Abstract
The induction of proinflammatory cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha is crucial for the early control of bacterial infections. Since interleukin-18 (IL-18) acts as a potent inducer of IFN-γ, it might play an important role in the induction of a protective immune response in listeriosis. We used a murine model of systemic Listeria monocytogenes infection to study the immune response to these intracellular bacteria in the absence of IL-18. For this purpose, IL-18-deficient mice and mice treated with anti-IL-18 neutralizing antibody were infected with L. monocytogenes, and their innate and adaptive immune responses were compared to those of control mice. Unexpectedly, we found that mice deficient in IL-18 were partially resistant to primary infection with L. monocytogenes. At day 3 after infection, the numbers of listeriae in the livers and spleens of control mice were up to 500 times higher than those in IL-18-deficient or anti-IL-18 antibody-treated mice. In addition, the level of proinflammatory cytokines was markedly reduced in IL-18-deficient mice. Enhanced resistance to L. monocytogenes infection in IL-18-deficient mice was accompanied by increased numbers of leukocytes and reduced apoptosis in the spleen 48 to 72 h after infection. In contrast, control and IL-18-deficient mice showed no significant differences in their abilities to mount a protective L. monocytogenes-specific T-cell response.
Interleukin-18 (IL-18) is a cytokine with pleiotropic functions in the immune system. One of its best-known functions is to act in synergism with IL-12 in the induction of gamma interferon (IFN-γ) production, mainly by natural killer (NK) and Th1 T cells (33). In addition to its recognized ability to act as a cofactor for IFN-γ production, IL-18 possesses several other biological activities, including the induction of IL-8, IL-1β, and tumor necrosis factor alpha (TNF-α) (41); the upregulation of adhesion molecule expression (31); T-cell, dendritic cell (DC), and neutrophil chemotaxis and migration (6, 21, 26); and the regulation of hepatocyte apoptosis (16). Although initially described as a Th1-polarizing cytokine, IL-18 has recently been shown to mediate both Th1- and Th2-driven immune responses (32). To gain its full bioactive functions, the intracellular IL-18 precursor has to be cleaved by caspase-1 (15). Recent reports have demonstrated that proteinase-3 can also generate biological activity from pro-IL-18 (47). Besides IL-18 production by various cells of the hematopoietic lineage, such as liver Kupffer cells, macrophages, T cells, DCs, astrocytes, and microglia (32, 44), IL-18 production by nonimmune cells, including intestinal and airway epithelial cells (5, 49), has been demonstrated.
In vivo, IL-18 production is rapidly induced after the infection of mice with various bacterial, fungal, or viral pathogens (3, 18, 25, 45, 50). In addition, protection against lipopolysaccharide-induced shock has also been reported to depend on the functions of IL-18 (23). Overproduction of IL-18 has been shown to promote inflammatory disorders including rheumatoid arthritis (38, 55), systemic lupus erythematosus (14), and inflammatory bowel disease (27).
A critical role for IL-18 was also reported in host defense against infection with intracellular bacteria. IL-18-deficient (IL-18 knockout [IL-18ko/ko]) mice infected with Mycobacterium tuberculosis or Mycobacterium bovis BCG develop marked granulomatous lesions in their lungs and spleens, with high bacterial loads in their lungs compared to what is seen for wild-type (WT) mice. Infected IL-18ko/ko mice showed lower levels of splenic IFN-γ than WT mice, although levels of IL-12 and nitric oxide (NO) production were normal (46, 48). When mice were treated in vivo with anti-IL-18 antisera, their resistance to Salmonella enterica serovar Typhimurium infection was also impaired, and bacterial levels in spleen and liver were increased (11, 29). These defects in host immunity against infections with Mycobacteria and Salmonella were attributed to the lower IFN-γ levels, suggesting that the major function of IL-18 in host resistance against these intracellular pathogens is the induction of IFN-γ.
The murine model of systemic Listeria monocytogenes infection has proven to be an excellent experimental system to study immune responses against intracellular pathogens. L. monocytogenes is a facultative, intracellular, gram-positive organism that invades the livers and spleens of hosts (12, 37). The early acute phase of L. monocytogenes infection is accompanied by extensive apoptotic death of lymphocytes in the white pulp of the spleen (30), which has been attributed to the action of listeriolysin O (7). However, the immunological relevance of this apoptosis is not clear. Via Toll-like receptors, L. monocytogenes products can directly activate macrophages, DCs, and NK-DCs to secrete TNF-α, IL-1β, IL-6, IFN-γ, and IFN-γ-inducing cytokines such as IL-12 and IL-18 (13, 17, 39, 42). An important role for IFN-γ in early resistance against L. monocytogenes infection was shown in studies using severe combined immunodeficient (SCID) mice (51).
Regarding its importance in IFN-γ induction, a critical role for IL-18 in resistance against infection with L. monocytogenes has been reported (34, 42, 52). However, considering its pleiotropic effects, functions of IL-18 beyond its potency as an IFN-γ inducer during infection with L. monocytogenes are still elusive.
In this study, we have taken a different approach to examine the early innate immune response as well as the adaptive response by use of IL-18ko/ko mice or neutralizing IL-18-specific antibodies. In contrast to the results published previously, we could demonstrate that after systemic infection of mice with L. monocytogenes, IL-18 deficiency leads to a reduction in bacterial burden in the spleen and liver, despite an overall reduction of IFN-γ levels. This enhanced resistance was not due to altered uptake of Listeria by IL-18ko/ko macrophages. Instead, we found that L. monocytogenes-infected IL-18ko/ko mice displayed reduced apoptotic cell death in the spleen at early stages after infection compared to what was seen for WT controls, leading to elevated numbers of leukocytes in the spleens of IL-18ko/ko mice.
MATERIALS AND METHODS
Mice and infection.
C57BL/6 and BALB/c mice were purchased from Harlan (Borchen, Germany) or were bred in our facility. IL-18ko/ko mice (23) were backcrossed for six generations on C57BL/6 mice. All mice were housed in the mouse facility of the Institute for Medical Microbiology, Immunology and Hygiene of the Technical University of Munich under specific-pathogen-free conditions. Virulent L. monocytogenes (WT strain or ovalbumin-expressing L. monocytogenes ova [40]) was grown in brain heart infusion (BHI). Mice were injected via the tail veins with 200 μl phosphate-buffered saline (PBS) with or without bacteria. Livers and spleens from infected mice were removed at different time points after infection with L. monocytogenes, and Listeria CFU were calculated by plating dilutions of tissue homogenates on BHI plates.
Anti-IL-18 treatment.
For anti-IL-18 treatment, C57BL/6 mice received single intravenous (i.v.) injections with 500 μg neutralizing anti-IL-18 monoclonal antibody (MAb) clone SK113AE4 (28) 1 to 3 days prior to L. monocytogenes infection. C57BL/6 control mice were treated with PBS. For recall experiments, mice received an additional injection of 500 μg anti-IL-18 MAb 3 days prior to secondary infection with L. monocytogenes.
Ex vivo Listeria infection of macrophages.
