Abstract
Splenic microarchitecture is substantially altered during acute malaria infections, which may affect the development and regulation of immune responses. Here we investigated whether engagement of host Toll-like receptor 2 (TLR2), TLR4, TLR9, and the adaptor protein MyD88 is required for induction of the changes and whether antibody responses are modified when immunization takes place during the period of splenic disruption. The alterations in splenic microarchitecture were maximal shortly after the peak of parasitemia and were not dependent on engagement of TLR2, TLR4, or TLR9, and they were only minimally affected by the absence of the MyD88 adaptor molecule. Although germinal centers were formed in infected mice, they did not contain the usual light and dark zones. Immunization of mice with chicken gamma globulin 2 weeks prior to acute Plasmodium chabaudi infection did not affect the quantity or avidity of the immunoglobulin G antibody response to this antigen. However, immunization at the same time as the primary P. chabaudi infection resulted in a clear transient reduction in antibody avidity in the month following immunization. These data suggest that the alterations in splenic structure, particularly the germinal centers, may affect the quality of an antibody response during a malaria infection and could impact the development of immunity to malaria or to other infections or immunizations given during a malaria infection.
The spleen has a central role in the immune response to malaria in humans (46) and rodents (20, 30, 67), and splenomegaly is one of the most striking features of malaria infection. Within the first week after infection the size of the spleen increases severalfold, due in part to an influx of lymphocytes in both human (14, 35, 46) and mouse (54, 68) infections. Additionally, malaria-associated splenomegaly has been associated with increased erythropoiesis in mice (41, 52, 61, 68). Malarial splenomegaly is accompanied by transient alterations in the microarchitecture during acute infection (2, 9, 29, 32, 54, 65, 67), which in mouse infections returns to normal several weeks after the acute parasitemia (1, 54, 67).
The alterations of the splenic microarchitecture observed during acute malaria infection are similar in some respects to those observed after administration of the Toll-like receptor 4 (TLR4) ligand lipopolysaccharide (LPS) (19). MyD88, an intracellular adaptor protein for TLR4 and other TLRs (for a review, see reference 55), as well as other pattern recognition or cytokine receptors (26), is involved in the initial host response to erythrocytic stages of Plasmodium (4, 28, 45). In addition, Plasmodium contains ligands that bind to at least three TLRs, including glycosylphosphatidylinositol anchors, which have been shown to bind TLR2 and to a lesser extent TLR4 (28), and DNA trapped within hemozoin, which can stimulate dendritic cells via TLR9 (13, 45). Therefore, it is possible that ligation of TLRs is involved in the changes in the spleen structure in malaria infections.
Changes in the location of plasma cells, B cells, T cells, and dendritic cells could affect access to antigen, interactions between these cells, and access to the chemokines necessary for correct migration of cells and thus could impede immune responses. In this regard, there is a wealth of information suggesting that immune responses are suppressed in malaria infections (10, 16, 37, 42, 48, 64, 69-71) and that both splenic T and B cells are lost from the spleen and undergo significant apoptosis (2, 22, 53, 71).
Here we investigated the role of TLRs and the adaptor protein MyD88 in the transient alterations in splenic microarchitecture that take place during Plasmodium chabaudi infection of mice and observed that splenomegaly and changes in spleen structure occurred independently of TLR2, TLR4, TLR9, or the MyD88 adaptor molecule. The significant changes in the spleen during the acute infection, including the lack of formation of dark and light zones within the germinal centers, did not appear to affect the magnitude of an immunoglobulin G (IgG) antibody response to an unrelated antigen (chicken gamma globulin [CGG]) administered 2 weeks before a primary infection, but affinity maturation of the anti-CGG antibody response was delayed in mice that received CGG and P. chabaudi concurrently.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice and TLR2−/−, TLR4−/−, TLR6−/−, TLR9−/−, and MyD88−/− mice (5, 23, 24, 57, 58) with a C57BL/6 background were bred in the specific-pathogen-free unit of the National Institute for Medical Research (NIMR) under the NIMR guidelines for animal husbandry. They were infected or immunized when they were 6 to 12 weeks old. For experimental use, all mice were conventionally housed with sterile bedding, food, and water. All experiments with mice were carried out under a United Kingdom Home Office license according to United Kingdom law and were approved by the NIMR Ethical Review Panel.
Parasites and immunization.
P. chabaudi chabaudi AS (66) was maintained as a frozen stock and was passaged in mice as described previously (36). For experiments, mice were inoculated with 105 parasitized red blood cells (pRBC) diluted in 100 μl Kreb's saline containing glucose (11 mM) intraperitoneally (i.p.). Infections were monitored by microscopic examination of Giemsa-stained thin blood films as described previously (31).
To obtain irradiated parasites, mice were exsanguinated at a parasitemia of approximately 30% pRBC. Infected blood, diluted 1:10 in Kreb's saline, was irradiated (30,000 rads) and resuspended in Kreb's saline at a concentration of approximately 5 × 108 pRBC/ml. Mice were inoculated with three 200-μl doses of the pRBC suspension given 2 days apart. Spleens were removed for histological examination 3 days after the final dose. To determine that irradiation had prevented parasite replication, Giemsa-stained thin blood films from the inoculated mice were assessed for the subsequent 10 days for the presence of pRBC and were found to be negative.
Groups of mice (six mice per group) were immunized i.p. once with 25 μg of CGG in alum. One group received CCG in alum and was not infected, one group was given CGG at the time of infection with 105 pRBC, and one group was given CGG in alum 2 weeks prior to initiation of the P. chabaudi infection. Blood used to obtain plasma was taken 1, 2, and 3 months after immunization.
Mice were given three 2-μg doses of LPS (Alexis) i.p. in 100 μl phosphate-buffered saline (PBS) on days 0, 2, and 5, and spleens were removed for histological examination (see below) on day 7.
ELISAs for measurement of antibodies to CGG.
