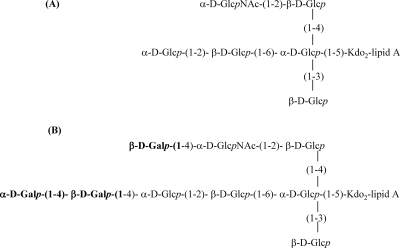Abstract
Lipooligosaccharide (LOS) from Moraxella catarrhalis has the potential to elicit bactericidal antibodies against the pathogen. We generated LOS-based conjugate vaccines that elicited bactericidal antibodies in animal models. However, epitopes on the LOS recognized by the functional anti-LOS antibodies remain unidentified. In this study, a mutant strain, D4, which lost the recognition by a bactericidal anti-LOS rabbit serum in Western blotting was generated from a serotype C strain 26404 by random transposon mutagenesis. Sequence analysis revealed there was an insertion of a kanamycin resistance gene in the lgt2 gene of D4, which encodes β(1-4)-galactosyltransferase. An isogenic lgt2 mutant, 26404lgt2, was constructed. Structural analysis indicated that the mutant strain produced a truncated LOS lacking terminal galactoses from 4- and 6-linked oligosaccharide chains of strain 26404. Further studies showed that the antiserum lost the recognition of both mutant cells and LOSs in Western blotting, an enzyme-linked immunosorbent assay (ELISA), or a flow cytometry assay. The antiserum also lost the ability to kill the mutant strain in a bactericidal assay, whereas it showed a bactericidal titer of 1:80 to strain 26404. In an inhibition ELISA, d-(+)-galactose or 26404lgt2 LOS showed no inhibition. However, the 26404 LOS and a serotype A O35E LOS with terminal galactoses on its 6-linked oligosaccharide chain showed >90% inhibition, while a serotype B 26397 LOS showed >60% inhibition. These studies suggest that the terminal α-Gal-(1→4)-β-Gal on the 6-linked oligosaccharide chain of 26404 LOS plays a critical role in forming the epitope recognized by the bactericidal antiserum induced by immunization with our conjugate vaccine.
Moraxella catarrhalis is a gram-negative human respiratory tract pathogen that causes 15 to 20% of acute otitis media in children, or a total of 3 to 4 million cases annually in the United States (37). The organism's only known host is the human, and its importance as a host-adapted pathogen is now widely recognized. In addition to causing childhood ear infections, this bacterium is also responsible for 10 to 35% of lower respiratory tract infections in adults with chronic obstructive pulmonary disease, the fourth leading cause of death in the United States (25). The present treatment of these diseases has relied largely on antimicrobial agents. However, with growing antibiotic resistance observed in clinical isolates all over the world (10) and the emergence of fatal neonatal meningitis (4), attention has been focused on the possibility of vaccination to protect humans from M. catarrhalis infections (3, 24).
There have been a number of putative vaccine candidates described for M. catarrhalis, and most of them are outer membrane proteins (12, 16, 23). Lipooligosaccharide (LOS) is another prominent surface component of M. catarrhalis. It has been implicated as a virulence factor important in the pathogenesis of this organism (5, 11, 28). Clinical investigations demonstrated that serum antibodies to LOS developed in patients with respiratory tract infections (31), while the bactericidal activity of the convalescent-phase anti-LOS immunoglobulin G (IgG) from patients was against M. catarrhalis (34). Serological properties of the LOSs in humans reveal a less variable structure among three serotypes of LOS (31) accounting for 95% of clinical isolates (A, 61%; B, 29%; and C, 5%) (36). No correlation between serotype and severity of infection has been found (36). No phase-variable LOS biosynthesis genes have been identified to date, though a sequence motif which is related to the phase variation of Haemophilus influenzae was found in M. catarrhalis (27). By using mouse monoclonal antibodies (MAbs) (13, 20, 26, 32) and rabbit polyclonal antibodies (1, 30, 36), serotype-specific and cross-reactive epitopes have been identified. Our early study showed that a specific MAb against serotype A and C LOSs was bactericidal and able to inhibit M. catarrhalis adherence to human epithelia and to promote clearance in a mouse pulmonary model after an aerosol challenge (20). However, a recent study revealed that a mouse bactericidal MAb was to a common LOS epitope in all three serotype strains (13). Structural studies showed that the LOSs from three serotypes were branched with a common oligosaccharide (OS) inner core and the terminal tetrasaccharide α-d-Galp-(1→4)-β-d-Galp-(1→4)-α-d-Glcp-(1→2)-β-d-Glcp-(1→ at the 6-linked position of the trisubstituted glucose residue (6-8, 17). The differences among the three serotype LOSs are mainly limited to the 4-linked position of the trisubstituted glucose residue; types C and A contain α-d-GlcpNAc, while type B contains α-d-Glcp in its place. Only serotype C contains additional terminal galactoses, while in serotype A the α-d-GlcpNAc is the terminal sugar.
We previously synthesized LOS-based conjugate vaccines from all serotypes by detoxification of the LOSs and conjugation of the detoxified LOSs (dLOSs) to protein carriers (14, 40, 41). The vaccines elicited high levels of anti-LOS IgG antibodies with bactericidal activity in mice or rabbits. Rabbit bactericidal anti-LOS sera elicited by serotype A or C conjugates were highly cross-reactive among serotype A and C strains and moderately cross-reactive among serotype B strains (14, 41), while the rabbit bactericidal anti-LOS sera elicited by serotype B conjugates were rather specific and showed cross-reactivity to only some serotype A/C strains (40). Active or passive immunization with the serotype A conjugates or their antisera generated protection against homologous and heterologous serotype A or C but not B strains in a mouse model of pulmonary clearance (19, 21). These lines of evidence indicate that the conjugate vaccine-generated immune responses against LOS may play an important role in the elimination of bacteria interacting with the host, resulting in resolution of infections. However, the main epitope on the LOS recognized by the above-described functional anti-LOS antibodies from the conjugate vaccination remains unknown. To address this question, we generated one LOS mutant, D4, from a serotype C strain, 26404, using transposon mutagenesis followed by Western blotting with a bactericidal anti-LOS rabbit serum elicited by a serotype C dLOS-tetanus toxoid (TT) conjugate vaccine (41). The mutation was confirmed by sequence analysis to be in the lgt2 gene. An isogenic mutant strain, 26404lgt2, was then constructed and characterized.
MATERIALS AND METHODS
Bactericidal anti-LOS rabbit serum, strains, plasmids, and growth conditions.
