Abstract
Enterococcus faecium is a multidrug-resistant opportunist causing difficult-to-treat nosocomial infections, including endocarditis, but there are no reports experimentally demonstrating E. faecium virulence determinants. Our previous studies showed that some clinical E. faecium isolates produce a cell wall-anchored collagen adhesin, Acm, and that an isogenic acm deletion mutant of the endocarditis-derived strain TX0082 lost collagen adherence. In this study, we show with a rat endocarditis model that TX0082 Δacm::cat is highly attenuated versus wild-type TX0082, both in established (72 h) vegetations (P < 0.0001) and for valve colonization 1 and 3 hours after infection (P ≤ 0.0002), making Acm the first factor shown to be important for E. faecium pathogenesis. In contrast, no mortality differences were observed in a mouse peritonitis model. While 5 of 17 endocarditis isolates were Acm nonproducers and failed to adhere to collagen in vitro, all had an intact, highly conserved acm locus. Highly reduced acm mRNA levels (≥50-fold reduction relative to an Acm producer) were found in three of these five nonadherent isolates, including the sequenced strain TX0016, by quantitative reverse transcription-PCR, indicating that acm transcription is downregulated in vitro in these isolates. However, examination of TX0016 cells obtained directly from infected rat vegetations by flow cytometry showed that Acm was present on 40% of cells grown during infection. Finally, we demonstrated a significant reduction in E. faecium collagen adherence by affinity-purified anti-Acm antibodies from E. faecium endocarditis patient sera, suggesting that Acm may be a potential immunotarget for strategies to control this emerging pathogen.
Infective endocarditis (IE), an infection of the endocardial surface of the heart, is among the most severe complications of enterococcal bacteremia. Enterococci are ranked third in causing this disease overall (14) and first (5) or second (11) in causing health care-associated endocarditis. In patients of ≤60 years of age, 5 to 8% of cases of native valve endocarditis are due to enterococci, and for patients aged >60 years, enterococci account for 14 to 17% of cases (14). In addition, enterococcal endocarditis is a frequent problem in patients with prosthetic valve replacement (14).
Although Enterococcus faecalis is a more common cause of endocarditis, Enterococcus faecium is more problematic because this species has increasingly acquired resistance to multiple antimicrobials, including ampicillin, vancomycin, and aminoglycosides (12, 14). E. faecium, previously thought to be a harmless commensal and, in fact, used as a probiotic for decades, can spread beyond its gastrointestinal niche into the bloodstream and other sites, with subsequent attachment and initiation of infection. Thus, adherence of E. faecium to collagen-rich heart valves at sites of valvular injury could be important to its ability to cause endocarditis.
In an earlier study, we reported that some E. faecium clinical isolates exhibit collagen adherence, and we identified a homologue of Cna (a collagen adhesin shown to be important for Staphylococcus aureus endocarditis [8]), which we named Acm, for adhesin of collagen from E. faecium, in our draft genome sequence of the human endocarditis E. faecium isolate TX0016 (referred to elsewhere as strain DO) (22). Acm has typical characteristics of an MSCRAMM (microbial surface components recognizing adhesive matrix molecules) (24), including the DE variant of an immunoglobulin (Ig)-like fold (17). Introduction of an intact acm gene into two natural acm mutant E. faecium strains resulted in up to 11-fold enhancement in their capacity to adhere to collagen (22) but not to fibronectin and fibrinogen. In subsequent genetic analyses, we generated isogenic acm mutants of two endocarditis-derived E. faecium isolates and showed that they had substantially reduced collagen type I adherence, confirming that Acm mediates E. faecium adherence to collagen (19). Data from our companion paper (20) show that the acm locus is very widely distributed but is found as a functional gene almost exclusively in clinically derived multidrug-resistant E. faecium isolates, particularly those of the globally spread clonal complex 17 (9) and endocarditis-derived isolates, and occurs as a transposon-interrupted pseudogene in approximately one quarter of the community-derived and animal isolates examined. However, a role for Acm in E. faecium pathogenesis has not been demonstrated.
The present study was aimed at testing our hypotheses that (i) the absence of Acm influences the outcome in animal models (rat endocarditis and mouse peritonitis models) of infection, (ii) the acm genes of all endocarditis-derived strains are intact and may be regulated at the transcriptional or posttranscriptional level in nonadhering strains, and (iii) acm is expressed during experimental IE even if it is not produced under in vitro growth conditions.
MATERIALS AND METHODS
Bacterial strains and culture media.
The eight endocarditis-derived E. faecium isolates used in this study are listed in Table 1. Collagen adherence phenotypes of these isolates were assessed elsewhere (20, 22). An allelic replacement acm deletion mutant, TX6051 (TX0082 Δacm::cat; resistant to chloramphenicol [10 μg/ml]), with no adherence to collagen has been described previously (19). Probing of Northern blots of wild-type E. faecium strain TX0082 total RNA with acm showed a single RNA band of ∼2.4 kb, consistent with the expected size of a monocistronic acm mRNA transcript and confirming the nonpolar nature of the acm deletion (data not shown). Brain heart infusion (BHI) broth and agar (Difco Laboratories, Detroit, MI) were used for routine E. faecium growth.
TABLE 1.
Characteristics of bacterial isolates used in this study
| Isolate name | Phenotype or genotypea | Origin; place of isolation; yr of isolation | PFGE typeb | Reference |
|---|---|---|---|---|
| TX0082 | Vanr Ampr Cn-Adh+ | Endocarditis isolate; Houston, TX; 1999 | PF1 | 19 |
| TX6051 | TX0082 Δacm::cat; acm deletion mutant of TX0082; Chlr Cn-Adh− | 19 | ||
| TX0016 (DO) | Vms Apr Cn-Adh− | Endocarditis isolate; Houston, TX; 1992 | PF3 | 22, 26 |
| TX0068 | Vms Apr Cn-Adh− | Endocarditis isolate; Worcester, MA; 1996 | PF5 | 20 |
| TX0080 | Vmr Apr Cn-Adh− | Endocarditis isolate; Worcester, MA; 1996 | PF7 | 20, 22 |
| TX0110 | Vmr Apr Cn-Adh− | Endocarditis isolate; Houston, TX; 2002 | PF2 | 20 |
| TX0111 | Vmr Apr Cn-Adh− | Endocarditis isolate; Phoenix, AZ; 2002 | PF2 | 20 |
| TX2535 | Vmr Apr Cn-Adh+ | Endocarditis isolate; Houston, TX; 1995 | PF1 | 20, 22 |
| TX0074 | Vmr Apr Cn-Adh+ | Endocarditis isolate; Valhalla, NY; 1995 | PF6 | 20 |
Ampr, ampicillin resistant; Chlr, chloramphenicol resistant; Vanr, vancomycin resistant; Vans, vancomycin susceptible; Cn-Adh+, adherence to collagen type I; Cn-Adh−, no adherence to collagen type I.
