Abstract
Secondary transporters of citrate in complex with metal ions belong to the bacterial CitMHS family, about which little is known. The transport of metal-citrate complexes in Streptomyces coelicolor has been investigated. The best cofactor for citrate uptake in Streptomyces coelicolor is Fe3+, but uptake was also noted for Ca2+, Pb2+, Ba2+, and Mn2+. Uptake was not observed with the Mg2+, Ni2+, or Co2+ cofactor. The transportation of iron- and calcium-citrate makes these systems unique among the CitMHS family members reported to date. No complementary uptake akin to that observed for the CitH (Ca2+, Ba2+, Sr2+) and CitM (Mg2+, Ni2+, Mn2+, Co2+, Zn2+) systems of Bacillus subtilis was noted. Competitive experiments using EGTA confirmed that metal-citrate complex formation promoted citrate uptake. Uptake of free citrate was not observed. The open reading frame postulated as being responsible for the metal-citrate transport observed in Streptomyces coelicolor was cloned and overexpressed in Escherichia coli strains with the primary Fe3+-citrate transport system (fecABCDE) removed. Functional expression was successful, with uptake of Ca2+-citrate, Fe3+-citrate, and Pb2+-citrate observed. No free-citrate transport was observed in IPTG (isopropyl-β-d-thiogalactopyranoside)-induced or -uninduced E. coli. Metabolism of the Fe3+-citrate and Ca2+-citrate complexes, but not the Pb2+-citrate complex, was observed. Rationalization is based on the difference in metal-complex coordination upon binding of the metal by citrate.
Citrate is a primary metabolite that is ubiquitously used as a source of carbon and energy by most living organisms. While there has been extensive research conducted on citrate transport across membranes (2), there has been a relative dearth of research on membrane protein systems that can transport complexed citrate (21). Currently, there is predicted to be over 90 members in the so-called CitMHS family of secondary transporters, according to the UC San Diego Transport Classification Database (http://www.tcdb.org). Members of this superfamily are found in gram-positive bacteria and are predicted in gram-negative bacteria. It is believed that these organisms take up complexed citrate, because it is predominantly available as such in their environment, or they allow access to critical metal ions, such as iron. To date, the only functionally characterized systems for metal-citrate transport in this family are those of Bacillus subtilis (21), Streptococcus mutans (20), and, most recently, Enterococcus faecalis (5). These members of the CitMHS family transport metal-citrate complexes in symport with one H+ ion per M2+ (metal)-citrate complex. Krom et al. demonstrated that CitM from B. subtilis transported citrate in complex with Mg2+, Ni2+, Mn2+, Co2+, and Zn2+ but not in complex with Ca2+, Ba2+, or Sr2+ (21). CitH, also from B. subtilis, transported citrate and isocitrate complexed with Ca2+, Ba2+, and Sr2+ but not with Mg2+, Ni2+, Mn2+, Co2+, or Zn2+ (28). Clearly, the group of metal ions transported by CitM includes the smaller cations, with a Pauling radius of less than ∼0.80 Å. The ions transported by CitH of B. subtilis have radii larger than 0.98 Å. Neither transporter was shown to transport free citrate or metal complexes of other tricarboxylates (or similar dicarboxylates), such as cis-aconitate and tricarballylate. More recently, Korithoski et al. functionally characterized the CitM homolog from S. mutans (20). Citrate complexed with Fe3+ and Mn2+ was transported with S. mutans, but citrate complexed with Ca2+, Mg2+, and Ni2+ was not. Korithoski et al., in fact, state that iron is the most efficient cofactor for citrate uptake in S. mutans (20). This suggests the intriguing possibility, given that S. mutans is considered the major etiological agent of dental cavities, that the organism may use the system to access essential iron, and so the system may play a role in pathogenesis. Because members of the CitMHS family are postulated to occur in bacteria such as Bacillus anthracis, Mycobacterium tuberculosis, and Corynebacterium diphtheriae, further dimension is added to our desire to understand these systems and their possible contribution to pathogenicity.
The CitH transporter of Enterococcus faecalis was characterized in 2006 (5). High amino acid (AA) sequence homology to the sequence of S. mutans led researchers to believe that it could use a CitMHS transporter to access iron. In fact, this was shown not to be the case. The system was shown to be a functional CitM (B. subtilis) homolog, with metals with larger ionic radii, such as Ca2+, Sr2+, Cd2+, and Pb2+, but not Fe2+ or Fe3+, involved in transport. This unpredictability clearly demonstrates our limited understanding of these systems, which drove us to investigate the postulated transporter in Streptomyces coelicolor. The genome of S. coelicolor was sequenced in 2002 (4). This effort identified an unprecedented number of genes encoding membrane-spanning transporters and gene sets that encode enzymes for utilizing complex nutrients. Among the possible functions of these genes was the putative ability to take up citrate in a complexed, metal-bound form. A comprehensive phylogenetic tree of the CitMHS family reported by Blancato et al. shows this S. coelicolor CitMHS member on a branch well separated from those systems investigated to date (5). The transporters on S. coelicolor's branch actually share only between 35 and 45% AA sequence homology with those transporters investigated to date, compared to the 60 to 83% AA homology between the B. subtilis, E. faecalis, and S. mutans transporters. Given the importance of S. coelicolor in the environment, the weak AA sequence homology (sequence identity does not warrant the same substrate specificity) (1) to all CitMHS transporters studied to date, and the fact that it may serve as a model organism for disease-causing members of the actinomycete family, such as M. tuberculosis, we set out to investigate this CitMHS family member and evaluate its contribution, if any, to the pathogenicity of bacteria by iron acquisition.