Enzyme-linked immunosorbent assays (ELISAs) were carried out essentially as described previously (3), except that 5 μg/ml CGG in 0.2 M sodium bicarbonate (pH 9.6) was used as the coating antigen and alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology) (with p-nitrophenyl phosphate [Sigma] as the substrate) was used as the detecting reagent. Optical densities at 450 nm were determined. A pooled plasma sample taken from mice 2 weeks after the third immunization with 25 μg of CGG was used as a standard, and normal mouse plasma was used as the negative control. Antibody amounts were expressed in arbitrary units of specific antibody relative to the standard (1,000 U).
Measurement of antibody avidity.
The avidity of CGG-specific antibody was measured by determining the effect on the formation of an antibody-antigen complex of 1 M ammonium thiocyanate (NH4SCN). CGG at a concentration of 5 μg/ml diluted in 15 mM Na2CO3-34 mM NaHCO3 (pH 9.4) was used as a coating antigen. ELISAs were carried out as described above using twofold dilutions of plasma from CGG-immunized mice, starting with a dilution predetermined to give maximum binding to the plate-bound antigen, in the absence of NH4SCN; the test series was diluted in 1 M NH4SCN in PBS, and a control series was diluted in 1% bovine serum albumin-PBS-Tween 20. After washing, bound antibody was detected with an anti-mouse IgG-horseradish peroxidase conjugate (Dako) and the o-phenylenediamine dihydrochloride (Sigma) substrate. Optical densities at 490 nm were determined. The titration curves were plots of absorption (A) versus log reciprocal dilution (D). Binding curves were fitted to the equation A = Amax · [1 − D/(D + Ke)] + B, where A is the observed absorption at dilution 1/D, Amax is the absorption when CGG is saturated with antibody, B is the blank value, and Ke is the midpoint titer of the serum [the estimated dilution giving 50% binding corresponding to the inflection point in the plot of A versus log(D)]. Fitting was carried out by least-squares minimization using the solver attachment in Excel, and the goodness of fit was tested by regression analysis (r2 > 0.7 was considered acceptable). The titration curve was shifted to the left by increasing concentrations of NH4SCN (47), and the avidity of the anti-CGG antibody response was defined as the ratio of the fitted K estimate (KC) in the presence of 1 M NH4SCN to the K estimate in the absence of chaotrope (K0).
Flow cytometry.
Spleen cells from naïve or P. chabaudi-infected mice were mashed through a 0.7-μm sieve (Falcon) to create a single-celled suspension. Erythrocytes were lysed with red blood cell lysis buffer (Sigma), and the cell pellet was washed and resuspended in FACS buffer (1% [wt/vol] bovine serum albumin, 5 mM EDTA, and 0.01% sodium azide in PBS). Cells were enumerated with a hemocytometer and trypan blue (Sigma) exclusion or with a Coulter Counter. Before addition of specific fluorescently labeled antibodies, 5 × 105 cells were preincubated at room temperature for 10 min with anti-Fc receptor antibody (63) to prevent nonspecific binding via the FcR. All other preparations with antibodies were incubated for 20 min on ice. Cells were analyzed with a BD FACScalibur.
Histology.
Five-micrometer spleen sections were prepared as described previously (2). Immunofluorescence staining was carried out by using a method modified from the method described by Achtman et al. (2). All antibody incubations were carried out in the dark for 1 h. The primary antibodies were rat anti-mouse CD8, CD138, biotinylated hamster anti-mouse CD11c (BD), rat anti-mouse CD3, IgM, CD169/sialoadhesin/MOMA-1, MAdCAM-1 (Serotec), hamster anti-mouse CD11c Alexa Fluor 488 (Caltag), sheep anti-mouse IgD (The Binding Site), biotinylated peanut agglutinin (Vector Labs), rat anti-mouse ER-TR9 (BMA), rat anti-mouse F4/80 fluorescein isothiocyanate (FITC) (eBioscience), and goat anti-mouse IgM FITC (Sigma). The secondary antibodies used were donkey anti-sheep Alexa Fluor 647, donkey anti-sheep Alexa Fluor 568, goat anti-rat IgG Alexa Fluor 488, chicken anti-rat Alexa Fluor 647, Neutravidin Texas Red (Molecular Probes), donkey anti-sheep horseradish peroxidase (The Binding Site), biotinylated rabbit anti-rat IgG (Dako), and streptavidin FITC (BD). Slides were mounted with fluorescence mounting medium (Dako). Nonfluorescent histological examination was carried out as described previously (2).
Statistics.
Differences in splenocyte and follicle numbers between wild-type, TLR−/−, and MyD88−/− mice and differences in antibody avidities (K0 − K1M) were analyzed by using the Mann-Whitney test. Parasitemias for strains of mice were compared by using a one-way analysis of variance. Avidity binding curve fitting was carried out by least-squares minimization, and the goodness of fit was tested by regression analysis.
RESULTS
Alterations in splenic structure during acute malaria infection.
The splenic microarchitecture was investigated during the first 10 days of P. chabaudi infection, up to and including the peak of parasitemia (see Fig. S1A in the supplemental material). As described previously (2), P. chabaudi infection induced an increase in spleen size and cellularity (see Fig. S1B in the supplemental material), as well as a transient loss of clearly defined follicles including CD3+ T-cell and IgD+ B-cell areas and transient increases in the number of CD138+ plasma cells and the size of PNA+ germinal centers (see Fig. S1C and D in the supplemental material).