All isolates and PF types except TX0074 (PF6) belong to CC17.
General techniques.
Chromosomal DNAs from E. faecium isolates were prepared following the hexadecyltrimethyl ammonium bromide method described earlier (35). PCRs and sequencing were performed using previously described primers (22). Agarose plugs containing genomic DNA were digested with SmaI, and pulsed-field gel electrophoresis was performed using a previously described method (13).
Experimental endocarditis.
Vegetations were produced in male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) by inserting a polyethylene catheter via the right carotid artery across the aortic valve and into the left ventricle (21). Twenty minutes later, wild-type endocarditis-derived E. faecium strain TX0082 (belonging to the globally spread clonal complex 17) and its isogenic acm deletion mutant, TX6051, with their optical densities (ODs) adjusted with the goal that the wild type would be ≤50% of the inoculum (to avoid bias toward the wild type), were thoroughly mixed and injected intraventricularly through the catheter; because ODs do not always predict the exact CFU, the mixture was also plated for colony counts to precisely determine the ratio of wild type to mutant in the inoculum (see below). At ∼72 h, the animals were sacrificed, and the vegetations were excised, weighed, and homogenized in 1 ml of saline. Because our initial results for five rats (not included in the analysis) showed silencing of the chloramphenicol resistance gene in some TX6051 colonies from vegetations, we subsequently determined the ratio of wild type to mutant by plating serial dilutions of homogenized vegetations onto BHI agar. Randomly picked colonies from each vegetation were replica plated onto BHI agar, with or without 10 μg/ml chloramphenicol, along with TX0082 and TX6051 controls, and these colonies were also tested by colony hybridization using acm and cat probes to confirm the strain identities and to calculate the proportions of the wild type and the allelic replacement strain, TX6051. Data were expressed as percentages of wild type and mutant per vegetation. For vegetations showing only wild-type TX0082 colonies, i.e., for example, when 46/46 colonies were cat negative and acm positive and 0/46 colonies were cat positive and acm negative, the proportion of TX6051 in the vegetation was assigned the value of 1/47 (2.1%) (i.e., as if the next colony picked had been a mutant).
To evaluate differences in the initial adherence step, the wild type and the acm deletion mutant were inoculated as an equal mixture in the above rat endocarditis model, and CFU of viable bacteria from heart valves of these animals were measured at 1 h and 3 h postinoculation.
Similar to the method previously described for E. faecalis endocarditis using a mixed infection (21), the mean virulence index of the mutant relative to the wild type was calculated using the following equation:
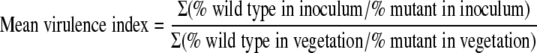 |
Mouse peritonitis model.
E. faecium strains TX0082 and TX6051 grown in BHI broth were individually injected intraperitoneally into outbred ICR mice (Harlan Sprague Dawley) as four inocula (2.5 × 108 to 2.8 × 109) premixed with 50% sterile rat fecal extract, using six mice per inoculum, and mice were observed for 5 days by our previously published method (31). Preapproved guidelines of the Animal Welfare Committee of the University of Texas Health Science Center at Houston were followed throughout the animal experiments.
Analysis of acm expression. (i) Extraction of total RNA.
Total RNA from E. faecium grown to exponential phase was isolated using an RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocol, except that 10 mg/ml lysozyme was used for the lysis step. RNAs were treated three times with 20 U of RQ1 DNase (Promega, Madison, WI) for 30 min at 37°C. RNA concentrations were determined using spectrophotometry. Part of each sample was electrophoresed through an agarose-formaldehyde gel in 3-[N-morpholino]propanesulfonic acid (MOPS) buffer as previously described (29).
(ii) RT-PCR.
Total RNA (between 2 ng and 250 ng) was reverse transcribed with acm primers (AcmF4 and AcmR5) (22), using a SuperScript one-step reverse transcriptase PCR (RT-PCR) kit with Platinum Taq (Invitrogen, San Diego, CA). As an internal control, a 509-bp fragment of gyrA (encoding gyrase) was amplified using the FmGyrF (5′-TATTACCTGGACCAGATTTTCCAA-3′) and FmGyrR (5′-TTCTAAGATGTGTGCTCTTGCTTC-3′) primers. Reactions without RT were performed as controls to detect DNA contamination in the total RNA preparations.
(iii) Real-time qRT-PCR.
Amplification, detection, and real-time analysis were performed using an ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA). Primers designed to produce amplicons of approximately equivalent lengths were selected using Primer Express software (Applied Biosystems). The primers used for quantitative RT-PCR (qRT-PCR) were AcmQrtF (5′-GGCCAGAAACGTAACCGATA-3′), AcmQrtR (5′-AACCAGAAGCTGGCTTTGTC-3′), 23S-rRNAFmF (5′-AGGCTAAGGAATGCAGACGA-3′), and 23S-rRNAFmR (5′-TTATTTCCCTCCCCATCACA-3′). For each sample, cDNA synthesis and PCR amplification were performed in a two-step process as previously described for E. faecalis (15). Relative quantification of gene expression was performed using 23S rRNA as the internal standard. The ΔΔCT method (10) was used to calculate the relative amount of specific RNA present in a sample, from which the level of induction of transcription of the gene was estimated by comparing it to the value for strain TX0074. Data were expressed as means ± standard deviations. Amplifications were performed on three independent RNA samples.
Protein extraction and chemiluminescent Western blotting.
Surface protein extracts from E. faecium isolates were prepared using mutanolysin (Sigma Chemical Co., St. Louis, MO) or 0.2% Zwittergent 3-12 (Calbiochem, La Jolla, CA) as described earlier (16, 37). Five units of mutanolysin was used for the number of cells equivalent to 1 OD600 value. Protein concentrations were estimated by bicinchoninic acid assay (Pierce, Rockford, IL). For preparing total cell lysates, 0.5 ml of bacterial culture washed in phosphate-buffered saline (PBS) was resuspended in 100 μl of 4× lithium dodecyl sulfate sample buffer (106 mM Tris HCl, 141 mM Tris base, 2% lithium dodecyl sulfate, 10% glycerol, 0.51 mM EDTA, 0.22 mM Serva blue G250, 0.175 mM phenol red, pH 8.5) and 10 μl of 500 mM dithiothreitol and boiled for 10 min. After centrifugation at 13,000 × g, 20-μl supernatants diluted twice with PBS were applied to the gels. Protein extracts were electrophoresed in 4 to 12% NuPAGE bis-Tris gels (Invitrogen) under reducing conditions in MOPS buffer and transferred to a polyvinylidene difluoride membrane. Membranes were then incubated with either affinity-purified anti-Acm A-domain-specific Igs (20) or preimmune rabbit serum (PRS) Igs (antibody I), followed by horseradish peroxidase-conjugated goat anti-rabbit antibodies (antibody II). Affinity purification of anti-Acm A-domain-specific Igs is described in our companion paper (20). The blots were then developed with Supersignal West Pico chemiluminescent substrate (Pierce). Purified recombinant Acm A domain was used as a positive control.