A rabbit serum elicited by three doses of a serotype C 26404 LOS conjugate vaccine consisting of dLOS-TT plus Ribi adjuvant was used, and the serum showed 59,049 enzyme-linked immunosorbent assay (ELISA) units and a bactericidal titer of 1:80 (41). Bacterial strains, plasmids, and primers are described in Table 1. M. catarrhalis strains were cultured on chocolate agar plates (Remel, Lenexa, KS) at 37°C with 5% CO2 or shaken in 3% tryptic soy broth (BD, Sparks, MD) at 37°C and 250 rpm. Mutant strains were selected on brain heart infusion (BHI) agar (Difco, Detroit, MI) supplemented with kanamycin at 20 μg/ml. Escherichia coli was grown on Luria-Bertani agar plates or broth with antibiotic supplementation (kanamycin, 30 μg/ml; ampicillin, 50 μg/ml).
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli TOP 10 | Cloning strain | Invitrogen |
| M. catarrhalis | ||
| O35E | Serotype A wild-type strain | 29 |
| 26397 | Serotype B wild-type strain | CCUGa |
| 26404 | Serotype C wild-type strain | CCUG |
| D4 | Strain 26404 with EZ::TN transposon insertion in lgt2 gene | This study |
| 26404lgt2 | lgt2 knockout mutant strain | This study |
| Plasmids | ||
| pCR2.1 | TOPO TA cloning vector | Invitrogen |
| pBluescript II KS(+) | Cloning vector | Stratagene |
| pUC4K | Kanamycin resistance cassette | Amersham |
| pCR-lgt2 | lgt2 cloned into pCR2.1 | This study |
| KS-lgt2 | EcoRI-SalI lgt2 fragment cloned into KS(+) | This study |
| KS-lgt2-Kan | EcoRI-blunted kanamycin resistance cassette inserted into blunted HindIII site of KS-lgt2 | This study |
| Primers | ||
| lgt2-F | 5′-CTCGTCGACTGTGAATTTGGCAAACAAGG-3′b | This study |
| lgt2-R | 5′-CTCGAATTCACGCTTACCAATGCTTCGAT-3′c | This study |
| lgt587 | 5′-GCCGAAAATAATCACGCAAC-3′ | This study |
| lgt1482 | 5′-TGGCATCAAAAATCACATGG-3′ | This study |
| lgt1754 | 5′-CCCAAATAGACATCGTCCTCA-3′ | This study |
| lgt2188 | 5′-CCAGCGATGAGTGATGTGTT-3′ | This study |
| lgtR1071 | 5′-CGGTCAAATTGTGCCACATC-3′ | This study |
| lgtR2002 | 5′-TGCCACAAAAAGAAGAGAACA-3′ | This study |
| lgtR3472 | 5′-AAAATTGCCCAAAGTCATGG-3′ | This study |
| KAN-2 RP-1 | 5′-GCAATGTAACATCAGAGATTTTGAG-3′ | Epicenter |
CCUG, Culture Collection of the University of Goteborg, Goteborg, Sweden.
SalI site underlined.
EcoRI site underlined.
General DNA methods.
Taq DNA polymerase, DNA restriction endonuclease, T4 DNA ligase, and Klenow fragment (DNA polymerase I large fragment) were purchased from Fermentas (Hanover, MD). Qiagen plasmid maxikits and QIAquick PCR purification kits from Qiagen (Santa Clarita, CA) were used for the preparation of plasmids and the purification of PCR products or digested DNA fragments. Bacterial chromosomal DNA was isolated using the Wizard genomic DNA purification kit (Promega, Madison, WI). DNA nucleotide sequences were obtained with a 3070xl DNA analyzer (Applied Biosystems, Foster City, CA) and analyzed with DNASTAR software (DNASTAR Inc., Madison, WI).
Transposon mutagenesis and identification of the mutant.
Two hundred milliliters of logarithmic-phase culture of strain 26404 (optical density at 600 nm [OD600] = 0.5 to 0.7) was harvested by centrifugation and washed three times with ice-cold 10% (vol/vol) glycerol in distilled water. The final cell pellet was suspended in 1 to 2 ml of washing buffer to make the final cell concentration of 1 × 1010 to 3 × 1010 cells/ml. A 20-μl portion of this cell suspension was mixed with 1 μl of EZ::TN<KAN-2>Tnp Transposome (Epicenter, Madison, WI) and electroporated using a field strength of 3.0 kV over a 1-mm distance for 4 ms (Micropulser; Bio-Rad, Hercules, CA). After the electroporation, the cell suspension was added to 1 ml of BHI broth, shaken at 37°C with 250 rpm for 3 h, and plated on BHI agar plates containing kanamycin. After 24 h of incubation, the resulting kanamycin-resistant (Kanr) colonies were screened by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting for changed binding to the bactericidal anti-LOS rabbit serum. The clone was selected for chromosomal DNA purification and nucleotide sequence analysis. The first primer, KAN-2 RP-1, was provided in the EZ::TN<KAN-2>Tnp Transposome kit; primers lgt1482 and lgt1754 were designed according to the results of nucleotide sequence analysis (Table 1). The lgt2 homologue from strain 26404 was identified by BLAST searches in GenBank of the National Center for Biotechnology Information.
Cloning of the lgt2 homologue and construction of the isogenic lgt2 mutant.
The putative lgt2 homologue was amplified from chromosomal DNA of strain 26404 using primers lgt2-F and lgt2-R (Table 1; Fig. 1). This PCR product was cloned into pCR2.1 using a TOPO TA cloning kit (Invitrogen, Carlsbad, CA) to obtain pCR-lgt2. The insertion was released by EcoRI-SalI digestion and then subcloned into an EcoRI-SalI site of KS(+) to form KS-lgt2. A Kanr cassette (1,282 bp) obtained from pUC4K by EcoRI digestion was subsequently inserted into the lgt2 gene at the HindIII site to form KS-lgt2-Kan. After verification by sequence analysis, the mutagenic construct (containing an insertion of the Kanr cassette within the lgt2 coding region) was amplified by PCR using primers lgt2-F and lgt2-R. The PCR-generated DNA product was purified and used for electroporation. After 24 h of incubation, the resulting Kanr colonies were selected for PCR analysis of chromosomal DNA. The mutant with an inactivated lgt2 gene was verified by nucleotide sequence analysis and named 26404lgt2.
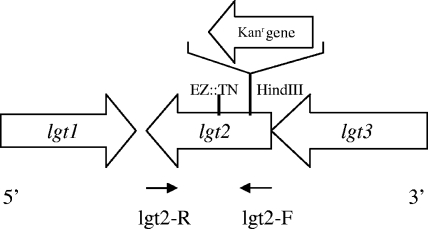
FIG. 1.
Genetic organization of the lgt cluster in the genome of M. catarrhalis strain 26404. Large arrows represent the direction of transcription, the site of the transposon insertion identified in D4 is denoted EN::TN, and the Kanr gene was inserted at the HindIII cleavage site in mutant strain 26404lgt2. The small arrows represent the directions of the primers (Table 1).
LOS purification.
