Abstract
Secretory immunoglobulin A (SIgA) antibodies directed against the O antigen of lipopolysaccharide (LPS) are the primary determinants of mucosal immunity to gram-negative enteric pathogens. However, the underlying mechanisms by which these antibodies interfere with bacterial colonization and invasion of intestinal epithelial cells are not well understood. In this study, we report that Sal4, a protective, anti-O5-specific monoclonal IgA, is a potent inhibitor of Salmonella enterica serovar Typhimurium flagellum-based motility. Using video light microscopy, we observed that Sal4 completely and virtually instantaneously “paralyzed” laboratory and clinical strains of serovar Typhimurium. Sal4-mediated motility arrest preceded and occurred independently of agglutination. Polyclonal anti-LPS IgG antibodies and F(ab)2 fragments were as potent as was Sal4 at impeding bacterial motility, whereas monovalent Fab fragments were 5- to 10-fold less effective. To determine whether motility arrest can fully account for Sal4's protective capacity in vitro, we performed epithelial cell infection assays in which the requirement for flagellar motility in adherence and invasion was bypassed by centrifugation. Under these conditions, Sal4-treated serovar Typhimurium cells remained noninvasive, revealing that the monoclonal IgA, in addition to interfering with motility, has an effect on bacterial uptake into epithelial cells. Sal4 did not, however, inhibit bacterial uptake into mouse macrophages, indicating that the antibody interferes specifically with Salmonella pathogenicity island 1 (SPI-1)-dependent, but not SPI-1-independent, entry into host cells. These results reveal a previously unrecognized capacity of SIgA to “disarm” microbial pathogens on mucosal surfaces and prevent colonization and invasion of the intestinal epithelium.
Salmonella enterica serovar Typhimurium is an invasive, pathogenic, gram-negative bacterium. In humans these bacteria cause acute gastroenteritis, whereas in mice serovar Typhimurium cells proliferate in the intestinal mucosa and then spread systemically to the liver and spleen, eliciting a disease that resembles typhoid fever (23, 35). Several key attributes of serovar Typhimurium underlie its capacity to successfully colonize and invade the intestinal epithelium. Foremost is lipopolysaccharide (LPS), the major constituent of the outer leaflet of the bacterial outer membrane. In particular, the O-antigen component of LPS aligns laterally to form a protective coat surrounding the bacterium that confers resistance against antimicrobial agents present in intestinal secretions (15, 39). Serovar Typhimurium is also highly motile due to the presence of flagella, which act in concert to propel the bacterium through liquid and viscous environments (4, 28). This motility is postulated to enable the bacterium to penetrate the thick mucus coat that covers the intestinal mucosa, as well as to promote contact with epithelial cell surfaces (29, 48). Finally, serovar Typhimurium cells express a type 3 secretion system (T3SS) encoded by Salmonella pathogenicity island 1 (SPI-1) which allows the bacteria to specifically invade intestinal epithelial cells (14, 21, 23). Once serovar Typhimurium cells have breached the epithelial barrier (at least in the mouse), the bacteria disseminate systemically and reside primarily within macrophages.
In the intestinal tract, secretory immunoglobulin A (SIgA) antibodies directed against the O antigen of serovar Typhimurium are sufficient to prevent mucosal infection (11, 26, 36, 47). This was first demonstrated experimentally by Kraehenbuhl and Neutra and Michetti and colleagues who produced and characterized a collection of B-cell hybridomas isolated from the Peyer's patches of mice immunized with an attenuated strain of serovar Typhimurium (32, 36). From this screen, Michetti and colleagues identified Sal4, an anti-O-antigen-specific, dimeric monoclonal IgA antibody (IgA) that when delivered into the intestinal lumen by normal receptor-mediated transepithelial transport was sufficient to protect mice against a lethal oral challenge with serovar Typhimurium (36). Using an in vitro model system, it was subsequently demonstrated that Sal4 (∼5 μg/ml) prevented serovar Typhimurium from invading polarized epithelial cell monolayers (37). Sal4 did not protect mice against a systemic challenge with serovar Typhimurium, revealing that the monoclonal antibody's mechanism of protection was mucosal specific (36).
It is generally assumed that secretory antibodies function by “immune exclusion,” a term which refers to the ability of polyvalent IgA to promote bacterial agglutination, entrapment in mucus, and clearance via peristalsis (9, 42). While immune exclusion may account for some of the protection conferred by Sal4 in vivo, it cannot explain the capacity of Sal4 to prevent the invasion of polarized epithelial cell monolayers by serovar Typhimurium in vitro. The epithelial cell lines used in these previous studies do not produce detectable amounts of mucus, nor are they able to mediate mechanical clearance (i.e., peristalsis) (37). Agglutination is also unlikely to explain Sal4-mediated immunity, as others have shown that cross-linking of Salmonella enterica serovar Enteritidis cells with antiflagellin (anti-H) antibodies has no effect on their ability to invade epithelial cells in vitro (25).
Therefore, we postulated that Sal4 has additional “effector” function(s) which account for its capacity to inhibit serovar Typhimurium invasion of epithelial cells. In this study, we undertook an examination of the effects of Sal4 on bacterial processes known to be involved in invasion of the intestinal mucosa. We put forth evidence demonstrating that Sal4, at concentrations previously shown to prevent bacterial entry into epithelial cells, is a potent inhibitor of both serovar Typhimurium flagellum-based motility and SPI-1-mediated entry into host cells. These data suggest a possible mechanism to explain Sal4's protective capacity in vitro and in vivo and challenge our previous assumptions about how secretory antibodies interfere with microbial pathogenesis at mucosal surfaces.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Serovar Typhimurium strains JS107 (zjg8101::kan), JS93 (zjg8101::kan oafA126::Tn10d-Tc), JS481 (ΔSPI-1), and JS534 (fkpA-lacZ) are derivatives of strain ATCC 14028 (17, 31) and were kindly provided by James Slauch (University of Illinois at Urbana-Champaign). Serovar Typhimurium strains SJF3 (Δrfc) and SJF10 (zjg8101::kan oafA126::Tn10d-Tc fkpA-lacZ) are isogenic derivatives of ATCC 14028. SJF3 was created by using Lambda Red-mediated recombination with the primer set 5′TTACGCTTCAGAGCCAAATAAAACGGCGGCATTGCCGCCGTATAACTTAATTCCGGGGATCCGTCGACC3′ and 5′GCCTGATGGTAATATTTTTAATACTAAGCATTTTTTCTAAAGGCTCTATATGTGTAGGCTGGAGCTGCTTCG3′, with pKD13 as the antibiotic resistance marker template (16). Appropriate insertion of the antibiotic resistance marker was confirmed by PCR. SJF10 was created by P22 HT int-mediated transduction of fkpA-lacZ from strain JS534 into strain JS93. Strains BJ11 (cheA52 zea::Tn10) and BJ32 (motB275 zea-2::Tn10) are isogenic derivatives of strain SL1344 and were a gift from Brad Jones (University of Iowa) (30). Bacterial strains were routinely cultured at 37°C in Luria-Bertani (LB) broth. When necessary, media were supplemented with antibiotics (Sigma Co., St. Louis, MO) at the following concentrations: kanamycin, 50 μg/ml, and tetracycline, 20 μg/ml.