Periodate treatment of bacteria.
Periodate treatment of E. faecium was based on a method described previously for Listeria monocytogenes (7). In brief, bacteria washed in PBS were incubated with 10 mM sodium m-periodate in sodium acetate buffer (pH 4.5) for 1 h at room temperature in the dark. Cells were then centrifuged, washed once in PBS, and incubated for 30 min in 50 mM sodium borohydride in PBS. Following two washes with PBS, cells were stained with either affinity-purified anti-Acm specific Igs (20) or PRS Igs (negative control) for flow cytometry detection as described in our companion paper (20).
In vivo expression of Acm determined by flow cytometry.
Sterile vegetations were produced in four rats as described above, and three were injected intraventricularly with TX0016. Vegetations harvested after 72 h from the three infected and one noninfected rat heart valve were processed to remove host tissue debris, similar to a method described earlier for staphylococcally infected rabbit vegetations (36), with some modifications. Vegetations homogenized in saline were sonicated (ultrasonic dismembrator; Fisher Scientific, Hampton, NH) with a microtip (10 to 20 s; ∼25 W), followed by low-speed centrifugation (120 × g for 10 min) and filtration of the lysate through 35-μm-pore-size filter-capped tubes (Becton Dickinson and Company, Franklin Lakes, NJ) to trap residual host cell debris. Filtrates containing infected E. faecium cells were pelleted by high-speed centrifugation (13,000 × g for 6 min), and the pellets were washed twice with PBS. Processed samples from three infected vegetations were mixed and then divided into four aliquots for labeling with different antibodies. One aliquot was labeled with PRS Igs (negative control), and another was labeled with Igs purified from antiserum raised against formalin-killed TX0016 whole cells (positive control) (26). The remaining two aliquots were labeled with affinity-purified anti-Acm specific Igs (20). Goat anti-rabbit IgG conjugated with F(ab′)2 fragment-specific R-phycoerythrin (Jackson Immunoresearch, West Grove, PA) was used for flow cytometry detection as described in our companion paper (20). We analyzed forward scatter (for analysis of particle sizes in the sample) and side scatter (for analysis of cell granularity or internal complexity) of vegetation-based processed cells and compared them with those for in vitro-grown E. faecium TX0016 cells. The noninfected vegetation sample was probed with affinity-purified anti-Acm specific Igs to assess possible cross-reactivity of anti-Acm Igs with host tissue.
Enrichment of Acm-specific Igs eluted from E. faecium endocarditis patient serum and their effect on adherence.
Recombinant Acm A domain protein was electrophoresed in 10% NuPAGE bis-Tris gels (Invitrogen), transferred to a polyvinylidene difluoride membrane, and incubated with serum from an E. faecium endocarditis patient infected with E. faecium strain TX0016; elution of Acm A-specific Igs was performed by a procedure described elsewhere (18).
To test inhibition of collagen adherence by Igs, labeled bacteria were preincubated with 1 or 5 μg/ml of the four different Igs mentioned below for 1 h at 37°C, and 50 μl of these cells was then added to collagen- or bovine serum albumin-coated wells in an adherence assay described earlier (22). The TX0082 cells used in this assay were grown to entry into stationary phase, the phase at which we detected Acm on the surfaces of >75% of cells by flow cytometry (20). Results are presented as percentages of cells adhering to collagen (radioactivity of bound cells/radioactivity of total cells added × 100).
Igs used for inhibition studies were (i) total Igs from normal human sera (NHS) from healthy volunteers, purified by affinity chromatography on a protein G column (Pierce); (ii) specific anti-Acm Igs from a patient with E. faecium endocarditis, eluted against the recombinant Acm A domain; (iii) total Igs from PRS (17); and (iv) specific anti-Acm37 Igs from rabbit antisera raised against the Acm A domain and eluted against the high-affinity binding segment, rAcm37 (17). Rabbit-derived specific anti-Acm37 Igs, previously found to be the most effective in inhibiting E. faecium TX0082 adhesion to collagen, causing ∼80% inhibition at 20 μg/ml (17), were used here for comparison purposes.
Statistical analyses.
The percentages of wild-type TX0082 in the inoculum versus wild-type TX0082 in vegetations/heart valves for individual rats were analyzed for significance by the paired t test. The decreased collagen adherence percentages from the anti-Acm Ig inhibition assay were analyzed for significance by the unpaired t test. P values of <0.05 were considered significant. Statistical tests were performed by using GraphPad Prism v4 for Windows.
RESULTS
Acm of E. faecium contributes to experimental rat endocarditis.
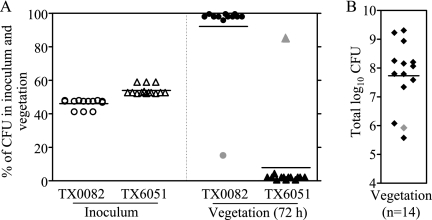
To determine if Acm has an in vivo role during IE, we tested E. faecium TX0082 and its isogenic acm deletion mutant TX6051 (TX0082 Δacm::cat) (19) in a rat endocarditis model. Fourteen rats were given an approximately equal mixture of the wild type and the acm deletion mutant (Fig. 1A). The CFU of wild-type TX0082 and the mutant TX6051 in the inoculum were 4.5 × 106 (47.4% of the inoculum) and 5 × 106 (52.6%), respectively, for seven animals; 1.7 × 107 (41.1%) and 2.4 × 107 (58.9%), respectively, for three animals; 2.75 × 107 (47.8%) and 3 × 107 (52.2%), respectively, for three animals; and 7 × 107 (46.7%) and 8 × 107 (53.3%), respectively, for one animal (overall average, 45.4% for TX0082 and 54.6% for TX6051). Vegetations recovered at autopsy 72 h after intraventricular injection contained 3.8 × 105 to 2.0 × 109 CFU/g (Fig. 1B), and vegetations from 10 of 14 rats had no mutants recovered. After assigning a minimum detection value of 0.5 to 4.2%, depending on the number of bacteria analyzed, the mean percentage of TX0082 in the total CFU of bacteria recovered from the 14 vegetations was 92.1%, compared to 46.1% in the inoculum (Fig. 1A) (P < 0.0001 by the paired t test), demonstrating a considerable advantage of the wild type versus the mutant. The mean virulence index (21) of the acm mutant relative to the wild type in vegetations was 0.012; this indicates that acm has an important role in this endovascular infection.
