Abstract
Strain superinfection affects the dynamics of epidemiological spread of pathogens through a host population. Superinfection has recently been shown to occur for two genetically distinct strains of the tick-borne pathogen Anaplasma marginale that encode distinctly different surface protein variants. Superinfected animals could serve as a reservoir for onward transmission of both strains if the tick vector is capable of acquiring and transmitting both strains. Whether competition among strains during development within the tick vector, which requires sequential invasion and replication events, limits colonization and subsequent transmission to a single strain is unknown. We tested this possibility by acquisition feeding Dermacentor andersoni ticks on a reservoir host superinfected with the genetically distinct St. Maries and EMΦ strains. Although the St. Maries strain consistently maintained higher bacteremia levels in the mammalian host and the EMΦ strain had an early advantage in colonization of the tick salivary glands, individual ticks were coinfected, and there was successful transmission of both strains. These results indicate that a genetically distinct A. marginale strain capable of superinfecting the mammalian host can subsequently be cotransmitted and become established within the host population despite the presence of an existing established strain.
Superinfection occurs when a second, genetically distinct pathogen strain infects a host already carrying a primary strain of the same pathogen species. Superinfection allows the second strain to become established in the host and compete with the primary strain for onward transmission. For antigenically variant microbial pathogens this requires that the second strain encode a unique set of variants. This hypothesis has recently been tested and accepted using the tick-borne bacterial pathogen Anaplasma marginale as a model (5). Strains of A. marginale that encode distinct variants of the immunodominant surface protein Msp2 are capable of superinfection despite the establishment of long-term infection and broad immunity against the variants encoded by the primary strain. Consequently, superinfected animals maintain an infection with two genetically distinct strains and, in theory, serve as reservoir hosts of both strains for subsequent tick transmission to naïve animals.
Whether two distinct strains can be simultaneously acquired by the tick vector and subsequently transmitted is unknown, and this represents a gap in our knowledge that is relevant to pathogen strain structure in reservoir host populations. If only a single pathogen strain can be acquired and transmitted, then the new strain (either generated by genetic change or introduced) needs to have a significant competitive advantage to become established in the reservoir host population. Conversely, transmission of both strains to naïve animals would progressively expand the representation of the second strain in the population.
A. marginale undergoes complex development within the tick, and pathogen strain fitness for transmission is affected at at least two steps. The initial infection and a first round of intracellular replication occur within the tick midgut epithelium (7, 9, 10). The ability to enter and replicate in the midgut epithelium has been shown to be a determinant of A. marginale strain transmission fitness in the tick Dermacentor andersoni (16). Whether simultaneous acquisition of two A. marginale strains results in competition between the strains has not been tested, but entry of a first A. marginale strain into the midgut has been postulated to prevent establishment of infection by a second strain via a process termed “infection exclusion” (3). The second event required for transmission is pathogen entry into and replication within the tick salivary glands (7, 9-11). There are strain-specific differences in colonization within the salivary gland, and the strain transmission fitness of D. andersoni is also affected at the level of the salivary gland (16). Consequently, competition between strains at either step (initial midgut invasion and replication or subsequent invasion and replication within the salivary gland) may limit transmission to a single A. marginale strain despite tick acquisition feeding on a reservoir host superinfected with two strains. Here we describe testing this hypothesis and present the findings in the context of determinants of pathogen strain transmission fitness and the implications for strain structure within the reservoir host population.
MATERIALS AND METHODS
Strain superinfection.
The St. Maries (2, 4) and EMΦ (12) strains are genetically distinct strains with nonoverlapping genomic repertoires of msp2 pseudogene alleles (13), and they have been shown to establish superinfection regardless of which strain is the primary infecting strain and which strain is the superinfecting strain (5). Superinfection was established by primary infection of naïve Holstein calves with the St. Maries strain, followed >12 months later by tick transmission of the EMΦ strain. The presence and level of each strain during superinfection in the reservoir host and at the time of tick acquisition feeding were determined by strain-specific PCR and strain-specific quantitative PCR as described in detail below.
Strain-specific detection and quantification.
DNA was extracted either from washed erythrocytes using a Puregene DNA isolation kit (Gentra) and the whole-blood protocol or from individual tick salivary gland pairs as previously described (11, 14). Strain-specific PCR targeted the msp1α gene, which has a set of repeats that vary in sequence and number near the 5′ end (1). The St. Maries strain-specific primers were forward primer 5′-CAGCAGAGTATGTGTCCTCC-3′ and reverse primer 5′-CATTGGAGCGCATCTCTTGC-3′, and the EMΦ strain-specific primers were forward primer 5′-TAGCAGAGTGTGTGTCCG-3′ and reverse primer 5′-GCCTGACCGCTTTGAGATGA-3′. These strain-specific primer sets amplify fragments that are different sizes, as follows: for strain St. Maries, 319 bp; and for strain EMΦ, 179 bp. Infection status was determined using PCR followed by Southern hybridization with the msp1α gene as a probe. Digoxigenin-labeled probes were synthesized using a Roche PCR DIG probe synthesis kit and the msp1α primers described above. The PCR consisted of one cycle of 96°C for 5 min, followed by 30 cycles of 96°C for 30 s, 62°C for 30 s, and 72°C for 1 min and then a final extension at 72°C for 7 min. The PCR fragments were separated on a 1.2% agarose gel prior to Southern blotting as previously described (13). The level of infection with each strain was quantified by real-time PCR using the strain-specific msp1α primers described above and Taqman FAM-490 strain-specific probes. The St. Maries probe was 5′-TCAGCTGATAGCTCGTTAGCGGGT-3′, and the EMΦ probe was 5′-CCAGCTGATAGCTCGTCAGCGAGT-3′. Each strain was assayed independently of the other strain, and then the results were compared after adjustment using each standard curve. A standard curve was constructed for each strain-specific assay by cloning the msp1α gene from each strain into the PCR-4 TOPO vector (Invitrogen Corporation, Carlsbad, CA). The assay consisted of one cycle of 95°C for 10 min, followed by 50 cycles of 95°C for 30 s, 60°C for 15 s, and 72°C for 1 min and a final extension at 72°C for 7 min.
