Abstract
Streptococcus pyogenes does not produce catalase, but it can grow in aerobic environments and survive in the presence of peroxide. One of the stress proteins of this organism, peroxide resistance protein (Dpr), has been studied to examine its role in resistance to hydrogen peroxide, but the protective mechanism of Dpr is not clear. The aim of this study was to characterize the dpr gene and its role in dealing with different stresses. A dpr deletion mutant was constructed by double-crossover mutagenesis. The dpr mutant was more sensitive to H2O2, and complementation could partially restore the defect in the mutant. Pretreatment with the iron chelator deferoxamine mesylate rescued the survival activity of the mutant under oxidative stress conditions. The dpr mutant also showed a low survival rate in the long-term stationary phase, when it was treated with extreme acids, and under alkaline pH conditions compared to the wild-type strain. The growth of the dpr mutant was slower than that of the wild-type strain in iron-limiting conditions. The dpr mutant showed high sensitivity to iron and zinc but not to manganese, copper, nickel, and calcium. Recombinant Dpr protein was purified and showed iron-binding activity, whereas no DNA-binding activity was found. These data indicate that an iron-binding protein, Dpr, provides protection from hydrogen peroxide stress by preventing the Fenton reaction, and Dpr was identified as a novel stress protein that protects against several stresses in group A streptococci.
Oxidative stress is one of the common stresses in bacteria. Bacteria encounter oxidative stress by exposure to reactive oxygen species (ROS) present in the aerobic environment and immune responses (3). ROS, such as superoxide (·O2), hydrogen peroxide (H2O2), and the hydroxyl radical (·OH), cause severe damage to DNA, proteins, and lipids (16, 22). Bacteria have developed complex strategies to protect themselves from injury and to prevent exposure to oxidants. Multiple factors participate in the protection of bacteria from ROS damage, such as catalase, superoxide dismutase, NADH oxidase, alkyl hydroperoxide reductase, and DNA-binding protein from starved cells (Dps) (29, 35). The toxicity of H2O2 for microorganisms is mild, but this compound can be transformed into the highly toxic hydroxyl radical in the presence of iron by the Fenton reaction (H2O2 + Fe2+ → ·OH + OH− + Fe3+) (36). In order to prevent the toxicity of H2O2, Dps, a ferritinlike protein, can chelate the excess free iron and interfere with the formation of highly toxic hydroxyl radicals by Fenton reactions (17, 35, 47). Furthermore, Dps also protects cells against ROS resulting from its binding to DNA nonspecifically in Escherichia coli (2, 23). Therefore, Dps family proteins are vital in preventing hydrogen peroxide stress.
Most proteins belonging to the Dps family can bind to iron, but some of them cannot bind to DNA to protect cells against oxidative stress. (5, 35, 39). In addition, Dps homologues in Salmonella enterica serovar Typhimurium (14) and Listeria monocytogenes (26) and NapA in Helicobacter pylori have been shown to be associated with virulence (31). All of these molecules are important for protecting against hydrogen peroxide stress (14, 17, 25, 26, 35, 46). A Dps homologue is also present in Streptococcus pyogenes (group A streptococcus [GAS]) and is designated Dpr (Dps-like peroxide resistance) or MrgA (6). The expression of dpr in GAS is elevated in the mouse infection model and in human saliva, as determined by microarray analysis (12, 34). It has been suggested that dpr of GAS is an important factor for protecting the organisms against oxidative stress and may have roles in adaptation to the host environment.
Brenot et al. have shown that Dpr mutants are hypersensitive to hydrogen peroxide and that the Dpr promoter, containing a Per box, is recognized by the PerR regulator (6). However, the protective mechanism and biological functions of Dpr are not well known. In this study, we demonstrated the mechanism of hypersensitivity to hydrogen peroxide of a dpr mutant in GAS and its role in multiple stresses.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Wild-type GAS strain A-20 (serotype M1) used in this study has been described previously (40). E. coli DH5α was used for cloning, and E. coli BL21(DE3) served as the host for the expression of His6-tagged Dpr protein. Plasmids pSF152 and pDL278 have been described previously (43). Plasmid pET21b was purchased from Invitrogen, Cergy-Pontoise, France. All GAS strains were cultivated in tryptic soy broth supplemented with 0.5% yeast extract (TSBY) without agitation at 37°C. E. coli strains were grown with agitation at 37°C in Luria-Bertani broth supplemented with spectinomycin (100 μg/ml), ampicillin (100 μg/ml), or chloramphenicol (25 μg/ml) as necessary. For preparation of iron-limiting medium, TSBY was depleted of iron by adding 16 mM nitrilotriacetic acid (trisodium salt) (NTA) and supplemented with MgCl2, CaCl2, MnCl2, and ZnCl2 (1 mM each).
Construction of a dpr mutant and a complementation strain.
A dpr deletion mutant was constructed by using GAS strain A-20 as the parental strain. Oligonucleotide primers (forward primer 5′-CCGGAATTCGCGACAAAATAAAGCCCAAA-3′ and reverse primer 5′-CGCGGATCCTCAGCCGCCATGAAAATGTC-3′; EcoRI and BamHI restriction sites are underlined) were used for amplification of the upstream 947 bp and downstream 1,021 bp of the dpr region. The 2,496-bp PCR product was digested with BamHI and EcoRI and cloned into vector pSF152, yielding plasmid pMW410. The dpr gene was digested at the Eco0109I and XcmI restriction enzyme sites; then the sticky ends were filled in and a chloramphenicol resistance cassette was inserted to disrupt the dpr region, and the resulting construct was designated plasmid pMW411. The proper construct was confirmed by PCR and restriction enzyme digestion. Plasmid pMW411 was introduced into A-20 by electroporation at 1.8 kV, and bacteria were resuspended in 1 ml TSBY immediately after electroporation. The sample was incubated for 2 h at 37°C and plated on TSBY agar plates supplemented with 3 μg/ml chloramphenicol to select mutants. The plates were incubated at 37°C for 24 to 48 h, and the mutant was confirmed by PCR and Southern blot analysis. For complementation of the dpr mutant, 1,000 bp containing the dpr gene and promoter region was amplified with forward primer 5′-CCGGAATTCTGCCCGAACATATACTAAAA-3′ and reverse primer 5′-CGCGGATCCATAAAGACGTTTGCCAAGGT-3′ (EcoRI and BamHI restriction sites are underlined). The purified DNA, digested with BamHI and EcoRI, was cloned into appropriately digested shuttle vector pDL278, and the resulting plasmid was designated pMW409. Plasmid pMW409 was electroporated into the dpr mutant, resulting in a dpr complementation strain.