LOSs were purified from strains 26404lgt2, 26404, O35E (serotype A), and 26397 (serotype B) by phenol-water extraction as described previously (14). The protein and nucleic acid contents of the LOSs were shown to be less than 2% (33, 38).
Western blotting and silver staining.
One milliliter of culture of each logarithmic-phase bacterial strain (OD540 = 0.65) was centrifuged, and the cell pellets were suspended in 50 μl of sample buffer (1 M Tris [pH 6.8], 2% SDS, 4% 2-mercaptoethanol, 10% glycerol, and 0.004% bromophenol blue) and boiled at 100°C for 10 min for Western blotting as previously described (20) with modifications. Ten microliters of each sample or 100 ng of purified LOS was subjected to SDS-PAGE on a Mini Protein II gel system (Bio-Rad, Richmond, CA) with 15% polyacrylamide in the separating gel. After electrophoresis at 100 V for 2 h, the samples were transferred onto a pure nitrocellulose membrane (Bio-Rad) using a semidry transfer cell (Bio-Rad) at 15 V for 15 min. The membrane was blocked with 3% bovine serum albumin in phosphate-buffered saline (PBS) for 2 h and incubated with 10 ml of a 1/1,500 dilution of a dLOS-TT rabbit antiserum (41) for 2 h and then with a goat anti-rabbit IgG-alkaline phosphatase conjugate (1/3,000 dilution) (Sigma Chemical Co., St. Louis, MO) for 1 h. It was developed in 5-bromo-4-chloro-3-indolyl phosphate (Sigma). A duplicate gel was silver stained for observation of the LOS pattern after SDS-PAGE as described previously (35).
ELISA and inhibition.
ELISA was performed for testing the recognition of the purified LOSs by the bactericidal anti-LOS rabbit serum as described previously (14) with modifications. Briefly, after overnight coating with a 100-μl sample of LOS from strain 26404lgt2 or 26404 at 10 μg/ml in PBS (pH 7.4) containing 10 mM MgCl2, the plate was blocked with 1% bovine serum albumin for 2 h. The bactericidal anti-LOS rabbit serum was serially diluted, added, and left for 2 h, followed by treatment with alkaline phosphatase-conjugated goat anti-rabbit IgG for 1.5 h. The OD405 was read by using a microplate autoreader after 1 h with a substrate. For the inhibition assay, the antiserum was diluted (1/5,000) and incubated with an equal volume of inhibitor of d-(+)-galactose (Sigma) at concentrations of 20,000, 4,000, 800, 160, 32, and 6.4 μg/ml and of LOS from mutant 26404lgt2 or strain O35E, 26397, or 26404 at concentrations of 20,000, 4,000, 800, 160, 32, and 6.4 ng/ml. The inhibition was performed at 37°C with 160 rpm for 2 h and was followed by ELISA as described above. The percent inhibition was calculated as follows: (OD405 from serum without inhibitor − OD405 from serum with inhibitor)/OD405 from serum without inhibitor × 100%.
Flow cytometry.
To further investigate the recognition of strain 26404lgt2 or 26404 by the bactericidal anti-LOS rabbit serum, flow cytometry was performed. Three milliliters of logarithmic-phase bacterial culture was washed three times with PBS and centrifuged. The pellets were resuspended in 1 ml of the bactericidal anti-LOS or preimmune rabbit serum in PBS (1:50) and incubated at 37°C for 1 h. The cells were then pelleted and resuspended in 500 μl of fluorescein isothiocyanate-labeled goat anti-rabbit IgG (1 μg/million cells; Advanced Targeting Systems, San Diego, CA) and incubated at 37°C for 1 h. After three washings with PBS, the cells were suspended with 1 ml of PBS for flow cytometry (Coulter XL-MCL; Beckman Coulter, Miami, FL). The forward and side scatters were set up using unstained bacterial particles. The threshold was a forward scatter of ≥5,000 events. Stained bacterial particles were gated on forward scatter versus side scatter. Approximately 10,000 cells were counted, and their relative fluorescence was measured.
Bactericidal assay.
The bactericidal anti-LOS rabbit serum was inactivated at 56°C for 30 min and tested for bactericidal activity against strain 26404lgt2 or 26404 by a microbactericidal assay as described previously (40).
Bacterial pulmonary clearance in mice.
Female BALB/c mice (8 weeks of age) were obtained from Taconic Farms Inc. (Germantown, NY). The mice were housed and underwent procedures in accordance with the guidelines of the National Institutes of Health under animal study protocol 1072-05. Bacterial aerosol challenges were carried out in mice using the same OD540 value for mutant strain 26404lgt2 (7.5 × 108 CFU/ml) or strain 26404 (5.5 × 108 CFU/ml) in 10 ml PBS containing calcium magnesium and 0.1% gelatin as previously described (18). The number of bacteria present in the lungs was measured at 0, 3, 6, and 24 h postchallenge. The minimum detectable number of viable bacteria was 100 CFU per lung. Clearance of M. catarrhalis was expressed as the percentage of bacterial CFU at each time point compared with the number deposited at time zero. The clearance percentages were analyzed with a chi-square test.
Isolation of OS and de-O-acylated LOS.
LOS from strain 26404lgt2 or 26404 was hydrolyzed in aqueous 1% (vol/vol) acetic acid for 2 h at 100°C. The hydrolysate was centrifuged at 10,000 × g for 20 min, and the supernatant was collected. The pellet was washed once with water and centrifuged again. The water wash was added to the supernatant, and the total aqueous phase, containing the OS, was lyophilized. The lyophilized OSs were dissolved in milliQ water, passed through a Dionex H+ cartridge to remove any salt present in the OSs, and then lyophilized.
The LOS samples were also de-O acylated according to the procedure of Helander et al. (15). Approximately 0.5 mg of LOS from 26404lgt2 or 26404 was incubated with 0.2 ml of anhydrous hydrazine for 20 min at 37°C. The solution was cooled to −20°C, and 1 ml of chilled acetone was added dropwise to precipitate the dLOS. The sample was then centrifuged at 12,000 × g for 20 min at 4°C. The supernatant was removed, and the pellet was washed again with cold acetone and centrifuged. The precipitated de-O-acylated LOS was then resuspended in 1 ml of water and lyophilized.
Glycosyl composition and linkage analysis.
The glycosyl composition of each OS was determined by the preparation and analysis of trimethylsilyl methylglycosides (39). Briefly, the samples were methanolyzed with 1 M methanolic HCl at 80°C for 18 h, followed by re-N acetylation of methylglycosides using pyridine-acetic anhydride in the presence of methanol at 100°C for 1 h. The free hydroxyl groups of re-N-acetylated methylglycosides were trimethylsilylated with Tri-Sil reagent (Pierce) at 80°C for 20 min. The volatile trimethylsilyl methylglycosides were then analyzed by combined gas-liquid chromatography-mass spectrometry (GLC-MS) using a DB-1 capillary column (J&W Scientific) (30 m by 0.25 mm), and detection was done with a mass-selective detector (Hewlett-Packard HP 5890 series II GC interfaced to a 5971A mass-selective detector).