FIG. 1.
Attenuation of an acm deletion mutant in a rat endocarditis model. (A) Percentages of wild-type TX0082 and the acm deletion mutant TX6051 recovered from the initial inocula (left) and from vegetations 72 h after infection of 14 rats (right) are shown. In 10 rats, zero colonies in the vegetation were mutants and were assigned individualized minimum detection values (see the text for details). Horizontal lines indicate means (P < 0.0001 [by paired t test] for percentage of total bacteria in the vegetation versus that in the inoculum). (B) Log10 CFU/g of vegetations from rats infected with a mixture of wild-type E. faecium TX0082 and its isogenic acm deletion mutant TX6051. The horizontal line indicates the geometric mean. Empty circles and triangles represent inoculum percentages, and solid circles and triangles represent vegetation or valve percentages of wild-type TX0082 and TX6051, respectively. The single rat in which the mutant outnumbered the wild type is shaded in gray.
It is of interest that the single vegetation with 15.2% wild-type TX0082 and 84.8% TX6051 had 1.95 log10 fewer CFU than the geometric mean CFU/g (7.5 × 107) of the other 13 vegetations and suggested the possibility that a nonfunctioning acm gene had emerged in TX0082 in this vegetation. Multiple wild-type TX0082 colonies derived from this vegetation were verified for strain identity by pulsed-field gel electrophoresis. Colonies were then screened by PCR for possible interruption of the acm gene by insertion elements (those commonly found in E. faecium strains were previously reported to interrupt the acm locus in some E. faecium isolates) (22). The complete acm loci of a few colonies were then sequenced to detect possible mutations. We also screened for surface Acm by flow cytometry. Results showed neither insertions/mutations in the acm locus nor a defect in the surface expression of Acm (data not shown).
Acm enhances initial adherence of E. faecium to damaged heart valves.
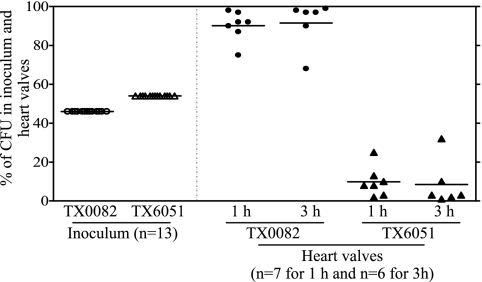
To test whether initial valvular adherence is altered in the acm mutant, 13 additional rats were evaluated, and for these, the CFU of viable bacteria from heart valves obtained at 1 h (7 rats) and 3 h (6 rats) postinoculation were determined. The CFU of wild-type TX0082 and the mutant TX6051 in the inoculum were 5.2 × 107 (46.4% of the inocula) and 6 × 107 (53.6%), respectively, for three animals sacrificed at 1 h and three animals sacrifice at 3 h; 3.0 × 107 (46.2%) and 3.5 × 107 (53.8%), respectively, for three animals sacrificed at 1 h and three animals sacrificed at 3 h; and 1.54 × 107 (46.4%) and 1.78 × 107 (53.6%), respectively, for one animal. Figure 2 shows the percentages of CFU of the wild type and the acm deletion mutant in the inoculum (time zero) (46%) and in heart valves at 1 h (90%) and 3 h (92%) (P < 0.0001 for 1 h and P = 0.0002 for 3 h by paired t test versus inoculum), consistent with CFU ratios in vegetations recovered 72 h after inoculation.
FIG. 2.
The acm mutant TX6051 is defective in colonization of damaged aortic valves. The percentages of wild-type TX0082 and the acm deletion mutant TX6051 recovered from heart valves 1 h and 3 h after infection of 13 rats and from the initial inocula are shown. Horizontal lines indicate means (P < 0.0001 for 1 h and P = 0.0002 for 3 h versus 0 h). Empty circles and triangles represent inoculum percentages, and solid circles and triangles represent vegetation or valve percentages of wild-type TX0082 and TX6051, respectively.
Both the wild-type strain and the acm mutant caused mortality at similar rates in a mouse peritonitis model.
To test the influence of Acm in other in vivo systems, we also tested wild-type TX0082 and the isogenic acm mutant in a mouse peritonitis model. In contrast to the endocarditis results, which strongly favored wild-type TX0082, there was no evidence of attenuation of TX6051 in this model, as TX0082 and TX6051 caused mortality at similar rates over 120 h in mice (P ≥ 0.814 for four inocula by log rank test) (data not shown).
All endocarditis-derived E. faecium isolates have an intact and highly conserved acm gene.
Because 5 (including TX0016) of the 17 endocarditis strains previously studied did not show in vitro adherence to collagen or surface Acm (20, 22), we sequenced and analyzed the complete acm loci of 4 strains and found an intact acm gene in each; we previously reported an intact acm gene sequence for the fifth strain, TX0016 (22).
Since it is known that MSCRAMMs have a significant degree of sequence divergence within different strains (e.g., the collagen adhesin Ace of E. faecalis) (18), which may account for the variability of their adherence phenotypes, we compared these five acm sequences with sequences of four additional endocarditis isolates exhibiting various levels of in vitro collagen adherence. Among the acm gene sequences of the nine endocarditis isolates compared, there was only a single nucleotide difference in TX0068, indicating that Acm is highly conserved.
Lack of in vitro Acm production by some endocarditis-derived isolates can be explained by differences in gene expression.
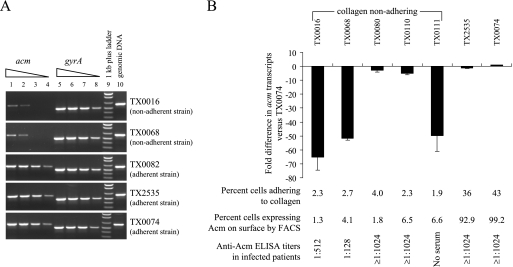
To further explore the reason(s) for the lack of collagen binding by the five nonadhering endocarditis isolates (all of which have an intact acm gene), we first performed semiquantitative RT-PCR with RNAs from two non-collagen-adhering and three collagen-adhering endocarditis isolates. Abundant acm mRNA was detected in all three Acm surface-expressing (and collagen-adhering) strains, whereas a reduced amount of acm mRNA was detected in each nonadhering strain (Fig. 3A). The levels of control gyrA mRNA from all five strains were almost equal (Fig. 3A).