Cloning and purification of recombinant His6-tagged Dpr and Dps proteins.
The full-length dpr gene (528 bp) was amplified from A-20 genomic DNA by PCR with a forward primer containing an EcoRI site (5′-CCGGAATTCCATGACAAACACACTCGTTGA-3′) and a reverse primer containing a XhoI site (5′-CCGCTCGAGGAGTGCTGGGCCTTGTCCAC-3′) (restriction sites are underlined). The dps gene was amplified from an E. coli K-12 strain by PCR using primers CCGGAATTCCATGAGTACCGCTAAATTAGT and CCGCTCGAGTTCGATGTTAGACTCGATAA (restriction sites are underlined). The PCR products of the dpr and dps genes were digested with EcoRI-XhoI and cloned into pET-21b to generate Dpr and Dps expression plasmids pMW408 and pMW524, respectively.
Cultures of E. coli BL21 harboring the recombinant plasmids were grown to an optical density at 600 nm of 0.6 prior to induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h. Cells were harvested by centrifugation at 8,000 rpm, and each cell pellet was resuspended in 20 mM Tris-HCl-100 mM NaCl (pH 7.5). The cells were disrupted by two passages through a French pressure cell, and the cell debris was removed by centrifugation at 12,000 rpm. Purification of the His6-tagged protein was performed by Ni2+ affinity chromatography (GE Amersham, Uppsala, Sweden). The purified recombinant Dpr (rDpr) and recombinant Dps (rDps) proteins were dialyzed with 20 mM Tris-HCl-100 mM NaCl (pH 7.5) and concentrated by using an ultrafiltration cell (Amicon Corp., Lexington, MA).
H2O2 sensitivity assays.
H2O2 sensitivity was examined and modified by using liquid culture methods previously described by Brenot et al. (6). Overnight cultures of GAS were reinoculated (1:100) into fresh TSBY and incubated at 37°C for 5 h. An aliquot of each culture was removed, and the cell pellet was suspended in 1× phosphate-buffered saline. A 100-μl aliquot was removed (time zero), and 5 mM (final concentration) H2O2 was added to the bacterial culture. The mixture was incubated at 37°C for 1 h. Appropriate bacterial dilutions were plated onto solid TSBY agar plates for determination of the numbers of CFU. The viable cells were counted, and the percentage of surviving cells was calculated by dividing the number of CFU at 1 h by the initial number of CFU at time zero. In the iron chelator complementation analysis of H2O2 hypersensitivity, the method described by Ishikawa et al. was used, with modifications (17). The bacteria were pretreated with different concentrations of deferoxamine mesylate (DFOM) (Sigma Chemical Co., St. Louis, MO) for 30 min at 37°C and then challenged with 5 mM H2O2 for 1 h without agitation. The results were expressed as the averages and standard errors of the means of at least three independent experiments.
Iron staining.
Iron staining was performed as described elsewhere (8, 17, 45). Different concentrations of rDpr, rDps, and bovine serum albumin (BSA) were incubated with 1 mM Fe(NH4)2(SO4)·6H2O on ice for 30 min. The mixtures were resolved by 8% nondenaturing polyacrylamide gel electrophoresis (PAGE). Iron-binding proteins were visible in a gel stained with 1 mM 3-(2-pyridyl)-5,6-bis(2-[5-furyl sulfonic acid])-1,2,4-triazine (Ferene S; Sigma Chemical Co.) and 15 mM thioglycolic acid (Sigma Chemical Co.) in 2% (vol/vol) acetic acid. A gel was stained with Coomassie brilliant blue as a loading amount control.
DNA-binding assay.
The DNA-binding assay was performed as described previously (2, 17, 46). Different amounts of the rDpr and rDps proteins were added to plasmid pUC18 DNA containing Fe(NH4)2(SO4). The mixtures were incubated for 1 h on ice and electrophoresed using a 1.0% agarose gel in Tris-acetate buffer. DNA on the gel was detected by staining it with ethidium bromide.
Spontaneous mutation rates.
The method used to determine spontaneous mutation rates was the method described by Poyart et al., with modifications (29). Overnight cultures of GAS were reinoculated (1:100) into fresh TSBY and incubated at 37°C for 5 h. Then strains were treated with 0.1 mM H2O2 for 2 h. Appropriate dilutions of the strains were plated on TSBY agar with or without 0.1 μg/ml rifampin (Sigma Chemical Co.). The mutation rate was calculated by dividing the number of rifampin-resistant CFU by the total number of CFU of GAS.
Long-term growth assays.
To investigate long-term survival of GAS strains, overnight GAS cultures were inoculated (1:100) into fresh TSBY. The viable cell counts were periodically determined by plating the appropriate dilution on a TSBY agar plate.
pH stress assays.
Aliquots of overnight stationary-phase cultures of GAS strains were removed. Cells were incubated in medium whose pH was adjusted to 4 or 11 at 37°C for 1 h. The viable cell counts were determined, and the percentage of survival was calculated by dividing the number of CFU at 1 h by the initial number of CFU at time zero.