Antibodies.
The murine hybridoma secreting the monoclonal, polymeric IgA antibody Sal4 was obtained from Marian Neutra (Children's Hospital, Boston, MA) (36). The hybridoma was cultured in CD hybridoma serum-free, protein-free medium (Gibco-Invitrogen, Carlsbad, CA) without antibiotics and was maintained at 37°C in a 5% CO2-95% air atmosphere. Hybridoma supernatants were dialyzed against phosphate-buffered saline (PBS) by means of a Slide-A-Lyzer (10,000-molecular-weight cutoff; Pierce Scientific, Rockford, IL) prior to use. The concentration of Sal4 was determined by sandwich enzyme-linked immunosorbent assay, with IgA TEPC-15 (Sigma Co.) as a standard. Rabbit polyclonal antisera against Salmonella O antigens (group B factors 1, 4, 5, and 12), H antigens (single factor 2 and factor i), and Vibrio cholerae O1 Ogawa were purchased from BD Difco (Franklin Lakes, NJ). All antibody preparations were dialyzed against PBS for >16 h to remove any possible traces of azide or other preservatives prior to being used in the assays described in this study. Dialysis was performed by using 10,000-molecular-weight-cutoff Slide-A-Lyzer dialysis cassettes (Pierce, Rockford, IL).
The murine monoclonal IgA antibody TEPC-15, directed against phosphoryl choline (Sigma Co.), was used as an isotype control for Sal4 in these studies. TEPC-15 has no detectable reactivity with serovar Typhimurium as determined by enzyme-linked immunosorbent assay, nor did it have any effect on bacterial motility (see below). TEPC-15 was routinely reconstituted at 1 mg/ml and dialyzed against PBS prior to use in serovar Typhimurium motility and invasion experiments described herein.
F(ab)2 and Fab fragments were produced essentially as described previously (24). Salmonella O antiserum (100 μl) was mixed with protein A on Sepharose beads (50 μl; Sigma) and incubated with rocking for 2 h at room temperature. For F(ab)2 production, the antibody-protein A bead complexes were collected by centrifugation (2 min at 3,000 × g), washed in PBS, and then suspended in 100 mM sodium citrate (pH 3.5 to 4.0) buffer containing ∼38 units of pepsin bound to agarose (Sigma Co.). Digestion was achieved by incubation at 37°C for 8 h with gentle rocking. The reaction was stopped by the addition of 3 M Tris (pH 8.8). The mixture was subjected to centrifugation (2 min at 3,000 × g) to separate the Fc-protein A agarose beads (pellet) from the F(ab)2 products (supernatant). For Fab production, the antibody-protein A bead complexes were collected by centrifugation (2 min at 3,000 × g), washed in PBS, and then suspended in digestion buffer consisting of 20 mM EDTA, 20 mM l-cysteine (pH 6.3), and 1 unit of papain bound to agarose beads. Digestion was achieved by incubation at 37°C for 12 h with gentle shaking. The Fab products were separated from the Fc-protein A beads and papain-agarose beads by centrifugation, as described above. The purity of the F(ab)2 and Fab-containing supernatants was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with goat anti-rabbit IgG-horseradish peroxidase antibody (heavy plus light chain [H+L] specific; Southern Biotech). F(ab)2 and Fab preparations were dialyzed against PBS (pH 7.4) prior to use in motility and invasion assays.
Bacterial motility assays using video light microscopy.
For agar motility assays, LB agar plates (0.3 g Bacto agar/100 ml LB) containing antibodies (5 μg/ml) were stab inoculated with 1 μl of an overnight culture of serovar Typhimurium. The plates were incubated upright at 37°C for 8 h, and the diameter of bacterial spread was measured every hour. For video light microscopy, bacteria in mid-log phase were diluted into PBS (∼1 × 106 CFU in a volume of 10 μl) at room temperature, mixed with the relevant monoclonal or polyclonal antibodies, and then immediately spotted onto a microscope slide. The droplet was mounted with a coverslip (22 by 22 mm) and imaged at room temperature by using a Nikon TE2000 inverted microscope equipped with differential interference contrast optics, a 40× (0.9 numerical aperture) objective lens, and an HQ camera (Roper Scientific, Trenton, NJ). The images were captured at approximately 7 Hz with RS Image software (Roper Scientific) at 100 frames of 100 ms each. The movements of individual bacteria over time were tracked in sequential video frames by overlaying the video screen with transparency film and highlighting bacteria with an ink pen. In some cases, bacterial motility was monitored with ImagePro software (Media Cybernetics, Silver Springs, MD). Typically, there were 30 to 60 bacteria per field (range, 19 to 123 bacteria); at this density, bacterial “collisions,” and thereby agglutination, were minimized.
For video light microscopy, motility was expressed as percentage of control motility: (% motile bacteria in experimental sample/% motile bacteria in control samples) × 100. TEPC-15 (1 to 10 μg/ml) was used as an IgA isotype control for Sal4 in these experiments. We routinely verified that that the percentage of motile bacteria in TEPC-15-treated cultures did not change over the course of the experiments. For each experimental condition, we analyzed 30 to 40 videos, each containing 30 to 60 bacteria. Three to six replicates were performed per experimental and control conditions. The Student t test was used to determine statistical significance between control and experimental sample results.
Invasion and attachment assays.