FIG. 3.
Analysis of acm transcripts from E. faecium strains. (A) Semiquantitative RT-PCR analysis of acm, using RNAs extracted from three collagen-adhering and two nonadhering E. faecium endocarditis isolates. Results are presented as amplification products electrophoresed in ethidium bromide-stained agarose gels. The first and fifth lanes for each strain represent 250 ng of total mRNA, and lanes 2 to 4 and 6 to 8 show 50, 10, and 2 ng (fivefold serial dilutions) of RNA before RT-PCR. The genomic DNAs (lane 10) used as a control for PCR were extracted from the respective isolates. RT-PCRs performed with two independent sample preparations showed similar results. (B) Relative quantitation of acm expression by real-time RT-PCR. acm transcripts (means ± standard deviations) were quantitated from one collagen-adhering and five nonadhering E. faecium endocarditis strains relative to transcripts of TX0074, a strain that showed surface Acm on >99% of cells (20). 23S rRNA transcripts were used as an endogenous control. The percentages of cells adhering to collagen, the percentages of cells expressing Acm on their surfaces in vitro, and anti-Acm titers of the respective infected patient sera from the companion study (20) are given at the bottom of the figure.
To further examine the correlation between acm transcripts and Acm production, we next measured acm transcript levels of all five non-collagen-adhering strains (including two studied above) and of two surface Acm-expressing endocarditis isolates by qRT-PCR. Three of the nonadhering endocarditis isolates showed 50- to 65-fold reductions in acm mRNA (Fig. 3B) compared to TX0074 (an isolate that expresses Acm on 99% of cells) (20), demonstrating that acm transcription is reduced in vitro in some isolates. In contrast, for two of the nonadhering strains (TX0080 and TX0110), there were only 2.8- and 5-fold reductions, suggesting that there is yet another mechanism for controlling Acm production in these isolates.
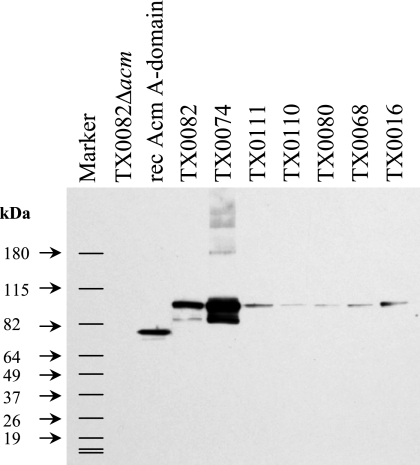
To investigate other possibilities for the lack of surface Acm on TX0080 and TX0110 (e.g., masking of Acm by other surface components, such as polysaccharides), cell lysates and surface protein preparations of these isolates were analyzed by Western blotting using affinity-purified anti-Acm specific Igs. No Acm bands were detected in either cell lysate or Zwittergent protein extract blots (data not shown), although a weak Acm band was detected in mutanolysin blots of these isolates (Fig. 4). The highly reduced levels of Acm (compared to that in TX0074) detected in these strains were similar to or lower than the levels for three endocarditis isolates that showed ≥50-fold less acm mRNA (Fig. 4).
FIG. 4.
Western blots of mutanolysin surface protein extracts of E. faecium isolates probed with anti-Acm A-domain-specific Igs. Ten micrograms of mutanolysin extracts of E. faecium isolates and 10 ng of recombinant Acm A domain were used for each lane. TX0074 and TX0082 are adherent strains, while TX0016, TX0068, TX0080, TX0110, TX0111, and TX6051 are nonadherent strains.
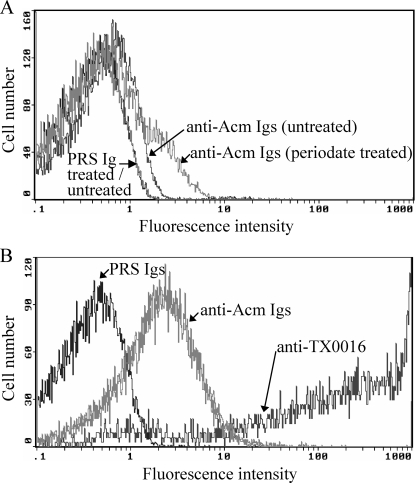
We next compared surface Acm levels of periodate-treated and untreated cells of two selected strains, TX0016 and TX0110, by fluorescence-activated cell sorting (FACS) analysis. Periodate-treated TX0016 cells showed a ≥6-fold increase in the percentage of cells expressing surface Acm versus untreated cells (Fig. 5A). In contrast, no differences were observed between periodate-treated and untreated cells of TX0110 (data not shown).
FIG. 5.
FACS analysis of Acm surface expression in E. faecium cells. (A) Analysis of Acm surface expression in E. faecium TX0016 cells after sodium m-periodate treatment. Cells were incubated with control PRS Igs or anti-Acm A-domain-specific Igs, followed by incubation with F(ab′)2 fragments of goat anti-rabbit IgG (H+L) conjugated to R-phycoerythrin (antibody II). (B) Flow cytometry analysis of Acm surface expression in E. faecium cells derived from vegetations of rat experimental IE. Processed vegetations were incubated with control PRS Igs, anti-Acm A-domain-specific Igs, or Igs purified from antiserum raised against formalin-killed TX0016 whole cells (anti-TX0016), followed by incubation with antibody II. Specific binding by anti-Acm or anti-TX0016 antibodies is indicated as log fluorescence intensity on the x axis. Each histogram represents 50,000 bacteria (A) or 10,000 to 38,000 events (bacterium-sized particles) (B).
TX0016 expresses Acm on its surface after growth in experimental endocarditis vegetations but not after growth in vitro.
To determine if the acm gene of TX0016 is expressed during in vivo infection, we performed FACS analysis on extracts directly processed from vegetations infected with TX0016. Comparison of forward and side scatter patterns of particles from processed vegetations and those of in vitro-grown E. faecium TX0016 cells suggested that most host tissue particles were removed from the vegetation after the processing steps described in Materials and Methods. While PRS Igs did not exhibit any measurable binding, Igs from antiserum raised against formalin-killed TX0016 whole cells (our positive control) bound 85% of the bacterium-sized particles with very high fluorescence intensities (Fig. 5B). Using affinity-purified anti-Acm specific Igs, Acm was found on ∼40% of bacterium-sized particles from rat IE vegetations infected with TX0016 (Fig. 6), which is approximately sevenfold more cells than that seen with periodate-treated cells. Similarly processed sterile vegetations probed with anti-Acm specific Igs did not result in labeling of the bacterium-sized particles (data not shown), thus confirming the specificity of anti-Acm Igs.