Peritoneal exudate cells (PEC) were obtained from thioglycolate-injected mice by peritoneal lavage and plated in RPMI 1640 plus 10% fetal calf serum supplemented with penicillin-streptomycin on 12-mm glass coverslips in 24-well plates. PEC were plated at 3 × 106 cells per well and incubated for 3 h, washed three times with PBS, and cultured for an additional hour with complete RPMI without antibiotics. The remaining cells were typical adherent macrophages. Cells were infected with 1.5 × 106 listeriae per well in 1 ml of antibiotic-free medium for 30 min. Medium was then changed to medium containing 5 μg/ml gentamicin, capable of killing any remaining extracellular bacteria. Plates were kept at 37°C during the course of the experiment.
At different time points after infection, coverslips were taken for immunofluorescence staining. Coverslips were washed with PBS, and cells were fixed for 10 min in 10% buffered formalin. Cells were permeabilized with Tris-buffered saline containing 0.1% Triton X-100 (TBS-Tx) and 0.1% bovine serum albumin (BSA) for 15 min. Cells were stained with rabbit anti-Listeria polyclonal serum (serotypes 1 and 4; Difco) diluted 1:200 in TBS-Tx plus 0.1% BSA for 1 h. Secondary staining was then performed using anti-rabbit immunoglobulin G-biotin (1:300) in TBS-Tx plus 0.1% BSA for 45 min followed by streptavidin-Cy3 (Molecular Probes) diluted 1:200 in TBS-Tx for 30 min. Staining was performed at room temperature. Coverslips were washed with TBS-Tx and mounted in Vectashield (Vector). Cells were observed either with a standard fluorescent microscope or with a Zeiss LSM510 confocal laser scanning microscope.
Flow cytometry.
To assess leukocyte frequencies and total cell numbers, cells from spleens were labeled at 4°C with the following reagents: anti-Ly-6G-fluorescein isothiocyanate (FITC) (Gr-1, clone RB6-8C5; Pharmingen), anti-CD11b-phycoerythrin (PE) (clone M1/70; Pharmingen), anti-CD11c-allophycocyanin (APC) (clone HL3; Pharmingen), anti-NK-FITC (clone DX5; Pharmingen), anti-CD45R-PE (B220, clone RA-36B2; Pharmingen), anti-T-cell receptor-biotin (clone H57-597, Pharmingen) or streptavidin-APC (Pharmingen).
For intracellular cytokine staining, 2 ml RPMI 1640 containing 1 × 107 splenocytes per ml was added per well of a 24-well plate and incubated for 5 h at 37°C in the presence of 10−6 M peptide (Ova258-264 [SIINFEKL] or LLO190-201 [NEKYAQAQPNVS]; Affina, Berlin, Germany); for the last 3 h, brefeldin A (GolgiPlug; Pharmingen) was added. Peptide-stimulated cells were subsequently incubated for 20 min with 1 μM ethidium monazide bromide (EMA; Molecular Probes, The Netherlands) and Fc block (Pharmingen). After a washing step, EMA bound to the DNA of dead cells was photocross-linked by brief light exposure. Cells were then stained for the surface antigens CD8α (clone 53-6.7; Pharmingen) and CD4 (clone L3T4; Pharmingen) for 20 min at 4°C, followed by staining for intracellular TNF-α (clone MP6-XT22; Pharmingen) or IFN-γ (clone XMG1.2; Pharmingen) by use of the Cytofix/Cytoperm plus kit (Pharmingen).
All data were acquired on a FACSCalibur (Becton Dickinson) and further analyzed with FlowJo software (TreeStar).
Tetramer production and staining.
Major histocompatibility complex (MHC) tetramer H2-Kb-SIINFEKL was generated using streptavidin-PE (Molecular Probes) as described previously (4). In brief, a specific biotinylation site (27) was added to the COOH terminus of a truncated H2-Kb heavy chain. This fusion protein and beta 2 microglobulin were expressed in large amounts as recombinant proteins in Escherichia coli. Purified heavy chain and beta 2 microglobulin were dissolved in 8 M urea and diluted into refolding buffer containing high concentrations of synthetic SIINFEKL peptide to generate monomeric, soluble H2-Kb-peptide complexes. MHC-peptide complexes were purified by gel filtration and then tetramerized by the addition of PE-conjugated streptavidin (Molecular Probes, Eugene, OR) at a molar ratio of 4:1.
Cells were first treated with Fc block and EMA as described above and then triply stained with anti-CD62L-FITC (clone MEL-14; Pharmingen), PE-conjugated tetramer, and anti-CD8α-APC (clone CD8α; Caltag) for 1 h on ice. After three washes, cells were fixed in 1% paraformaldehyde-PBS (pH 7.45) and analyzed by use of a fluorescence-activated cell sorter (FACS) as described above.
Histochemistry and terminal deoxynucleotidyltransferase-mediated dUTP- biotin nick end labeling (TUNEL) analysis.
After removal, tissues were shock frozen in liquid nitrogen. Sections (7 μm) were prepared on a Leica cryostat. For immunohistochemistry, sections were fixed for 10 min with ice-cold acetone. To stain neutrophils in the liver, rat anti-mouse neutrophil antibody (clone 7/4; Linaris) was used at a 10-fold dilution as described elsewhere (22).
For TUNEL analysis, sections were fixed in 4% PBS-buffered paraformaldehyde for 25 min. The TUNEL assay was performed with the Dead End colorimetric TUNEL system (Promega) according to the manufacturer's protocol. In brief, sections were permeabilized in 0.2% Triton X-100 in PBS for 10 min, washed three times in PBS, and preincubated for 10 min in equilibration buffer (Promega). Sections were incubated with 50 μl of reaction mix (Promega) for 60 min in a humidified incubator at 37°C. The reaction was stopped by treatment with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 15 min. After three washes with PBS, sections were incubated for 30 min with a streptavidin-horseradish peroxidase conjugate (1:300 in PBS; Dako Cytomation), washed three times with PBS, and subsequently stained with NovaRed (Vector Laboratories) for 15 min. Sections were mounted with Vectamount (Vector Laboratories) and analyzed on a Leica DMRBE microscope using Zeiss Axiovision software.
Cytokine and NO measurement.
To determine IFN-γ, TNF-α, and IL-12 cytokine concentrations in the supernatants of cell cultures as well as IL-18 in the sera of mice, OptEIA mouse enzyme-linked immunosorbent assay kits were used according to the manufacturer's protocol. Serum samples were assayed simultaneously for levels of IFN-γ, IL-12p70, TNF-α, monocyte chemoattractant protein-1, and IL-6 by use of the mouse inflammation cytometric bead array kit (BD Biosciences) according to the manufacturer's instructions. Data were acquired on a FACSCalibur (BD) and analyzed using BD Biosciences analysis software.
NO concentrations in the supernatants of cell cultures were determined using Griess reagent (19). In brief, a 1:1 mixture of 0.2% n-(1-naphtyl)ethylene diamine dihydrochloride in H2O and 2% sulfanilamide in 5% H3PO4 was added to the supernatants (1:1), and NO concentrations were determined 10 min later by measuring the absorbance at an optical density of 540 nm. A NaNO2 solution was used as the standard.
