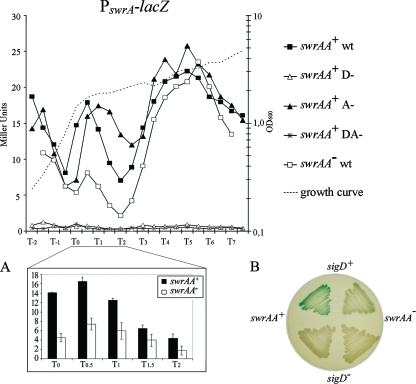
To verify our findings in vivo, we constructed transcriptional fusions to lacZ of the entire PswrA region shown in Fig. 1, in the pJM783 plasmid (27). A fragment of 700 bp containing the pksA gene was also cloned upstream of PswrA, in the opposite transcriptional orientation, to direct plasmid integration into the nonessential pks locus. Transcriptional fusion constructs were also generated using the promoter deletions indicated in Fig. 1 both alone and in combination. Strains integrating these constructs in pks will be identified as PswrAWT-lacZ, PswrAA−-lacZ, and PswrAD−-lacZ, for the wild-type sequence and the single mutations, and as PswrADA−-lacZ for the double mutant. Each construct was inserted in both swrAA+ (PB5249) and swrAA mutant (PB5370) isogenic strains. The transcriptional fusions were assayed in liquid LB medium during a prolonged growth curve (shown by a dotted line in Fig. 3). This medium was chosen because it is used for standard swimming and swarming motility assays (13) and allowed a direct comparison with data obtained from motility experiments (see below). As shown in Fig. 3, in swrAA+ strains β-galactosidase is produced at comparable levels by PswrAWT-lacZ and PswrAA−-lacZ; the mutation of the sigD promoter, PswrAD−-lacZ, completely abolished transcription, as occurs with the double mutant PswrADA−-lacZ. Thus, under these conditions, swrA is an operon exclusively transcribed by σD.
FIG. 3.
PswrA-lacZ is transcribed by the σD-dependent promoter. Transcriptional fusions in degUwt strains were assayed during growth in liquid LB medium, and activities from the wt and mutant swrA promoters are compared. The swrAA+ strains are PB5400 for the wt promoter, PB5402 for D−, PB5401 for A−, and PB5403 for DA−. Strain PB5404 carries PswrAWT-lacZ and is an swrAA mutant. β-Galactosidase units are shown on the left axis; time, in hours before and after the transition phase (T0), is shown on the x axis. The typical growth curve for these strains is represented by a dotted line, and the corresponding OD600 values are on the right axis. An enlargement from T0 to T2 is shown in panel A: β-galactosidase units of PswrAWT-lacZ in swrAA+ and swrAA mutant strains are displayed. Bars refer to the means ± standard errors of the means (σn−1/n1/2). The same strains shown in panel A are displayed in the upper part of the plate in panel B: PB5400 (swrAA+ sigD+) is on the left and PB5404 (swrAA mutant sigD+) is on the right. In the lower part of the plate the strains carry a sigD deletion: PB5427 (swrAA+ ΔsigD) is on the left and PB5426 (swrAA mutant ΔsigD) on the right. Note that transcription from PswrA(A) is not perceptible even on solid plates. The LB plate contains 1.5% agar and 100 μg/ml X-Gal and was incubated for 8 h at 37°C, followed by a 72-h incubation at room temperature.
It has been shown that SwrAA stimulates transcription of the fla/che operon and of sigD contained therein, thus increasing transcription of σD-dependent genes (14). Therefore, according to what we observed, SwrAA should indirectly stimulate its own expression. Indeed, at T0.5 β-galactosidase levels from PswrAWT-lacZ are higher in swrAA+ than in swrAA mutant strains, as can be better appreciated from insets A and B in Fig. 3. The same is true for the σD-dependent fliDST promoter PfliDST (4, 9), which shows higher activity in swrAA+ than in swrAA mutant strains (data not shown).