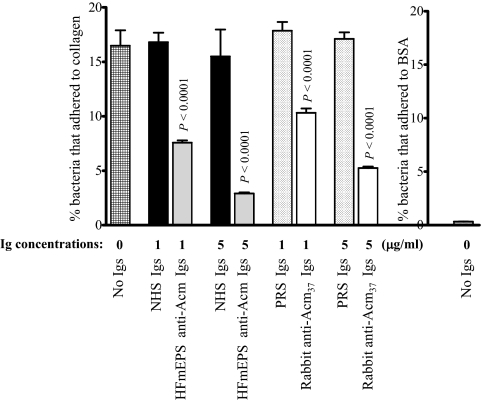
FIG. 6.
Inhibition of adherence of E. faecium strain TX0082 to collagen type I by Acm A-specific Igs eluted from E. faecium endocarditis patient serum. 35S-labeled bacteria were incubated with 1 or 5 micrograms/ml of eluted Acm A-specific antibodies for 1 h at 37°C. Adherence was tested in wells coated with 1 μg of collagen. Bars represent the percentages of cells bound (means ± standard deviations) for 6 to 12 wells. Results are representative of three independent experiments. NHS Ig: total Igs from NHS from healthy volunteers; HFmEPS anti-Acm Ig, human E. faecium endocarditis patient serum-derived specific anti-Acm Igs eluted against the recombinant Acm A domain; PRS Ig, total Igs from PRS; rabbit anti-Acm37 Ig, specific anti-Acm Igs from rabbit antisera raised against the Acm A domain and eluted against the high-affinity binding segment, rAcm37 (17).
Human anti-Acm Igs purified from a patient with E. faecium endocarditis substantially reduce adherence of E. faecium strain TX0082 to collagen type I.
We previously reported reductions in adherence of two vancomycin-resistant endocarditis-derived E. faecium isolates, TX2535 and TX0082, to collagen after preincubation of rabbit anti-Acm Igs purified against different subsegments of Acm (17). Here we examined the inhibitory ability of human-derived Acm A-domain-specific Igs (from an E. faecium endocarditis patient serum) on adherence of E. faecium strain TX0082 to collagen. As shown in Fig. 6, incubation of human-derived anti-Acm Igs reduced adherence of TX0082 to collagen by 54% and 82% with 1- and 5-μg/ml concentrations, respectively, compared to a 2% increase and 6% decrease with similar concentrations of NHS Igs (P < 0.0001). As in our earlier report (17), rabbit derived anti-Acm37 Igs also caused reductions in collagen adherence of TX0082 (37% and 68% with 1- and 5-μg/ml concentrations, respectively), but they were less potent than human anti-Acm Igs at both concentrations tested.
DISCUSSION
An estimated 15,000 to 20,000 new cases of IE are diagnosed each year in the United States (3). Among these, IE cases due to isolates of multidrug-resistant E. faecium, which have been on the rise since the late 1980s, pose a major clinical challenge, with high mortality rates due to very limited therapeutic options (12, 14). To develop alternative therapeutic or preventive measures, such as active or passive immunization against E. faecium IE, we believe it is important to identify surface antigens which function as adhesins to heart valves and also to understand the mechanism(s) underlying these adhesive functions.
Damaged cardiac endothelium predisposes patients to the formation of sterile thrombotic vegetations formed by platelets and fibrin matrix (1). Bacterial infection of the endocardial lining occurs following bacteremia, which may be transient and occult, and adherence of microorganisms to the matrix components of damaged endothelium and/or to the vegetation. Collagen (the most abundant protein in vertebrates) has been reported to be found in sterile vegetations (1), in addition to being enriched in valvular and aortic tissues, and is a possible host target for bacteria to colonize at these sites and contribute to endocarditis. In support of the notion that collagen adhesins contribute substantially to endocarditis, in addition to several types of other localized infections, such as septic arthritis, compelling data were generated from S. aureus animal models (4, 8, 25, 27, 38) relating to the role of the S. aureus collagen adhesin gene, cna, which is present in only 38 to 56% of S. aureus strains (28, 32, 33).
The above observations from S. aureus pathogenesis studies, together with our detection of anti-Acm antibodies in all E. faecium endocarditis patient sera tested (20), led us to investigate whether the acm gene contributes to E. faecium endocarditis in rats with catheter-induced aortic vegetations. To test the role of Acm in experimental endocarditis, we used a mixed-inoculum model in which TX0082 and TX6051 were inoculated via catheter at an approximately 1:1 ratio. We prefer mixing experiments because this method requires fewer animals compared to 50% infective dose estimation with monoinfections, overcomes the considerable rat-to-rat difference in total numbers of bacteria per vegetation, and has been shown to be sensitive for detecting virulence differences of E. faecalis and other gram-positive organisms (8, 21, 23). For the competition assay, 14 rats were challenged with a mixture of approximately equal numbers of the wild type and its acm deletion mutant (total inocula ranged from 9.5 × 106 to 1.5 × 108); all showed vegetations, and the percentages of the wild type recovered from vegetations were significantly higher (average of 92.1% in vegetations versus 46.1% in the inoculum; P < 0.0001 by paired t test) than the percentages of the acm mutant (7.9% versus 53.9%), indicating that acm is important in this endovascular infection.
The highly reduced infectivity of the acm deletion mutant compared to the wild type at 72 h postinfection could be attributed to reduced adherence to damaged valves, a reduced ability to multiply in infected vegetations, or both. Subsequent CFU analysis with heart valves obtained at 1 h and 3 h postinoculation from 13 additional rats indicated that the acm mutant was hindered in the early adherence step in the pathogenesis of endocarditis, i.e., at the stage of colonization of the damaged valves. The construct in which we introduced the functional acm gene on a plasmid to complement the natural acm mutant E. faecium strain was found to be unstable in the absence of antibiotic selection (22), thus impeding in vivo complementation studies. Since acm deletion has no polar effect on downstream genes, we infer that the difference in virulence of TX6051 is likely due to its inability to adhere to collagen, but we cannot exclude the possibility of another ligand or another function of this protein.
We also tested the wild type and the acm mutant in the mouse peritonitis model. In this model, TX6051 did not show attenuation versus the wild type in either total death or time to death over a period of 5 days. These results indicate an apparent need for adhesins to initiate endocarditis and a lack of this need in peritonitis (21, 30) and also suggest that there is not a biologically significant in vivo growth or survival defect of the mutant, at least in this model.