For quantitative reverse transcriptase PCR, spleens were snap-frozen, and RNA was isolated using Trizol (Invitrogen) reagent, followed by RNA purification using the RNeasy Mini kit (Qiagen) according to the manufacturer's protocols. cDNA was produced using random primers and Superscript III (Invitrogen). Quantitative PCR was performed for IFN-β and glyceraldehyde-3-phosphate dehydrogenase (both primers from SuperArray) by use of SYBR green PCR master mix (SuperArray) on a Bio-Rad Cromo4 LightCycler. Data were analyzed by Opticon Monitor 3 software.
RESULTS
IL-18 deficiency renders mice less susceptible to infection with L. monocytogenes.
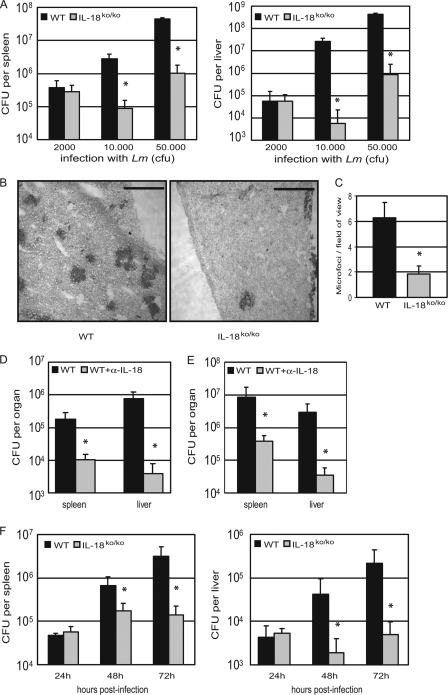
To explore the role of IL-18 in host defense against L. monocytogenes infection, we first injected WT and IL-18ko/ko mice with different doses of L. monocytogenes (2,000, 10,000, or 50,000 PFU, corresponding to 0.1, 0.5, or 2.5 × the 50% lethal dose [LD50], respectively). There was no difference in the numbers of viable bacteria recovered 3 days after infection from the spleens and livers of WT and IL-18 KO mice infected with 2,000 PFU of L. monocytogenes. After infection with higher doses of L. monocytogenes, however, the numbers of viable bacteria in spleens and livers of WT mice were up to 500 times greater than those from IL-18ko/ko mice (Fig. 1A). This was also reflected by a strong increase in neutrophil-containing microfoci in livers of WT mice 3 days after infection with 50,000 PFU of L. monocytogenes. The frequency of these neutrophilic microfoci was about five times higher in liver sections from infected WT mice than in those from IL-18ko/ko mice (Fig. 1B and C).
FIG. 1.
Lower bacterial burden in IL-18ko/ko mice in the early phase after infection with L. monocytogenes. (A) C57BL/6 WT and IL-18ko/ko mice were infected i.v. with 2,000, 10,000, or 50,000 CFU of L. monocytogenes (Lm). On day 3 after infection, spleen and liver homogenates were plated on BHI plates, and CFU were counted. (B) C57BL/6 and IL-18ko/ko mice were infected with 50,000 listeriae. Three days after infection, livers were removed and sections immunostained for neutrophils as described in Materials and Methods. Bar, 100 μm. (C) The number of microfoci was counted in 15 randomly selected fields of view per group. (D) Anti-IL-18 MAb-treated C57BL/6 mice (WT+α-IL-18) and PBS-treated C57BL/6 control mice (WT) were infected with 10,000 CFU of L. monocytogenes. Numbers of viable bacteria were determined on day 3 as described above. (E) Untreated BALB/c or anti-IL-18 MAb-treated BALB/c mice were infected i.v. with 20,000 CFU of L. monocytogenes. On day 3 after infection, spleen and liver homogenates from five mice per group were plated on BHI plates, and CFU were counted. (F) C57BL/6 and IL-18ko/ko mice were infected i.v. with 50,000 listeriae. After 24, 48, and 72 h infection, spleen and liver homogenates were plated on BHI plates, and CFU were counted. Asterisks indicate significant differences (P < 0.05) determined using Student's t test. For all experiments, data are shown for three to four mice per group from one representative out of at least three experiments.
To ensure that the increased resistance to listeriosis in IL-18ko/ko mice was not due to adaptive changes in the immune systems of these animals, we also tested WT mice treated with a neutralizing anti-IL-18 MAb (28). We found that bacterial numbers in the spleens and livers of mice infected for 3 days with L. monocytogenes were up to 200 times greater in control mice than in mice treated with the neutralizing antibody (Fig. 1D). Similar results were obtained for C57BL/6 (Fig. 1D) and BALB/c (Fig. 1E) mice. All further experiments shown were performed with C57BL/6 mice. Taken together, these results demonstrate that IL-18 exerts an unexpected inhibitory function in the early immune response against L. monocytogenes infection.
The clear difference in bacterial loads in the spleens and livers of IL-18ko/ko and WT mice on day 3 prompted us to perform experiments to more closely investigate the kinetics of bacterial replication in these mice (Fig. 1F). For this purpose we infected mice with high doses of L. monocytogenes (∼2.5 × LD50) to enable the detection of viable bacteria at early time points. Bacterial titers from spleens and livers 24 h after infection with L. monocytogenes were equivalent for the two strains. However, while there was a steady increase in the bacterial titers for the spleens and livers of WT mice after 48 and 72 h, differences with those found for IL-18ko/ko mice became apparent after 48 h: at this time point, listerial titers in spleens and livers were already 5 to 30 times lower in IL-18ko/ko or anti-IL-18 MAb-treated mice than in WT mice. At 72 h after infection, the differences in the bacterial titers in the spleen were larger than those seen at 48 h (about 30 times lower in IL-18ko/ko mice). The difference in bacterial load at 72 h was even more pronounced in the liver (50 to 100 times lower in IL-18ko/ko mice) (Fig. 1F).
Equal uptake and outgrowth levels of L. monocytogenes in macrophages from WT and IL-18ko/ko mice.
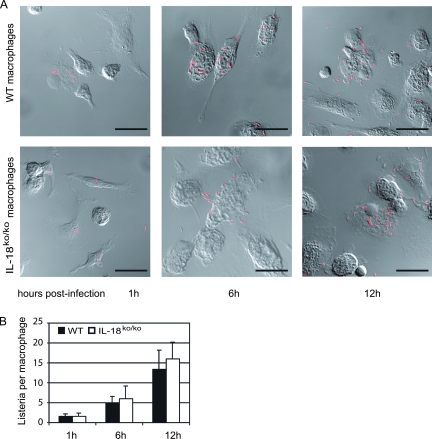
To assess whether the lower bacterial burden found in the organs of IL-18ko/ko mice is due to altered uptake or outgrowth of Listeria in macrophages, we measured Listeria growth in PEC, mainly comprising macrophages, isolated from thioglycolate-treated WT or IL-18ko/ko mice. The cells were infected with a fivefold excess of L. monocytogenes and then treated with gentamicin at a concentration sufficient to kill extracellular bacteria but having no effect on intracellular bacterial growth. After 1 h of infection, only low numbers of listeriae in macrophages from both IL-18ko/ko and WT mice could be observed by confocal microscopy. Rapid replication of the organism was visible 6 h postinfection, with bacteria starting to spread out and infect neighboring macrophages, leading to the infection of most macrophages in the culture after 12 h (Fig. 2A).