To that end, we have functionally characterized metal-citrate transport in S. coelicolor and successfully performed heterologous expression in Escherichia coli of the transporter (designated here S. coelicolor Cit [CitSc]).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Streptomyces coelicolor A3(2) was obtained from the John Innes Centre, Norwich, United Kingdom, and the American Type Culture Collection (ATCC BAA-471). S. coelicolor A3(2) stocks were grown in yeast extract-malt extract broth at 28°C in 125-ml baffled flasks to an optical density at 600 nm (OD600) of ∼2.0. Glycerol (20%) was added to the cells in a 1:1 (vol/vol) ratio, and the cells were chilled on ice for 1 h and placed at −80°C still in the ice container. After 24 h, the cells were taken out of the ice container and left at −80°C temperature. Streptomyces coelicolor was grown in Streptomyces minimal medium (SMM) broth for functional-characterization studies. SMM was prepared as previously reported (18) with the following modifications: l-asparagine was replaced with ammonium sulfate (1 g liter−1), agar was eliminated, and either 56 mM sodium citrate (SMMC) or 56 mM glucose (SMMG) was added as the sole carbon source. Fifty milliliters of SMMC or SMMG broth was inoculated with 1 ml of S. coelicolor A3(2) stock suspensions, grown for 48 to 72 h at 28°C, and centrifuged at 300 rpm with shaking in 125-ml baffled flasks.
Cloning was conducted in E. coli DH5α cells purchased from Invitrogen (Carlsbad, CA). E. coli JW4251-2 ΔfecA758 cells were obtained from the Molecular Cellular and Developmental Biology Department at Yale University (New Haven, CT). E. coli AA93 Δfec cells were obtained from the Institute of Microbiology II at the University of Tuebingen (Tuebingen, Germany). Strains were grown at 37°C and centrifuged at 250 rpm in Luria-Bertani (LB) broth. AA93 Δfec cells harboring the pET-27b(+)-CitSc plasmid were grown in the presence of 35 μg/ml kanamycin. JW4251-2 ΔfecA758 cells harboring the pET-25b(+)-CitSc plasmid were grown in the presence of 50 μg/ml ampicillin.
Media systems.
Buffer and broth ingredients were purchased from Sigma-Aldrich, Becton Dickerson and Company, or Merck and were of biological grade. [14C]sodium citrate was purchased from Sigma-Aldrich (St. Louis, MO). Metal salts were purchased from Sigma-Aldrich and were of 99% purity or higher. DNA was isolated using the Promega Wizard Plus SV miniprep kit. Buffers and broths were made using water that was distilled and deionized to 18.6 MΩ using a Barnstead Diamond ultrapurification system. Chelex was added to all buffers at 15 g/liter, and the resulting suspension was stirred overnight. The Chelex was then removed by vacuum filtration. This was to ensure the removal of metal ion impurities in the buffers. All cells were incubated in a Barnstead MaxQ 5000 shaker with a digital temperature display. Antibiotics came from EMD or Sigma-Aldrich. The pET-27b(+) and pET-25b(+) expression vectors were purchased from Novagen. All primers were ordered from Integrated DNA Technologies (Coralville, IA). Cells were centrifuged with a Sorvall Legend RT tabletop centrifuge at 4,000 rpm for 10 min at 25°C. Cloning was performed with a Techne TC-312 thermal cycler. All restrictive enzymes were purchased from or provided pro gratis by Promega (Madison, WI). Scintillation fluid used was PerkinElmer Ultima Gold. Scintillation vials were purchased from Laboratory Product Sales and VWR. All radiation was detected by a PerkinElmer tri-carbon 2900 TR liquid scintillation analyzer. Culture density was determined by OD600 measurements by using a Cary 50 Bio UV-visible-light spectrophotometer in a 1-ml quartz cuvette.
Cloning of a putative ORF for CitSc.
The S. coelicolor A3(2) cosmid St10A9 containing the open reading frame (ORF) encoding CitSc was obtained from the John Innes Centre, Norwich, United Kingdom. The ORF was cloned by PCR using Deep Vent polymerase (New England Biolabs) and the following primers: 5′-CAGCCATGGCACTGACCATCCTCGGCCTTCG-3′ (forward) and 5′-GATGGATCCTCAGATGATGCCGAAC-3′ (reverse). PCR conditions were as follows: 34 cycles of denaturing at 95°C, primer annealing at 63.0°C, and PCR at 74.0°C.