To reinforce these results, we determined that PswrAWT activity in a ΔsigD background is extremely low (Fig. 3B), giving an average of 1.29 MU (range, 0.70 to 2.04 MU) between T−0.5 and T7 (not shown). These data confirm the results obtained by runoff experiments; altogether they indicate that the identified consensus is in fact recognized by σD, which is the sole promoter driving swrA expression in these conditions, and prove the existence of a transcriptional feedback loop between swrA and fla/che operons.
It is conceivable that swrAA (yvzD) has never been identified as a σD-dependent gene in array experiments (14, 32) because the level of swrA promoter activity, measured as β-galactosidase MU, is low. At T0.5 it reaches a maximum of 16.55 MU (Fig. 3).
Extending our examination up to T7 (7 h after the transition phase) and by using LB medium, we were able to reproducibly detect three main time intervals of σD activity (Fig. 3). We detected a peak of activity centered around T0.5, which has already been described for σD-dependent promoters, and a later activation burst around T5, which could be medium specific and which, to our knowledge, has never been described before. We verified that sporulation did not begin at T7 (data not shown). The activity observed at T−2 is also unclear and could depend on medium composition (for a substantial discussion on this matter, see reference 21); indeed, the same profile can be obtained with the σD-dependent fliDST promoter (4, 9) (not shown).
Transcription of swrA is regulated by degU.
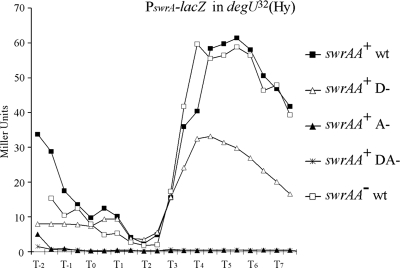
Since σD-directed transcription is shut off in the presence of the degU32(Hy) mutation, due to direct repression of Pfla/che(A) (1, 36), swrA transcription should also be silenced in degU32(Hy) strains. In fact, strains bearing the degU32(Hy) mutation are nonmotile (1, 23, 37). Therefore, to confirm the σD dependence of PswrA, we introduced the degU32(Hy) allele in all strains carrying PswrA-lacZ and assessed β-galactosidase activity in these conditions. Surprisingly, in contrast to what happens with the well-characterized σD-dependent fliDST promoter (data not shown), transcription of PswrA is not shut off. We found a consistent enhancement in β-galactosidase activity clearly visible on LB plates containing X-Gal (data not shown). When the activity was measured during a growth curve in liquid medium, this enhancement was localized around T5, where 61.42 MU was measured (Fig. 4). Using the mutant promoters, we established that PswrA activity is no longer dependent on SigD. In fact, there is no difference in PswrAWT activity measured in the single mutant degU32(Hy) strains (Fig. 4) or in the double mutant degU32(Hy) ΔsigD strains (data not shown), confirming that SigD is not involved. Rather, transcription depends on the putative σA-dependent promoter whose deletion leads to the loss of β-galactosidase expression (indicated as A− in Fig. 4). Thus, the second promoter, silent in liquid medium in a degUwt background and not recognized by EσA in vitro, becomes active in the presence of DegU∼P in conditions in which σD-directed transcription is blocked. It can be excluded that induction of the putative σA-dependent PswrA is due to the lack of interference created by SigD binding, since in degUwt strains such a promoter is not used even in the PswrAD−-lacZ mutant (Fig. 3). These data are supported by microarray and Northern blotting data obtained by Kobayashi, in which the swrA operon is shown to be positively regulated by degU and degS (15).
FIG. 4.
PswrA-lacZ is transcribed by the putative σA-dependent promoter in degU32(Hy) strains. The assay is the same as that shown in Fig. 3, but all strains carry a degU32(Hy) mutant allele. The swrAA+ strains are PB5408 for the wt promoter, PB5410 for D−, PB5409 for A−, and PB5411 for DA−. Strain PB5412 carries PswrAWT-lacZ and is an swrAA mutant.