The glycosyl linkage analyses were performed using the NaOH method (2) with modifications. OS samples (300 μg) were dissolved in dimethyl sulfoxide (200 μl) and methylated by sequential addition of an NaOH slurry in dimethyl sulfoxide (120 mg/ml, 100 μl; 30 min) and three additions of methyl iodide (10, 20, and 50 μl) at 10-min intervals. After an additional 20 min, the methylated glycans were recovered in the organic phase after addition of CHCl3 (200 μl, three times) and 1 M sodium thiosulfate (200 μl). The permethylated products were further purified by reversed-phase chromatography on a Sep-Pak C18 cartridge (39). The permethylated glycans were hydrolyzed with 2 M trifluoroacetic acid (120°C, 2 h), reduced with NaBD4, acetylated, and analyzed by GLC-MS using a 30-m DB-5 capillary column (J & W Scientific).
MALDI-TOF MS analysis.
The molecular masses of both de-O-acylated LOS and OS were analyzed by matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) MS using the QSTAR XL system (Applied Biosystems) with the MALDI 2 source and high-performance quadrupole TOF MS. The de-O-acylated LOSs or OSs were dissolved in milliQ water at a final concentration of 2 μg/μl, and 1 μl of each was mixed with 1 μl of a matrix of 1,2-dihydroxybenzoic acid in 50% methanol for analysis. Spectra were acquired in negative-ion modes.
Nucleotide sequence accession number.
The nucleotide sequence of the lgt2 gene in M. catarrhalis strain 26404 has been deposited in GenBank under accession number EF372584.
RESULTS
Identification and cloning of the lgt2 gene from M. catarrhalis serotype C strain 26404.
M. catarrhalis mutants were constructed by random transposon mutagenesis. The resulting Kanr colonies were screened with a bactericidal anti-LOS rabbit serum by Western blotting assay. One clone that showed no detectable LOS band was selected and named D4. The chromosomal DNA of D4 was purified and subjected to nucleotide sequence analysis. The analysis showed that the EZ::TN transposon was inserted at bp 452 of the DNA fragment within a single open reading frame of 765 bp (Fig. 1). BLAST searches in GenBank with the deduced polypeptide sequence revealed 99% identity compared with the lgt2 amino acid sequences of strains RS10 (serotype C) (7) and 7169 (serotype B) and 61% identity with the lgt2 amino acid sequences of strains 2951 and 25238 (serotype A).
Construction of isogenic mutant strain 26404lgt2.
To confirm the function of the predicted lgt2 gene, an lgt2 knockout mutant was constructed by allelic exchange with an insertion of a Kanr cassette into the HindIII site of the lgt2 gene. Primers lgt2-F and lgt2-R were used to amplify the lgt2 gene. The sequence analysis of PCR products confirmed that the cassette was inserted into the lgt2 gene of the strain 26404 chromosomal DNA at the predicted position (Fig. 1). The clone was named 26404lgt2.
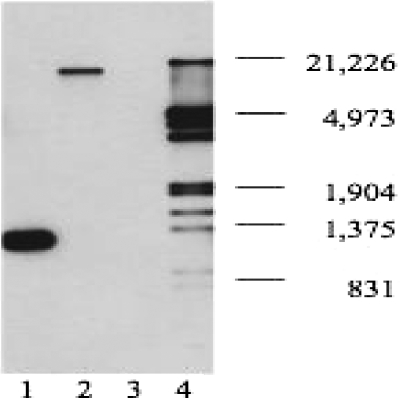
To test whether a single copy of the Kanr gene was inserted into the genome of strain 26404, Southern blotting was performed (28). Only one band was detected in the chromosomal DNA from mutant strain 26404lgt2 (Fig. 2, lane 2), showing that there was a single insertion in the genome. No binding band was detected in the chromosomal DNA from strain 26404 (Fig. 2, lane 3). A positive control of the Kanr gene from pUC 4K showed the binding band at the molecular size of 1,282 bp (Fig. 2, lane 1).
FIG. 2.
Detection of the kanamycin resistance gene inserted into mutant strain 26404lgt2 chromosomal DNA by Southern blotting. Lane 1, 10 ng of pUC4K (positive control, plasmid with a kanamycin resistance cassette) digested with EcoRI; lane 2, 800 ng of chromosomal DNA from mutant strain 26404lgt2 digested with EcoRV; lane 3, 800 ng of chromosomal DNA from strain 26404 digested with EcoRV; lane 4, 1 μl of digoxigenin-labeled DNA molecular weight marker III (Roche) (the sizes are shown in base pairs). Each digested sample was resolved on a 0.7% agarose gel, and Southern blotting was performed using a digoxigenin-labeled kanamycin resistance gene probe.
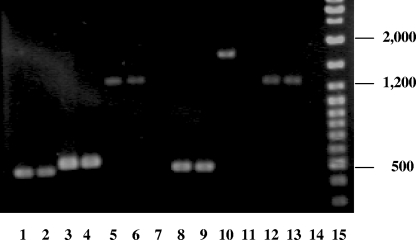
To determine whether the insertion had a polar effect on the downstream gene, total RNA isolated from strain 26404lgt2 or 26404 was subjected to reverse transcription-PCR (RT-PCR) analysis using primer sets (Table 1) designed for genes lgt1 (lgt587 and lgtR1071), lgt2 (lgt1482 and lgtR2002), and lgt3 (lgt2188 and lgtR3472). Compared to strain 26404 (Fig. 3), the insertion of the Kanr gene in mutant 26404lgt2 disrupted only the transcription of the lgt2 gene (lane 11) and had no effect on transcription of the upstream (lgt1; lane 9) and downstream (lgt3; lane 13) genes.
FIG. 3.
Detection of lgt2 gene expression by RT-PCR. The RT-PCR was performed using the following nucleic acid templates: total RNA from strain 26404 was used for lanes 2, 4, 6 and 7; total RNA from mutant strain 26404lgt2 was used for lanes 9, 11, 13, and 14; chromosomal DNA from strain 26404 was used for lanes 1, 3, and 5; and chromosomal DNA from mutant strain 26404lgt2 was used for lanes 8, 10, and 12. Reaction sets contained the following primers: primers lgt587 and lgtR1071 were used for lanes 1, 2, 8, and 9; primers lgt1482 and lgtR2002 were used for lanes 3, 4, 10, and 11; and primers lgt2188 and lgtR3472 were used for lanes 5, 6, 12, and 13. The controls (lanes 7 and 14) used total RNA as the nucleic acid template without activation of the RT. GeneRuler DNA ladder mix (Fermentas) was used for the molecular size standards (in base pairs) (lane 15).