The forward primer introduced an NcoI restrictive site overlapping the ATG start codon of CitSc. The reverse primer introduced a BamHI site immediately downstream of the stop codon. The PCR product was digested with NcoI and BamHI and ligated (T4 ligase, 16°C, 1 h) into the pET-27b(+) and pET-25b(+) plasmids, which had previously been digested with NcoI, BamHI, and calf intestinal phosphatase. The resulting plasmids, designated pET-27b(+)-CitSc and pET-25b(+)-CitSc, code for CitSc fused to the pelB leader sequence at the N terminus. The correct insertion was confirmed by sequencing performed by Vickie McKee at the SUNY Upstate Medical University's DNA Core Facility, Syracuse, NY. The pET-27b(+)-CitSc plasmid was chemically transformed (30 min at 4°C, 45 seconds at 42°C, 2 min on ice, recovery for 1 h in SOC broth at 37°C) into AA93 Δfec cells for expression and functional characterization. The pET-25b(+)-CitSc plasmid was chemically transformed (30 min at 4°C, 45 seconds at 42°C, 2 min on ice, recovery for 1 h in SOC broth at 37°C) into E. coli JW4251-2 ΔfecA cells for expression and functional characterization.
Metal speciation.
Speciation for all transport assays was calculated using the Visual MINTEQ 2.51 program designed by the Environmental Protection Agency (14). MINTEQ values were calculated at a pH of 6.5 and a temperature of 30°C or 37.0°C, as indicated in Tables 1 and 2, and were calculated to be appropriate to the bacterium.
TABLE 1.
Percentages of metal-citrate speciation at pH 6.5 and at 30°C for S. coelicolor experimentsa
| Metal ion | Concn of:
|
% Metal-citrate speciation | |
|---|---|---|---|
| Metal (mM) | Citrate (μM) | ||
| Ba2+ | 10 | 4.4 | 94.244 |
| Ca2+ | 10 | 4.4 | 98.261 |
| Co2+ | 1 | 4.4 | 99.625 |
| Fe3+ | 0.075 | 4.4 | 94.730 |
| Mg2+ | 1 | 4.4 | 96.191 |
| Ni2+ | 1 | 4.4 | 99.647 |
| Pb2+ | 1 | 4.4 | 98.652 |
Percentages were calculated using the MINTEQ program (13). The excess of metal relative to the citrate level is calculated to optimize metal-citrate complex formation under set experimental conditions.
TABLE 2.
Percentages of metal-citrate speciation at pH 6.5 and at 37°C for E. coli experimentsa
| Metal ion | Concn of:
|
% Metal-citrate speciation | |
|---|---|---|---|
| Metal (mM) | Citrate (μM) | ||
| Ba2+ | 10 | 4.4 | 94.103 |
| Ca2+ | 10 | 4.4 | 98.110 |
| Co2+ | 1 | 4.4 | 99.262 |
| Fe3+ | 0.075 | 4.4 | 94.787 |
| Mg2+ | 1 | 4.4 | 96.380 |
| Ni2+ | 1 | 4.4 | 99.656 |
| Pb2+ | 1 | 4.4 | 98.619 |
Percentages were calculated using the MINTEQ program (13). The excess of metal relative to the citrate level is calculated to optimize metal-citrate complex formation under set experimental conditions.
[1,5-14C]citrate transport assay in whole cells. (i) Transport in Streptomyces coelicolor A3(2).
S. coelicolor A3(2) cells were grown from stocks for 48 to 72 h at 28°C and centrifuged at 300 rpm. Cells were collected via centrifugation and washed twice with 50 mM Chelex-treated piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (pH 6.5). Cells were resuspended to an OD of 0.2 after the second wash. Cells (100 μl) were added to 2.2-ml microcentrifuge tubes. Five microliters of metal as the chloride salt was then added from the following stock solution concentrations: 220 mM CaCl2 or BaCl2; 22 mM MgCl2, NiCl2, CoCl2, PbCl2, and MnCl2; and 1.65 mM FeCl3. Different molar amounts of metals were used to maximize metal-citrate complex formation as per MINTEQ calculations (14) and successfully used in previous literature reports (5, 20, 21). Cells were then incubated at 30°C for 8 min with shaking. After 8 min, 5 μl of 96.8 μM [1,5-14C]citrate was added to each sample to provide a final concentration of 4.4 μM. Uptake was stopped by the addition of 2 ml of 0.1 M LiCl. This was followed by immediate filtration through cellulose nitrate filters (0.45 μm, 2.1-cm diameter). The filters were washed twice with 2 ml of 0.1 M LiCl solution, and radioactivity was recorded using a liquid scintillation counter. In every experimental run, background radioactivity was gauged by adding 2 ml of LiCl prior to the addition of the radiolabeled citrate. As before, cells were then immediately filtered and washed twice with 2 ml of 0.1 M LiCl, and radioactivity was monitored. This served as the background and was subtracted from the final data. Experiments were performed using at least three independent cultures, and samples were collected in triplicate at each time point per run. Cells (100 μl; four samples) from each run were filtered directly through preweighed cellulose nitrate filter paper and dried to determine average cell sample weight. Transport assays were also “stopped” using 10 mM HEPES-glucose buffer instead of 0.1 M LiCl to check for the occurrence of “leaking,” which has been postulated to occur with the use of LiCl (23). No noticeable difference was observed across multiple tested cultures of either S. coelicolor or E. coli (as described in the next section).
It is important to note that preincubating the “free” metal prior to the addition of [14C]citrate or synthesizing the complex and then adding it to the cells made no difference to experimental results, consistent with the work reported by Krom et al. (21), except in the case of Fe3+. Transport assays with Fe3+ were performed at 25.0°C, with the metal precomplexed with [14C]citrate to prevent Fe3+ precipitation in the aqueous buffer. The temperature and pH changes were accounted for in the MINTEQ calculations. All experiments using precomplexed mixtures were filtered using a 0.45-μm syringe filter (Fisher Scientific) prior to the addition to cells.