Unfortunately the binding of DegU or DegU32(Hy) proteins to PswrA could not be detected (not shown). Therefore, it cannot be excluded that degU activates PswrA indirectly.
Currently, there are no clear explanations for the reduced level of transcription obtained with the PswrAD−-lacZ construct, which could be due to a structural effect of the deletion on DNA conformation.
In vivo analysis of the effects of swrA promoter mutations in swimming and swarming.
The swrA operon not only is crucial for swarming motility but also improves swimming as a result of a dramatic increase in the number of flagella (3, 14, 31). We hence determined the effect of mutations in PswrA on motility phenotypes in swrAA+ strains, where the gene is functional.
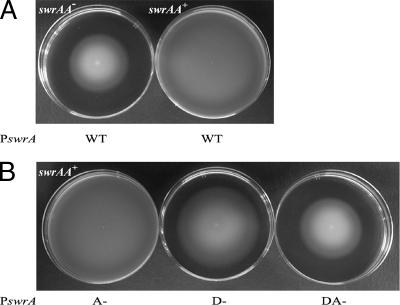
The WT, A−, D−, and DA− promoter mutations were inserted into the swrA locus by double crossover, driving transcription of the swrA operon. These strains will be identified hereafter as PswrAWT, PswrAD−, PswrAA−, and PswrADA−. Swimming plates were freshly prepared with LB medium supplemented with a low concentration of agar (0.2%). Strains were inoculated on the center of the surface with a toothpick and incubated at 30°C for 13 h. As shown in Fig. 5A, swrAA+ strains swim better than swrAA mutant strains and this ability is unaffected in the PswrAA− mutant (Fig. 5B), confirming that swimming depends mainly on a functional σD-dependent promoter. Unexpectedly, in the PswrAD− mutant swimming is only slightly reduced (Fig. 5B), suggesting that a minor role must be played by the remaining σA-dependent promoter, which is, although silent in β-galactosidase assays, partially active on swimming plates in vivo. As expected, the PswrADA− mutant loses swrAA activity and becomes indistinguishable from an swrAA mutant strain.
FIG. 5.
Swimming depends mainly on the σD-dependent promoter. (A) Swimming ability is different in swrAA+ (PB5392) and swrAA mutant strains (PB5396). (B) Swimming ability in swrAA+ strains carrying mutations in the putative σA-dependent swrA promoter (A−, PB5393), in the σD-dependent swrA promoter (D−, PB5394), and in both promoters (DA−, PB5395).
Since our laboratory strains are derived from strain 168 and are sfp°, it is possible to observe swarming migration on LB plates enriched with 0.7% agar upon addition of surfactin (13). Swarming motility is restricted to swrAA+ strains (Fig. 6A). As expected the PswrADA− mutation eliminates PswrA activity and thus eliminates swarming. Surprisingly, however, both the PswrAD− and PswrAA− single mutations have no effect on this behavior (Fig. 6). This finding strongly points to equivalent roles for the two promoters in swarming conditions. Thus, on semisolid surfaces the putative σA-dependent swrAA promoter is fully functional and can replace σD-directed swrAA transcription, indicating that either promoter alone is sufficient for swarming migration.
FIG. 6.
Each swrA promoter is sufficient for swarming. (A) Swarming ability in swrAA+ (PB5392) and swrAA mutant (PB5396) strains. (B) Swarming ability in swrAA+ strains carrying mutations in the putative σA-dependent swrA promoter (A−, PB5393), in the σD-dependent swrA promoter (D−, PB5394), and in both promoters (DA−, PB5395).
Kunst and Rapoport (17) analyzed the transcription of sacB, a degU-controlled gene, and reported that sacB expression is eightfold higher on agar plates than in liquid medium. In liquid medium a comparable level of expression could be obtained by the addition of 1 M NaCl, and the assay demonstrated that this effect was dependent on a functional DegS-DegU two-component system (17). We presume that the surface of the plates is exposed to evaporation, leading to a gradual increase in solute concentration. Salinity changes are thought to be perceived through the DegS-DegU system (17, 29, 34), leading to gradual phosphorylation of DegU, although characteristics other than salinity could vary as well between liquid and solid media.