Metal stress assays.
The procedures used for metal stress assays were modified from the procedures of Nair and Finkel (25). Overnight stationary-phase cultures of GAS strains were incubated at 37°C in the presence or absence of different metals at the following final concentrations: 30 mM FeSO4, 50 mM ZnCl2, 30 to 100 mM MnCl2, 0.1 to 1 mM CuSO4, 1 to 100 mM NiSO4, and 1 to 100 mM CaCl2. Viable cell counts were determined at different times. All metal stress assays were performed at least three times.
RESULTS
Construction of a dpr mutant and a complementation strain.
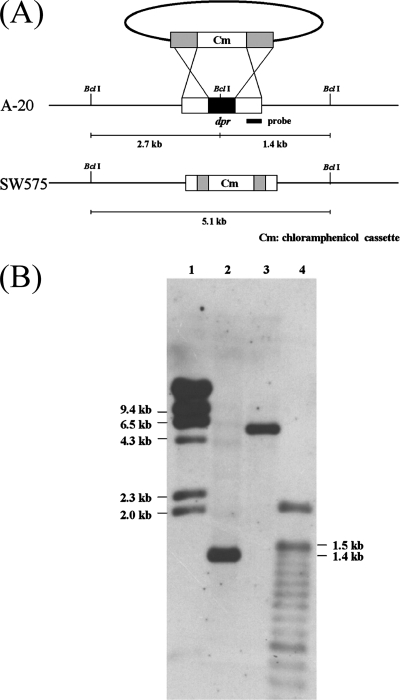
The role of the dpr gene in the stress response was examined by constructing a GAS dpr mutant. A dpr deletion mutant, SW575, was constructed by double-crossover mutagenesis and confirmed by Southern blotting (Fig. 1A). The chromosomal DNA was digested with BclI, and then the membrane was hybridized with the 0.5-kb fragment upstream of the dpr gene. The data showed that there was a 1.4-kb band in the wild-type strain, whereas a 5.1-kb band was present in the dpr mutant (Fig. 1B). The dpr complementation plasmid derived from shuttle vector pDL278 was introduced into SW575, resulting in a strain designated SW576. The growth curves of SW575 and SW576 were similar to that of the wild-type strain (data not shown).
FIG. 1.
Construction and confirmation of the dpr mutant. (A) Schematic diagram of construction of the dpr mutant by allelic exchange. The dpr gene was replaced by a 1.6-kb chloramphenicol resistance cassette. The DNA was introduced into GAS by electroporation. BclI restriction enzyme sites were present in the dpr locus. The thick line indicates the fragment from the dpr upstream region used as the probe for Southern blotting. The predicted hybridization sizes are shown. (B) Southern blot confirming the disruption of dpr. Chromosomal DNA from the wild type and the dpr mutant were digested with BclI and probed with the DNA upstream of dpr. Lane 1, λ/HindIII marker; lane 2, wild-type genomic DNA; lane 3, dpr mutant genomic DNA; lane 4, 100-bp marker.
Role of dpr in oxidative stress.
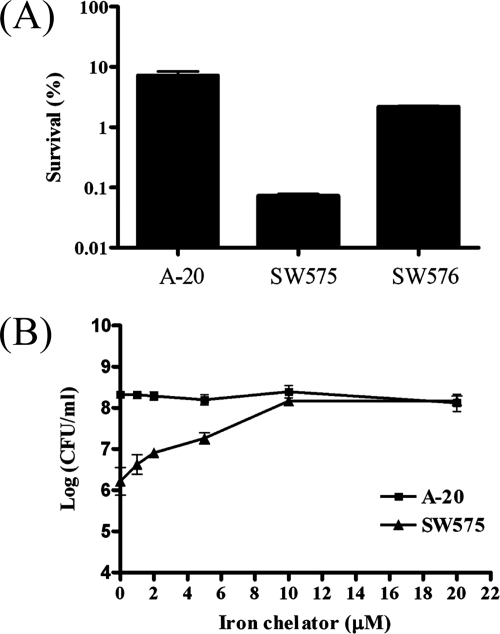
To determine the role of dpr in oxidative stress, A-20, SW575, and SW576 were treated with 5 mM H2O2 and incubated for 1 h. The results showed that the level of survival of the A-20 strain was 100-fold higher than that of SW575. The sensitivity to hydrogen peroxide was restored to nearly wild-type levels in the complementation strain SW576 (Fig. 2A). The sensitivity of the dpr mutant to hydrogen peroxide is similar to that described previously (6). However, the mechanism by which dpr confers resistance to oxidative stress in GAS is not known. To investigate the mechanism of resistance to H2O2 in GAS, the wild-type strain and mutant were pretreated with different concentrations of the iron chelator DFOM (Desferal) for 30 min and then challenged with 5 mM H2O2. As shown in Fig. 2B, the higher the concentration of the iron chelator used in the experiment, the greater the survival of the dpr mutant. The survival of SW575 increased 2 logs in the presence of 10 μM DFOM, whereas there was no significant increase in the survival of A-20 in the presence of 10 μM DFOM. When the two strains were pretreated with DFOM concentrations higher than 10 μM, the survival activity did not increase significantly. The results showed that an iron chelator, DFOM, can rescue hypersensitivity of a dpr mutant to H2O2, suggesting that Dpr serves as an iron chelator.
FIG. 2.
Roles of dpr in oxidative stress. (A) Effect of H2O2 treatments on GAS survival. After cells were freshly subcultured for 5 h in TSBY, H2O2 (5 mM) was added to 1-ml aliquots of the culture. After 60 min, viable counts were determined by plating on TSBY agar before and after the addition of H2O2. The data are the means and standard deviations of three independent experiments. (B) Effect of the iron chelator DFOM on the hypersensitivity to H2O2 of the dpr mutant. GAS A-20 and SW575 were pretreated with different concentrations of chelators for 30 min and then challenged with 5 mM H2O2 for 1 h. The numbers of viable bacteria were determined by serial dilution and plating on TSBY agar. The data are the means ± standard deviations of three independent experiments.