(ii) Transport in E. coli.
E. coli AA93 and JW4251-2 cultures were grown in LB broth as described above. Protein expression for transport assays was induced at 30.0°C or 4°C (specifically for Fe3+-citrate only [see Fig. 5]) with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at an OD600 of 1.0 for 1 h. Subsequent flux assays followed the protocol described above for S. coelicolor and the protocol previously noted in the literature (21) with the following adaptations: cells were resuspended with 10 ml of PIPES buffer at a pH of 6.5 for all metals, and the transport assay was conducted at 37.0°C except with iron (25°C). Noninduced controls were included in all runs. Concentrations of metal ion and citrate were chosen to maximize the formation of the desired metal-citrate complex (Table 2). Experiments were performed using at least three independent cultures, and samples at each time point were collected in triplicate per run. Incubation of the metal ion (except for Fe3+ as described above), and precomplexation of the metal with [14C]citrate prior to cell addition yielded similar results.
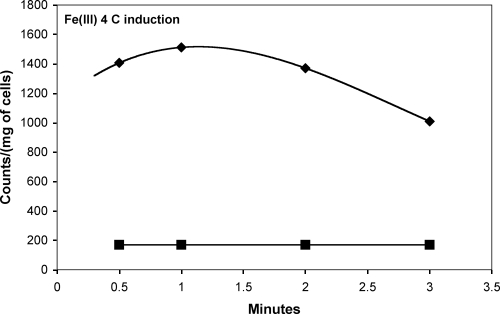
FIG. 5.
Uptake and metabolism of Fe3+-citrate with CitSc induced with IPTG at 4°C in E. coli.
RESULTS
While CitSc was designated a putative metal-citrate transporter, the evidence for homology (based on AA sequences) was weak compared to that for any CitMHS member functionally characterized to date (35% identity with the Cit of E. faecalis, 39% with the Cit of S. mutans, 42% with CitH of B. subtilis, and 45% with CitM of B. subtilis).
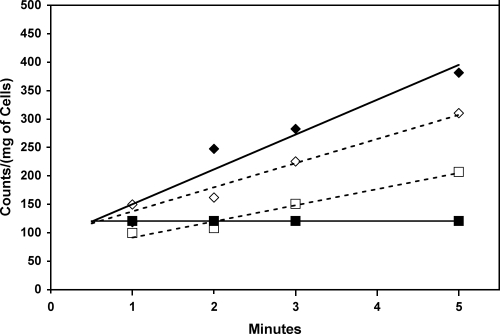
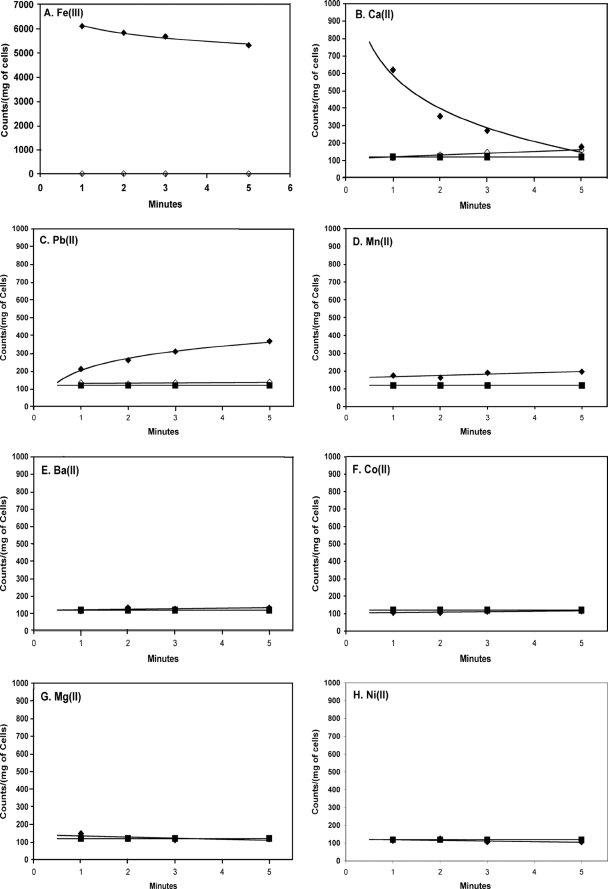
In S. coelicolor grown in broth containing citrate as the sole carbon source, no uptake of free citrate was observed. Rather, uptake was dependent on the presence of metal ions such as Fe3+ and, to a lesser extent, Mn2+, Ca2+, Ba2+, or Pb2+ (Fig. 1). Concentrations of metal ions were used to maximize the formation of the metal-citrate complex under the assay conditions (Table 1). When metals such as Mg2+, Ni2+, or Co2+ were added to the reaction mixture, the rate of uptake of citrate was no greater than the rate of uptake without metal ions. In the presence of the different concentrations of the calcium chelator EGTA, the rate of uptake of citrate in the presence of 10 mM Ca2+ decreased to zero at concentrations of EGTA over 10 mM (Fig. 2). This, combined with the fact that no metal-free citrate uptake was noted, supports the idea that free citrate is not transported but, rather, that the presence of select metal ions is necessary for uptake.