The low level of activity of PswrA(A) in degUwt backgrounds impairs measurements of its activity by β-galactosidase assays even on solid media (e.g., in Fig. 3B). Nevertheless, the σA promoter becomes functionally more active in media with increasing agar concentrations (0.2% and 0.7%) as shown in Fig. 5 and 6.
DegU∼P and SwrAA work together to activate swarming motility.
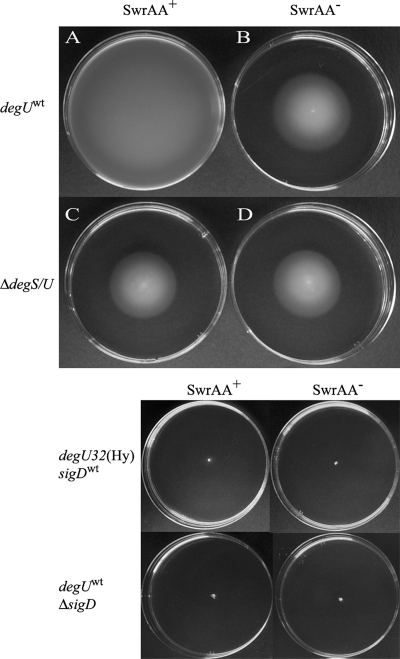
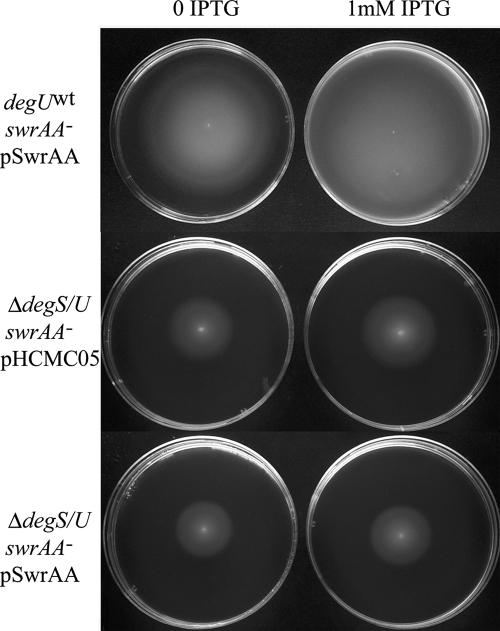
Swarming requires a low level of DegU∼P (15, 37), although the constitutively active degU32(Hy) mutant is completely nonmotile (1, 15, 23, 37). When a degS-degU deletion (ΔdegS/U) was introduced in our swrAA+ and swrAA mutant strains, not only was there an impairment in swarming, as already reported (15, 37), but swimming was affected as well (Fig. 7). The positive effect exerted by swrAA (compare A and B in Fig. 7) was completely lost, and the swimming halos of swrAA+ and swrAA mutant strains became indistinguishable (compare C and D in Fig. 7). This demonstrates that also in swimming the copresence of DegU and SwrAA is required for full motility. As the putative σA-dependent swrAA promoter is stimulated by DegU∼P (Fig. 4), we asked if the requirement of DegU∼P in swarming could be related to the activation of swrA transcription. If this were the case, SwrAA overexpression from an IPTG-inducible promoter would complement the motility defect in strains lacking DegU. Therefore, swrAA was overexpressed from the Pspac promoter of the multicopy plasmid pHCMC05 (24) in ΔdegS/U strains, overcoming the dependence of swrAA transcription on degU. Although this construct is able to complement an swrAA mutant strain, both in swimming (Fig. 8) and in swarming (not shown), SwrAA overexpression is not sufficient to complement the swimming (Fig. 8) and swarming (not shown) defect in ΔdegS/U strains. Therefore, the DegU∼P requirement in swimming (Fig. 7 and 8) and swarming (15, 37) is not to be attributed solely to its contribution to swrA transcription.