Characterization of the Dpr protein.
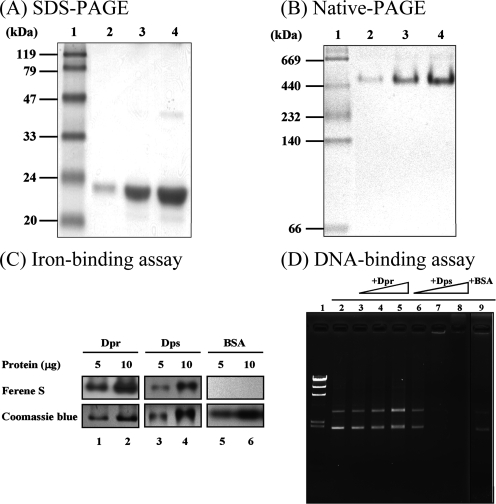
In order to clarify the protective mechanism and roles of Dpr in oxidative stress, His6-tagged rDpr proteins were overexpressed in E. coli and purified under native conditions using a nickel-chelating column. The predicted molecular mass of the rDpr protein with T7 and His6 tags is 22 kDa. More interestingly, workers have described Dpr homologs that can form a spherical structure, like ferritin, which is composed of 12 identical subunits (35). The molecular mass of the rDpr protein was determined by nondenaturing PAGE and denaturing sodium dodecyl sulfate-PAGE. The rDpr protein was resolved at 22 kDa under denaturing conditions (Fig. 3A), whereas the molecular mass was more than 400 kDa as determined by nondenaturing PAGE (Fig. 3B). Thus, a polymeric form of Dpr was present in our purified preparation. In order to determine the iron-binding property of Dpr, different concentrations of the rDpr and rDps proteins were pretreated with Fe(NH4)2(SO)4, and the mixtures were subjected to nondenaturing PAGE. The iron-protein complex could be found after the gels were stained with Ferene S, an iron-specific staining dye (Fig. 3C). These results demonstrated that Dpr is similar to Dps and is an oligomeric protein having iron-binding activity. The rDpr and rDps proteins were also used to perform a gel mobility shift assay with plasmid pUC18 to determine whether rDpr possesses DNA-binding activity. The results showed that for the rDpr mixture there was no band shift in the DNA-binding assay (Fig. 3D, lanes 3 to 5), whereas Dps-DNA complexes displayed decreased mobility during electrophoresis (Fig. 3D, lanes 7 and 8). Taken together, the results indicate that Dpr cannot bind to DNA.
FIG. 3.
Characterization of the rDpr protein. Different amounts of rDpr were electrophoresed on a 15% sodium dodecyl sulfate (SDS)-PAGE gel (A) and an 8% nondenaturing polyacrylamide gel (B). Lane 1, marker; lanes 2 to 4, 1, 5, and 10 μg of rDpr, respectively. (C) Different amounts of recombinant proteins and BSA preincubated with or without Fe(NH4)2(SO4) were electrophoresed on nondenaturing PAGE gels. A visible iron-protein complex was evident after the gels were stained with Ferene S. Coomassie blue staining of the gel served as a loading amount control. (D) DNA-binding activities of rDpr. Recombinant proteins and BSA were incubated with plasmid pUC18 containing Fe(NH4)2(SO4) on ice for 1 h. The mixtures were electrophoresed using a 1% Tris-acetate agarose gel, and the gel was stained with ethidium bromide. Lane 1, DNA marker; lane 2, plasmid pUC18 alone; lanes 3 to 5, pUC18 with rDpr (1, 5, and 10 μg, respectively); lanes 6 to 8, pUC18 with rDps (0.5, 1, and 5 μg, respectively); lane 9, pUC18 with 10 μg BSA.
Effect of dpr on DNA damage.
ROS derived from H2O2 by the Fenton reaction can partially damage DNA, and most antioxidants can protect cells against oxidative DNA damage that results in DNA mutations (11). To determine whether Dpr not only protects GAS against H2O2 killing but also prevents mutation, the bacterial cultures were treated with H2O2 and selected on rifampin plates. The results showed that the mutation frequency to rifampin resistance of the dpr mutant (2.4 × 10−6 ± 5.07 × 10−7) was 17-fold higher than that of A-20 (1.4 × 10−7 ± 5.13 × 10−8) or SW576 (3.1 × 10−7 ± 1.12 × 10−7). The data indicate that dpr can minimize DNA damage caused by ROS in GAS.
Effect of dpr in the long-term stationary phase.
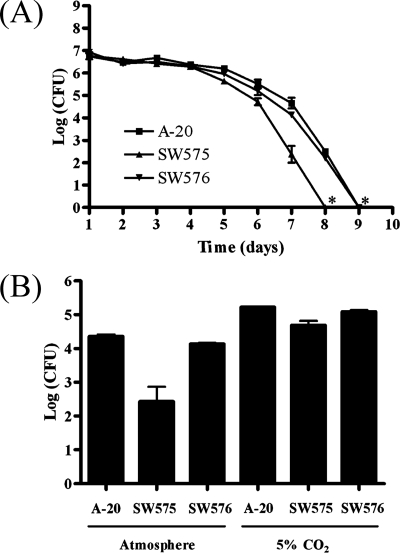
It has been reported that GAS can persist in long-term stationary-phase cultures (44). To determine whether dpr had a role in the long-term stationary phase, GAS were cultured in vitro for 9 days, and the numbers of viable GAS were determined by plating on TSBY agar. The survival of the dpr mutant was found to have decreased by 1 log after 6 days and by 3 logs after 7 days compared with the wild-type strain (Fig. 4A). The plasmid in SW576 restored the defect in survival in the long-term stationary phase, and the plasmid could be extracted from the complementation strain after 7 days of incubation (Fig. 4A). No surviving cells could be detected in dpr mutant cultures after 8 days of incubation, while surviving cells in the wild-type and complemented cultures were not detectable after 9 days. The initial pH of the fresh TSBY was 6.9, and the pH was consistently determined to be between 5.7 and 5.8 throughout the 9-day course of the experiment (data not shown). Therefore, the survival defect in the long-term stationary phase of the dpr mutant was not due to the pH shift. However, a higher survival rate was found when GAS strains were cultured in the presence of 5% CO2 than when they were cultured in a normal atmosphere (Fig. 4B). We suggest that the suddenly decreased survival of SW575 was probably due to the ROS derived from oxygen and that dpr is an important factor for GAS survival in the long-term stationary phase.