Characterization of LOS from strain 26404lgt2 or 26404 with bactericidal anti-LOS rabbit serum.
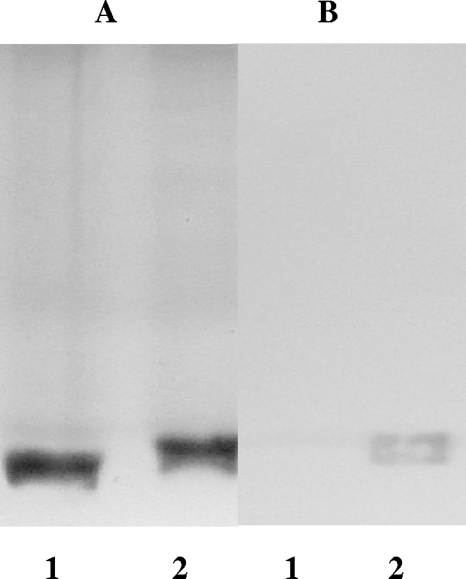
In a silver-stained gel after SDS-PAGE, the 26404lgt2 LOS had quantities similar to but more electrophoretic mobility than the 26404 LOS, suggesting a smaller molecule for the 26404lgt2 LOS (Fig. 4A). In Western blotting, however, the 26404lgt2 LOS did not show a detectable band binding to the bactericidal anti-LOS rabbit serum, as the 26404 LOS did (Fig. 4B). In ELISA, the 26404lgt2 LOS did not show a detectable binding reaction with the rabbit antiserum at the 1:500 dilution; however, the 26404 LOS showed a positive binding at a 1:100,000 dilution, at least a 200-fold difference.
FIG. 4.
LOS patterns on SDS-PAGE followed by silver staining (A) or Western blotting (B) of 26404lgt2 (lanes 1) and 26404 (lanes 2). Each lane represents 100 ng purified LOS. Bactericidal anti-LOS rabbit serum elicited from serotype C M. catarrhalis conjugate dLOS-TT was used at a 1/1,500 dilution for Western blotting.
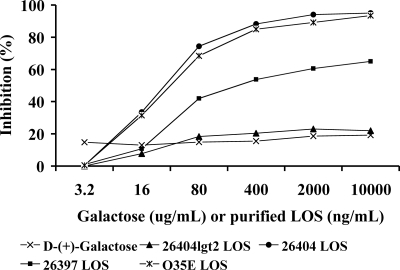
Further ELISA inhibition assays revealed that d-(+)-galactose (up to 10 mg/ml) or 26404lgt2 LOS (up to 10 μg/ml) showed no significant inhibition in the assay (Fig. 5). However, LOSs from strains 26404, O35E (serotype A), and 26397 (serotype B) showed 74%, 68%, and 42% of the inhibition at the concentration of 0.08 μg/ml and 95%, 94%, and 65% of the inhibition at the concentration of 10 μg/ml, respectively.
FIG. 5.
ELISA inhibition by inhibitors of d-(+)-galactose, LOS from mutant strain 26404lgt2, and LOS from strain 26404 (serotype C), O35E (serotype A), or 26397 (serotype B), respectively. The percent inhibition was calculated as follows: (OD405 from serum without inhibitor − OD405 from serum with inhibitor)/OD405 from serum without inhibitor.
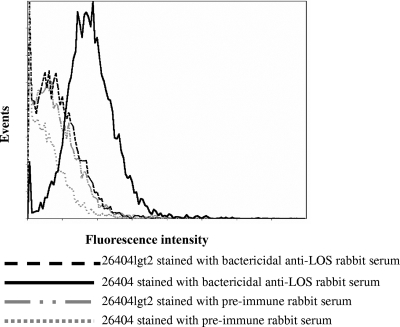
To determine whether the LOS binding epitope is surface exposed on the bacteria, strain 26404lgt2 or 26404 was stained with a preimmune serum or the bactericidal anti-LOS rabbit serum, followed by flow cytometry analysis. The mutant 26404lgt2 did not show strong fluorescence intensity, similar to the isotype controls of both strains stained with preimmune rabbit serum (Fig. 6); however, strain 26404 stained with the bactericidal anti-LOS rabbit serum showed very strong fluorescence intensity.
FIG. 6.
Strain 26404lgt2 or 26404 whole-cell bacteria were incubated with preimmune or bactericidal anti-LOS rabbit serum for 1 h. The bacteria were incubated for another 1 h with fluorescein isothiocyanate-labeled goat anti-rabbit IgG secondary antibody. Samples were read on a Coulter XL-MCL flow cytometer, and data were analyzed with ADC 32 software.
To further study whether the mutant 26404lgt2 can be recognized and lysed like its parental strain by the bactericidal anti-LOS rabbit serum, a bactericidal assay was performed. The bactericidal anti-LOS rabbit serum did not show any detectable bactericidal titer to the mutant 26404lgt2; however, the antiserum showed a bactericidal titer of 1:80 to strain 26404.
Characterization of the mutant 26404lgt2 in a mouse clearance model.
To study whether the mutant 264040lgt2, which retains a truncated LOS, has changes in resistance or pathogenesis in vivo, a murine model of bacterial pulmonary clearance by aerosol changes with viable bacteria was performed (Table 2). In mouse lungs the mutant 26404lgt2 showed a significantly accelerated clearance rate compared to the wild-type strain 26404 at 3 h (76.4% versus 43.8%; P < 0.01) or 6 h (87.1% versus 68.5%; P < 0.01) postchallenge.
TABLE 2.
Pulmonary clearance patterns in mice after an aerosol challenge with M. catarrhalis mutant strain 26404lgt2 and its parental strain 26404
| Challenge straina | Bacterial recoveryb [CFU/mouse (SD range)] or reductionc (%) at:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 h (recovery) | 3 h
|
6 h
|
24 h
|
||||
| Recovery | Reduction | Recovery | Reduction | Recovery | Reduction | ||
| 26404lgt2 | 34,142 (24,258-48,053) | 8,064 (5,269-12,343) | 76.4** | 4,407 (2,572-7554) | 87.1** | 152 (107-218) | 99.6 |
| 26404 | 7,945 (6,455-9,779) | 4,464 (3,778-5,276) | 43.8* | 2,505 (1,883-3,334) | 68.5* | 308 (177-534) | 96.1 |
Mice were challenged by aerosol with 10 ml of 26404lgt2 at 2.3 × 109 CFU/ml or 26404 at 7.5 × 108 CFU/ml.
Lungs were collected for bacterial counts, and the result for each time point represents the geometric mean bacterial CFU for eight mice.