MDCK II cells were obtained from the ATCC (CCL-34) and were maintained in Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum at 37°C under a 5% CO2-95% air atmosphere. MDCK II cells were seeded in 96-well plates (∼5 × 104 cells/well in 0.1 ml) and incubated for 7 to 10 days to permit the formation of polarized monolayers. HeLa cells were obtained from the Wadsworth Center cell culture core facility and were maintained as described for the MDCK II cells. HeLa cells were used for invasion assays 24 to 48 h after seeding in 96-well plates (∼70 to 90% confluence). Competitive (and noncompetitive) invasion assays were performed essentially as described previously (37). Serovar Typhimurium strains JS107 and SF10 or JS93 were grown to mid-log phase in LB with aeration, adjusted with fresh LB to equivalent optical densities (optical density at 600 nm, ∼0.7), diluted 1:10, and mixed at equal proportions (∼107 total CFU in 0.2 ml). The exact ratio of the two strains was determined by replica plating. The bacteria were then incubated with Sal4 (5 μg/ml) for either 15 min (to minimize agglutination) or 120 min (to permit agglutination) before being applied to epithelial cells (multiplicity of infection of 10) in 96-well microtiter plates. As necessary, the microtiter plates were subjected to low-speed centrifugation (10 min at 1,000 × g) at 4°C to promote bacterium-epithelial cell contact. The number of invasive bacteria was determined via a gentamicin protection assay (37). The competitive index (CI) was calculated as CI = [(% strain A recovered/% strain B recovered)/(% strain A inoculated/% strain B inoculated)] (17). The Student t test was used to determine statistical significance between control and experimental sample results. For attachment assays, HeLa cells were washed three times in cold Hanks balanced salt solution immediately following centrifugation to remove unbound bacteria and then lysed with a 1% Triton X-100 detergent solution. Numbers of CFU in HeLa cell lysates were determined by plating on LB agar.
The murine macrophage cell line J774A.1 was obtained from the ATCC (TIB-67) and maintained in Dulbecco's minimal essential medium with 10% fetal bovine serum, as recommended by the vendor. For macrophage invasion assays, cells were seeded into 96-well microtiter plates (2 × 105 to 5 × 105 cells per well in 0.2 ml) and used 18 to 40 h later, as described previously (10).
RESULTS
Sal4 arrests flagellum-based motility of serovar Typhimurium, independent of agglutination.
The mucus overlying the intestinal epithelium is one of the first barriers encountered by serovar Typhimurium during the infection process and is a site where SIgA antibodies have been proposed to entrap enteric pathogens (43). We used a standard soft agar motility assay as a model system in which to examine the effects of Sal4 on the ability of serovar Typhimurium to migrate through a semiviscous medium (1). Stab inoculation of wild-type serovar Typhimurium strain JS107 into 0.3% LB agar containing a control IgA antibody, TEPC-15, resulted in measurable migration (∼8 cm) of the bacteria away from the initial site of inoculation over a period of 8 h (Fig. 1). The addition of Sal4 (5 μg/ml) to the medium resulted in an ∼60% reduction in bacterial migration (Fig. 1). This effect on motility was specific, as revealed by the fact that the migration of serovar Typhimurium strain JS93 was unaffected by Sal4 in this assay (data not shown). Strain JS93 is an isogenic derivative of JS107 which, due to a null mutation in the oafA gene, lacks the O5 epitope recognized by Sal4 (50).
FIG. 1.
Measurement of bacterial migration in agar motility assays. Strain JS107 was stab inoculated into 0.3% LB agar containing TEPC-15 (5 μg/ml) or Sal4 (5 μg/ml) and incubated at 37°C for 8 h. The diameter (cm) of the migration was measured at hourly intervals. The results shown are the averages of the results for two plates.
While the results of the soft agar motility assays described above suggested that Sal4 was capable of impairing serovar Typhimurium cells' ability to swim, we were unable to discern whether this was a direct effect on flagellum-based motility or simply the result of antibody-induced agglutination. To distinguish between these two possibilities, we used video light microscopy to visualize and quantitate the motility of individual bacteria in the absence and presence of Sal4 under nonagglutinating conditions. By video light microscopy, we observed that wild-type serovar Typhimurium strain JS107 demonstrated normal swimming behavior, which consisted of “runs” (directional forward movement for ∼1 s) and “tumbles” (∼0.1-s period of random reorientation) (4). In a typical overnight culture, approximately 75% of the bacteria were motile.
Exposure of serovar Typhimurium strain JS107 to TEPC-15 (1 to 15 μg/ml) had no measurable effect on bacterial motility or swimming behavior. In contrast, within minutes of the addition of Sal4 (1 to 5 μg/ml), serovar Typhimurium stopped swimming (Fig. 2). Sal4-mediated motility arrest was both time and dose dependent. Sal4-treated bacteria appeared “paralyzed” and were phenotypically indistinguishable from a serovar Typhimurium nonmotile mutant (data not shown). These bacteria were, however, subject to Brownian motion, indicating that they were not somehow tethered by Sal4 to the surface of the slide or coverslip. Finally, the antibody's effects were antigen specific, because the motility of JS93, which lacks the O5 epitope due to a mutation in the oafA gene, was unchanged upon treatment with Sal4 (Fig. 2).
FIG. 2.
Sal4 arrests motility of serovar Typhimurium cells in a dose- and time-dependent manner. Wild-type serovar Typhimurium cells (strain JS107) or an oafA mutant (strain JS93) were mixed with Sal4 at indicated concentrations, spotted on glass microscope slides, and imaged by video light microscopy at the indicated time points. Motility was expressed as percentage of control motility: (% motile bacteria in experimental sample/% motile bacteria in control samples) × 100. Control samples were treated with TEPC-15 (10 μg/ml) for 15 min, as described in Materials and Methods. Each value represents the average (with standard error) of the results from four to six independent video recordings. An asterisk indicates a statistically significant (P ≤ 0.01) reduction in motility in comparison to that of TEPC-15-treated bacteria.
To address the possibility that these effects were specific to strain JS107 (a derivative of ATCC 14028), we examined the outcome of Sal4 exposure on other serovar Typhimurium O5 serotypes. Sal4 arrested flagellum-based motility of all O5 strains tested, including two common laboratory strains, SL1344 and LT2, as well as five clinical isolates which were obtained from the Bacteriology Laboratory at the Wadsworth Center (data not shown). Interestingly, a serovar Typhimurium cheA mutant which swims constitutively due to a mutation in the chemotaxis signaling pathway (30) was halted upon exposure to Sal4, revealing that Sal4's effects occur independent of chemotaxis.