FIG. 4.
Survival of GAS in the long-term stationary phase. (A) Overnight cultures of GAS were subcultured in fresh TSBY for 9 days at 37°C without agitation. Viable bacteria were counted every day. An asterisk indicates that no surviving GAS were detected. (B) The wild-type strain, SW575, and SW576 were cultured in an incubator containing a normal atmosphere and an incubator containing 5% CO2 at 37°C for 7 days.
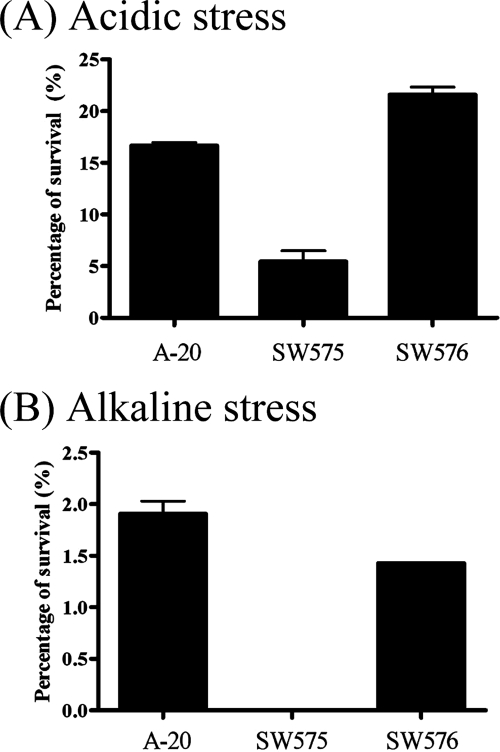
Effects of dpr at extremely alkaline and acid pHs.
To examine the role of Dpr in extreme pH stress conditions, overnight GAS cultures were shifted to TSBY medium with different pH values for 1 h. The survival rates of the wild-type strain were similar in the pH range from 5 to 10 (data not shown). However, the dpr mutant had a threefold-lower survival rate at pH 4 (extremely acidic conditions) (Fig. 5A) and a 100-fold-lower survival rate at pH 11 (extremely alkaline conditions) (Fig. 5B) than the wild-type strain after 1 h of incubation.
FIG. 5.
Influence of extreme pH conditions on the survival of GAS. A-20, SW575, and SW576 were harvested from overnight cultures, and bacteria were suspended in pH 4 (A) and pH 11 (B) TSBY. The bacterial suspensions were incubated for 1 h at 37°C, and the numbers of remaining viable bacteria were determined by plating serial dilutions on TSBY agar plates. The results are representative of three separate experiments.
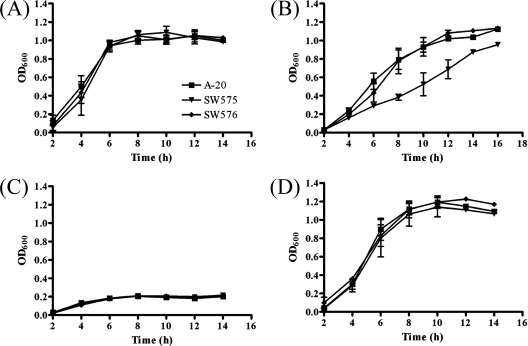
Effect of dpr in iron-limiting conditions.
It has been reported that GAS has three iron acquisition transporters used to acquire heme, ferric iron, and ferrichrome (15). This suggests that the iron-binding protein, Dpr, and the metal transporters are involved in iron homeostasis and growth. To investigate the survival rate of a dpr mutant of GAS, we compared the abilities of wild-type and mutant bacteria to grow in TSBY media (Fig. 6A) and in iron-limiting medium that was pretreated with 16 mM NTA (Fig. 6B). The growth of the dpr mutant was significantly reduced under iron-limiting conditions compared to the growth of the wild-type strain and the complementation strain. None of the strains could grow after the concentration of the iron chelator was increased to 18 mM (Fig. 6C). However, the defect could be restored in medium supplemented with FeSO4 (Fig. 6D).
FIG. 6.
Growth of GAS strains in TSBY containing different amounts of iron chelator and ferrous sulfate. (A) Wild-type strain A-20, SW575, and SW576 were incubated in TSBY without any treatment at 37°C, and the optical density at 600 nm (OD600) was determined during growth. Strains were also incubated in TSBY with 16 mM NTA (B), 18 mM NTA (C), and18 mM NTA plus 10 mM ferrous sulfate (D).