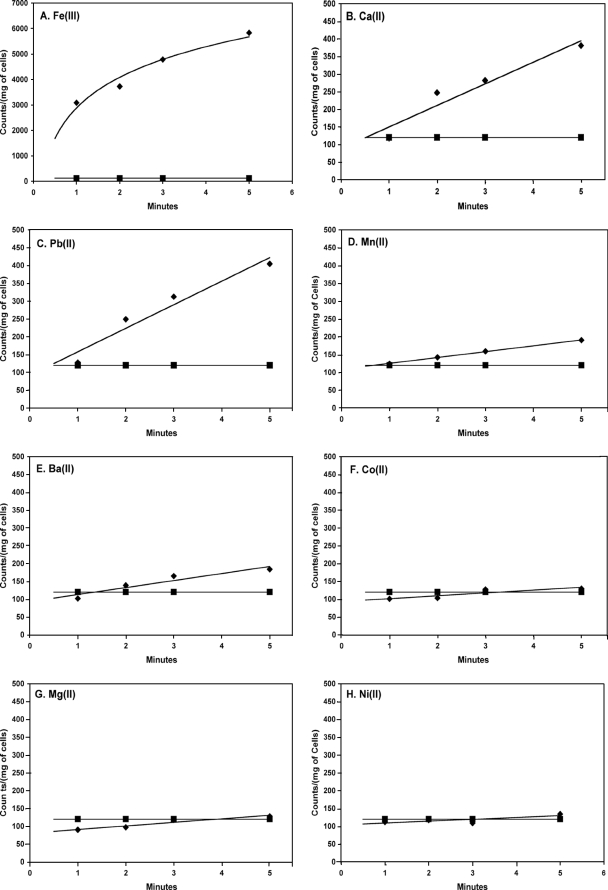
FIG. 1.
Uptake of [1,5-14C]citrate by Streptomyces coelicolor in the presence of different metal ions (⧫). The uptake was measured in 50 mM Chelex-washed PIPES buffer with a 75 μM concentration of Fe3+ (A), 10 mM concentrations of Ca2+ (B), Pb2+ (C), and Ba2+ (E), and 1 mM concentrations of Mn2+ (D), Co2+ (F), Mg2+ (G), and Ni2+ (H). S. coelicolor was grown in SMMC broth. Results with metal-free controls (i.e., “free” citrate) (▪) are shown as a contrast to the results with metal ions in each plot.
FIG. 2.
Effect of the addition of increasing concentrations of the chelating agent EGTA on citrate uptake in the presence of 10 mM Ca2+. At Ca2+ concentrations greater than 10 mM, no uptake greater than that of “free” citrate (▪) is observed. ⧫, 0.1 mM EGTA; ⋄, 1.0 mM EGTA; □, 10.0 mM EGTA.
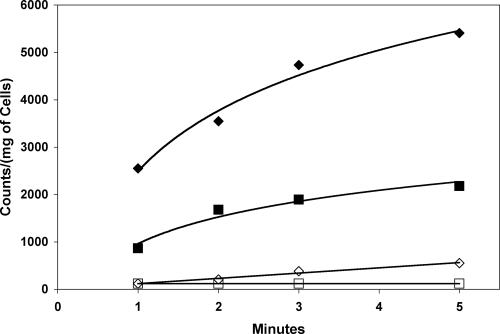
No uptake of any “free” citrate or metal-citrate species (Mg2+, Co2+, Ni2+, Ba2+, Ca2+, Pb2+) above background was observed when more-readily-metabolized carbon sources such as glucose were used in place of, or in combination with, citrate, indicating that expression does not occur in the presence of glucose. The exception to this was with media containing Fe3+. A significantly higher uptake was observed for iron-citrate than for “free” citrate when S. coelicolor was grown in SMMG broth supplemented with 36 μM FeCl3 · 6H2O (Fig. 3). This implies that the system may be used not only as an alternate energy source but also as an uptake system for iron. Transcriptional control, then, may be through downregulation in the presence of glucose or through upregulation in the presence of both iron and citrate.
FIG. 3.
Fe3+-citrate uptake in S. coelicolor grown in SMM broth containing glucose as the sole carbon source. ⧫, S. coelicolor grown with 36 μM Fe3+ in growth broth; ⋄, no iron added to growth broth; ▪ “free”-citrate uptake in cells grown in SMMG broth with 36 μM Fe3+ added to growth broth; □, “free”-citrate uptake in SMMG broth with no added Fe3+ to growth broth.
In addition, the growth of S. coelicolor was arrested in Chelex-washed, metal-free SMMC broth. This arrest could be prevented by the addition of Fe3+ (or Ca2+), but not Mg2+ or Ni2+, to the SMMC broth.