To investigate whether motility arrest is a general response of serovar Typhimurium bacteria to anti-LPS antibodies, bacteria were treated with commercially available polyclonal rabbit anti-O antiserum (group B factors 1, 4, 5, and 12), which is used routinely in clinical laboratories for serotyping. As shown in Table 1, serovar Typhimurium strain JS107 stopped swimming within minutes of being treated with rabbit anti-O antibodies. In fact, anti-O antiserum was as effective as anti-H (i.e., antiflagellum) antiserum in inhibition of motility (Table 1). It should be noted that, even at the light-microscopic level, the mechanisms by which these antibody preparations immobilized serovar Typhimurium could be seen to differ. Bacteria exposed to anti-H antiserum underwent a “twitching phase” prior to motility arrest. This twitching phase likely represents movement in which flagellum rotation is partially impaired due to flagellum cross-linking and/or bundling (5). In contrast, bacteria treated with Sal4 or anti-O antisera became almost instantaneously immobilized in the absence of any detectable twitching or aberrant swimming behavior. These observations suggest that the mechanisms by which anti-H and anti-O antibodies alter serovar Typhimurium motility are in fact distinct.
TABLE 1.
Effect of anti-LPS and antiflagellum antibodies on serovar Typhimurium motilitya
| Time (min) | Motility with indicated antibody
|
||||||
|---|---|---|---|---|---|---|---|
| TEPC-15 | Sal4 | Anti-O | Anti-Hb | F(ab′)2 | Fab | Fab plus IgGc | |
| ∼1-2 | +++ | ++ | + | ++/+ | ++ | +++ | + |
| 5 | +++ | + | − | +/− | + | ++ | − |
| 10 | +++ | − | − | − | − | ++ | − |
| 15 | +++ | − | − | − | − | ++ | − |
Bacteria (∼1 × 108 per ml) were mixed with antibodies (this was designated time zero) and immediately spotted (10 μl) onto a glass microscope slide, mounted with a coverslip, and visualized by light microscopy. Antibodies were used at a final concentration of 5 μg/ml, except for TEPC-15, which was at 10 μg/ml. Motility was assessed by light microscopy and scored as follows: +++, more than 60% of the bacteria were motile; ++, between 30 and 60% of the bacteria were motile; +, less than 30% of the bacteria were motile; −, none of the bacteria were motile.
Anti-H antiserum reacts with both phase 1 and 2 flagellins.
Bacteria were exposed to Fab fragments (5 μg/ml) for 2 min and then mixed with rabbit anti-goat IgG (H+L) immediately prior to being spotted onto glass microscope slides.
The fact that Sal4 is polymeric in nature (i.e., primarily a dimer) seemed of little consequence with respect to its ability to interfere with serovar Typhimurium motility, as anti-LPS IgG antibodies (which consist exclusively of monomers) were equally capable at arresting bacterial swimming. However, both IgG and IgA are bivalent molecules capable of binding to two adjacent O-antigen side chains and thereby are potentially cross-linking LPS molecules. To determine the importance of LPS cross-linking in motility arrest, we performed bacterial motility assays in the presence of monovalent antigen binding fragments (Fab fragments). Fab fragments were generated from rabbit polyclonal anti-LPS antisera rather than Sal4, because murine IgA Fab fragments are unstable due to the lack of an interchain (H+L) disulfide bond (37). At 5 μg/ml, Fab fragments had a slight effect on bacterial motility, whereas IgG antibodies at the same concentration completely stopped bacterial movement (Table 1). However, when anti-LPS Fab fragments (5 μg/ml) were incubated with serovar Typhimurium and then artificially cross-linked by the addition of goat anti-rabbit polyclonal antibodies, bacterial motility was arrested almost instantaneously (Table 1). Monovalent Fab fragments were capable of fully inducing arrest, but only at concentrations of ≥15 μg/ml. As expected, anti-LPS F(ab)2 fragments, which retain bivalent binding activity but lack the IgG Fc region, were as effective as whole IgG antibodies in arresting motility (Table 1). We conclude that cross-linking enhances, but is not essential for, the ability of anti-LPS antibodies to arrest serovar Typhimurium motility.
Finally, we wished to examine the possibility that the mechanism by which Sal4 arrests the motility of serovar Typhimurium involves physical (“steric”) interference in flagella rotation (i.e., the Fc region of Sal4 may extend beyond the LPS and physically impede flagella rotation). This was accomplished by testing the effect of Sal4 on the motility of a serovar Typhimurium Δrfc strain. Whereas LPS in wild-type serovar Typhimurium cells consists of 20 to 35 O-antigen repeats and is ∼20 to 30 nm in length, the LPS of the Δrfc strain has only a single O-antigen unit and extends ∼6 nm from the outer membrane. As a dimeric IgA, Sal4 would be expected to be ∼28 nm from one end to the other and would not project sufficiently far from the cell surface that it could effectively interfere either with the flagella filament or the hook (7). Indeed, the motility of the Δrfc strain was impaired by the addition of Sal4 or anti-LPS Fab fragments (data not shown). These and other data (see Discussion) suggest that it is highly unlikely that Sal4, when bound to LPS on the surface of serovar Typhimurium cells, physically interferes with flagellum rotation.
Sal4 inhibits serovar Typhimurium invasion of epithelial cells, even when the requirement for motility is bypassed through centrifugation.
Motility is required for serovar Typhimurium cells to gain entry into epithelial cells in vitro, as evidenced by the fact that nonmotile mutants are 20 to 50 times less invasive than are wild-type controls (30). Therefore, Sal4's effect on serovar Typhimurium's flagellum-based motility might account for the previously reported capability of Sal4 to inhibit bacterial invasion of polarized epithelial cell monolayers (37). This possibility is testable, as the requirement for motility in the invasion process can be overcome through the use of centrifugation to artificially promote bacterium-host cell contacts (30). If Sal4 acts solely by interfering with the motility of serovar Typhimurium cells, then we would expect that centrifugation of Sal4-treated bacteria onto polarized epithelial cell monolayers would restore invasion to levels observed for untreated bacteria. On the other hand, if Sal4 interferes with another step in the entry process, then we would expect that Sal4-treated bacteria would remain noninvasive, even when contact with host cells is enhanced by centrifugation.