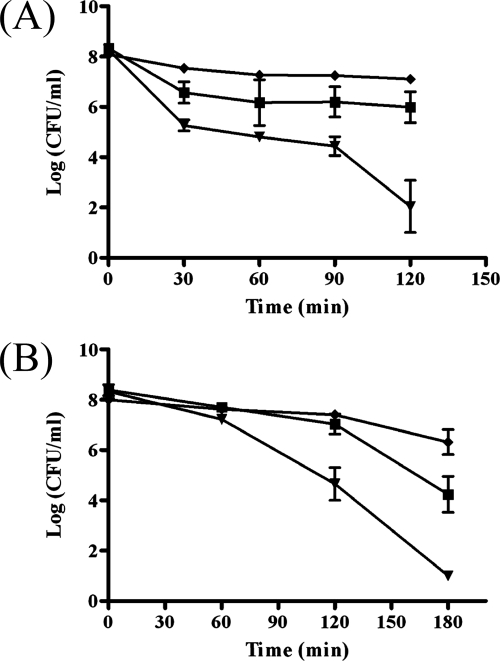
Effect of dpr on metal stresses.
Metal is vital for bacterial growth, but an excess is toxic to bacteria. Overnight cultures of A-20, SW575, and SW576 were treated with different amounts of metals, and the results showed that there were no significant differences in the survival rates after treatment with manganese, copper, nickel, and calcium (data not shown). However, when these strains were treated with 30 mM ferrous sulfate, the survival of the dpr mutant was 1.5 logs lower than that of the wild-type strain after 30 min of incubation, and the plasmid in the complementation strain fully restored the survival (Fig. 7A). After 2 h of incubation, the dpr mutant was found to be 4 logs more susceptible to iron stress than the wild-type strain (Fig. 7A). In addition to iron, it was found that the dpr mutant was hypersensitive to zinc. After 2 h of treatment with 50 mM zinc sulfate, the density of the wild-type strain was 107 CFU/ml, whereas the density of the dpr mutant was reduced to ∼104 CFU/ml (Fig. 7B). The dpr mutant was not detectable after 3 h of incubation, whereas the density of the wild-type strain was ∼104 CFU/ml.
FIG. 7.
Survival of GAS strains after metal stresses. Overnight cultures of wild-type strain A-20 (▪), SW575(▾), and SW576 (♦) were challenged with 30 mM ferrous sulfate (A) or 50 mM zinc sulfate (B) at 37°C without agitation. The remaining viable bacterial counts were determined at different time points by determining the number of CFU, and the data are means ± standard deviations.
DISCUSSION
In this study, we characterized the iron-binding ability of the Dpr protein and showed that Dpr could protect GAS against ROS made by the Fenton reaction. Moreover, a strain defective in Dpr has less resistance to multiple stresses. To our knowledge, this is the first demonstration that Dpr is vital for several stress responses in GAS.
Homologues of a Dps-like protein have been identified in several bacteria, and the roles of these homologues are diverse (35). Dps-like proteins in intracellular bacteria, such as ferritinlike Dps in S. enterica serovar Typhimurium and Fri in L. monocytogenes, are important virulence factors (14, 26). The iron-binding activities of Dps-like proteins are found in most bacteria (17, 21, 30, 35, 46), but the DNA-binding activities are present in only some bacteria, like H. pylori, Mycobacterium smegmatis, and Porphyromonas gingivalis (10, 13, 35, 41). Most of the dpr homologues are required for resistance to hydrogen peroxide (35). The GAS dpr mutant was hypersensitive to hydrogen peroxide stress, whereas if it was pretreated with 10 μM DFOM, an iron chelator, the survival rate increased to nearly that of the wild-type strain (Fig. 2B). In addition, the iron-binding activity of Dpr in GAS was also characterized by Ferene S staining (Fig. 3C). These data suggest that Dpr binds free iron in the cytosol to prevent toxicity through the Fenton reaction.
We also found that Dpr can protect GAS from extreme pH stress. Since ion pumps and ion transporters play important roles in controlling the pH homeostasis in bacteria and most of these factors are regulated by metalloregulators (1, 7), Dpr might control the intracellular concentration of iron and regulate ion transporter genes in GAS.
Iron is an essential factor for bacterial growth and during infection. A multimetal transport system (mts) and a streptococcal metal transport repressor (mtsR) were identified previously in GAS (4, 19, 20). MtsABC is an ABC transporter which possesses the ability to accumulate iron and zinc, and MtsR is a metallorepressor that represses the streptococcal iron acquisition (sia) operon (4). Moreover, strains with mtsABC or mtsR mutations exhibit impaired growth in iron-depleted conditions (4, 20). However, in this study, the dpr mutant also grew more slowly in the iron-restricted conditions than the wild-type strain. Our results suggest that the iron-binding protein, Dpr, plays an important role under iron-limiting conditions. Whether dpr is also related to mtsABC or mtsR requires further study.
ROS can be generated through Fenton-like or autooxidation reactions by some heavy metals in addition to iron (37). Appropriate concentrations of metals are important for bacterial growth, but excess metals are lethal to bacteria. Heavy metals not only bind to free thiol groups, destroying protein function, but also compete with cofactors in proteins (9, 37). In this study, we analyzed several heavy metal stresses, and the results showed that Dpr plays a vital role in iron and zinc stress but not in manganese, copper, nickel, or calcium stress. The amino acid sequences of Dpr in Streptococcus mutans and S. pyogenes are 72% identical. It has also been reported that Dpr binds not only to iron but also to zinc in S. mutans (46). The concentrations of zinc in serum and lung tissue have been reported to be around 15.3 and 229.4 μM, respectively (42). However, the concentrations of zinc are increased in blood and other body sites; e.g., they are increased three- to fourfold in the liver during inflammation (24, 38).
GAS can cause asymptomatic infections for weeks to months and then result in tonsillitis when there is a defect in immunity (27, 28, 32). Shelburne et al. found that GAS is able to persist in human saliva (33, 34). When gene expression in human saliva is analyzed, dpr is one of the upregulated genes (34). It seems reasonable to suppose that Dpr plays a role in persistence in human saliva. Survival in long-term stationary phase may reflect persistence in the host environment. The long-term stationary growth assays were performed under aerobic conditions and produced ROS like hydrogen peroxide (18). Here, we found that the dpr mutant had a defect in the ability to survive after 6 days of incubation. This may suggest that H2O2 is produced by GAS in long-term stationary-phase conditions and that mutants lacking Dpr are killed quickly by ROS derived from hydrogen peroxide.