To further investigate the role of the CitSc gene, a number of expression systems were constructed for attempted expression in E. coli (known to be devoid of a “free”-citrate uptake protein under aerobic conditions) (11). Expression of CitSc in E. coli proved highly toxic in systems tested prior to the use of plasmids utilizing the pelB leader sequence [pET-27b(+) and pET-25b(+)]. Cells containing either pET-27b(+)-CitSc (E. coli AA93 Δfec) or pET-25b(+)-CitSc (E. coli JW4251-2 ΔfecA) were successfully induced with 0.5 mM IPTG for 1 h in LB broth in late log phase, with uptake of [1,5-14C]citrate observed with Fe3+, Ca2+, and Pb2+ metal ions (Fig. 4). The presence of Mg2+, Ni2+, or Co2+ as a cofactor did not result in citrate uptake in E. coli. Uncomplexed citrate was not transported. These results are consistent with uptake in S. coelicolor. No transport was observed when CitSc was not induced by IPTG. It should be noted that E. coli has a primary transport system specifically for Fe3+-citrate transport. This system actively binds and transports Fe3+-citrate in E. coli and is ATP dependent (7). As a result, transport assays for Fe3+ needed to be conducted with E. coli without a functioning fec system. The two strains used in transport assays here had either the cell surface fecA gene (JW4251-2) removed or the entire fecABCDE operon (AA93) knocked out. In either case, the results were the same and indicated that Fe3+ is a cofactor for citrate transport through the secondary transporter of the CitMHS family.
FIG. 4.
Effect of CitSc expression in E. coli on the uptake of [1,5-14C]citrate in the presence (⧫) and absence (▪) of different metal ions. Graphs represent results from strain JW4251-2 and are characteristic of data collected from both E. coli JW4251-2 and E. coli AA93. Note that the y axis for panel A is greater than those for panels B to H. Noninduced controls (⋄) are shown except where obscured by free-citrate controls. The uptake was measured in 50 mM Chelex-washed PIPES buffer (pH 6.5) with a 75 μM concentration of Fe3+ (A), 10 mM concentrations of Ca2+ (B), Pb2+ (C), and Ba2+ (E), and 1 mM concentrations of Mn2+ (D), Co2+ (F), Mg2+ (G), and Ni2+ (H).
In the case of Ca2+- and Fe3+-citrate uptake, there is evidence of rapid uptake and metabolism of the transported complex, but the metabolic rates for each complex are notably different. Metabolic differences can be attributed to stability constants and the reduction of iron to the 2+ oxidation state for release. Ca2+-citrate has a stability constant of 103.5, which allows for intracellular decomplexation to rapidly occur in E. coli. Fe3+-citrate has a stability constant of 1011.85, preventing decomplexation from readily occurring. Instead, E. coli uses systems such as flavins to reduce Fe3+-citrate to Fe2+-citrate, which has a stability constant of 103.2, significantly lower than that for Fe3+-citrate and similar to that of the calcium complex (8, 26). Initial rapid metabolism of Fe3+-citrate and subsequent metabolic decreases can be seen in Fig. 4A. The change in metabolic rates may be accredited to the change in the concentration of reduced flavin. Reduced flavin formation occurs enzymatically by NAD(P)H:flavin oxidoreductase in E. coli, which converts flavin into reduced flavin at a kcat of 63 ± 2 s−1. Due to the low kcat, the rate of reduced flavin formation is below the rate of reduced flavin usage, accounting for the change in metabolic rates (8). A reduction of the induction temperature to 4°C and collection of samples from time points prior to 1 min “catch” the E. coli cultures in a period where the transportation of Fe3+-citrate exceeds metabolic activity, yielding a positive initial slope, which solidifies the notion that CitSc is responsible for metal-citrate transportation (Fig. 5).
Once transported, it is likely that oxidative decarboxylation, as part of the tricarboxylic acid cycle, decomplexes the metal-citrate system and converts the citrate to oxaloacetate and, with subsequent amination, aspartate. This would serve to lower radioactivity counts inside the cells by evolving [14C]CO2 (5). Interestingly, while uptake was also observed for Pb2+, no reduction in the postuptake concentration indicative of metabolism was observed. Seminal work by Francis et al. on the metabolism of metal-citrate complexes can be used to rationalize these results (10, 11). Those researchers found that metal-citrate complexes formed as mononuclear, bidentate species (such as those of Fe3+ and Ca2+) could be metabolized by Pseudomonas fluorescens but that tridentate species (such as those of Pb2+) could be transported but were not metabolized. The key that they proposed was the presence of an uncomplexed citrate hydroxyl group that may be critical for recognition/binding with aconitase and so for subsequent incorporation into the tricarboxylic acid cycle (11).
DISCUSSION
We believe that this is only the second example of a gram-positive citrate transporter that preferentially uses ferric ions as a vital cofactor for transport. It is the first such member of the CitMHS family to be successfully functionally characterized outside the native organism. This is also, we believe, the first study to show preferential metabolism of the transported Fe3+-citrate species (over Pb2+-citrate, for example) when it is expressed heterologously. The Fe-citrate species was also the only species transported above the free-citrate background when S. coelicolor was grown in SMM containing glucose and iron. The absence of iron from the broth in the same experiment greatly reduced subsequent Fe3+-citrate uptake. This suggests that S. coelicolor uses this system to acquire iron. While Fe3+ was the dominant vital cofactor for transport, other metal ions with ionic radii of ∼80 pm or greater (Mn2+, Ca2+, Pb2+, and Ba2+) were also transported. Assuming a high-spin, octahedral-complex form for Fe3+-citrate (15), this means an ionic radius of ∼79 pm for iron. With the metal ions Mg2+, Ni2+, and Co2+ not transported and having radii of less than 72 pm, it appears then that the ionic radius has a role in determining transporter specificity (Table 3).
TABLE 3.