We used a competitive in vitro invasion assay (see Materials and Methods) to distinguish between these two possibilities. Using this method, we confirmed that Sal4 reduced the ability of serovar Typhimurium bacteria to invade polarized epithelial cell monolayers by more than 10-fold (Fig. 3A). Identical results were obtained whether invasion assays were performed under bacteria-agglutinating conditions (data not shown), as done previously by Michetti and colleagues (37), or under nonagglutinating conditions (Fig. 3A). We then introduced centrifugation into the invasion assay protocol. Under these conditions, Sal4-treated bacteria attached to epithelial cells as well as control bacteria (Fig. 4). However, in spite of the addition of centrifugation, Sal4-treated serovar Typhimurium remained noninvasive (Fig. 3A). To ensure that the centrifugation protocol was sufficient to restore the invasiveness of a nonmotile mutant of serovar Typhimurium, as originally reported by Jones and colleagues (29), we performed invasion assays using an motB mutant. The motB strain of serovar Typhimurium assembles flagella normally on the cell surface, but these flagella are unable to rotate due to a defect in the motor protein. As expected, the motB mutant was highly attenuated for entry into polarized epithelial cell monolayers, and this defect could be overcome by centrifugation (Fig. 3B). From the results of these experiments, we conclude that Sal4, in addition to inhibiting flagellum-based motility, affects a step downstream of attachment in the SPI-1-dependent entry of serovar Typhimurium bacteria into epithelial cells.
FIG. 3.
Sal4 blocks serovar Typhimurium invasion of epithelial cells, even when the requirement for motility is bypassed through centrifugation. (A) Wild-type (strain JS107) and oafA mutant (strain JS93) serovar Typhimurium cells were mixed 1:1 and incubated for 15 min with 5 μg/ml TEPC-15 or Sal4 before being applied to MDCK II cell monolayers in 96-well microtiter plates and subjected (or not) to brief centrifugation, as described in Materials and Methods, so as to promote (or not promote) bacterium-epithelial cell contact. The results shown are the average values (with standard errors) from three independent experiments, each done in triplicate. An asterisk indicates a statistically significant (P ≤ 0.05) reduction in invasion in the presence of Sal4 in comparison to that in the TEPC-15 control. (B) Centrifugation enhances uptake of a nonmotile mutant of serovar Typhimurium. Cells of the wild type and a nonmotile motB serovar Typhimurium strain were mixed 1:1 before being applied to MDCK II cell monolayers in 96-well microtiter plates and subjected (or not) to brief centrifugation. The results shown are the average values (with standard errors) from three independent experiments, each done in triplicate. An asterisk indicates that bacterial invasion in the presence of centrifugation (+) was significantly (P ≤ 0.05) greater than in the absence of centrifugation (−).
FIG. 4.
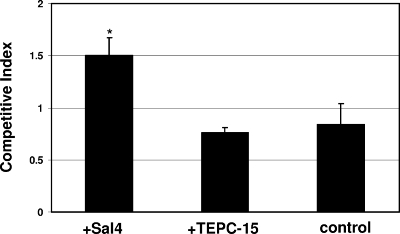
Sal4 does not impede serovar Typhimurium attachment to epithelial cells. The wild-type or ΔSPI-1 strain of serovar Typhimurium was mixed with the oafA mutant at a 1:1 ratio, incubated without antibody (white bars) or with Sal4 (5 μg/ml; black bars) for 15 min, applied to HeLa cells seeded in 96-well microtiter plates, and then subjected to centrifugation (10 min at 4°C) to promote bacterium-epithelial cell adherence. Immediately thereafter, the HeLa cells were washed to remove unbound bacteria and then lysed with 1% Triton X-100. The number of bacteria in the lysates (CFU) was determined by serial dilution onto LB. The CI reflects the attachment of wild-type or ΔSPI-1 strain cells to HeLa cells relative to the attachment of oafA mutant cells. Sal4 marginally enhanced attachment of both the wild-type (P = 0.06) and the ΔSPI1 (P = 0.02) serovar Typhimurium strain to HeLa cells, possibly due to antibody-mediated agglutination that may have occurred during centrifugation.
While these data strongly suggested that agglutination by Sal4 is not a determinant in the blockage of bacterial entry, we could not exclude the possibility that some antibody-mediated cross-linking occurred on the surfaces of epithelial cells immediately following centrifugation. To address this concern, we performed invasion assays in the presence of Fab fragments derived from polyclonal anti-LPS IgG antiserum, as described above. We found that monovalent Fab fragments (14 μg/ml) blocked serovar Typhimurium entry into epithelial cells as effectively as did Sal4 (5 μg/ml) or anti-LPS F(ab)2 (5 μg/ml) (Fig. 5). The extent of invasion observed following antibody treatment was similar to that observed for an ΔSPI-1 strain, which lacks the SPI-1 T3SS and effector proteins (17). These data demonstrate conclusively that agglutination is not integral to Sal4's capability to block serovar Typhimurium entry into epithelial cells.
FIG. 5.
Anti-LPS antibodies inhibit invasion by serovar Typhimurium cells of epithelial cells independent of bacterial agglutination. Serovar Typhimurium strain JS107 was incubated with TEPC-15 (5 μg/ml), Sal4 (5 μg/ml), polyclonal anti-LPS F(ab)2 (5 μg/ml), or Fab fragments (14 μg/ml) for 15 min and then applied to HeLa cells seeded in 96-well microtiter plates. The noninvasive ΔSPI-1 strain was used in parallel as a control. Due to the reactivity of polyclonal anti-O antibodies with the oafA strain of serovar Typhimurium, we were unable to perform competitive invasion assays in this experiment. Therefore, invasion is expressed as percentage of initial inoculum. The data shown are the average values (with standard errors of the means) from a single representative experiment done in triplicate. An asterisk indicates a statistically significant (P ≤ 0.05) reduction in invasion in the presence of Sal4 in comparison to that of the TEPC-15 control. +, present.
Sal4 interferes with SPI-1-dependent, but not SPI-1-independent, entry of serovar Typhimurium into host cells.
Serovar Typhimurium entry into intestinal epithelial cells is strictly dependent on the SPI-1 T3SS and its effector proteins (21). In contrast, bacterial uptake by phagocytic cells, such as macrophages, is fundamentally different; it occurs by host cell-mediated phagocytosis and does not require SPI-1. We wished to examine whether Sal4 blocks SPI-1-independent, as well as SP-1-dependent, uptake of serovar Typhimurium into host cells. This was accomplished by means of a J774 macrophage invasion assay (10). Whereas Sal4 blocked serovar Typhimurium invasion of epithelial cells (Fig. 3), Sal4 did not interfere with bacterial entry into J774 cells. In fact, we observed that under agglutinating conditions, Sal4 slightly enhanced bacterial entry into J774 cells (Fig. 6), possibly because the macrophages were able to engulf bacteria-antibody aggregates. It should be noted that J774 cells do not express the recently described Fcα/μ receptor (49). From these data, we conclude that Sal4 inhibits SPI-1-dependent, but not -independent, entry of serovar Typhimurium bacteria into host cells.