To test whether the collagen adherence variability of different endocarditis isolates (20, 22) is due to mutations in acm sequence, we compared the acm genes of nine selected endocarditis strains. Sequence analysis of the acm loci of the 5 nonadherent endocarditis strains (20, 22) identified an intact copy of the acm gene in each, whereas our earlier study had identified an acm pseudogene in many E. faecium strains of nonendocarditis origin (22); thus, of the 17 endocarditis isolates tested, all had an intact acm gene, which was demonstrated by sequencing (9 strains) and/or by finding Acm on the surface (12 strains) (20). The highly conserved nature of Acm (of nine endocarditis strains sequenced, there was only a single nucleotide difference in TX0068) demonstrates that the observed variation in collagen adherence of individual strains is not caused by structural variations of Acm.
Flow cytometry results for surface Acm of the five nonadhering and four adhering E. faecium endocarditis isolates reported in our companion paper (20) demonstrated that the level of collagen adherence is related to the amount of Acm on cell surfaces as well as the percentage of cells expressing Acm. To investigate the reason(s) for the lack of Acm surface expression by the five nonadhering E. faecium endocarditis isolates, acm transcripts of these strains were analyzed by real-time qRT-PCR. Highly reduced levels of acm transcripts were found in three of these five endocarditis isolates, indicating that acm transcription is downregulated in these isolates under standard in vitro growth conditions, as seen with the cna gene of some S. aureus isolates (6) and as is characteristic of ace of E. faecalis (15). Two of the E. faecium isolates (TX0080 and TX0110) that lacked surface Acm had only modestly reduced levels of acm mRNA versus TX0074. Analysis of different cell components of these isolates by Western blotting showed highly reduced levels of Acm compared to those for TX0074 in mutanolysin surface extracts, suggesting that there may be other posttranscriptional factors regulating Acm production in these isolates. Furthermore, surface Acm analysis of periodate-treated cells of one of these isolates (TX0110) ruled out a carbohydrate masking effect in this isolate, paralleling the Western blot results. Unlike the case with TX0110, there appeared to be some masking of Acm of TX0016 by polysaccharides. Consistent with this observation, our earlier studies identified electron-dense small clumps of polysaccharide adjacent to the cell wall, resembling a capsule, in electron micrographs of TX0016 (2, 26).
To determine if Acm is produced by endocarditis isolates during in vivo growth, even when it is not detected in vitro, we carried out FACS analysis on extracts processed directly from vegetations infected with TX0016, a strain producing little to no detectable Acm in vitro. Acm surface expression was detectable in ∼40% of rat IE vegetation-derived TX0016 cells, which is much higher even than that after periodate treatment; this result is consistent with our finding of anti-Acm antibodies in a human endocarditis patient serum infected by TX0016 (20). We then grew three endocarditis-derived E. faecium strains that did not express Acm in vitro (but had an intact acm gene) in BHI and in 40% horse serum in BHI. Analysis of Acm surface expression of these isolates showed no obvious differences between cells grown in BHI or BHI plus serum (data not shown), unlike Ace of E. faecalis, which showed enhanced expression after growth in BHI plus serum (15). To test the inhibitory ability of serum from the endocarditis patient infected by TX0016, we eluted Acm-specific Igs from the recombinant Acm A domain lane of a Western blot incubated with this serum and used these antibodies in our adherence assay. The results showed a substantial reduction in adherence of E. faecium TX0082 to collagen by these human-derived anti-Acm Igs, but not by Igs from NHS, raising the possibility that Acm may have potential as a prophylactic agent, even for strains, such as TX0016, that make little or no exposed Acm in vitro but have an intact gene. Our ongoing protection studies using the rat endocarditis model may provide some proof in this direction.
At least three major conclusions can be drawn from this study. First, our endocarditis model results provide experimental evidence that acm contributes to E. faecium pathogenesis, consistent with the observation of a functional gene in all endocarditis isolates from our collection; this is the first demonstration of any gene's importance in experimental E. faecium infections. A second conclusion is that variation in acm gene expression, posttranscriptional regulation, and also antigen masking (e.g., by polysaccharide in TX0016) appear to be explanations for the lack of detectable Acm production in vitro for some endocarditis isolates, all of which have an intact gene. Finally, the highly conserved nature of Acm, along with data showing that human antibodies against Acm inhibit collagen adherence of E. faecium (17), underscores the possibility of using Acm as a target for immunoprophylaxis or in combination with antibiotics (34) for treating endocarditis.
Acknowledgments
We thank Karen Jacques-Palaz for her technical help.
This work was supported by NIH grant R01 AI 67861 from the Division of Microbiology and Infectious Diseases, NIAID, to B.E.M.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Angrist, A. A., and M. Oka. 1963. Pathogenesis of bacterial endocarditis. JAMA 183249-252. [DOI] [PubMed] [Google Scholar]
- 2.Arduino, R. C., K. Jacques-Palaz, B. E. Murray, and R. M. Rakita. 1994. Resistance of Enterococcus faecium to neutrophil-mediated phagocytosis. Infect. Immun. 625587-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer, A. S., A. F. Bolger, K. A. Taubert, W. Wilson, J. Steckelberg, A. W. Karchmer, M. Levison, H. F. Chambers, A. S. Dajani, M. H. Gewitz, J. W. Newburger, M. A. Gerber, S. T. Shulman, T. J. Pallasch, T. W. Gage, and P. Ferrieri. 1998. Diagnosis and management of infective endocarditis and its complications. Circulation 982936-2948. [DOI] [PubMed] [Google Scholar]
- 4.Elasri, M. O., J. R. Thomas, R. A. Skinner, J. S. Blevins, K. E. Beenken, C. L. Nelson, and M. S. Smeltzer. 2002. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone 30275-280. [DOI] [PubMed] [Google Scholar]
- 5.Giannitsioti, E., I. Skiadas, A. Antoniadou, S. Tsiodras, K. Kanavos, H. Triantafyllidi, and H. Giamarellou. 2007. Nosocomial vs. community-acquired infective endocarditis in Greece: changing epidemiological profile and mortality risk. Clin. Microbiol. Infect. 13763-769. [DOI] [PubMed] [Google Scholar]