Reduction compared with time point zero. * versus **, significant difference in bacterial reduction between 26404 and 26404lgt2 (P < 0.01).
Structural analysis.
Strain 26404 OS contains a major (85%) decasaccharide and a minor undecasaccharide, similar to the case for the serotype C strain RS10 (7). By GLC-MS analysis, the OS of strain 26404 was found to be composed of glucose, galactose, N-acetylglucosamine, and 2-keto-3-deoxyoctulosonic acid (KDO) as major monosaccharide constituents, while the mutant OS lacked the galactose component (Table 3). Further glycosyl linkage analyses of both OSs by GC-MS are shown in Table 3.
TABLE 3.
Monosaccharide composition and methylation analysis of OSs isolated from 26404lgt2 and 26404a
| OS | mol%
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monosaccharidesb
|
Methylation
|
||||||||||
| d-Glc | d-Gal | d-GlcNAc | T-Glc | T-Gal | 2-Glc | 4-Glc | 4-Gal | 3,4,6-Glc | T-GlcNAc | 4-GlcNAc | |
| 26404lgt2 | 84 | 16 | 34 | 36 | 16 | 14 | |||||
| 26404 | 50 | 38 | 12 | 12 | 20 | 22 | 10 | 18 | 10 | 8 | |
Abbreviations: d-Glc, d-glucose; d-Gal, d-galactose; d-GlcNAc, d-glucosamine; T-Glc, 1,5-di-O-acetyl-1-deuterio-2,3,4,6-tetra-O-methyl-d-glucitol; T-Gal, 1,5-di-O-acetyl-1-deuterio-2,3,4,6-tetra-O-methyl-d-galactitol; 2-Glc, 1,2,5-tri-O-acetyl-1-deuterio-3,4,6-tri-O-methyl-d-glucitol; 4-Glc, 1,4,5-tri-O-acetyl-deuterio-2,3,6-tri-O-methyl-d-glucitol; 4-Gal, 1,4,5-tri-O-acetyl-deuterio-2,3,6-tri-O-methyl-d-galactitol; 3,4,6-Glc, 1,3,4,5,6-penta-O-acetyl-1-deuterio-2-O-methyl-d-glucitol; T-GlcNAc, 1,5-di-O-acetyl-2-(acetylmethylamino)-2-deoxy-1-deuterio-3,4,6-tri-O-methyl-d-glucitol; 4-GlcNAc, 1,4,5-tri-O-acetyl-2-(acetylmethylamino)-2-deoxy-1-deuterio-3,4,6-tri-O-methyl-d-glucitol.
KDO is also present but was not quantified.
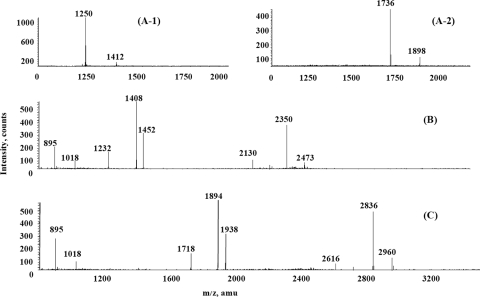
Analysis of the molecular masses of the de-O-acylated LOSs by negative-ion MALDI-TOF MS showed heterogeneity and was consistent with the glycosyl composition and linkage analysis results. The mass spectrum of strain 26404 de-O-acylated LOS corresponds with a previously published structure of RS10 LOS (Fig. 7C) (7), yielding several deprotonated (M − H) ions. The ions with m/z 2836 and 2960 are consistent with an LOS containing Gal3Glc5GlcNAcKDO2-lipid A or Gal3Glc5GlcNAcKDO2-lipid A-phosphatidylethanolamine (PEA). The ion that was observed at m/z 2616 corresponds to a loss of KDO. The ions that were observed at m/z 1938 and 1894 correspond to the OS region containing Gal3Glc5GlcNAcKDO2. Loss of CO2 was observed from one KDO residue. The peak at m/z 1718 also corresponds to OSs losing one KDO residue. The signals at m/z 895 and 1018 correspond to a de-O-acylated lipid A species with one PEA group attached to it. Negative-ion MALDI-TOF MS analysis of strain 26404 OS revealed the presence of major signals at m/z 1736 corresponding to Gal3Glc5GlcNAcKDO (Fig. 7A-2). Similarly, mutant strain 26404lgt2 de-O-acylated LOS shows structural heterogeneity, and negative-ion MALDI-TOF MS spectra are shown in Fig. 7B. The species at m/z 2350 and 2473 correspond to Glc5GlcNAcKDO2-lipid A or Glc5GlcNAcKDO2-lipid A-PEA, as no galactose is present. Negative-ion MALDI-TOF MS analysis of mutant strain 26404lgt2 OS revealed the presence of signals at m/z 1250 corresponding to Glc5GlcNAcKDO (Fig. 7A-1). The proposed major structures of the LOSs from strains 26404lgt2 (96%) and 26404 (85%) are shown in Fig. 8.
FIG. 7.
Negative-ion MALDI-TOF MS analysis of OS derived from strain 26404lgt2 (A-1) or 26404 (A-2) and LOS (de-O acylated) derived from strain 26404lgt2 (B) or 26404 (C). Data were acquired in both positive and negative modes.
FIG. 8.
Proposed major chemical structures of the LOSs from strain 26404lgt2 (A) and 26404 (B). The LOS from mutant strain 26404lgt2 lacks the terminal galactose residues (bold) from 4-linked and 6-linked carbohydrate chains of the LOS from 26404.
DISCUSSION
In an effort to identify functional epitopes on the M. catarrhalis LOS, a main component of conjugate vaccines, we found a serotype C LOS lgt2 mutant that lost binding activity against a bactericidal anti-LOS rabbit serum elicited by a serotype C LOS-based conjugate vaccine. On the basis of a report of a serotype B 7169 LOS, the lgt2 gene encodes β(1-4)-galactosyltransferase (9). Subsequently, the isogenic lgt2 mutant 26404lgt2 was generated and characterized for its structural features and biological activities. The structural analysis showed that the LOS from strain 26404lgt2 lacked the terminal galactose residues in both 4- and 6-linked carbohydrate chains of the 26404 LOS. Further aerosol challenges of mice with the mutant and its parental strain showed that the mutant 26404lgt2 showed accelerated clearance in mouse lungs, suggesting that the terminal galactose residues of the LOS may be related to the resistance or pathogenesis of M. catarrhalis in vivo. Importantly, the data on additional biological activities demonstrated that the mutant 26404lgt2 or its LOS lost binding in Western blotting, an ELISA, and flow cytometry and was not killed by the bactericidal anti-LOS rabbit serum in a complement-mediated bactericidal assay. These data suggest that the terminal galactose residues on the 26404 LOS play an important role in forming the epitope recognized by the rabbit functional antibodies.