In summary, this study demonstrated that an iron-binding protein, Dpr, prevents hydrogen peroxide stress by preventing the Fenton reaction. Dpr was identified as a novel stress protein that is active in allowing GAS to tolerate several stresses.
Acknowledgments
We are very grateful to Robert M. Jonas and Ching-Hao Teng for helpful comments on the manuscript.
This work was supported in part by grants NSC94-2320-B-006-085 and NSC95-2320-B-006-024 from the National Science Council and by grant NHRI-EX95-9429SP from National Health Research Institutes, Taiwan.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 9 June 2008.
REFERENCES
- 1.Agranoff, D., and S. Krishna. 2004. Metal ion transport and regulation in Mycobacterium tuberculosis. Front. Biosci. 92996-3006. [DOI] [PubMed] [Google Scholar]
- 2.Almiron, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 62646-2654. [DOI] [PubMed] [Google Scholar]
- 3.Babior, B. M. 1978. Oxygen-dependent microbial killing by phagocytes (first of two parts). N. Engl. J. Med. 298659-668. [DOI] [PubMed] [Google Scholar]
- 4.Bates, C. S., C. Toukoki, M. N. Neely, and Z. Eichenbaum. 2005. Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect. Immun. 735743-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzi, M., G. Mignogna, S. Stefanini, D. Barra, C. Longhi, P. Valenti, and E. Chiancone. 1997. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J. Biol. Chem. 2723259-3265. [DOI] [PubMed] [Google Scholar]
- 6.Brenot, A., K. Y. King, and M. G. Caparon. 2005. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol. Microbiol. 55221-234. [DOI] [PubMed] [Google Scholar]
- 7.Brenot, A., B. F. Weston, and M. G. Caparon. 2007. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol. Microbiol. 631185-1196. [DOI] [PubMed] [Google Scholar]
- 8.Chung, M. C. 1985. A specific iron stain for iron-binding proteins in polyacrylamide gels: application to transferrin and lactoferrin. Anal. Biochem. 148498-502. [DOI] [PubMed] [Google Scholar]
- 9.Connell, D. W., and G. J. Miller. 1984. Chemistry and ecotoxicology of pollution. John Wiley & Sons, New York, NY.
- 10.Cooksley, C., P. J. Jenks, A. Green, A. Cockayne, R. P. Logan, and K. R. Hardie. 2003. NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J. Med. Microbiol. 52461-469. [DOI] [PubMed] [Google Scholar]
- 11.Farr, S. B., R. D'Ari, and D. Touati. 1986. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc. Natl. Acad. Sci. USA 838268-8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, M. R., K. Virtaneva, S. F. Porcella, D. J. Gardner, R. D. Long, D. M. Welty, W. T. Barry, C. A. Johnson, L. D. Parkins, F. A. Wright, and J. M. Musser. 2006. Analysis of the transcriptome of group A streptococcus in mouse soft tissue infection. Am. J. Pathol. 169927-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta, S., and D. Chatterji. 2003. Bimodal protection of DNA by Mycobacterium smegmatis DNA-binding protein from stationary phase cells. J. Biol. Chem. 2785235-5241. [DOI] [PubMed] [Google Scholar]
- 14.Halsey, T. A., A. Vazquez-Torres, D. J. Gravdahl, F. C. Fang, and S. J. Libby. 2004. The ferritin-like Dps protein is required for Salmonella enterica serovar Typhimurium oxidative stress resistance and virulence. Infect. Immun. 721155-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanks, T. S., M. Liu, M. J. McClure, M. Fukumura, A. Duffy, and B. Lei. 2006. Differential regulation of iron- and manganese-specific MtsABC and heme-specific HtsABC transporters by the metalloregulator MtsR of group A streptococcus. Infect. Immun. 745132-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henle, E. S., and S. Linn. 1997. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J. Biol. Chem. 27219095-19098. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa, T., Y. Mizunoe, S. Kawabata, A. Takade, M. Harada, S. N. Wai, and S. Yoshida. 2003. The iron-binding protein Dps confers hydrogen peroxide stress resistance to Campylobacter jejuni. J. Bacteriol. 1851010-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen, W. T., M. Bolm, R. Balling, G. S. Chhatwal, and R. Schnabel. 2002. Hydrogen peroxide-mediated killing of Caenorhabditis elegans by Streptococcus pyogenes. Infect. Immun. 705202-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janulczyk, R., J. Pallon, and L. Bjorck. 1999. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol. Microbiol. 34596-606. [DOI] [PubMed] [Google Scholar]
- 20.Janulczyk, R., S. Ricci, and L. Bjorck. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 712656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauko, A., A. T. Pulliainen, S. Haataja, W. Meyer-Klaucke, J. Finne, and A. C. Papageorgiou. 2006. Iron incorporation in Streptococcus suis Dps-like peroxide resistance protein Dpr requires mobility in the ferroxidase center and leads to the formation of a ferrihydrite-like core. J. Mol. Biol. 36497-109. [DOI] [PubMed] [Google Scholar]
- 22.Luo, Y., Z. Han, S. M. Chin, and S. Linn. 1994. Three chemically distinct types of oxidants formed by iron-mediated Fenton reactions in the presence of DNA. Proc. Natl. Acad. Sci. USA 9112438-12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez, A., and R. Kolter. 1997. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J. Bacteriol. 1795188-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milanino, R., M. Marrella, R. Gasperini, M. Pasqualicchio, and G. Velo. 1993. Copper and zinc body levels in inflammation: an overview of the data obtained from animal and human studies. Agents Actions 39195-209. [DOI] [PubMed] [Google Scholar]