FIG. 2.
In vitro infection of PEC with L. monocytogenes. PEC (2 × 105) from thioglycolate-treated C57BL/6 or IL-18ko/ko mice were infected with 1 × 106 L. monocytogenes bacteria for 30 min in medium without antibiotics. The medium was then replaced by medium containing gentamicin (5 μg/ml) to exclude extracellular growth of bacteria. (A) Intracellular growth of bacteria was monitored at the indicated time points by confocal microscopy after staining with fluorescently labeled anti-Listeria antibody. Bar, 10 μm. (B) Numbers of intracellular bacteria were determined in PEC cultures. For each indicated time point, numbers of macrophages and intracellular bacteria were counted for 10 randomly selected fields of view. Data represent the average number of intracellular listeriae per cell (±standard error of the mean).
Direct microscopic quantification of L. monocytogenes in such cultures revealed a steady increase in the average number of listeriae per macrophage (1 or 2 listeriae/macrophage at 1 h; 13 to 16 listeriae/macrophage at 12 h), with no significant differences between the cultures from WT and IL-18ko/ko mice (Fig. 2B). This was in accordance with equivalent absolute numbers of organisms in L. monocytogenes CFU assays from all cultures (not shown).
Reduced amounts of proinflammatory cytokines in IL-18ko/ko mice during the early phase of infection with L. monocytogenes.
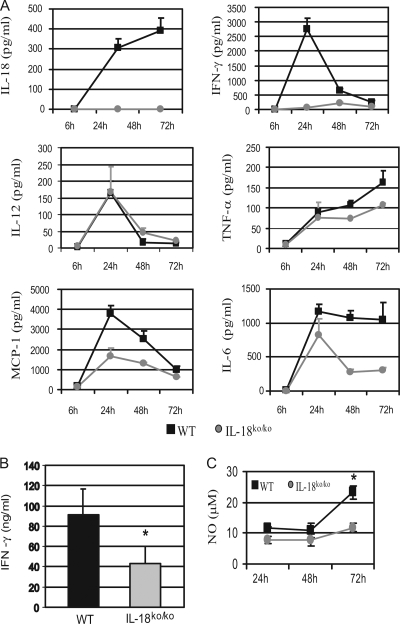
During the initial phase, the antilisterial immune response is mediated by mechanisms of the innate branch of the immune system and is characterized by a complex interplay between cytokines (such as IL-12, TNF-α, and IFN-γ) and macrophages, neutrophils, and NK cells (53). To assess the influence of IL-18 deficiency on the cytokine network, we determined the concentrations of several cytokines in the sera of infected mice at different time points after infection with L. monocytogenes (Fig. 3A). First, we confirmed that infection with L. monocytogenes induces a robust production of IL-18 in WT mice (Fig. 3A). IL-6, IL-12p70, and TNF-α were induced equally in both groups of mice 24 h after infection. However, at later time points (48 to 72 h), lower levels of IL-6 and TNF-α were detectable in the sera of IL-18ko/ko mice, probably reflecting the lower number of CFU per organ at that time point (Fig. 3A). Most striking were the differences in IFN-γ concentrations: while strong IFN-γ production was detected in sera from WT mice, with a peak at 24 h, only very low levels of IFN-γ were found in the sera of IL-18ko/ko mice at all time points, which is in agreement with the well-described function of IL-18 as a potent inducer of IFN-γ (36). Yet, IFN-γ is essential for controlling L. monocytogenes infection by enhancing macrophage killing of L. monocytogenes (12), and the decreased IFN-γ in the sera of IL-18ko/ko mice cannot explain their enhanced resistance to the pathogen. To test whether the low amounts of IFN-γ found in the sera reflect a general inability of the IL-18ko/ko mice to induce IFN-γ in response to L. monocytogenes infection, we directly measured IFN-γ production in a primary site of L. monocytogenes infection, the spleen. For this purpose, splenic cell suspensions were prepared from mice 3 days after infection with L. monocytogenes and restimulated with heat-killed Listeria (HKL) for an additional 24-hour period. High levels of IFN-γ (91 ± 26 ng/ml) were detectable in the cultures from WT mice, whereas IFN-γ levels about 50% lower (43 ± 17 ng/ml) were detected in cultures from IL-18ko/ko mice (Fig. 3B). Nevertheless, these results show that splenocytes from IL-18ko/ko mice are capable of producing substantial amounts of IFN-γ in response to L. monocytogenes infection, although at levels lower than those seen for their WT counterparts.
FIG. 3.
Cytokine pattern in IL-18ko/ko mice during the early phase of L. monocytogenes infection. (A) C57BL/6 and IL-18ko/ko mice were infected i.v. with 50,000 listeriae. Serum cytokine concentrations of these mice were assessed at the indicated time points after infection. The sera of four mice per group were pooled, and cytokine concentrations were determined by cytometric bead assay. (B) Three days after infection with 50,000 listeriae, 3 × 106 splenic cells from infected mice were restimulated in vitro with 5 × 107 HKL cells for 24 h, and the IFN-γ concentration in the supernatant was measured by enzyme-linked immunosorbent assay (*, P < 0.05). Cytokine levels in cultures which were not restimulated with HKL were below the detection limit. (C) After 24, 48, and 72 h of infection, splenic cells were restimulated with HKL for 24 h, and NO concentrations in the supernatants were determined as described in Materials and Methods. Data show one experiment representative of three performed.
NO is a critical molecule in early resistance to L. monocytogenes and is mainly produced by macrophages in an IFN-γ-dependent manner (2). To assess differences in NO production in our model, we purified splenocytes at early time points after infection with L. monocytogenes and restimulated them in vitro with HKL. We found that splenocytes taken from WT and IL-18ko/ko mice 24 h and 48 h after infection with L. monocytogenes produced comparable amounts of NO (Fig. 3C). Significantly high production of NO by splenocytes from WT mice compared to that of their IL-18ko/ko counterparts was detected only 72 h after infection, a finding which might be explained by their higher bacterial load at that time point.
Increased numbers of leukocytes and less apoptosis in the spleens of IL-18ko/ko mice during the early phase of infection.
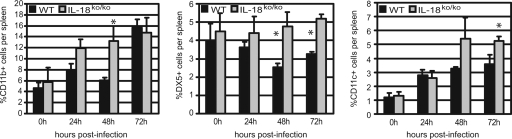
A variety of cell types are essential for early host defense. Bacteria cleared from the bloodstream by the liver are bound extracellularly by Kupffer cells and killed by immigrating neutrophils (20). In the spleen, macrophages together with neutrophils and NK cells are critically involved in capturing and destroying L. monocytogenes. In our experiments, we noted significantly higher frequencies of splenic leukocyte subpopulations in IL-18ko/ko mice than in WT mice (Fig. 4). For macrophages, the difference was most significant at 24 to 48 h; for NK cells and DCs, differences were greatest 48 to 72 h after infection with L. monocytogenes. This was also reflected by significant differences in total cell numbers in the spleens of IL-18ko/ko and anti-IL-18 MAb-treated mice compared to those of WT mice 48 to 72 h after infection with L. monocytogenes (Table 1). We found that total numbers of macrophages and polymorphonuclear cells were elevated in the spleens of both IL-18ko/ko and anti-IL-18 MAb-treated mice, most notably after 48 h of infection. Numbers of NK cells and DCs were also significantly higher in these mice 72 h after infection. In addition, we found significantly high numbers of T cells and B cells in the spleens of IL-18ko/ko and anti-IL-18 MAb-treated mice compared to what was seen for WT mice at 48 to 72 h.