To confirm the above investigation and locate which terminal galactose residues were involved in the epitope, an inhibition ELISA was performed. The data revealed that similarly to the 26404 LOS but not the 26404lgt2 LOS, a serotype A O35E LOS with terminal galactoses only on its 6-linked carbohydrate chain (29) showed >90% inhibition, while a serotype B 26397 LOS showed >60% inhibition. By comparing the similarities and specificities of the known LOS structures and the reactivities with the bactericidal anti-LOS rabbit serum of strains 26404 and 26404lgt2 as well as strains of serotype A O35E and B 26397, we conclude that the terminal α-Gal-(1→4)-β-Gal on the 6-linked carbohydrate chain of the 26404 LOS, which is common among LOSs of all three serotypes, is critical for forming the epitope recognized by the functional antiserum from the conjugate vaccination. This is the first report to identify such a bactericidal epitope on the LOS from M. catarrhalis vaccination. The epitope was previously suggested to be a virulence factor in M. catarrhalis's resistance to bactericidal activity mediated by normal human serum (42), and MAbs to the epitope reacted with a similar one in type IV pili of Neisseria meningitidis (32). Our current data from a mouse model of pulmonary clearance also indicate that such an epitope might be related to the resistance or pathogenesis of M. catarrhalis in vivo. Therefore, the identification of the functional epitopes on the LOS will benefit vaccine design and the prevention of M. catarrhalis infections because synthetic saccharide-based vaccines with a specific epitope(s) can be developed. Since there are natural M. catarrhalis strains with an OS structure similar to that of the 26404lgt2 OS (i.e., lacking the terminal galactose residues), such as B6, B7, and B8 OSs from serotype B LOS (8) and C8 OS from serotype C LOS (7), identification of an additional protective epitope(s) on those LOSs may be necessary in order to develop a more efficient synthetic carbohydrate vaccine that covers most clinical isolates.
From the studies described above, it is still not clear why the serotype B LOS showed only 60% inhibition with the bactericidal rabbit antiserum, how many sugars are needed for the functional epitope on the serotype C LOS, and whether the epitope is conformational. Previous studies of type 14 pneumococcal polysaccharide showed that the induction of osponically active antibodies is critically dependent on chain length and the expression of a conformational epitope (22). Similarly, the protective glycotope of type III group B streptococcal polysaccharide is a conformationally dependent, helical structure requiring extended chain lengths for expression (43). In the case of M. catarrhalis LOS, all three major LOS serotype structures are available, which show both common and specific structures with variation within each strain (6-8). Recently, several genes, including galE and the lgt cluster, that encode enzymes responsible for the biosynthesis of LOSs in M. catarrhalis have been reported (9, 42). A series of stepwise-truncated LOS mutants can therefore be established by knocking out some of the genes, and then the biological activities of the different truncated LOS mutants can be compared to decipher potential roles of each moiety of the LOS. To elucidate more details on the protective epitope of the M. catarrhalis LOS, further investigations will include (i) constructing additional isogenic mutants such as lgt2 and lgt5 mutants from different serotypes to test recognition by the bactericidal anti-LOS rabbit serum, (ii) performing inhibition studies using those mutant LOSs as well as terminal synthetic saccharides if possible, and (iii) performing structural analysis of the serotype B 26397 LOS.
Acknowledgments
We thank Eric J. Hansen and John C. McMichael (retired from Wyeth Vaccines, New York) for kindly supplying Moraxella catarrhalis strains.
This research was supported by the intramural research program of the NIH, NIDCD.
Editor: A. Camilli
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Burman, L. A., M. Leinonen, and B. Trollfors. 1994. Use of serology to diagnose pneumonia caused by nonencapsulated Haemophilus influenzae and Moraxella catarrhalis. J. Infect. Dis. 170220-222. [DOI] [PubMed] [Google Scholar]
- 2.Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131209-217. [Google Scholar]
- 3.Cripps, A. W., and D. C. Otczyk. 2006. Prospects for a vaccine against otitis media. Expert Rev. Vaccines 5517-534. [DOI] [PubMed] [Google Scholar]
- 4.Daoud, A., F. Abuekteish, and H. Masaadeh. 1996. Neonatal meningitis due to Moraxella catarrhalis and review of the literature. Ann. Trop. Paediatr. 16199-201. [DOI] [PubMed] [Google Scholar]
- 5.Doyle, W. J. 1989. Animal models of otitis media: other pathogens. Pediatr. Infect. Dis. J. 81(Suppl.)S45-S47. [PubMed] [Google Scholar]
- 6.Edebrink, P., P. E. Jansson, M. M. Rahman, G. Widmalm, T. Holme, M. Rahman, and A. Weintraub. 1994. Structural studies of the O-polysaccharide from the lipopolysaccharide of Moraxella (Branhamella) catarrhalis serotype A (strain ATCC 25238). Carbohydr. Res. 257269-284. [DOI] [PubMed] [Google Scholar]
- 7.Edebrink, P., P. E. Jansson, M. M. Rahman, G. Widmalm, T. Holme, and M. Rahman. 1995. Structural studies of the O-antigen oligosaccharides from two strains of Moraxella catarrhalis serotype C. Carbohydr Res. 266237-261. [DOI] [PubMed] [Google Scholar]
- 8.Edebrink, P., P. E. Jansson, G. Widmalm, T. Holme, and M. Rahman. 1996. The structures of oligosaccharides isolated from the lipopolysaccharide of Moraxella catarrhalis serotype B, strain CCUG 3292. Carbohydr. Res. 295127-146. [DOI] [PubMed] [Google Scholar]
- 9.Edwards, K. J., S. Allen, B. W. Gibson, and A. A. Campagnari. 2005. Characterization of a cluster of three glycosyltransferase enzymes essential for Moraxella catarrhalis lipooligosaccharide assembly. J. Bacteriol. 1872939-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felmingham, D., and J. Washington. 1999. Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens-findings of the Alexander Project 1992-1996. J. Chemother. 11(Suppl. 1)5-21. [DOI] [PubMed] [Google Scholar]
- 11.Fomsgaard, J. S., A. Fomsgaard, N. Hoiby, B. Bruun, and C. Galanos. 1991. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect. Immun. 593346-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsgren, A., M. Brant, M. Karamehmedovic, and K. Riesbeck. 2003. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect. Immun. 713302-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gergova, R. T., I. D. Iankov, I. H. Haralambieva, and I. G. Mitov. 2007. Bactericidal monoclonal antibody against Moraxella catarrhalis lipooligosaccharide cross-reacts with Haemophilus spp. Curr. Microbiol. 5485-90. [DOI] [PubMed] [Google Scholar]