FIG. 7.
Both swrAA and degU are required for full motility. Swimming in LB medium for strains swrAA+ degUwt (PB5249) (A), swrAA mutant degUwt (PB5370) (B), swrAA+ ΔdegS/U (PB5343) (C), and swrAA mutant ΔdegS/U (PB5342) (D). There is no difference in the swimming abilities of swrAA mutant and swrAA+ ΔdegS/U strains. Nonmotile strains are shown below as controls: they are PB5408 [degU32(Hy) swrAA+ sigDwt], PB5412 [degU32(Hy) swrAA mutant sigDwt], PB5425 (degUwt swrAA+ ΔsigD), and PB5426 (degUwt swrAA mutant ΔsigD) as indicated.
FIG. 8.
SwrAA overexpression does not complement the swimming defect in a ΔdegS/U mutant. Plasmid pSwrAA was transformed into a degUwt swrAA mutant strain (PB5370) and into a ΔdegS/U swrAA mutant strain (PB5342). As a control, the empty plasmid pHCMC05 was used. Swimming plates contained antibiotics and were supplemented with 1 mM IPTG, where indicated. Identical results were obtained with swrAA+ strains (not shown).
DISCUSSION
We described the characteristics of the swrA operon transcription driven by two promoters: a σD-dependent promoter, used in liquid growth (Fig. 3 and 5), and a putative σA-dependent promoter, triggered in the presence of DegU∼P (Fig. 4) and on surface growth (Fig. 6). Currently, there is no explanation for the lack of use of the putative σA-dependent PswrA, both in vitro and, more importantly, during liquid growth in a degUwt background; despite the close resemblance to a σA-directed promoter, there is no formal proof that it is indeed recognized by EσA. It is an issue that requires further experimental investigation.
Our findings demonstrate that in planktonic conditions SwrAA is transcribed solely by the σD-dependent promoter and closes an autoregulatory loop, where it stimulates fla/che transcription (14), raising SigD levels and consequently flagellar gene expression and motility, besides enhancing its own transcription (Fig. 3A). The existence of a mechanism of positive autoregulation for motility genes, sufficient to set up the phenomenon of bistability (7), had already been predicted (14). However, further experiments are needed to prove that SwrAA is involved in this mechanism.
On swarming plates the levels of DegU phosphorylation are probably moderately raised due to the environmental conditions. This results in a change in swrA transcription: namely, the second PswrA promoter becomes active (Fig. 6). It should be kept in mind that in degUwt strains the σD-dependent swrA promoter remains functional (Fig. 6), as the level of DegU∼P is not as high as in degU32(Hy) mutants, and this allows the maintenance of the positive feedback action.
Furthermore, our data enhance the importance of degU, demonstrating that beyond its requirement for swarming in undomesticated and laboratory strains of B. subtilis (15, 37), it plays a role also in swimming (Fig. 7), at least in swrAA+ domestic strains. SwrAA expression does not simply counteract the negative effect of DegU∼P on Pfla/che, as in this case a ΔdegS/U swrAA mutant strain should fully swim and swarm, while the copresence of DegU∼P and SwrAA is necessary to achieve complete motility. To reinforce this scenario, there is at least another phenotype dependent on the concerted action of degU32(Hy) and SwrAA, which is the activation of the pgs operon, driving the synthesis of γ-polyglutamic acid (Amati et al., unpublished). Unfortunately, preliminary pull-down experiments could not demonstrate any physical interactions between SwrAA and DegU.
The same model holds true if a third player is involved, which can be envisaged as the product of a gene regulated by SwrAA and DegU∼P together. Future studies will be focused on determining if such a factor exists.
Acknowledgments
This work was supported by the Italian Ministero dell'Università e della Ricerca, grant no. 2005058814-001.