FIG. 4.
Frequencies of leukocytes in the spleens of WT and IL-18ko/ko mice during the early phase of L. monocytogenes infection. Mice were treated as described for Fig. 3A and sacrificed 24, 48, and 72 h after infection. The frequencies of macrophages (CD11b+), NK cells (DX5+), and DCs (CD11c+) were determined by flow cytometry. Data are shown for one representative out of two experiments with three mice per group. Asterisks indicate significant differences (P < 0.05) determined using Student's t test.
TABLE 1.
Total cell numbers in spleens of WT versus IL-18ko/ko and control versus anti-IL-18 MAb-treated mice at early time points after L. monocytogenes infectiona
| Mouse group | Time postinfection (h) | Total no. (106) of cells in indicated splenic leukocyte subpopulationb
|
|||||
|---|---|---|---|---|---|---|---|
| Macrophages | PMNsc (Gr1+) | DCs (CD11c+) | NK cells (DX5+) | B cells (B220+) | T cells (TCRβ+d) | ||
| WT | 48 | 9.0 ± 1.4 | 1.7 ± 0.1 | 2.2 ± 0.3 | 6.4 ± 0.6 | 36.2 ± 5.7 | 23.3 ± 2.0 |
| IL-18ko/ko | 12.5 ± 1.0 | 2.6 ± 0.3 | 2.9 ± 0.5 | 9.0 ± 0.6 | 51.8 ± 8.2 | 27.5 ± 3.4 | |
| Control | 4.4 ± 1.3 | 0.8 ± 0.4 | 2.8 ± 1.0 | 3.7 ± 1.0 | 20.4 ± 7.0 | 11.0 ± 3.9 | |
| α-IL-18 MAb treated | 7.9 ± 1.6 | 1.2 ± 0.1 | 3.5 ± 0.8 | 7.0 ± 1.4 | 27.6 ± 7.8 | 16.8 ± 4.2 | |
| WT | 72 | 2.0 ± 0.4 | 0.7 ± 0.1 | 2.4 ± 0.4 | 3.4 ± 0.8 | 14.1 ± 2.5 | 7.2 ± 2.4 |
| IL-18ko/ko | 6.6 ± 3.3 | 1.4 ± 0.3 | 4.2 ± 2.4 | 9.1 ± 2.2 | 26.3 ± 6.0 | 12.7 ± 4.9 | |
| Control | 7.5 ± 3.1 | 0.7±0.2 | 4.0 ± 2.1 | 7.9 ± 2.8 | 19.9 ± 8.3 | 8.8 ± 2.7 | |
| α-IL-18 MAb treated | 10.1 ± 1.4 | 3.2 ± 0.5 | 8.9 ± 1.2 | 8.1 ± 1.4 | 43.4 ± 3.8 | 25.2 ± 3.1 | |
Mice were infected with 50,000 listeriae. Spleens of four mice per group were removed 48 and 72 h after infection. Total cell numbers were counted, and numbers of splenic leukocyte subpopulations were subsequently assessed by FACS analysis.
Numbers in bold indicate significant differences in total cell numbers between WT and IL-18ko/ko mice or control and antibody-treated mice (P < 0.05; Student's t test). Data are from one experiment representative of two.
PMNs, polymorphonuclear leukocytes.
TCRβ, T-cell receptor β.
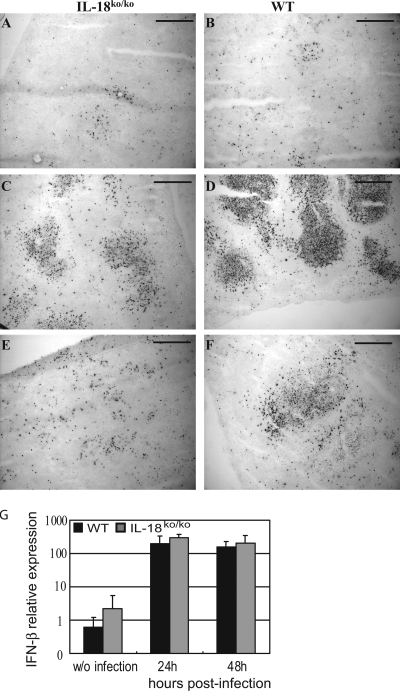
It is well known that lymphocytes undergo apoptosis during the early phase of L. monocytogenes infection (30). Therefore, we reasoned that enhanced apoptosis in the spleens of WT mice might be responsible for the lower numbers of splenocytes found in these mice 48 to 72 h after infection. Examination of splenic tissue from infected mice by TUNEL assay revealed massive apoptosis, as indicated by the abundance of TUNEL-positive cells in the spleens of WT mice 48 h after infection with L. monocytogenes (Fig. 5D). As has been reported for infection with high doses of Listeria (30), apoptotic cells in WT mice were widely distributed throughout the white pulp, affecting both the T-cell and the B-cell zones. Although some apoptosis induction was visible in the spleens of IL-18ko/ko mice 48 h after infection, the extent was clearly reduced compared to what was seen for WT mice (Fig. 5C). The number of TUNEL-positive cells decreased rapidly after the 48-h time point in IL-18ko/ko mice, and after 72 h only a few apoptotic cells were visible in the spleen (Fig. 5E). In contrast, spleens of WT mice still contained substantial numbers of TUNEL-positive cells at 72 h (Fig. 5F). Since endogenous factors such as type I IFN can sensitize lymphocytes to apoptosis induced by LLO during the course of Listeria infection (9, 35), we assessed the levels of type I IFN in spleens of WT and IL-18ko/ko mice 24 and 48 h after infection. Although the expression of type I IFN was strongly induced by the infection, we did not detect quantitative differences between WT and IL-18ko/ko mice (Fig. 5G).
FIG. 5.
Apoptotic cell death is reduced in IL-18ko/ko mice. C57BL/6 and IL-18ko/ko mice were infected with 50,000 listeriae. Spleens were collected at different time points, and the degree of apoptosis was determined by TUNEL staining as described in Materials and Methods. TUNEL-positive cells stain dark brown. Spleens were collected 24 h (A and B), 48 h (C and D), and 72 h (E and F) after infection with L. monocytogenes. Bar, 100 μm. (G) C57BL/6 or IL-18ko/ko mice were infected with 5,000 listeriae, and spleens were taken at the indicated time points after infection. Mice without (w/o) infection were used as controls. RNA was isolated, and IFN-β levels were determined in triplicate using quantitative reverse transcriptase PCR. Relative expression is shown using glyceraldehyde-3-phosphate dehydrogenase as a standard. Data are derived from three mice per group.