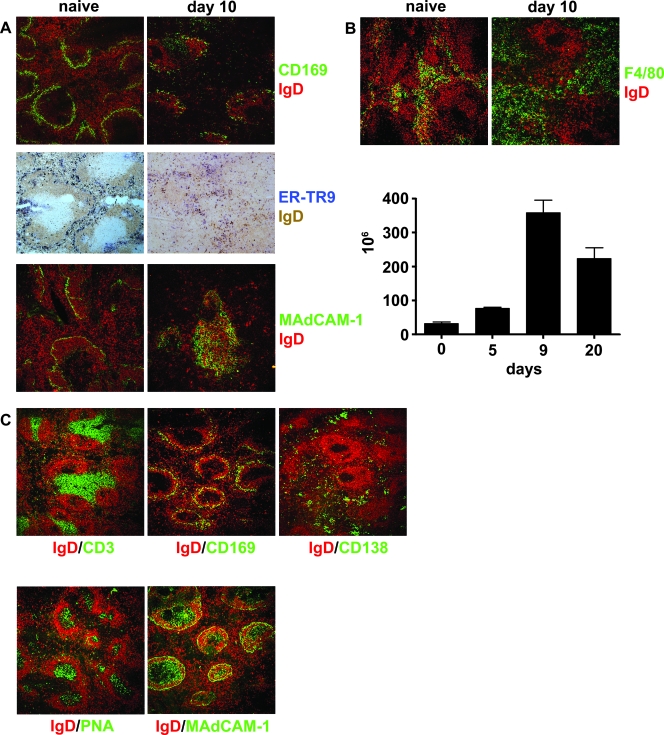
Changes in spleen structure were first visible at day 5 postinfection, and the maximum alterations were observed on day 10, 2 to 3 days after the peak of infection. The clear marginal zones surrounding the follicles seen in normal spleens disappeared, and there was loss of CD169+ marginal metallophilic macrophages (MMM) and ER-TR9+ marginal zone macrophages (MZM) (Fig. 1A). Expression of the adhesion molecule MAdCAM-1, normally seen as a defined ring surrounding the follicles and white pulp (27), was increased and appeared as diffusely stained areas over the white pulp (Fig. 1A). There was also a large increase in the number of F4/80+ macrophages, which remained in the red pulp throughout infection (Fig. 1B). Normal splenic structure returned by day 60, when the parasitemia subsided to subpatent levels (2, 3, 8, 29, 54, 67; data not shown).
FIG. 1.
Changes in splenic microarchitechure and composition during the acute stage of a primary infection with P. chabaudi and after injection of irradiated parasites. Five-micrometer-thick cryostat sections of spleens from naïve mice and from mice on day 10 postinfection were stained with IgD (red or brown) and either (A) CD169 (also known as sialoadhesin or MOMA-1) (green), ER-TR9 (blue), or MAdCAM-1 (green) or with (B, upper panel) IgD (red) and F4/80 (green). (B, lower panel) Graph showing quantification of the F4/80 cells enumerated by flow cytometry during the first 20 days of infection. The bars indicate the medians and ranges for three mice. (C) Mice were inoculated with irradiated parasites as described in Materials and Methods. Spleens were removed 3 days after the final injection, sectioned, and stained for IgD (red) and for CD3 (green), CD169 (green), CD138 (green), peanut agglutinin (green), or MAdCAM-1 (green). The sections in panels A to C are representative of three mice. Final magnification, ×120.
The majority of the alterations in splenic microarchitecture were the result of a live infection, as administration of three doses of irradiated parasites at 2-day intervals (a total of 3 × 108 parasites) to mice did not disrupt the segregation of B- and T-cell zones or result in loss of marginal zone cells (Fig. 1C). However, compatible with exposure to an immunogen(s), there was development of both plasma cells and germinal centers, as well as increased expression of MAdCAM-1 over the white pulp.
TLR2, TLR4, TLR6, or TLR9 or the adaptor protein MyD88 is not necessary for alterations of the splenic microarchitecture during infection.
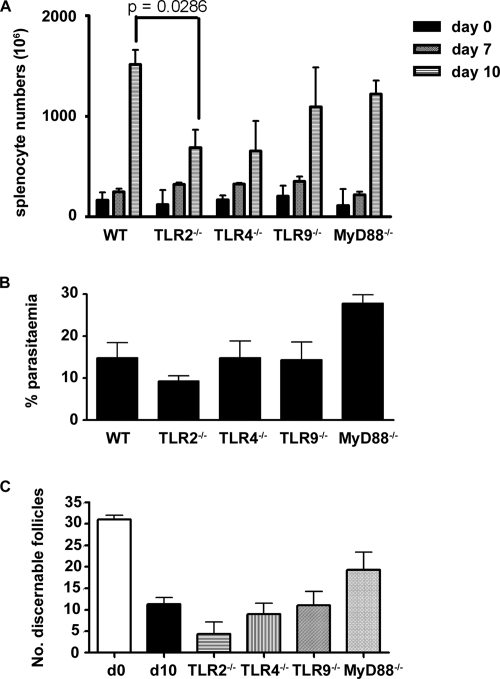
TLR2−/−, TLR4−/−, TLR9−/−, and MyD88−/− mice were infected with P. chabaudi, and their spleens were subjected to a histological examination at day 10 postinfection, when alterations of the splenic microarchitecture were maximal. Spleens of uninfected wild-type mice and MyD88−/− and TLR−/− mice contained comparable number of cells (Fig. 2A) and had similar architecture (see Fig. S2 in the supplemental material), although the TLR2−/− mouse spleens contained significantly fewer cells at day 10 postinfection than the spleens of wild-type mice (P = 0.0286, Mann-Whitney test).
FIG. 2.
P. chabaudi parasitemia, total spleen cell numbers, and numbers of discernible splenic follicles at 10 days postinfection. C57BL/6 (WT), TLR2−/−, TLR4−/−, TLR9−/−, and MyD88−/− mice were used in the experiments. (A) Total spleen cell numbers in naïve mice on days 7 and 10 postinfection. (B) Percentage of parasitemia at day 10 postinfection. (C) Number of discernible splenic follicles in 4,720 μm2 at day 10 postinfection (d10). The bars and error bars indicate the medians and ranges for three mice. D0, day 0.
The increase in spleen size or cellularity after infection with P. chabaudi was not dependent on the presence of MyD88, TLR4, or TLR9 despite the slightly higher parasitemia at day 10 in infected MyD88−/− mice compared with the other groups of TLR−/− mice (for MyD88−/− and TLR2−/− mice, P < 0.01; for MyD88 and TLR9−/− mice, P < 0.05 [one-way analysis of variance]) (Fig. 2B).