We thank Alessandra Albertini for the gift of the recombinant σA; Andrew A. Fulvini for critically reading the manuscript; and Elisabetta Andreoli, Elena Lovato, and Giulia Grimaldi for their invaluable help.
Footnotes
Published ahead of print on 20 June 2008.
REFERENCES
- 1.Amati, G., P. Bisicchia, and A. Galizzi. 2004. DegU-P represses expression of the motility fla-che operon in Bacillus subtilis. J. Bacteriol. 1866003-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertero, M. G., B. Gonzales, C. Tarricone, F. Ceciliani, and A. Galizzi. 1999. Overproduction and characterization of the Bacillus subtilis anti-sigma factor FlgM. J. Biol. Chem. 27412103-12107. [DOI] [PubMed] [Google Scholar]
- 3.Calvio, C., F. Celandroni, E. Ghelardi, G. Amati, S. Salvetti, F. Ceciliani, A. Galizzi, and S. Senesi. 2005. Swarming differentiation and swimming motility in Bacillus subtilis are controlled by swrA, a newly identified dicistronic operon. J. Bacteriol. 1875356-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L., and J. D. Helmann. 1994. The Bacillus subtilis σD-dependent operon encoding the flagellar proteins FliD, FliS, and FliT. J. Bacteriol. 1763093-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y. F., and J. D. Helmann. 1995. The Bacillus subtilis flagellar regulatory protein σD: overproduction, domain analysis and DNA-binding properties. J. Mol. Biol. 249743-753. [DOI] [PubMed] [Google Scholar]
- 6.Dahl, M. K., T. Msadek, F. Kunst, and G. Rapoport. 1992. The phosphorylation state of the DegU response regulator acts as a molecular switch allowing either degradative enzyme synthesis or expression of genetic competence in Bacillus subtilis. J. Biol. Chem. 26714509-14514. [PubMed] [Google Scholar]
- 7.Dubnau, D., and R. Losick. 2006. Bistability in bacteria. Mol. Microbiol. 61564-572. [DOI] [PubMed] [Google Scholar]
- 8.Estacio, W., S. S. Anna-Arriola, M. Adedipe, and L. M. Márquez-Magaña. 1998. Dual promoters are responsible for transcription initiation of the fla/che operon in Bacillus subtilis. J. Bacteriol. 1803548-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredrick, K., T. Caramori, Y.-F. Chen, A. Galizzi, and J. D. Helmann. 1995. Promoter architecture in the flagellar regulon of Bacillus subtilis: high-level expression of flagellin by the σD RNA polymerase requires an upstream promoter element. Proc. Natl. Acad. Sci. USA 922582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredrick, K., and J. D. Helmann. 1996. FlgM is a primary regulator of σD activity, and its absence restores motility to a sinR mutant. J. Bacteriol. 1787010-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, X., K. L. Fredrick, and J. D. Helmann. 1998. Promoter recognition by Bacillus subtilis σW: autoregulation and partial overlap with the σX regulon. J. Bacteriol. 1803765-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearns, D. B., F. Chu, R. Rudner, and R. Losick. 2004. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol. Microbiol. 52357-369. [DOI] [PubMed] [Google Scholar]
- 13.Kearns, D. B., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49581-590. [DOI] [PubMed] [Google Scholar]
- 14.Kearns, D. B., and R. Losick. 2005. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 193083-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi, K. 2007. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol. Microbiol. 66395-409. [DOI] [PubMed] [Google Scholar]
- 16.Kunst, F., M. Pascal, J. Lepesant-Kejzlarova, J. A. Lepesant, A. Billault, and R. Dedonder. 1974. Pleiotropic mutations affecting sporulation conditions and the syntheses of extracellular enzymes in Bacillus subtilis 168. Biochimie 561481-1489. [DOI] [PubMed] [Google Scholar]