- 25.Nair, S., and S. E. Finkel. 2004. Dps protects cells against multiple stresses during stationary phase. J. Bacteriol. 1864192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen, K. N., M. H. Larsen, C. G. Gahan, B. Kallipolitis, X. A. Wolf, R. Rea, C. Hill, and H. Ingmer. 2005. The Dps-like protein Fri of Listeria monocytogenes promotes stress tolerance and intracellular multiplication in macrophage-like cells. Microbiology 151925-933. [DOI] [PubMed] [Google Scholar]
- 27.Osterlund, A., and L. Engstrand. 1997. An intracellular sanctuary for Streptococcus pyogenes in human tonsillar epithelium—studies of asymptomatic carriers and in vitro cultured biopsies. Acta Otolaryngol. 117883-888. [DOI] [PubMed] [Google Scholar]
- 28.Osterlund, A., R. Popa, T. Nikkila, A. Scheynius, and L. Engstrand. 1997. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107640-647. [DOI] [PubMed] [Google Scholar]
- 29.Poyart, C., E. Pellegrini, O. Gaillot, C. Boumaila, M. Baptista, and P. Trieu-Cuot. 2001. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect. Immun. 695098-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulliainen, A. T., S. Haataja, S. Kahkonen, and J. Finne. 2003. Molecular basis of H2O2 resistance mediated by streptococcal Dpr. Demonstration of the functional involvement of the putative ferroxidase center by site-directed mutagenesis in Streptococcus suis. J. Biol. Chem. 2787996-8005. [DOI] [PubMed] [Google Scholar]
- 31.Satin, B., G. Del Giudice, V. Della Bianca, S. Dusi, C. Laudanna, F. Tonello, D. Kelleher, R. Rappuoli, C. Montecucco, and F. Rossi. 2000. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J. Exp. Med. 1911467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sela, S., R. Neeman, N. Keller, and A. Barzilai. 2000. Relationship between asymptomatic carriage of Streptococcus pyogenes and the ability of the strains to adhere to and be internalised by cultured epithelial cells. J. Med. Microbiol. 49499-502. [DOI] [PubMed] [Google Scholar]
- 33.Shelburne, S. A., III, C. Granville, M. Tokuyama, I. Sitkiewicz, P. Patel, and J. M. Musser. 2005. Growth characteristics of and virulence factor production by group A streptococcus during cultivation in human saliva. Infect. Immun. 734723-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelburne, S. A., III, P. Sumby, I. Sitkiewicz, C. Granville, F. R. DeLeo, and J. M. Musser. 2005. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc. Natl. Acad. Sci. USA 10216037-16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, J. L. 2004. The physiological role of ferritin-like compounds in bacteria. Crit. Rev. Microbiol. 30173-185. [DOI] [PubMed] [Google Scholar]
- 36.Stadtman, E. R., and B. S. Berlett. 1991. Fenton chemistry. Amino acid oxidation. J. Biol. Chem. 26617201-17211. [PubMed] [Google Scholar]
- 37.Teitzel, G. M., A. Geddie, S. K. De Long, M. J. Kirisits, M. Whiteley, and M. R. Parsek. 2006. Survival and growth in the presence of elevated copper: transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J. Bacteriol. 1887242-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurnham, D. I., A. S. Mburu, D. L. Mwaniki, and A. De Wagt. 2005. Micronutrients in childhood and the influence of subclinical inflammation. Proc. Nutr. Soc. 64502-509. [DOI] [PubMed] [Google Scholar]
- 39.Tonello, F., W. G. Dundon, B. Satin, M. Molinari, G. Tognon, G. Grandi, G. Del Giudice, R. Rappuoli, and C. Montecucco. 1999. The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Mol. Microbiol. 34238-246. [DOI] [PubMed] [Google Scholar]
- 40.Tsai, P. J., C. F. Kuo, K. Y. Lin, Y. S. Lin, H. Y. Lei, F. F. Chen, J. R. Wang, and J. J. Wu. 1998. Effect of group A streptococcal cysteine protease on invasion of epithelial cells. Infect. Immun. 661460-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueshima, J., M. Shoji, D. B. Ratnayake, K. Abe, S. Yoshida, K. Yamamoto, and K. Nakayama. 2003. Purification, gene cloning, gene expression, and mutants of Dps from the obligate anaerobe Porphyromonas gingivalis. Infect. Immun. 711170-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Versieck, J. 1985. Trace elements in human body fluids and tissues. Crit. Rev. Clin. Lab. Sci. 2297-184. [DOI] [PubMed] [Google Scholar]
- 43.Wang, C. H., C. Y. Lin, Y. H. Luo, P. J. Tsai, Y. S. Lin, M. T. Lin, W. J. Chuang, C. C. Liu, and J. J. Wu. 2005. Effects of oligopeptide permease in group A streptococcal infection. Infect. Immun. 732881-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood, D. N., M. A. Chaussee, M. S. Chaussee, and B. A. Buttaro. 2005. Persistence of Streptococcus pyogenes in stationary-phase cultures. J. Bacteriol. 1873319-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto, Y., M. Higuchi, L. B. Poole, and Y. Kamio. 2000. Role of the dpr product in oxygen tolerance in Streptococcus mutans. J. Bacteriol. 1823740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto, Y., L. B. Poole, R. R. Hantgan, and Y. Kamio. 2002. An iron-binding protein, Dpr, from Streptococcus mutans prevents iron-dependent hydroxyl radical formation in vitro. J. Bacteriol. 1842931-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao, G., P. Ceci, A. Ilari, L. Giangiacomo, T. M. Laue, E. Chiancone, and N. D. Chasteen. 2002. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J. Biol. Chem. 27727689-27696. [DOI] [PubMed] [Google Scholar]