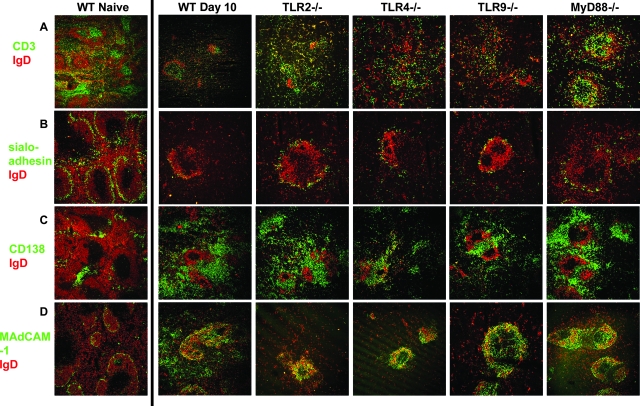
The loss of definition of B- and T-cell areas in the TLR−/− mice was comparable to that in wild-type C57BL/6 mice. Similarly, marginal zone structure was disturbed, as determined by loss of sialoadhesin-positive MMM (sialoadhesin is equivalent to CD169/MOMA-1 shown in Fig. 1A). The changes in MAdCAM-1 expression and production of CD138+ plasma cells in the TLR−/− and MyD88−/− mice were also similar in all cases to the changes observed in infected C57BL/6 mice (Fig. 3), suggesting that ligation of the individual TLRs by parasite moieties was not responsible for the changes in the marginal zones. There appeared to be more discernible follicles (number of follicles per 4,720 μm2) in the spleens of infected MyD88−/− mice than in the spleens of the infected TLR−/− and wild-type mice (Fig. 2C). However, the differences were not significant (e.g., for MyD88−/− mice versus wild-type mice on day 10, P = 0.2286 [Mann-Whitney test]; for MyD88−/− mice versus TLR2−/− mice, P = 0.1).
FIG. 3.
Alteration in splenic microarchitecture during an acute P. chabaudi infection is not affected by a lack of MyD88, TLR2, TLR4, or TLR9. Five-micrometer cryostat sections of spleens from naïve C57BL/6 (WT Naive) and day 10 postinfection C57BL/6 (WT), TLR2−/−, TLR4−/−, TLR9−/−, and MyD88−/− mice were stained with IgD (red) and (A) CD3 (green), (B) sialoadhesin (also known as CD169 or MOMA-1) (green), (C) CD138 (green), or (D) MAdCAM-1 (green). Final magnification, ×120. Representative areas for three mice are shown.
Administration of multiple doses of the TLR4 ligand LPS (three 2-μg doses, with the spleens removed 3 days after the final dose) caused emigration of T cells from the white pulp to the red pulp, as described previously (19). MAdCAM-1 expression was induced over the white pulp, but in contrast to malaria infection, there was no loss of MMM from the marginal zone; instead, there was a thickening of the MMM ring in the marginal zone (see Fig. S3 in the supplemental material).
Germinal center formation and IgG antibody responses take place despite changes in the splenic structure.
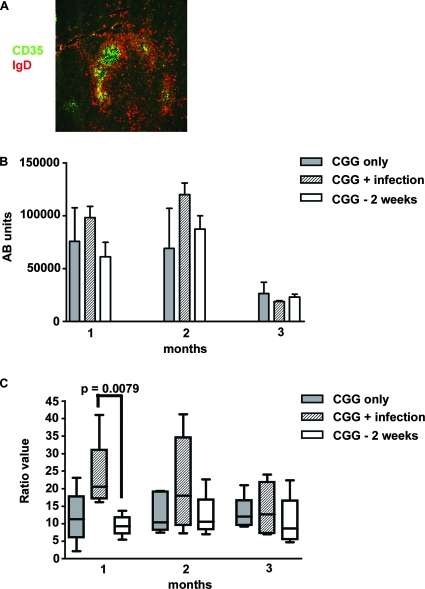
The loss of marginal zones and clear definition of T- and B-cell areas may be indications of adverse alterations in NF-κB and chemokine signaling (21, 62), which could affect correct cell migration (39, 40) and thus immune responses in acutely infected mice. Germinal centers did develop during the primary P. chabaudi infection (2) (see Fig. S1 in the supplemental material), but distinct dark and light zones (detected by antibodies to CD35 [6]) within these germinal centers, which are normally seen in germinal centers after immunization (6, 7), were not observed in spleen sections obtained from mice infected with P. chabaudi at 10 days postinfection (Fig. 4A) (typically, 12 germinal centers in a 4,720-μm2 area were counted). It is therefore possible that IgG antibody responses arising from germinal center B cells could be impaired during infection. Despite the abnormal appearance of the germinal centers in infected mice, the magnitude of the IgG antibody response to CGG that was administered before (14 days) or at initiation of the acute infection was not noticeably reduced (Fig. 4B). However, the avidity of the anti-CGG IgG antibody response at 1 month postimmunization was significantly lower (greater K0/KC ratio) in mice immunized with CGG at the same time that the P. chabaudi infection was initiated than in mice immunized with CGG 2 weeks prior to infection (P = 0.0079, Mann-Whitney test). This effect was transient as by 2 and 3 months after immunization, the avidities of the CGG antibodies were similar in all groups (Fig. 4C).
FIG. 4.
Avidity, but not magnitude, of the antibody response to CGG is reduced by infection with P. chabaudi. (A) Dark and light zones of germinal center in a P. chabaudi-infected mouse at day 10 postinfection. Five-micrometer cryostat sections of spleens from three mice were stained with IgD (red) and CD35 (green). (B and C) Amount (B) and avidity (C) of CGG-specific IgG antibodies of six mice immunized with CGG in alum (gray bars), mice immunized with CGG in alum given at the same time as primary infection with 105 P. chabaudi parasites (cross-hatched bars), and mice immunized with CGG in alum given 2 weeks prior to initiation of a P. chabaudi infection (open bars). Antibody levels and avidity were determined 1 to 3 months postimmunization. Each box and whisker plot in panel C indicates the range and 95% confidence interval of the values (K0/KC ratio, as described in Materials and Methods) obtained for five mice. The horizontal line in each bar indicates the median. Ab, antibody.