Ionic radii for all metal ions investigateda
| Metal ion | Ionic radius (pm) |
|---|---|
| Mg2+ | 66 |
| Ni2+ | 69 |
| Co2+ | 72 |
| Fe3+ | 79 |
| Mn2+ | 80 |
| Ca2+ | 99 |
| Pb2+ | 119 |
| Ba2+ | 134 |
Metal ions with radii greater than 79 pm were transported by CitSc.
Clearly, specificity does not correlate with the formation of bi- or tridentate complex formation since tridentate Pb2+ and bidentate Ca2+ and Fe3+ are all transported. This is in agreement with the works of Warner and Lolkema (28) and Francis et al. (10, 11). Blancato et al. have instead determined that a “size criterion” that is the result of the physical size of the protein binding site plays a major role in determining specificity (5). The difference between the transport of Mn2+ and that of Fe3+, however, despite the similar ionic radii of the two metal ions, suggests that complex speciation and/or coordination geometry may still play a part, with the presence of an Fe3+-bound hydroxyl group in the [Fe(III)(OH)-citrate]− complex (which is not found in the Mn2+-citrate complex, for example) playing a role (e.g., as the H-bond donor in the binding pocket). The two most common citrate-to-metal binding motifs are a linear citrate backbone and a “bent” citrate backbone. The manganese is found with the linear arrangement (24). Iron-citrate structures described by Matzapetakis et al. describe the citrate in the “bent” arrangement for Fe3+ (25). This may also explain the difference between Fe3+ uptake and Mn2+ uptake. Another potential effect may arise from complex charge with the manganese complex monovalent and the Fe(III)(OH)2-citrate complex both a mono- and a divalent species. The presence of both monovalent (68.2%) and divalent (31.8%) Fe-citrate species was calculated using the MINTEQA2 program (14). Interaction with the additional negative charge of the [Fe(III)(OH)2-citrate]2− complex may play some role in the transport of iron being greater than that of manganese and indeed greater than that of other metals forming monovalent complexes (Ca2+, Pb2+, Ba2+, Mg2+, Co2+, Ni2+) in general. Interestingly, it is the divalent species that was metabolized in studies of P. fluorescens conducted by Joshi-Tope and Francis (16).
S. coelicolor transports Fe3+-citrate when grown in SMMG broth containing Fe3+ but does not transport Fe3+-citrate when Fe3+ is omitted from the growth media. This suggests that the CitSc protein system may be used primarily to access iron and not to access citrate. Given that S. coelicolor is a dominant bacterium in the soil environment, it must be capable of surviving in such an environment. The ability to access trace vital elements such as Fe3+ is critical for survival and would give S. coelicolor an advantage in such a competitive environment. Korithoski et al. postulated this with their work on S. mutans, a major cause of dental caries (20). They suggested that S. mutans uses the S. mutans Cit (CitSm) transporter to gain access to Fe3+ in humans. Blancato et al. (5) and Warner and Lolkema (28) attempted to find a similar iron uptake result with E. faecalis, which they noted had 75% amino acid sequence homology to the CitSm Fe3+-citrate transporter of S. mutans. They found instead the system to be predominantly Ca2+-citrate with no iron uptake. Clearly these systems are being controlled by an as-yet-unknown subtle mechanism that controls for such exquisite metal-citrate selectivity (12). The importance of this work on S. coelicolor lies in the importance of iron limitation in blocking infection by pathogenic bacteria. For example, a single injection of iron was shown to decrease the lethal dose of Pseudomonas aeruginosa (in a murine infection model) from more than 104 organisms to fewer than 10 (9, 17). While not a pathogenic bacterium, S. coelicolor is a member of the actinomycete family, which also includes Mycobacterium tuberculosis. The ability to access iron from iron citrate found in blood plasma would give M. tuberculosis a possible route for overcoming iron-based bacteriostasis (3, 6, 27). High iron concentrations are also necessary for biofilm formation, which implies that access to higher levels of iron would promote pathogenesis in a biofilm-forming bacterial genus such as Mycobacterium (3). Chemical-speciation models actually indicate that, among the naturally occurring low-molecular-mass ligands, a dominant Fe3+-citrate species is formed at concentrations as low as 1 mM in serum (19). Also, a sequence homology study of the CitSc AA sequence and the B. anthracis genome found a sequence with 48% homology based on amino acid sequence. We have recently begun work on this gene to investigate if it is indeed a member of the CitMHS family and to compare it to CitSc and CitSm. We have also begun mutagenesis studies of CitSc to attempt to elucidate the mechanism behind this extraordinary uptake process. This work will be reported in due course.
Acknowledgments
We acknowledge Syracuse University, the iLEARN Program, and Promega Biotechnology (Madison, WI) for funding.
We thank Ann Meany, Radiation Safety Technician, Environmental Health Office, Syracuse University, for assistance with the radiation data collection. We thank Ann M. Valentine (Department of Chemistry, Yale University), who was the inspiration for this work.