FIG. 6.
Serovar Typhimurium uptake by murine macrophages in the presence of Sal4. A 1:1 mixture of wild-type and oafA strain serovar Typhimurium cells was incubated with Sal4 (5 μg/ml), TEPC-15 (5 μg/ml), or PBS and then applied to J774 cells seeded in 96-well microtiter plates. The CI, which reflects the ratio of wild-type serovar Typhimurium cells to oafA strain cells that were taken up into J774 cells, was determined as described in Materials and Methods. The results shown are from a single representative experiment done in quadruplicate. The asterisk indicates that Sal4 enhanced (P ≤ 0.05) the uptake of serovar Typhimurium cells into macrophages in comparison to that of TEPC-15-treated bacteria, possibly due to the fact that Sal4 was used under agglutinating conditions in this assay. +, present.
DISCUSSION
Despite the recognized importance of anti-LPS SIgA antibodies in preventing the colonization and invasion of epithelial cells by enteric pathogens (3, 9, 12, 27, 36, 37, 42, 44, 53), the underlying mechanisms by which these antibodies function in mucosal immunity remains unclear. In this study, we have demonstrated that Sal4, an anti-LPS monoclonal IgA that was previously shown to protect mice against oral challenge with serovar Typhimurium bacteria, is a potent inhibitor of serovar Typhimurium flagellum-based motility. Sal4's inhibitory effects were rapid (<15 min) and occurred independently of bacterial agglutination. The concentration of Sal4 (3 to 5 μg/ml) required to arrest motility is identical to what Michetti and colleagues previously reported as being necessary to block serovar Typhimurium invasion of intestinal epithelial cells (37). In addition to its effect on motility, we also propose that Sal4 interferes with SPI-1-mediated type three secretion. This conclusion is based primarily on our observation that Sal4-treated serovar Typhimurium cells were unable to invade epithelial cells in vitro, even when the requirement for motility in the invasion process was obviated by centrifugation of antibody-treated bacteria directly onto host cells. While the underlying mechanism by which Sal4 interferes with these two microbial processes (i.e., flagellum-based motility and type three secretion) remains to be elucidated, the data presented in this study reveal a previously unrecognized capacity of SIgA to potentially “disarm” enteric pathogens in mucosal secretions and thereby prevent colonization and invasion of the intestinal epithelium.
The capacity of anti-LPS IgA and IgG antibodies to interfere with the flagellum-based motility of serovar Typhimurium is an observation that, to our knowledge, has not been previously described in the literature and which may potentially have important implications for understanding microbial physiology, as well as mucosal immunity (as discussed below). While anti-LPS antibodies have been used for decades in clinical and diagnostic laboratories for serotyping members of the Salmonella, the end point of these assays is typically agglutination, which is measured macroscopically. In this study, the effect of Sal4 on serovar Typhimurium motility was only revealed by light microscopy and when the ratio of antibody-to-bacteria was adjusted so as to minimize agglutination. However, an effect of anti-LPS antibodies on flagellar motility of an enteric pathogen is not completely unprecedented. It is has been recognized for more than 20 years that anti-LPS antiserum interferes with the motility of V. cholerae (18, 22). V. cholerae differs from serovar Typhimurium in that it has a single, polar flagellum that is sheathed with LPS. It has generally been assumed, in the case of V. cholerae, that anti-LPS antibodies interfere with motility by coating the flagellum and impeding rotation by physical drag or cross-linking (18, 22). This cannot be the case for serovar Typhimurium bacteria, as their flagella (4 to 6 per cell) are not sheathed and are not recognized by anti-LPS antibodies.
How does Sal4 (and anti-LPS antibodies in general) interfere with serovar Typhimurium flagellum-based motility? Theoretically, it is unlikely that Sal4, when bound to the O antigen, sterically interferes with flagellum rotation. Serovar Typhimurium flagella each consist of a basal body spanning the inner and outer membranes, a hook, and a helical filament (4, 28). The flagellar hook is a highly flexible structure ∼55 nm in length, while the filament is up to 10 μm long and can assume different polymorphic forms in response to strain (4, 51). As a dimeric immunoglobulin, Sal4 is nominally 28 nm from one end to the other and would not be expected to project sufficiently far from the cell surface that it could effectively interfere with either the hook or filament (7, 54). Fab fragments, which also affected motility (albeit at relatively high concentrations), are ∼7 nm in length. This theoretical argument is supported by the fact that Sal4 impaired the motility of an Δrfc strain of serovar Typhimurium, whose severely truncated O antigen extends less than 6 nm from the outer membrane. Additionally, we estimate that Sal4-mediated motility arrest occurred at antibody concentrations sufficient to occupy less than 5% of the LPS on the surface of serovar Typhimurium cells, assuming there are ∼3.5 ×106 LPS molecules per cell (40). It seems unlikely that Sal4 bound to only a small fraction of LPS molecules on the cell surface could impede the rotation of the flagella, especially when one considers that a single flagellum generates enough torque to rotate an entire bacterium (6).
Does the association of Sal4 with the O antigen trigger a signaling cascade that could account for motility arrest? While we expect that one or more so-called extracytoplasmic stress responses (ESR) in serovar Typhimurium may be induced as a consequence of Sal4 binding to the cell surface, there is little evidence in the literature to suggest that the activation of an ESR could be the cause of flagellar dysfunction. Cpx and σE are the two major ESRs in serovar Typhimurium: Cpx is induced by high pH, misfolded periplasmic proteins, and abnormalities in the inner membrane, whereas σE is triggered by heat, ethanol, unfolded outer membrane proteins, and abnormal LPS (2, 45, 46). Although there is a connection between these ESRs and flagellar assembly/biosynthesis, there is no known direct link between these regulons and control of flagellum rotation (34). Therefore, while binding of Sal4 to LPS could result in downregulation at the transcriptional level of the flagellar operons, it is unlikely that the activation of either Cpx or σE is directly responsible for the antibody-mediated motility arrest observed in this study.