Taken together, these findings confirm that the lower bacterial burden found in IL-18ko/ko and antibody-treated mice at early time points is reflected by less apoptosis of splenocytes and provides an explanation for the significantly lower numbers of lymphocytes found in WT mice 48 to 72 h after infection.
IL-18 deficiency has no effect on primary and secondary Listeria-specific T-cell responses.
It has been reported that the development of a Th1 cell response after injection of Propionibacterium acnes or Mycobacterium bovis is impaired in IL-18ko/ko mice (48), and a study by Neighbors et al. proposed a critical role for IL-18 in primary and memory effector responses to L. monocytogenes (34). In contrast, L. monocytogenes-specific acquired immunity was reportedly normal in caspase-1-deficient mice (52), arguing against a major role for IL-18 in the T-cell response against L. monocytogenes.
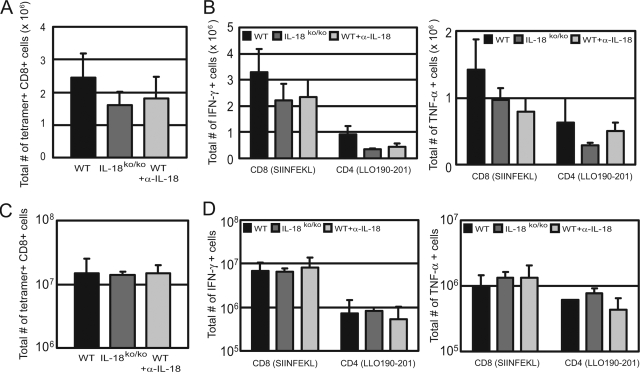
In this study, we directly determined the induction of L. monocytogenes-specific CD8+ T cells in the primary and memory immune responses after the infection of IL-18ko/ko mice with L. monocytogenes. For this purpose, we counted the total number of antigen-specific CD8+ T cells in the spleens of WT, anti-IL-18 MAb-treated, and IL-18ko/ko mice on day 7 after infection with an ovalbumin-expressing L. monocytogenes strain by use of H2-Kb-SIINFEKL tetramers. We found that all groups of mice were capable of inducing substantial numbers of tetramer-binding CD8+ T cells, with only marginally lower numbers in IL-18ko/ko and MAb-treated mice (P > 0.1) (Fig. 6A). To assess the development of effector CD4+ and CD8+ T cells, we restimulated splenocytes from infected mice on day 7 with the MHC class II-restricted LLO190-201 peptide and MHC class I-restricted SIINFEKL peptide, respectively. Afterwards, we performed intracellular cytokine staining and counted the total numbers of IFN-γ/TNF-α-positive antigen-specific CD4+ and CD8+ T cells per spleen. Again, we found induction of effector T cells in the spleens of mice from all groups, with only a modest reduction in the numbers of IFN-γ/TNF-α-positive CD4+ T cells in the spleens of IL-18ko/ko and antibody-treated mice. For tetramer-binding CD8+ T cells, this reduction did not reach statistical significance (P > 0.5) (Fig. 6B).
FIG. 6.
Listeria-specific T-cell response in IL-18ko/ko mice. Untreated C57BL/6 WT mice, IL-18ko/ko mice, and anti-IL-18 MAb-treated WT mice (WT+α-IL-18) were infected with 10,000 L. monocytogenes ova. (A) After 7 days of infection, total numbers of Listeria-specific CD8+ T cells per spleen were determined using SIINFEKL-restricted MHC class I tetramers. (B) Total numbers of IFN-γ- and TNF-α-producing CD8+/CD4+ T cells in the spleens of infected mice 7 days after infection. Cells were restimulated in vitro for 4 h with SIINFEKL peptide for CD8+ T cells and with LLO190-201 peptide for CD4+ T cells and subsequently stained for intracellular cytokines as described in Materials and Methods. Data are derived from three mice per group. (C) Five weeks after primary infection with 2,000 L. monocytogenes ova, C57BL/6 and IL-18ko/ko mice were reinfected with 200,000 L. monocytogenes ova. Depicted are total numbers of SIINFEKL tetramer-positive splenic CD8+ T cells 5 days after reinfection. (D) Total numbers of IFN-γ- and TNF-α-producing CD8+/CD4+ T cells in the spleens of mice after reinfection with 200,000 L. monocytogenes ova, determined as described for panel B. Data are shown for one out of two experiments with three mice per group.
For the memory response, mice were reinfected with a high dose (10 × LD50) of L. monocytogenes ova 5 weeks after primary infection with Listeria. Both IL-18ko/ko and antibody-treated mice showed normal memory responses (compared to those of WT mice), with comparable numbers of L. monocytogenes-specific CD8+ T cells and effector CD4+/CD8+ T cells found in mice of all groups (Fig. 6C and D). In addition, the mice were equally able to clear the infection, as no viable bacteria could be detected in the organs of reinfected mice from any group 5 days after reinfection (data not shown). These results indicate only a minor role for IL-18 in the development of a primary T-cell response and no effect of IL-18 deficiency on the L. monocytogenes-specific memory T-cell response.
DISCUSSION
Our experiments with IL-18ko/ko and anti-IL-18 MAb-treated mice demonstrate an inhibitory effect of IL-18 on the early clearance of Listeria following systemic infection. We found that in the absence of IL-18, infection of mice with L. monocytogenes resulted in low bacterial titers in the spleens and livers compared to what was seen for controls, especially during the first 2 days of infection. This is an unexpected finding, considering that IFN-γ levels in the sera of IL-18ko/ko mice were markedly reduced. Nevertheless, IL-18 deficiency did not lead to a reduction in IL-12 and TNF-α levels or NO production during the first 2 days of infection, suggesting that the most important effect of IL-18 on the cytokine network lies in the amplification of IFN-γ production. Production of IFN-γ in response to L. monocytogenes infection is a hallmark of the early innate immune response against Listeria. We could demonstrate that IL-18 deficiency does not lead to a complete inability to produce IFN-γ, as spleen cells from infected IL-18ko/ko mice produced substantial amounts of IFN-γ when restimulated in vitro with HKL. IL-18 deficiency nevertheless led to a substantial reduction in IFN-γ production after infection with L. monocytogenes, so it is surprising that IL-18 deficiency confers an advantage during early infection.
It has recently been shown by several groups that interference with the early apoptosis seen in cases of L. monocytogenes infection results in enhanced resistance to this pathogen. Mice deficient for the type I IFN receptor or IFN regulatory factor 3 as well as SCID mice exhibit profound resistance to L. monocytogenes infection (1, 8, 9, 35). The enhanced bacterial clearance was preceded by reduced apoptosis in the spleens of these mice. It was suggested that endogenous factors such as type I IFNs can sensitize lymphocytes to apoptosis induced by LLO in the course of Listeria infection. We were not able to demonstrate a direct link between IL-18 and type I IFNs in our study, as there were no significant differences between WT and IL-18ko/ko mice in levels of type I IFN expression detectable 24 and 48 h after infection (Fig. 5G). In another study by Zheng et al. using TNF-related apoptosis-inducing ligand-deficient mice, it was also demonstrated that the blockade of early apoptosis results in reduced listeriosis (56). Interestingly, we found that in the absence of IL-18, numbers of TUNEL+ cells in the white pulp of IL-18ko/ko mouse spleens were significantly diminished. A role for IL-18 in the regulation of apoptosis has been proposed. IL-18 upregulates perforin-dependent cytotoxic activity and the Fas-mediated induction of apoptosis (10, 16, 52). Although a role for Fas-induced apoptosis has been demonstrated for later phases of L. monocytogenes infection (24), its contribution to early apoptosis in the spleen is not clear. Together, our data indicate a role for IL-18 in enhancing apoptosis in L. monocytogenes infection, although we cannot distinguish between a direct IL-18-mediated effect on apoptosis and a secondary effect due to IL-18-mediated induction of other factors such as IFN-γ. In this scenario, the massive induction of proinflammatory cytokines after infection with high doses of L. monocytogenes may lead to enhanced sensitization of splenocytes toward apoptosis, thereby outweighing the beneficial effect.