- 14.Gu, X.-X., J. Chen, S. J. Barenkamp, J. B. Robbins, C.-M. Tsai, D. J. Lim, and J. Battery. 1998. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect. Immun. 661891-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helander, I. M., K. Nummila, I. Kilpelainen, and M. Vaara. 1995. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of polymyxin-resistant mutants of Salmonella typhimurium and Escherichia coli. Prog. Clin. Biol. Res. 39215-23. [PubMed] [Google Scholar]
- 16.Holm, M. M., S. L. Vanlerberg, D. D. Sledjeski, and E. R. Lafontaine. 2003. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect. Immun. 714977-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holme, T., M. Rahman, P. E. Jansson, and G. Widmalm. 1999. The lipopolysaccharide of Moraxella catarrhalis structural relationships and antigenic properties. Eur. J. Biochem. 265524-529. [DOI] [PubMed] [Google Scholar]
- 18.Hu, W. G., J. Chen, F. M. Collins, and X. X. Gu. 1999. An aerosol challenge mouse model for Moraxella catarrhalis. Vaccine 18799-804. [DOI] [PubMed] [Google Scholar]
- 19.Hu, W. G., J. Chen, J. F. Battey, and X. X. Gu. 2000. Enhancement of clearance of bacteria from murine lungs by immunization with detoxified lipooligosaccharide from Moraxella catarrhalis conjugated to proteins. Infect. Immun. 684980-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, W. G., J. Chen, J. C. McMichael, and X. X. Gu. 2001. Functional characteristics of a protective monoclonal antibody against serotype A and C lipooligosaccharides from Moraxella catarrhalis. Infect. Immun. 691358-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao, X., T. Hirano, Y. Hou, and X. X. Gu. 2002. Specific immune responses and enhancement of murine pulmonary clearance of Moraxella catarrhalis by intranasal immunization with a detoxified lipooligosaccharide conjugate vaccine. Infect. Immun. 705982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laferriere, C. A., R. K. Sood, J. M. de Muys, F. Michon, and H. J. Jennings. 1998. Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect. Immun. 662441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMichael, J. C. 2000. Vaccines for Moraxella catarrhalis. Vaccine 19S101-S107. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, T. F. 2005. Vaccine development for non-typeable Haemophilus influenzae and Moraxella catarrhalis: progress and challenges. Expert Rev. Vaccines 4843-853. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, T. F., A. L. Brauer, B. J. Grant, and S. Sethi. 2005. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am. J. Respir. Crit. Care Med. 172195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oishi, K., H. Tanaka, F. Sonoda, S. Borann, K. Ahmed, Y. Utsunomiya, K. Watanabe, T. Nagatake, M. Vaneechoutte, G. Verschraegen, and K. Matsumoto. 1996. A monoclonal antibody reactive with a common epitope of Moraxella (Branhamella) catarrhalis lipopolysaccharides. Clin. Diagn. Lab. Immunol. 3351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peak, I. R., M. P. Jennings, D. W. Hood, M. Bisercic, and E. R. Moxon. 1996. Tetrameric repeat units associated with virulence factor phase variation in Haemophilus also occur in Neisseria spp. and Moraxella catarrhalis. FEMS Microbiol. Lett. 137109-114. [DOI] [PubMed] [Google Scholar]
- 28.Peng, D., B. P. Choudhury, R. S. Petralia, R. W. Carlson, and X.-X. Gu. 2005. Roles of 3-deoxy-d-manno-2-octulosonic acid transferase from Moraxella catarrhalis in lipooligosaccharide biosynthesis and virulence. Infect. Immun. 734222-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng, D., W. G. Hu, B. P. Choudhury, A. Muszynski, R. W. Carlson, and X.-X Gu. 2007. Role of different moieties from the lipooligosaccharide molecule in biological activities of the Moraxella catarrhalis outer membrane. FEBS J. 2745350-5359. [DOI] [PubMed] [Google Scholar]
- 30.Rahman, M., and T. Holme. 1996. Antibody response in rabbits to serotype-specific determinants in lipopolysaccharides from Moraxella catarrhalis. J. Med. Microbiol. 44348-354. [DOI] [PubMed] [Google Scholar]
- 31.Rahman, M., T. Holme, I. Jonsson, and A. Krook. 1995. Lack of serotype-specific antibody response to lipopolysaccharide antigens of Moraxella catarrhalis during lower respiratory tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 14297-304. [DOI] [PubMed] [Google Scholar]
- 32.Rahman, M., A. B. Jonsson, and T. Holme. 1998. Monoclonal antibodies to the epitope alpha-Gal-(1-4)-beta-Gal-(1- of Moraxella catarrhalis LPS react with a similar epitope in type IV pili of Neisseria meningitidis. Microb. Pathog. 24299-308. [DOI] [PubMed] [Google Scholar]
- 33.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 15076-85. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka, H., K. Oishi, F. Sonoda, A. Iwagaki, T. Nagatake, and K. Matsumoto. 1992. Biochemical analysis of lipopolysaccharides from respiratory pathogenic Branhamella catarrhalis strains and the role of anti-LPS antibodies in Branhamella respiratory infections. J. Jpn. Assoc. Infect. Dis. 66709-715. [DOI] [PubMed] [Google Scholar]
- 35.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119115-119. [DOI] [PubMed] [Google Scholar]
- 36.Vaneechoutte, M., G. Verschraegen, G. Claeys, and A. M. Van Den Abeele. 1990. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J. Clin. Microbiol. 28182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verduin, C. M., C. Hol, A. Fleer, H. van Dijk, and A. van Belkum. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warburg, O., and W. Christian. 1942. Isolation and crystallization of enolase. Biochem. Z. 310384-421. [Google Scholar]
- 39.York, W. S., A. G. Darvill, M. McNeil, T. T. Stevenson, and P. Albersheim. 1985. Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 1183-40. [Google Scholar]
- 40.Yu, S., and X.-X. Gu. 2005. Synthesis and characterization of lipooligosaccharide-based conjugate vaccines for serotype B Moraxella catarrhalis. Infect. Immun. 732790-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu, S., and X.-X. Gu. 2007. Biological and immunological characteristics of lipooligosaccharide-based conjugate vaccines for serotype C Moraxella catarrhalis. Infect. Immun. 752974-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaleski, A., N. K. Scheffler, P. Densen, F. K. Lee, A. A. Campagnari, B. W. Gibson, and M. A. Apicella. 2000. Lipooligosaccharide P(k) (Galα1-4Galβ1-4Glc) epitope of Moraxella catarrhalis is a factor in resistance to bactericidal activity mediated by normal human serum. Infect. Immun. 685261-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou, W., R. Machenzie, L. Therien, T. Hirama, Q. Yang, M. A. Gidney, and H. J. Jennings. 1999. Conformational epitope of the type III group B Streptococcus capsular polysaccharide. J. Immunol. 163820-825. [PubMed] [Google Scholar]