While it is largely speculative at this point, we propose that the proton motive force (PMF) may be the link between Sal4, motility arrest, and inhibition of SPI-1-mediated entry into epithelial cells. In serovar Typhimurium, the PMF is the primary transmembrane proton electrochemical gradient across the inner membrane and is determined by both the transmembrane electrical potential and proton gradient (4). Flagellum-based motility is entirely dependent on the PMF, to the degree that flagellar motor speed varies linearly with the PMF gradient (19). In the presence of an intact PMF, the flagella are constantly rotating in a counterclockwise or clockwise manner, such that at any point in time serovar Typhimurium cells are either running or tumbling, respectively (4). “Paralysis” of serovar Typhimurium cells (as observed following Sal4 treatment) occurs only upon dissipation of the PMF (4). There is also increasing evidence that the export of effector proteins through T3SSs, such as SPI-1, requires an intact PMF (20, 52). While it is unknown at this time how Sal4, through its interaction with LPS, might affect bacterial bioenergetics, we postulate that the antibody may induce physical and/or mechanical stress on the bacterial envelope. It is known that physical/mechanical alterations in the curvature or tension of the bacterial envelope can perturb cytoplasmic homeostasis and result in reductions in bioenergetic potential (8). It should be underscored that Sal4 is neither bacteriostatic nor bactericidal, even in the presence of complement (36). Therefore, any effects on serovar Typhimurium physiology induced following antibody association with the outer leaflet of the outer membrane are certainly transient. This is in contrast to bactericidal IgG and IgM antibodies directed against certain surface-exposed outer membrane proteins of Borrelia species (13, 33) and Haemophilus influenza (38).
The results presented in this study are in accordance with Michetti and colleagues' original conclusion that Sal4 alone can prevent the adherence and invasion of serovar Typhimurium to epithelial cells in the absence of other immune or nonimmune protective mechanisms (36, 37). While these authors noted that Sal4's protective effects correlated with bacterial agglutination, they were careful to point out that agglutination per se was unlikely to explain the antibody's observed activity. We now propose that Sal4's protective effects are more likely due to the antibody's ability to interfere with motility and SPI-1 type three secretion. Indeed, anti-LPS SIgA antibodies are known to be protective against virtually all enteric pathogens, including Shigella flexneri, Escherichia coli, and Vibrio cholerae (3, 12, 27, 42, 44, 53), and the results of this and other studies (41) challenge us to rethink the long-held assumption that SIgA is primarily a physical barrier separating host and microbe.
Acknowledgments
We gratefully acknowledge Richard Cole (Advanced Light Microscopy Core Facility, Wadsworth Center) for technical assistance with the video microscopy. We extend a special thanks to James Slauch (University of Illinois Urbana-Champaign) for his helpful advice and technical suggestions, as well as his generosity in providing us with bacterial strains and plasmids. We thank Brad Jones (University of Iowa) for strains BJ11 and BJ32 and Marian Neutra (Children's Hospital Boston) for hybridoma Sal4. We thank Carolyn McGuinness, Tia Bumpus, and Elizabeth McCarthy for technical assistance.
N.J.M. wishes to recognize financial support from the Wadsworth Center and the Northeast Biodefense Center (NIH grant U54-AI057158).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Adler, J. 1966. Chemotaxis in bacteria. Science 153708-716. [DOI] [PubMed] [Google Scholar]
- 2.Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 52613-619. [DOI] [PubMed] [Google Scholar]
- 3.Apter, F. M., P. Michetti, L. S. D. Winner, J. A. Mack, J. J. Mekalanos, and M. R. Neutra. 1993. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect. Immun. 615279-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 7219-54. [DOI] [PubMed] [Google Scholar]
- 5.Berg, H. C., and R. A. Anderson. 1973. Bacteria swim by rotating their flagellar filaments. Nature 245380-382. [DOI] [PubMed] [Google Scholar]
- 6.Berg, H. C., and L. Turner. 1993. Torque generated by the flagellar motor of Escherichia coli. Biophys. J. 652201-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehm, M. K., J. M. Woof, M. A. Kerr, and S. J. Perkins. 1999. The Fab and Fc fragments of IgA1 exhibit a different arrangement from that in IgG: a study by X-ray and neutron solution scattering and homology modelling. J. Mol. Biol. 2861421-1447. [DOI] [PubMed] [Google Scholar]
- 8.Booth, I. R., M. D. Edwards, S. Black, U. Schumann, and S. Miller. 2007. Mechanosensitive channels in bacteria: signs of closure? Nat. Rev. Microbiol. 5431-440. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg, P. 2007. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 255467-5484. [DOI] [PubMed] [Google Scholar]
- 10.Buchmeier, N. A., and F. Heffron. 1989. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect. Immun. 571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chau, P. Y., R. S. Tsang, S. K. Lam, J. T. La Brooy, and D. Rowley. 1981. Antibody response to the lipopolysaccharide and protein antigens of Salmonella typhi during typhoid infection. II. Measurement of intestinal antibodies by radioimmunoassay. Clin. Exp. Immunol. 46515-520. [PMC free article] [PubMed] [Google Scholar]
- 12.Chowers, Y., J. Kirschner, N. Keller, I. Barshack, S. Bar-Meir, S. Ashkenazi, R. Schneerson, J. Robbins, and J. H. Passwell. 2007. Specific polysaccharide conjugate vaccine-induced IgG antibodies prevent invasion of Shigella into Caco-2 cells and may be curative. Proc. Natl. Acad. Sci. USA 1042396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly, S. E., and J. L. Benach. 2005. The versatile roles of antibodies in Borrelia infections. Nat. Rev. Microbiol. 3411-420. [DOI] [PubMed] [Google Scholar]
- 14.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304242-248. [DOI] [PubMed] [Google Scholar]
- 15.Dann, S. M., and L. Eckmann. 2007. Innate immune defenses in the intestinal tract. Curr. Opin. Gastroenterol. 23115-120. [DOI] [PubMed] [Google Scholar]
- 16.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellermeier, C. D., J. R. Ellermeier, and J. M. Slauch. 2005. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 57691-705. [DOI] [PubMed] [Google Scholar]
- 18.Fuerst, J. A., and J. W. Perry. 1988. Demonstration of lipopolysaccharide on sheathed flagella of Vibrio cholerae O:1 by protein A-gold immunoelectron microscopy. J. Bacteriol. 1701488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabel, C. V., and H. C. Berg. 2003. The speed of the flagellar rotary motor of Escherichia coli varies linearly with protonmotive force. Proc. Natl. Acad. Sci. USA 1008748-8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galan, J. E. 2008. Energizing type III secretion machines: what is the fuel? Nat. Struct. Mol. Biol. 15127-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galan, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444567-573. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson, B., and T. Holme. 1985. Rapid detection of Vibrio cholerae O:1 by motility inhibition and immunofluorescence with monoclonal antibodies. Eur. J. Clin. Microbiol. 4291-294. [DOI] [PubMed] [Google Scholar]