Splenic T cells and B cells have been described as the major targets for L. monocytogenes-induced early apoptosis (30). Nevertheless, it is likely that other cell types such as macrophages are also affected by L. monocytogenes-induced apoptosis (43). Consistent with this, we found enhanced numbers of CD11b+ and Gr-1+ cells in IL-18ko/ko spleens 2 days after L. monocytogenes inoculation. As previous reports have shown that the depletion of macrophages and neutrophils results in enhanced L. monocytogenes infection during the early states of the infection (54), the reduction of these cell types in the spleens of WT mice relative to those of IL-18ko/ko and anti-IL-18 MAb-treated mice may also contribute to the decreased immunity of WT mice.
Despite the effects of IL-18 deficiency on the early immune response, we could not observe an effect on the adaptive immune response. Both IL-18ko/ko and anti-IL-18-treated mice were able to mount efficient antigen-specific T-cell responses similar to that of their WT counterparts when infected with sublethal doses of L. monocytogenes. In contrast, Neighbors et al. reported a critical role for IL-18 in primary as well as memory effector responses to L. monocytogenes infection. In their study, treatment of BALB/c mice with an anti-IL-18 receptor antibody resulted in dramatically increased susceptibility toward L. monocytogenes infection, with higher bacterial load in organs and increased lethality of antibody-treated mice (34). Since BALB/c mice are more susceptible to L. monocytogenes infection than the C57BL/6 mice used in this study, we also treated BALB/c mice with anti-IL-18 MAb SK113AE-4 and confirmed the results we obtained using C57BL/6 mice (Fig. 1E). Therefore, the usage of anti-IL-18-receptor antibodies by Neighbors et al. is more likely to explain the discrepancy with our data. Such antibodies may have additional side effects, such as sensitizing cells for the induction of apoptosis, and might therefore dramatically influence the course of L. monocytogenes infection. We circumvented these problems by using IL-18ko/ko mice and an IL-18-neutralizing antibody that has no apoptosis-inducing potential.
In conclusion, our data suggest that IL-18, generally known as a proinflammatory cytokine, may also exert unexpected inhibitory functions on bacterial clearance in the early phase of infection. Therefore, the blockade of IL-18 may even exert beneficial functions in certain types of inflammatory reactions.
Acknowledgments
This study was supported by the VolkswagenStiftung, the Bundesministerium für Bildung und Forschung (01 GI0101) through Kompetenznetz CED, and the Deutsche Forschungsgemeinschaft through SFB 576 (to I.F. and D.H.B.) and FOR729 (to I.F.).
We are grateful to Klaus Pfeffer and Klaus Heeg for providing IL-18ko/ko mice, to Sabine Paul and Inge Brosch for expert technical help, and to Kristen Kerksiek for comments on the manuscript.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 23 June 2008.
REFERENCES
- 1.Auerbuch, V., D. G. Brockstedt, N. Meyer-Morse, M. O'Riordan, and D. A. Portnoy. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckerman, K. P., H. W. Rogers, J. A. Corbett, R. D. Schreiber, M. L. McDaniel, and E. R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J. Immunol. 150888-895. [PubMed] [Google Scholar]
- 3.Biet, F., C. Locht, and L. Kremer. 2002. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J. Mol. Med. 80147-162. [DOI] [PubMed] [Google Scholar]
- 4.Busch, D. H., I. M. Pilip, S. Vijh, and E. G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8353-362. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, L. A., R. A. Taha, A. Tsicopoulos, M. Kurimoto, R. Olivenstein, B. Wallaert, E. M. Minshall, and Q. A. Hamid. 1999. Airway epithelium expresses interleukin-18. Eur. Respir. J. 14553-559. [DOI] [PubMed] [Google Scholar]
- 6.Canetti, C. A., B. P. Leung, S. Culshaw, I. B. McInnes, F. Q. Cunha, and F. Y. Liew. 2003. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J. Immunol. 1711009-1015. [DOI] [PubMed] [Google Scholar]
- 7.Carrero, J. A., B. Calderon, and E. R. Unanue. 2004. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J. Immunol. 1724866-4874. [DOI] [PubMed] [Google Scholar]
- 8.Carrero, J. A., B. Calderon, and E. R. Unanue. 2006. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 203933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrero, J. A., B. Calderon, and E. R. Unanue. 2004. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dao, T., W. Z. Mehal, and I. N. Crispe. 1998. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J. Immunol. 1612217-2222. [PubMed] [Google Scholar]
- 11.Dybing, J. K., N. Walters, and D. W. Pascual. 1999. Role of endogenous interleukin-18 in resolving wild-type and attenuated Salmonella typhimurium infections. Infect. Immun. 676242-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelson, B. T., and E. R. Unanue. 2000. Immunity to Listeria infection. Curr. Opin. Immunol. 12425-431. [DOI] [PubMed] [Google Scholar]
- 13.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 1693869-3875. [DOI] [PubMed] [Google Scholar]
- 14.Esfandiari, E., I. B. McInnes, G. Lindop, F. P. Huang, M. Field, M. Komai-Koma, X. Wei, and F. Y. Liew. 2001. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J. Immunol. 1675338-5347. [DOI] [PubMed] [Google Scholar]
- 15.Fantuzzi, G., and C. A. Dinarello. 1999. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 191-11. [DOI] [PubMed] [Google Scholar]
- 16.Finotto, S., J. Siebler, M. Hausding, M. Schipp, S. Wirtz, S. Klein, M. Protschka, A. Doganci, H. A. Lehr, C. Trautwein, R. Khosravi-Far, D. Strand, A. Lohse, P. R. Galle, M. Blessing, and M. F. Neurath. 2004. Severe hepatic injury in interleukin 18 (IL-18) transgenic mice: a key role for IL-18 in regulating hepatocyte apoptosis in vivo. Gut 53392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 1642064-2069. [DOI] [PubMed] [Google Scholar]
- 18.Fujioka, N., R. Akazawa, K. Ohashi, M. Fujii, M. Ikeda, and M. Kurimoto. 1999. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J. Virol. 732401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126131-138. [DOI] [PubMed] [Google Scholar]
- 20.Gregory, S. H., and E. J. Wing. 2002. Neutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infections. J. Leukoc. Biol. 72239-248. [PubMed] [Google Scholar]
- 21.Gutzmer, R., K. Langer, S. Mommert, M. Wittmann, A. Kapp, and T. Werfel. 2003. Human dendritic cells express the IL-18R and are chemoattracted to IL-18. J. Immunol. 1716363-6371. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch, S., and S. Gordon. 1983. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics 18229-239. [DOI] [PubMed] [Google Scholar]