Footnotes
Published ahead of print on 13 June 2008.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandell, M., V. Ansanay, N. Rachidi, S. Dequin, and J. S. Lolkema. 1997. Membrane potential-generating malate (MleP) and citrate (CitP) transporters of lactic acid bacteria are homologous proteins. Substrate specificity of the 2-hydroxycarboxylate transporter family. J. Biol. Chem. 27218140-18146. [DOI] [PubMed] [Google Scholar]
- 3.Banin, E., M. L. Vasil, and E. P. Greenberg. 2005. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA 10211076-11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentley, S. D., K. F. Chater, A.-M. Cerdeño-Tárraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C.-H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M.-A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417141-147. [DOI] [PubMed] [Google Scholar]
- 5.Blancato, V. S., C. Magni, and J. S. Lolkema. 2006. Functional characterization and Me2+ ion specificity of a Ca2+-citrate transporter from Enterococcus faecalis. FEBS Lett. 2735121-5130. [DOI] [PubMed] [Google Scholar]
- 6.Brown, M. R. W., H. Anwar, and P. A. Lambert. 1984. Evidence that mucoid Pseudomonas aeruginosa in the cystic fibrosis lung grows under iron-restricted conditions. FEMS Microbiol. Lett. 21113-117. [Google Scholar]
- 7.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 2951715-1719. [DOI] [PubMed] [Google Scholar]
- 8.Fieschi, F., V. Nivière, C. Frier, J. Décout, and M. Fontecave. 1995. The mechanism of substrate specificity of the NADPH:flavin oxidoreductase from Escherichia coli. J. Biol. Chem. 27030392-30400. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg, C. M., and J. J. Bullen. 1972. The effect of passage and iron on the virulence of Pseudomonas aeruginosa. J. Clin. Pathol. 2565-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis, A. J., and C. D. Dodge. 1993. Influence of complex structure on the biodegradation of iron-citrate complexes. Appl. Environ. Microbiol. 59109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis, A. J., C. D. Dodge, and J. B. Gillow. 1992. Biodegradation of metal citrate complexes and implications for toxic-metal mobility. Nature 356140-142. [Google Scholar]
- 12.Glusker, J. P. 1980. Citrate conformation and chelation: enzymic implications. Acc. Chem. Res. 13345-352. [Google Scholar]
- 13.Gruber, T. M., and C. A. Gross. 2003. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57441-466. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson, J. P. 2006. Visual MINTEQ 2.51. Department of Land and Water Resources Engineering, KTH, Stockholm, Sweden.
- 15.Hao, X., Y. Wei, and S. Zhang. 2000. Synthesis, crystal structure and magnetic property of a binuclear iron(III) citrate complex. Transit. Metal Chem. 26384-387. [Google Scholar]
- 16.Joshi-Tope, G., and A. J. Francis. 1995. Mechanisms of biodegradation of metal-citrate complexes by Pseudomonas fluorescens. J. Bacteriol. 1771989-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneko, Y., M. Thoendel, O. Olakanmi, B. E. Britigan, and P. K. Singh. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 117877-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keiser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. J. Hopwood. 2000. Media, buffers and suppliers, p. 405-422. In Practical Streptomyces genetics. Crowes Press, Norwich, United Kingdom.
- 19.Konigsberger, L., E. Konigsberger, P. M. May, and G. T. Hefter. 2000. Complexation of iron(III) and iron(II) by citrate. Implications for iron speciation in blood plasma. J. Inorg. Biochem. 78175-184. [DOI] [PubMed] [Google Scholar]
- 20.Korithoski, B., K. Krastel, and D. G. Cvitkovich. 2005. Transport and metabolism of citrate by Streptococcus mutans. J. Bacteriol. 1874451-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krom, B. P., J. B. Warner, W. N. Konings, and J. S. Lolkema. 2000. Complementary metal ion specificity of the metal-citrate transporters CitM and CitH of Bacillus subtilis. J. Bacteriol. 1826374-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reference deleted.
- 23.Li, H., and A. M. Pajor. 2002. Functional characterization of CitM, the Mg2+-citrate transporter. J. Membr. Biol. 1859-16. [DOI] [PubMed] [Google Scholar]
- 24.Matzapetakis, M., N. Karlingiano, A. Bino, M. Dakanali, C. P. Raptopoulou, V. Tangoulis, A. Terzis, J. Giapintzakis, and A. Salifoglou. 2000. Manganese citrate chemistry: synthesis, spectroscopic studies, and structural characterizations of novel mononuclear, water-soluble manganese citrate complexes. Inorg. Chem. 394044-4051. [DOI] [PubMed] [Google Scholar]
- 25.Matzapetakis, M., C. P. Raptopoulou, A. Salifoglou, A. Tsohos, N. Moon, and V. Papaefthymiou. 1998. Synthesis, spectroscopic and structural characterization of the first mononuclear, water soluble iron-citrate complex, (NH4)5Fe(C6H4O7)2 · 2H2O. J. Am. Chem. Soc. 12013266-13267. [Google Scholar]
- 26.Nivière, V., F. Fieschi, J. Décout, and M. Fontecave. 1999. The NAD(P)H:flavin oxidoreductase from Escherichia coli. J. Biol. Chem. 27418252-18260. [DOI] [PubMed] [Google Scholar]
- 27.Singh, P. K., M. R. Parsek, E. P. Greenberg, and M. J. Welsh. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417552-555. [DOI] [PubMed] [Google Scholar]
- 28.Warner, J. B., and J. S. Lolkema. 2002. Growth of Bacillus subtilis on citrate and isocitrate is supported by the Mg2+-citrate transporter CitM. Microbiology 1483405-3412. [DOI] [PubMed] [Google Scholar]