- 17.Kunst, F., and G. Rapoport. 1995. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J. Bacteriol. 1772403-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäder, U., H. Antelmann, T. Buder, M. K. Dahl, M. Hecker, and G. Homuth. 2002. Bacillus subtilis functional genomics: genome-wide analysis of the DegS-DegU regulon by transcriptomics and proteomics. Mol. Genet. Genomics 268455-467. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 20.Mirel, D. B., and M. J. Chamberlin. 1989. The Bacillus subtilis flagellin gene (hag) is transcribed by the σ28 form of RNA polymerase. J. Bacteriol. 173095-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirel, D. B., W. F. Estacio, M. Mathieu, E. Olmsted, J. Ramirez, and L. M. Márquez-Magaña. 2000. Environmental regulation of Bacillus subtilis σD-dependent gene expression. J. Bacteriol. 1823055-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirel, D. B., P. Lauer, and M. J. Chamberlin. 1994. Identification of flagellar synthesis regulatory and structural genes in a σD-dependent operon of Bacillus subtilis. J. Bacteriol. 1764492-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Msadek, T., F. Kunst, D. Henner, A. Klier, G. Rapoport, and R. Dedonder. 1990. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J. Bacteriol. 172824-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen, Q. A., R. C. Ferreira, L. C. Ferreira, L. T. Tran, and W. Schumann. 2005. Construction of plasmid-based expression vectors for Bacillus subtilis exhibiting full structural stability. Plasmid 54241-248. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 26.Ogura, M., H. Yamaguchi, K. Yoshida, Y. Fujita, and T. Tanaka. 2001. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res. 293804-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perego, M. 1993. Insertional vectors for genetic manipulation in Bacillus subtilis, p. 615-625. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, DC.
- 28.Qi, Y., and F. M. Hulett. 1998. PhoP-P and RNA polymerase sigmaA holoenzyme are sufficient for transcription of Pho regulon promoters in Bacillus subtilis: PhoP-P activator sites within the coding region stimulate transcription in vitro. Mol. Microbiol. 281187-1197. [DOI] [PubMed] [Google Scholar]
- 29.Ruzal, S. M., and C. Sanchez-Rivas. 1998. In Bacillus subtilis DegU-P is a positive regulator of the osmotic response. Curr. Microbiol. 37368-372. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Senesi, S., E. Ghelardi, F. Pelandroni, S. Solvetti, E. Perisio, and A. Galizzi. 2004. Surface-associated flagellum formation and swarming differentiation in Bacillus subtilis are controlled by the ifm locus. J. Bacteriol. 1861158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serizawa, M., H. Yamamoto, H. Yamaguchi, Y. Fujita, K. Kobayashi, N. Ogasawara, and J. Sekiguchi. 2004. Systematic analysis of SigD-regulated genes in Bacillus subtilis by DNA microarray and Northern blotting analyses. Gene 329125-136. [DOI] [PubMed] [Google Scholar]
- 33.Sierro, N., Y. Makita, M. de Hoon, and K. Nakai. 2008. DBTBS: a database of transcriptional regulation in Bacillus subtilis containing upstream intergenic conservation information. Nucleic Acids Res. 36D93-D96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steil, L., T. Hoffmann, I. Budde, U. Völker, and E. Bremer. 2003. Genome-wide transcriptional profiling analysis of adaptation of Bacillus subtilis to high salinity. J. Bacteriol. 1856358-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stragier, P., C. Bonamy, and C. Karmazyn-Campelli. 1988. Processing of a sporulation factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52697-704. [DOI] [PubMed] [Google Scholar]
- 36.Tokunaga, T., M. H. Rashid, A. Kuroda, and J. Sekiguchi. 1994. Effect of degS-degU mutations on the expression of sigD, encoding an alternative sigma factor, and autolysin operon of Bacillus subtilis. J. Bacteriol. 1765177-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhamme, D. T., T. B. Kiley, and N. R. Stanley-Wall. 2007. DegU co-ordinates multicellular behaviour exhibited by Bacillus subtilis. Mol. Microbiol. 65554-568. [DOI] [PubMed] [Google Scholar]