DISCUSSION
An acute blood-stage malaria infection in mice results in profound changes in splenic architecture (1, 9, 29, 54, 67), which may impact the host's ability to mount a successful immune response to the parasite. In addition to the loss of marginal zones (MMM, marginal zone B cells, and MZM) and the general loss of follicular structure observed here in P. chabaudi infections and reported by other workers (1, 9, 54), there is also a clear change in the pattern of expression of the mucosal addressin adhesion molecule MAdCAM-1, particularly over the remnants of the follicles. These effects are transient, and the spleen returns to its normal size and structure within 6 weeks after the primary infection. However, during the early acute infection, as the splenic architecture is changing, affinity maturation of the antibody response to a third-party antigen, CGG, is delayed.
Some of the splenic alterations observed in this P. chabaudi infection, in particular the loss of B cells from the marginal zone, the redistribution of T cells throughout the red pulp, and the increase in red pulp macrophages, have also been observed in human Plasmodium falciparum infections (65). Such alterations of the splenic microarchitecture are, however, not confined to malaria infections. They have been observed to a greater or lesser degree in viral, bacterial, and other protozoan infections and after injection of LPS, a bacterial ligand for TLR2/4 (11, 17, 19). It is possible, therefore, that these alterations are initiated through pattern recognition receptor (PRR) recognition of pathogen-associated molecular patterns (PAMPs) as part of the host response to a systemic pathogen to allow the cells of the host's innate immune system to destroy the pathogen and to activate the appropriate acquired immune response. Plasmodium contains many membrane proteins with glycosylphosphatidylinositol anchors, which are recognized by TLR2 and TLR4 (28), and infected red blood cells contain hemozoin with trapped DNA, which is recognized by TLR9 (13, 44, 45). However, the absence of these TLRs or MyD88, one the major adaptor proteins in the signaling pathway of TLRs (for a review, see reference 56) had no significant effect on either the malaria-induced alterations in splenic microarchitecture or splenomegaly in P. chabaudi-infected mice. Ligation of TLRs can affect cell migration and expression of chemokines (43, 49); however, only a lack of TLR2 had a minor effect on splenomegaly in P. chabaudi-infected mice. Our results suggest several possibilities: signaling through other TLRs or several TLRs in concert is required for significant splenic changes, other PAMP-PRR interactions are involved, or the response is independent of PAMP-PRR interactions. This is in line with recent studies of P. chabaudi and Plasmodium berghei infections (18, 60; C. Voisine and J. Langhorne, unpublished data), which, although they did not examine splenomegaly and spleen structure, showed that there were only minor links or no association between MyD88, TLR2, TLR4, TLR6, or TLR9 expression and susceptibility to malaria infections. Similarly, they could not rule out the possibility of other PAMP-PRR interactions.
The changes in the spleen structure, particularly the loss of cells from the marginal zones, does not necessarily mean that the cells are no longer in the spleen or that they are not functional. Migration out of the marginal zones could be part of a normal protective host immune response, which is simply exaggerated in the case of malaria by the sustained high numbers of parasites, red cell loss, and increased hematopoesis. Marginal zone B cells, although no longer present in the marginal zones in acute P. chabaudi infection, are still present in the spleen (2) and thus could still participate in antiparasite responses. The location of MMM and MZM in the marginal sinus is ideal for allowing them to move into the red pulp to phagocytose blood-borne antigens, such as pRBC, removing them from the circulation. From other immunization studies these cells are known to be important contributors to the early antibody response to blood-borne antigens (for a review, see reference 34). Although MMM have been shown to colocalize with apoptotic bodies (9), indicating that they are lost from the spleen, it is not clear whether this is before or after phagocytosis of pRBC.
The increase in MAdCAM-1 is interesting. Expression of MAdCAM1, a ligand for L-selectin present on most leukocytes (for a review, see reference 59), is controlled by the alternative NF-κB pathway (21) and is upregulated in response to inflammation and agents such as LPS, tumor necrosis factor alpha, and interleukin-1 (50). Although MAdCAM-1 plays an important role in cell trafficking in the gut, no such role has ever been demonstrated for this molecule in the spleen, and the function that it has in the marginal sinus remains unknown (27). High levels of expression of this addressin are constitutively found on high endothelial venules (HEV) in Peyer's patches and mesenteric lymph nodes, but this molecule is otherwise normally only seen during the early development of HEV (25). Although fenestrated capillaries rather than HEV are present in the spleen, it is possible that elevated and extensive expression in an infected spleen reflects restructuring of capillaries and compartmentalization of distinct areas to maximize the chances of appropriate host responses and protection of the hematopoetic beds (67) in the red pulp (52).
On the other hand, the alterations of the splenic microarchitecture seen during acute malaria infection resemble those observed in mice lacking lymphotoxin-β expression on B cells (62), which have reduced numbers of B-cell follicles, little distinction between B- and T-cell zones, and few MMM, MZM, and marginal zone B cells in the marginal zone, as well as increased MAdCAM-1 expression over the white pulp area. A lack of lymphotoxin-β has been shown to increase susceptibility to Leishmania and viral infections, secondary to alterations in splenic microarchitecture (51), suggesting that the alterations seen in acute malaria infections may be detrimental to the host and may contribute to the lower B- and T-cell responses reported in several experimental malaria infections and in human infections (15, 38, 42, 64, 71).
Very little is known about whether the modified splenic structure in malaria infections affects immune responses and hematopoeisis. Although there was a substantial loss of clearly defined T- and B-cell areas, germinal centers were formed and persisted for up to 60 days postinfection (1), indicating that a T-cell-dependent B-cell response was able to take place. Indeed, the IgG antibody response to another antigen (CGG) given to mice either at the time of P. chabaudi infection or 2 weeks prior to infection was similar in magnitude to that of the antibody response to CGG given in the absence of infection. This contrasts somewhat with the results of Millington et al. (37), who showed that mice immunized with ovalbumin and LPS 6 h or 12 days after malaria infection had significantly reduced IgG responses to ovalbumin at 3 weeks postimmunization. This may have been due to differences in the timing of immunization and the adjuvant used, since in the study of these workers, when mice were immunized 4 days after malaria infection, no significant differences in IgG responses were observed. Thus, whatever effect malaria infection has on generation of antibody responses, the time of immunization matters. This may be a reflection of when splenic changes are initiated and their transient nature.