- 23.Hochholzer, P., G. B. Lipford, H. Wagner, K. Pfeffer, and K. Heeg. 2000. Role of interleukin-18 (IL-18) during lethal shock: decreased lipopolysaccharide sensitivity but normal superantigen reaction in IL-18-deficient mice. Infect. Immun. 683502-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen, E. R., A. A. Glass, W. R. Clark, E. J. Wing, J. F. Miller, and S. H. Gregory. 1998. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect. Immun. 664143-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami, K., Y. Koguchi, M. H. Qureshi, A. Miyazato, S. Yara, Y. Kinjo, Y. Iwakura, K. Takeda, S. Akira, M. Kurimoto, and A. Saito. 2000. IL-18 contributes to host resistance against infection with Cryptococcus neoformans in mice with defective IL-12 synthesis through induction of IFN-gamma production by NK cells. J. Immunol. 165941-947. [DOI] [PubMed] [Google Scholar]
- 26.Komai-Koma, M., J. A. Gracie, X. Q. Wei, D. Xu, N. Thomson, I. B. McInnes, and F. Y. Liew. 2003. Chemoattraction of human T cells by IL-18. J. Immunol. 1701084-1090. [DOI] [PubMed] [Google Scholar]
- 27.Lochner, M., and I. Forster. 2002. Anti-interleukin-18 therapy in murine models of inflammatory bowel disease. Pathobiology 70164-169. [DOI] [PubMed] [Google Scholar]
- 28.Lochner, M., H. Wagner, M. Classen, and I. Forster. 2002. Generation of neutralizing mouse anti-mouse IL-18 antibodies for inhibition of inflammatory responses in vivo. J. Immunol. Methods 259149-157. [DOI] [PubMed] [Google Scholar]
- 29.Mastroeni, P., S. Clare, S. Khan, J. A. Harrison, C. E. Hormaeche, H. Okamura, M. Kurimoto, and G. Dougan. 1999. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merrick, J. C., B. T. Edelson, V. Bhardwaj, P. E. Swanson, and E. R. Unanue. 1997. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am. J. Pathol. 151785-792. [PMC free article] [PubMed] [Google Scholar]
- 31.Morel, J. C., C. C. Park, J. M. Woods, and A. E. Koch. 2001. A novel role for interleukin-18 in adhesion molecule induction through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J. Biol. Chem. 27637069-37075. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 1253-72. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19423-474. [DOI] [PubMed] [Google Scholar]
- 34.Neighbors, M., X. Xu, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon gamma production. J. Exp. Med. 194343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Connell, R. M., S. K. Saha, S. A. Vaidya, K. W. Bruhn, G. A. Miranda, B. Zarnegar, A. K. Perry, B. O. Nguyen, T. F. Lane, T. Taniguchi, J. F. Miller, and G. Cheng. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okamura, H., H. Tsutsui, S. Kashiwamura, T. Yoshimoto, and K. Nakanishi. 1998. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 70281-312. [DOI] [PubMed] [Google Scholar]
- 37.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4812-823. [DOI] [PubMed] [Google Scholar]
- 38.Plater-Zyberk, C., L. A. Joosten, M. M. Helsen, P. Sattonnet-Roche, C. Siegfried, S. Alouani, F. A. van De Loo, P. Graber, S. Aloni, R. Cirillo, E. Lubberts, C. A. Dinarello, W. B. van Den Berg, and Y. Chvatchko. 2001. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J. Clin. Investig. 1081825-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plitas, G., U. I. Chaudry, T. P. Kingham, J. R. Raab, and R. P. DeMatteo. 2007. NL dendritic cells are innate immune responders to Listeria monocytogenes infection. J. Immunol. 1784411-4416. [DOI] [PubMed] [Google Scholar]
- 40.Pope, C., S. K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 1663402-3409. [DOI] [PubMed] [Google Scholar]
- 41.Puren, A. J., G. Fantuzzi, Y. Gu, M. S. Su, and C. A. Dinarello. 1998. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J. Clin. Investig. 101711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 1693863-3868. [DOI] [PubMed] [Google Scholar]
- 43.Stockinger, S., T. Materna, D. Stoiber, L. Bayr, R. Steinborn, T. Kolbe, H. Unger, T. Chakraborty, D. E. Levy, M. Muller, and T. Decker. 2002. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes. J. Immunol. 1696522-6529. [DOI] [PubMed] [Google Scholar]
- 44.Stoll, S., H. Jonuleit, E. Schmitt, G. Muller, H. Yamauchi, M. Kurimoto, J. Knop, and A. H. Enk. 1998. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur. J. Immunol. 283231-3239. [DOI] [PubMed] [Google Scholar]
- 45.Stuyt, R. J., M. G. Netea, I. Verschueren, G. Fantuzzi, C. A. Dinarello, J. W. Van Der Meer, and B. J. Kullberg. 2002. Role of interleukin-18 in host defense against disseminated Candida albicans infection. Infect. Immun. 703284-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugawara, I., H. Yamada, H. Kaneko, S. Mizuno, K. Takeda, and S. Akira. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 672585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugawara, S., A. Uehara, T. Nochi, T. Yamaguchi, H. Ueda, A. Sugiyama, K. Hanzawa, K. Kumagai, H. Okamura, and H. Takada. 2001. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J. Immunol. 1676568-6575. [DOI] [PubMed] [Google Scholar]
- 48.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8383-390. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi, M., Y. Nishizaki, O. Sano, T. Ohta, M. Ikeda, and M. Kurimoto. 1997. Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (“interleukin-18”) in mouse intestinal epithelial cells. Cell Tissue Res. 289499-503. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka-Kataoka, M., T. Kunikata, S. Takayama, K. Iwaki, K. Ohashi, M. Ikeda, and M. Kurimoto. 1999. In vivo antiviral effect of interleukin 18 in a mouse model of vaccinia virus infection. Cytokine 11593-599. [DOI] [PubMed] [Google Scholar]
- 51.Tripp, C. S., S. F. Wolf, and E. R. Unanue. 1993. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 903725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuji, N. M., H. Tsutsui, E. Seki, K. Kuida, H. Okamura, K. Nakanishi, and R. A. Flavell. 2004. Roles of caspase-1 in Listeria infection in mice. Int. Immunol. 16335-343. [DOI] [PubMed] [Google Scholar]
- 53.Unanue, E. R. 1997. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 935-43. [DOI] [PubMed] [Google Scholar]
- 54.Unanue, E. R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 15811-25. [DOI] [PubMed] [Google Scholar]
- 55.Wei, X. Q., B. P. Leung, H. M. Arthur, I. B. McInnes, and F. Y. Liew. 2001. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J. Immunol. 166517-521. [DOI] [PubMed] [Google Scholar]
- 56.Zheng, S. J., J. Jiang, H. Shen, and Y. H. Chen. 2004. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J. Immunol. 1735652-5658. [DOI] [PubMed] [Google Scholar]