- 23.Haraga, A., M. B. Ohlson, and S. I. Miller. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 653-66. [DOI] [PubMed] [Google Scholar]
- 24.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Iankov, I. D., D. P. Petrov, I. V. Mladenov, I. H. Haralambieva, R. Ivanova, V. R. Velev, and I. G. Mitov. 2002. Production and characterization of monoclonal immunoglobulin A antibodies directed against Salmonella H:g,m flagellar antigen. FEMS Immunol. Med. Microbiol. 33107-113. [DOI] [PubMed] [Google Scholar]
- 26.Iankov, I. D., D. P. Petrov, I. V. Mladenov, I. H. Haralambieva, O. K. Kalev, M. S. Balabanova, and I. G. Mitov. 2004. Protective efficacy of IgA monoclonal antibodies to O and H antigens in a mouse model of intranasal challenge with Salmonella enterica serotype Enteritidis. Microbes Infect. 6901-910. [DOI] [PubMed] [Google Scholar]
- 27.Iankov, I. D., D. P. Petrov, I. V. Mladenov, I. H. Haralambieva, and I. G. Mitov. 2002. Lipopolysaccharide-specific but not anti-flagellar immunoglobulin A monoclonal antibodies prevent Salmonella enterica serotype Enteritidis invasion and replication within HEp-2 cell monolayers. Infect. Immun. 701615-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iino, T. 1974. Assembly of Salmonella flagellin in vitro and in vivo. J. Supramol. Struct. 2372-384. [DOI] [PubMed] [Google Scholar]
- 29.Jones, B., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiated murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's Patches. J. Exp. Med. 18015-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, B. D., C. A. Lee, and S. Falkow. 1992. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect. Immun. 602475-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, M. L., and J. M. Slauch. 1999. Effect of acetylation (O-factor 5) on the polyclonal antibody response to Salmonella typhimurium O-antigen. FEMS Immunol. Med. Microbiol. 2683-92. [DOI] [PubMed] [Google Scholar]
- 32.Kraehenbuhl, J. P., and M. R. Neutra. 1992. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol. Rev. 72853-879. [DOI] [PubMed] [Google Scholar]
- 33.LaRocca, T. J., L. I. Katona, D. G. Thanassi, and J. L. Benach. 2008. Bactericidal action of a complement-independent antibody against relapsing fever Borrelia resides in its variable region. J. Immunol. 1806222-6228. [DOI] [PubMed] [Google Scholar]
- 34.Lin, D., C. V. Rao, and J. M. Slauch. 2008. The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J. Bacteriol. 19087-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackaness, G. B., R. V. Blanden, and F. M. Collins. 1966. Host-parasite relations in mouse typhoid. J. Exp. Med. 124573-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michetti, P., M. J. Mahan, J. M. Slauch, J. J. Mekalanos, and M. R. Neutra. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 601786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michetti, P., N. Porta, M. J. Mahan, J. M. Slauch, J. J. Mekalanos, A. Blum, J. P. Kraehenbuhl, and M. R. Neutra. 1994. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology 107915-923. [DOI] [PubMed] [Google Scholar]
- 38.Nelson, M. B., T. F. Murphy, H. van Keulen, D. Rekosh, and M. A. Apicella. 1988. Studies on P6, an important outer-membrane protein antigen of Haemophilus influenzae. Rev. Infect. Dis. 10(Suppl. 2)S331-S336. [DOI] [PubMed] [Google Scholar]
- 39.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikaido, H. 1996. Outer membrane. In F. C. Neidhardt, R. I. Curtiss, J. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. ASM Press, Washington, DC.
- 41.Peterson, D. A., N. P. McNulty, J. L. Guruge, and J. I. Gordon. 2007. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2328-339. [DOI] [PubMed] [Google Scholar]
- 42.Phalipon, A., A. Cardona, J. P. Kraehenbuhl, L. Edelman, P. J. Sansonetti, and B. Corthesy. 2002. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17107-115. [DOI] [PubMed] [Google Scholar]
- 43.Phalipon, A., and B. Corthesy. 2003. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2455-58. [DOI] [PubMed] [Google Scholar]
- 44.Phalipon, A., P. Michetti, M. Kaufmann, J. M. Cavaillon, M. Huerre, J. P. Kraehenbuhl, and P. J. Sansonetti. 1994. Protection against invasion of the mouse pulmonary epithelium by a monoclonal IgA directed against Shigella flexneri lipopolysaccharide. Ann. N. Y. Acad. Sci. 730356-358. [DOI] [PubMed] [Google Scholar]
- 45.Rowley, G., M. Spector, J. Kormanec, and M. Roberts. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4383-394. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz, N., D. Kahne, and T. J. Silhavy. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 457-66. [DOI] [PubMed] [Google Scholar]
- 47.Sarasombath, S., N. Banchuin, T. Sukosol, B. Rungpitarangsi, and S. Manasatit. 1987. Systemic and intestinal immunities after natural typhoid infection. J. Clin. Microbiol. 251088-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt, C. K., J. S. Ikeda, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, and A. D. O'Brien. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 695619-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shibuya, A., N. Sakamoto, Y. Shimizu, K. Shibuya, M. Osawa, T. Hiroyama, H. J. Eyre, G. R. Sutherland, Y. Endo, T. Fujita, T. Miyabayashi, S. Sakano, T. Tsuji, E. Nakayama, J. H. Phillips, L. L. Lanier, and H. Nakauchi. 2000. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 1441-446. [DOI] [PubMed] [Google Scholar]
- 50.Slauch, J. M., M. J. Mahan, P. Michetti, M. R. Neutra, and J. J. Mekalanos. 1995. Acetylation (O-factor 5) affects the structural and immunological properties of Salmonella typhimurium lipopolysaccharide O antigen. Infect. Immun. 63437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner, L., W. S. Ryu, and H. C. Berg. 2000. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 1822793-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilharm, G., V. Lehmann, K. Krauss, B. Lehnert, S. Richter, K. Ruckdeschel, J. Heesemann, and K. Trulzsch. 2004. Yersinia enterocolitica type III secretion depends on the proton motive force but not on the flagellar motor components MotA and MotB. Infect. Immun. 724004-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winner, L. D., J. Mack, R. Weltzin, J. J. Mekalanos, J. P. Kraehenbuhl, and M. R. Neutra. 1991. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect. Immun. 59977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woof, J. M., and M. A. Kerr. 2006. The function of immunoglobulin A in immunity. J. Pathol. 208270-282. [DOI] [PubMed] [Google Scholar]