Although antibody titers did not appear to be affected by malaria infection, there was a significant delay in affinity maturation when infection and immunization were carried out concurrently. Normal dark and light zones were not observed within the germinal centers in spleens of P. chabaudi-infected mice, similar to the results for germinal centers in P. berghei-infected mice (12). The light zones of germinal centers contain the network of follicular dendritic cells. Interaction of the immunoglobulin receptor of B cells with antigen displayed on their surface promotes somatic hypermutation of the receptor and thus affinity maturation of the antibody response (for a review, see reference 33). A detailed study of follicular dendritic cells and germinal center reactions together with an analysis of antibody affinity from individual B cells within germinal centers in malaria infections would elucidate whether the altered structure of germinal centers plays any role in the delayed affinity maturation.
The results obtained with the P. chabaudi malaria model have important implications in areas where transmission of malaria is endemic. Immunization during acute infection, particularly immunization of children, could result in antibodies with lower affinity. Studies are under way to establish whether immunization against a second infection during acute malaria leads to reduced protective efficacy.
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Council, United Kingdom, and was part of the activities of the BioMalPar European Network of Excellence supported by FP6 EU grant LSHP-CT-2004-503578. Emma T. Cadman received an MRC Ph.D. studentship, and Asmahan Abdallah was a recipient of FP6 EU-funded Marie Curie Action MalPar training (grant MEST-CT-2005-020492).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 16 June 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Achtman, A. H. 2003. The B cell response to Plasmodium chabaudi chabaudi malaria in the mouse model. Open University, London, United Kingdom.
- 2.Achtman, A. H., M. Khan, I. C. MacLennan, and J. Langhorne. 2003. Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J. Immunol. 171317-324. [DOI] [PubMed] [Google Scholar]
- 3.Achtman, A. H., R. Stephens, E. T. Cadman, V. Harrison, and J. Langhorne. 2007. Malaria-specific antibody responses and parasite persistence after infection of mice with Plasmodium chabaudi chabaudi. Parasite Immunol. 29435-444. [DOI] [PubMed] [Google Scholar]
- 4.Adachi, K., H. Tsutsui, S. Kashiwamura, E. Seki, H. Nakano, O. Takeuchi, K. Takeda, K. Okumura, L. Van Kaer, H. Okamura, S. Akira, and K. Nakanishi. 2001. Plasmodium berghei infection in mice induces liver injury by an IL-12- and Toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J. Immunol. 1675928-5934. [DOI] [PubMed] [Google Scholar]
- 5.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. [DOI] [PubMed] [Google Scholar]
- 6.Allen, C. D., K. M. Ansel, C. Low, R. Lesley, H. Tamamura, N. Fujii, and J. G. Cyster. 2004. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5943-952. [DOI] [PubMed] [Google Scholar]
- 7.Allen, C. D., T. Okada, and J. G. Cyster. 2007. Germinal-center organization and cellular dynamics. Immunity 27190-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves, H. J., W. Weidanz, and L. Weiss. 1996. The spleen in murine Plasmodium chabaudi adami malaria: stromal cells, T lymphocytes, and hematopoiesis. Am. J. Trop. Med. Hyg. 55370-378. [DOI] [PubMed] [Google Scholar]
- 9.Beattie, L., C. R. Engwerda, M. Wykes, and M. F. Good. 2006. CD8+ T lymphocyte-mediated loss of marginal metallophilic macrophages following infection with Plasmodium chabaudi chabaudi AS. J. Immunol. 1772518-2526. [DOI] [PubMed] [Google Scholar]
- 10.Bejon, P., J. Mwacharo, O. Kai, S. Todryk, S. Keating, B. Lowe, T. Lang, T. W. Mwangi, S. C. Gilbert, N. Peshu, K. Marsh, and A. V. Hill. 2007. The induction and persistence of T cell IFN-γ responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J. Immunol. 1794193-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedict, C. A., C. De Trez, K. Schneider, S. Ha, G. Patterson, and C. F. Ware. 2006. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog. 2e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho, L. J., M. F. Ferreira-da-Cruz, C. T. Daniel-Ribeiro, M. Pelajo-Machado, and H. L. Lenzi. 2007. Germinal center architecture disturbance during Plasmodium berghei ANKA infection in CBA mice. Malaria J. 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coban, C., K. J. Ishii, T. Kawai, H. Hemmi, S. Sato, S. Uematsu, M. Yamamoto, O. Takeuchi, S. Itagaki, N. Kumar, T. Horii, and S. Akira. 2005. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 20119-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crane, G. G., and D. S. Pryor. 1971. Malaria and the tropical splenomegaly syndrome in New Guinea. Trans. R. Soc. Trop. Med. Hyg. 65315-324. [DOI] [PubMed] [Google Scholar]
- 15.Dorfman, J. R., P. Bejon, F. M. Ndungu, J. Langhorne, M. M. Kortok, B. S. Lowe, T. W. Mwangi, T. N. Williams, and K. Marsh. 2005. B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J. Infect. Dis. 1911623-1630. [DOI] [PubMed] [Google Scholar]
- 16.Elliott, S. R., T. P. Spurck, J. M. Dodin, A. G. Maier, T. S. Voss, F. Yosaatmadja, P. D. Payne, G. I. McFadden, A. F. Cowman, S. J. Rogerson, L. Schofield, and G. V. Brown. 2007. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect. Immun. 753621-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engwerda, C. R., M. Ato, S. E. Cotterell, T. L. Mynott, A. Tschannerl, P. M. Gorak-Stolinska, and P. M. Kaye. 2002. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am. J. Pathol. 161429-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin, B. S., S. O. Rodrigues, L. R. Antonelli, R. V. Oliveira, A. M. Goncalves, P. A. Sales-Junior, E. P. Valente, J. I. Alvarez-Leite, C. Ropert, D. T. Golenbock, and R. T. Gazzinelli. 2007. MyD88-dependent activation of dendritic cells and CD4+ T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes Infect. 9881-890. [DOI] [PubMed] [Google Scholar]