- 6.Gillaspy, A. F., J. M. Patti, and M. S. Smeltzer. 1997. Transcriptional regulation of the Staphylococcus aureus collagen adhesion gene, cna. Infect. Immun. 651536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilot, P., P. Andre, and J. Content. 1999. Listeria monocytogenes possesses adhesins for fibronectin. Infect. Immun. 676698-6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hienz, S. A., T. Schennings, A. Heimdahl, and J. I. Flock. 1996. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J. Infect. Dis. 17483-88. [DOI] [PubMed] [Google Scholar]
- 9.Leavis, H. L., M. J. Bonten, and R. J. Willems. 2006. Identification of high-risk enterococcal clonal complexes: global dispersion and antibiotic resistance. Curr. Opin. Microbiol. 9454-460. [DOI] [PubMed] [Google Scholar]
- 10.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 11.McDonald, J. R., L. Olaison, D. J. Anderson, B. Hoen, J. M. Miro, S. Eykyn, E. Abrutyn, V. G. Fowler, Jr., G. Habib, C. Selton-Suty, P. A. Pappas, C. H. Cabell, G. R. Corey, F. Marco, and D. J. Sexton. 2005. Enterococcal endocarditis: 107 cases from the international collaboration on endocarditis merged database. Am. J. Med. 118759-766. [DOI] [PubMed] [Google Scholar]
- 12.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342710-721. [DOI] [PubMed] [Google Scholar]
- 13.Murray, B. E., K. V. Singh, J. D. Heath, B. R. Sharma, and G. M. Weinstock. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 282059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mylonakis, E., and S. B. Calderwood. 2001. Infective endocarditis in adults. N. Engl. J. Med. 3451318-1330. [DOI] [PubMed] [Google Scholar]
- 15.Nallapareddy, S. R., and B. E. Murray. 2006. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E. faecalis interaction. Infect. Immun. 744982-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nallapareddy, S. R., X. Qin, G. M. Weinstock, M. Hook, and B. E. Murray. 2000. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 685218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nallapareddy, S. R., J. Sillanpaa, V. K. Ganesh, M. Hook, and B. E. Murray. 2007. Inhibition of Enterococcus faecium adherence to collagen by antibodies against high-affinity binding subdomains of Acm. Infect. Immun. 753192-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nallapareddy, S. R., K. V. Singh, R. W. Duh, G. M. Weinstock, and B. E. Murray. 2000. Diversity of ace, a gene encoding a microbial surface component recognizing adhesive matrix molecules, from different strains of Enterococcus faecalis and evidence for production of Ace during human infections. Infect. Immun. 685210-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nallapareddy, S. R., K. V. Singh, and B. E. Murray. 2006. Construction of improved temperature-sensitive and mobilizable vectors and their use for constructing mutations in the adhesin-encoding acm gene of poorly transformable clinical Enterococcus faecium strains. Appl. Environ. Microbiol. 72334-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nallapareddy, S. R., K. V. Singh, P. C. Okhuysen, and B. E. Murray. 2008. A functional collagen adhesin gene, acm, in clinical isolates of Enterococcus faecium correlates with the recent success of this emerging nosocomial pathogen. Infect. Immun. 764110-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nallapareddy, S. R., K. V. Singh, J. Sillanpaa, D. A. Garsin, M. Hook, S. L. Erlandsen, and B. E. Murray. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Investig. 1162799-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nallapareddy, S. R., G. M. Weinstock, and B. E. Murray. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 471733-1747. [DOI] [PubMed] [Google Scholar]
- 23.Nannini, E. C., F. Teng, K. V. Singh, and B. E. Murray. 2005. Decreased virulence of a gls24 mutant of Enterococcus faecalis OG1RF in an experimental endocarditis model. Infect. Immun. 737772-7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48585-617. [DOI] [PubMed] [Google Scholar]
- 25.Patti, J. M., T. Bremell, D. Krajewska-Pietrasik, A. Abdelnour, A. Tarkowski, C. Ryden, and M. Hook. 1994. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect. Immun. 62152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakita, R. M., V. C. Quan, K. Jacques-Palaz, K. V. Singh, R. C. Arduino, M. Mee, and B. E. Murray. 2000. Specific antibody promotes opsonization and PMN-mediated killing of phagocytosis-resistant Enterococcus faecium. FEMS Immunol. Med. Microbiol. 28291-299. [DOI] [PubMed] [Google Scholar]
- 27.Rhem, M. N., E. M. Lech, J. M. Patti, D. McDevitt, M. Hook, D. B. Jones, and K. R. Wilhelmus. 2000. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect. Immun. 683776-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryding, U., J. I. Flock, M. Flock, B. Soderquist, and B. Christensson. 1997. Expression of collagen-binding protein and types 5 and 8 capsular polysaccharide in clinical isolates of Staphylococcus aureus. J. Infect. Dis. 1761096-1099. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and J. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Singh, K. V., S. R. Nallapareddy, and B. E. Murray. 2007. Importance of the endocarditis and biofilm-associated pilus (ebp) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J. Infect. Dis. 1951671-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh, K. V., X. Qin, G. M. Weinstock, and B. E. Murray. 1998. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 1781416-1420. [DOI] [PubMed] [Google Scholar]
- 32.Smeltzer, M. S., A. F. Gillaspy, F. L. Pratt, and M. D. Thames. 1997. Comparative evaluation of use of cna, fnbA, fnbB, and hlb for genomic fingerprinting in the epidemiological typing of Staphylococcus aureus. J. Clin. Microbiol. 352444-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Switalski, L. M., J. M. Patti, W. Butcher, A. G. Gristina, P. Speziale, and M. Hook. 1993. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol. Microbiol. 799-107. [DOI] [PubMed] [Google Scholar]
- 34.Vernachio, J., A. S. Bayer, T. Le, Y. L. Chai, B. Prater, A. Schneider, B. Ames, P. Syribeys, J. Robbins, and J. M. Patti. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 473400-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson, K. 1994. Preparation of genomic DNA from bacteria, p.2.4.1-2.4.2. In F. M. Ausubel, R. Brent, R. E. Kingston, D. M. David, J. G. Scidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. Green Publishing Associates, Brooklyn, NY. [DOI] [PubMed]
- 36.Xiong, Y. Q., W. Van Wamel, C. C. Nast, M. R. Yeaman, A. L. Cheung, and A. S. Bayer. 2002. Activation and transcriptional interaction between agr RNAII and RNAIII in Staphylococcus aureus in vitro and in an experimental endocarditis model. J. Infect. Dis. 186668-677. [DOI] [PubMed] [Google Scholar]
- 37.Xu, Y., L. Jiang, B. E. Murray, and G. M. Weinstock. 1997. Enterococcus faecalis antigens in human infections. Infect. Immun. 654207-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu, Y., J. M. Rivas, E. L. Brown, X. Liang, and M. Hook. 2004. Virulence potential of the staphylococcal adhesin CNA in experimental arthritis is determined by its affinity for collagen. J. Infect. Dis. 1892323-2333. [DOI] [PubMed] [Google Scholar]