- 19.Groeneveld, P. H., T. Erich, and G. Kraal. 1986. The differential effects of bacterial lipopolysaccharide (LPS) on splenic non-lymphoid cells demonstrated by monoclonal antibodies. Immunology 58285-290. [PMC free article] [PubMed] [Google Scholar]
- 20.Grun, J. L., C. A. Long, and W. P. Weidanz. 1985. Effects of splenectomy on antibody-independent immunity to Plasmodium chabaudi adami malaria. Infect. Immun. 48853-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo, F., D. Weih, E. Meier, and F. Weih. 2007. Constitutive alternative NF-κB signaling promotes marginal zone B-cell development but disrupts the marginal sinus and induces HEV-like structures in the spleen. Blood 1102381-2389. [DOI] [PubMed] [Google Scholar]
- 22.Helmby, H., G. Jonsson, and M. Troye-Blomberg. 2000. Cellular changes and apoptosis in the spleens and peripheral blood of mice infected with blood-stage Plasmodium chabaudi chabaudi AS. Infect. Immun. 681485-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 24.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1623749-3752. [PubMed] [Google Scholar]
- 25.Iizuka, T., T. Tanaka, M. Suematsu, S. Miura, T. Watanabe, R. Koike, Y. Ishimura, H. Ishii, N. Miyasaka, and M. Miyasaka. 2000. Stage-specific expression of mucosal addressin cell adhesion molecule-1 during embryogenesis in rats. J. Immunol. 1642463-2471. [DOI] [PubMed] [Google Scholar]
- 26.Janssens, S., and R. Beyaert. 2002. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem. Sci. 27474-482. [DOI] [PubMed] [Google Scholar]
- 27.Kraal, G., K. Schornagel, P. R. Streeter, B. Holzmann, and E. C. Butcher. 1995. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am. J. Pathol. 147763-771. [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnegowda, G., A. M. Hajjar, J. Zhu, E. J. Douglass, S. Uematsu, S. Akira, A. S. Woods, and D. C. Gowda. 2005. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J. Biol. Chem. 2808606-8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krucken, J., L. I. Mehnert, M. A. Dkhil, M. El-Khadragy, W. P. Benten, H. Mossmann, and F. Wunderlich. 2005. Massive destruction of malaria-parasitized red blood cells despite spleen closure. Infect. Immun. 736390-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar, S., M. F. Good, F. Dontfraid, J. M. Vinetz, and L. H. Miller. 1989. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J. Immunol. 1432017-2023. [PubMed] [Google Scholar]
- 31.Langhorne, J., S. Gillard, B. Simon, S. Slade, and K. Eichmann. 1989. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int. Immunol. 1416-424. [DOI] [PubMed] [Google Scholar]
- 32.Leisewitz, A. L., K. A. Rockett, B. Gumede, M. Jones, B. Urban, and D. P. Kwiatkowski. 2004. Response of the splenic dendritic cell population to malaria infection. Infect. Immun. 724233-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacLennan, I. C. 1994. Germinal centers. Annu. Rev. Immunol. 12117-139. [DOI] [PubMed] [Google Scholar]
- 34.Martin, F., and J. F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. 2323-335. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Peprah, R., I. Bates, G. Bedu-Addo, and D. P. Kwiatkowski. 2006. Investigation of familial segregation of hyperreactive malarial splenomegaly in Kumasi, Ghana. Trans. R. Soc. Trop. Med. Hyg. 10068-73. [DOI] [PubMed] [Google Scholar]
- 36.Meding, S. J., S. C. Cheng, B. Simon-Haarhaus, and J. Langhorne. 1990. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect. Immun. 583671-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millington, O. R., C. Di Lorenzo, R. S. Phillips, P. Garside, and J. M. Brewer. 2006. Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function. J. Biol. 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millington, O. R., V. B. Gibson, C. M. Rush, B. H. Zinselmeyer, R. S. Phillips, P. Garside, and J. M. Brewer. 2007. Malaria impairs T cell clustering and immune priming despite normal signal 1 from dendritic cells. PLoS Pathog. 31380-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moser, K., G. Muehlinghaus, R. Manz, H. Mei, C. Voigt, T. Yoshida, T. Dorner, F. Hiepe, and A. Radbruch. 2006. Long-lived plasma cells in immunity and immunopathology. Immunol. Lett. 10383-85. [DOI] [PubMed] [Google Scholar]
- 40.Moser, K., K. Tokoyoda, A. Radbruch, I. MacLennan, and R. A. Manz. 2006. Stromal niches, plasma cell differentiation and survival. Curr. Opin. Immunol. 18265-270. [DOI] [PubMed] [Google Scholar]
- 41.Nogueira, P. A., F. P. Alves, C. Fernandez-Becerra, O. Pein, N. R. Santos, L. H. Pereira da Silva, E. P. Camargo, and H. A. del Portillo. 2006. A reduced risk of infection with Plasmodium vivax and clinical protection against malaria are associated with antibodies against the N terminus but not the C terminus of merozoite surface protein 1. Infect. Immun. 742726-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ocana-Morgner, C., M. M. Mota, and A. Rodriguez. 2003. Malaria blood stage suppression of liver stage immunity by dendritic cells. J. Exp. Med. 197143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker, L. C., M. K. Whyte, S. N. Vogel, S. K. Dower, and I. Sabroe. 2004. Toll-like receptor (TLR)2 and TLR4 agonists regulate CCR expression in human monocytic cells. J. Immunol. 1724977-4986. [DOI] [PubMed] [Google Scholar]
- 44.Parroche, P., F. N. Lauw, N. Goutagny, E. Latz, B. G. Monks, A. Visintin, K. A. Halmen, M. Lamphier, M. Olivier, D. C. Bartholomeu, R. T. Gazzinelli, and D. T. Golenbock. 2007. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl. Acad. Sci. USA 1041919-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pichyangkul, S., K. Yongvanitchit, U. Kum-arb, H. Hemmi, S. Akira, A. M. Krieg, D. G. Heppner, V. A. Stewart, H. Hasegawa, S. Looareesuwan, G. D. Shanks, and R. S. Miller. 2004. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 1724926-4933. [DOI] [PubMed] [Google Scholar]
- 46.Pitney, W. R. 1968. The tropical splenomegaly syndrome. Trans. R. Soc. Trop. Med. Hyg. 62717-728. [DOI] [PubMed] [Google Scholar]
- 47.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 8683-87. [DOI] [PubMed] [Google Scholar]
- 48.Rockett, K. A., M. M. Awburn, E. J. Rockett, W. B. Cowden, and I. A. Clark. 1994. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 16243-249. [DOI] [PubMed] [Google Scholar]
- 49.Shaykhiev, R., J. Behr, and R. Bals. 2008. Microbial patterns signaling via Toll-like receptors 2 and 5 contribute to epithelial repair, growth and survival. PLoS ONE 3e1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sikorski, E. E., R. Hallmann, E. L. Berg, and E. C. Butcher. 1993. The Peyer's patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J. Immunol. 1515239-5250. [PubMed] [Google Scholar]
- 51.Spahn, T. W., H. P. Eugster, A. Fontana, W. Domschke, and T. Kucharzik. 2005. Role of lymphotoxin in experimental models of infectious diseases: potential benefits and risks of a therapeutic inhibition of the lymphotoxin-beta receptor pathway. Infect. Immun. 737077-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steiniger, B., and P. Barth. 2000. Microanatomy and function of the spleen. Springer, Berlin, Germany. [DOI] [PubMed]
- 53.Stephens, R., F. R. Albano, S. Quin, B. J. Pascal, V. Harrison, B. Stockinger, D. Kioussis, H. U. Weltzien, and J. Langhorne. 2005. Malaria-specific transgenic CD4+ T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood 1061676-1684. [DOI] [PubMed] [Google Scholar]
- 54.Stevenson, M. M., and G. Kraal. 1989. Histological changes in the spleen and liver of C57BL/6 and A/J. mice during Plasmodium chabaudi AS infection. Exp. Mol. Pathol. 5180-95. [DOI] [PubMed] [Google Scholar]
- 55.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 163-9. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi, O., and S. Akira. 2002. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270155-167. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11443-451. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13933-940. [DOI] [PubMed] [Google Scholar]
- 59.Tedder, T. F., D. A. Steeber, A. Chen, and P. Engel. 1995. The selectins: vascular adhesion molecules. FASEB J. 9866-873. [PubMed] [Google Scholar]
- 60.Togbe, D., L. Schofield, G. E. Grau, B. Schnyder, V. Boissay, S. Charron, S. Rose, B. Beutler, V. F. Quesniaux, and B. Ryffel. 2007. Murine cerebral malaria development is independent of Toll-like receptor signaling. Am. J. Pathol. 1701640-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsubata, S., K. Ebe, T. Kawamura, Y. Ishimoto, C. Tomiyama-Miyaji, H. Watanabe, H. Sekikawa, Y. Aoyagi, and T. Abo. 2005. Protection against malaria by anti-erythropoietin antibody due to suppression of erythropoiesis in the liver and at other sites. Immunol. Cell Biol. 83638-642. [DOI] [PubMed] [Google Scholar]
- 62.Tumanov, A. V., S. I. Grivennikov, A. N. Shakhov, S. A. Rybtsov, E. P. Koroleva, J. Takeda, S. A. Nedospasov, and D. V. Kuprash. 2003. Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol. Rev. 195106-116. [DOI] [PubMed] [Google Scholar]
- 63.Unkeless, J. C. 1979. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J. Exp. Med. 150580-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 40073-77. [DOI] [PubMed] [Google Scholar]
- 65.Urban, B. C., T. T. Hien, N. P. Day, N. H. Phu, R. Roberts, E. Pongponratn, M. Jones, N. T. Mai, D. Bethell, G. D. Turner, D. Ferguson, N. J. White, and D. J. Roberts. 2005. Fatal Plasmodium falciparum malaria causes specific patterns of splenic architectural disorganization. Infect. Immun. 731986-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walliker, D., R. Carter, and A. Sanderson. 1975. Genetic studies on Plasmodium chabaudi: recombination between enzyme markers. Parasitology 7019-24. [DOI] [PubMed] [Google Scholar]
- 67.Weiss, L. 1989. Mechanisms of splenic control of murine malaria: cellular reactions of the spleen in lethal (strain 17XL) Plasmodium yoelii malaria in BALB/c mice, and the consequences of pre-infective splenectomy. Am. J. Trop. Med. Hyg. 41144-160. [DOI] [PubMed] [Google Scholar]
- 68.Weiss, L., J. Johnson, and W. Weidanz. 1989. Mechanisms of splenic control of murine malaria: tissue culture studies of the erythropoietic interplay of spleen, bone marrow, and blood in lethal (strain 17XL) Plasmodium yoelii malaria in BALB/c mice. Am. J. Trop. Med. Hyg. 41135-143. [PubMed] [Google Scholar]
- 69.Wilson, N. S., G. M. Behrens, R. J. Lundie, C. M. Smith, J. Waithman, L. Young, S. P. Forehan, A. Mount, R. J. Steptoe, K. D. Shortman, T. F. de Koning-Ward, G. T. Belz, F. R. Carbone, B. S. Crabb, W. R. Heath, and J. A. Villadangos. 2006. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat. Immunol. 7165-172. [DOI] [PubMed] [Google Scholar]
- 70.Wykes, M. N., X. Q. Liu, S. Jiang, C. Hirunpetcharat, and M. F. Good. 2007. Systemic tumor necrosis factor generated during lethal Plasmodium infections impairs dendritic cell function. J. Immunol. 1793982-3987. [DOI] [PubMed] [Google Scholar]
- 71.Wykes, M. N., Y. H. Zhou, X. Q. Liu, and M. F. Good. 2005. Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. J. Immunol. 1752510-2516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.