Tick acquisition feeding and transmission.
Adult male D. andersoni ticks (n = 360) from the Reynolds Creek colony were allowed to attach and feed on animal 995 for 48 h (n = 60), 96 h (n = 60), and 168 h (n = 240). The presence of both strain St. Maries and strain EMΦ and the levels of these strains in the blood during the tick acquisition feeding period were determined using the PCR methodology described above. Following tick detachment, ticks were held at 26°C and 94% relative humidity for at least 120 h to ensure that there was complete digestion of the ingested bloodmeal and then placed on naïve (msp5 PCR-negative and competitive-inhibition enzyme-linked immunosorbent assay-seronegative) calves for transmission feeding. Ticks that acquisition fed for 48 or 96 h were transmission fed under separate patches on the same Holstein calf (animal 5854) for 48 h and then removed. Ticks that acquisition fed for 168 h were transmission fed on one of four Holstein calves (animals 5877, 5882, 5890, and 5896; 60 ticks per calf) for 168 h. Salivary gland pairs were dissected from individual ticks immediately following transmission feeding, and DNA was isolated for determination of the presence and levels of each A. marginale strain as described above. The calves were monitored for infection using microscopic examination of Giemsa-stained blood smears, msp5 PCR amplification, and seroconversion using the competitive-inhibition enzyme-linked immunosorbent assay (15). The presence and level of each strain following transmission were determined by PCR-Southern analysis and quantitative real-time PCR as described above.
RESULTS
Bacteremia levels of A. marginale strains in the superinfected reservoir host during tick acquisition feeding.
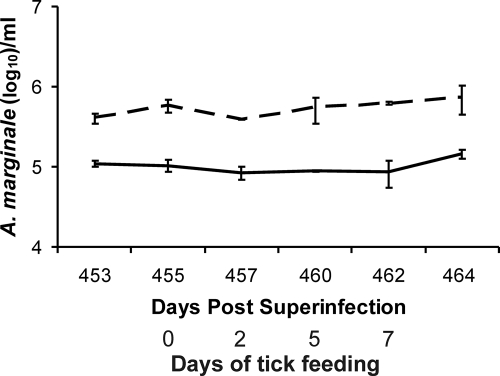
As previously reported, calves superinfected with the St. Maries and EMΦ strains contain higher levels of the St. Maries strain early after superinfection regardless of whether the St. Maries strain was the primary strain (n = 4; the level of St. Maries was 3 times higher) or the superinfecting strain (n = 4; the level of St. Maries was 5.2 times higher) (5). Consistent with this difference, animal 995 used for tick acquisition feeding had higher bacteremia levels with the St. Maries strain than with the EMΦ strain following superinfection (data not shown). The higher St. Maries strain bacteremia was confirmed at the time of acquisition tick feeding, 15 months following initial superinfection, and the levels were approximately fivefold greater than the EMΦ levels (Fig. 1).
FIG. 1.
Strain-specific bacteremia levels during acquisition feeding. The numbers of organisms of the EMΦ strain (solid line) and the St. Maries strain (dashed line) per milliliter of blood were determined by quantitative PCR during the period of tick acquisition feeding on animal 995 (15 months following the initial superinfection).
Prevalence of A. marginale strain-specific and strain coinfection in ticks.
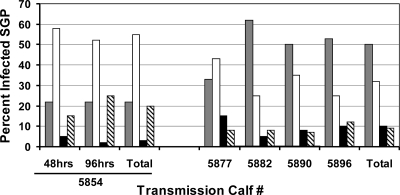
PCR amplification followed by Southern blotting provides highly sensitive and specific detection of A. marginale (6) and therefore was used to determine the infection status of the fed adult ticks (data not shown), which were assigned to one of four groups: uninfected, infected with the St. Maries strain, infected with the EMΦ strain, or infected with both strains (Fig. 2). Overall, 80% of the ticks acquisition fed for 48 and 96 h were infected, while 91% of the ticks fed for 168 h were infected. Of the infected ticks, 40% were coinfected with both the St. Maries and EMΦ strains, while 47% carried a single strain. The percentage of ticks coinfected with both strains was significantly greater for ticks acquisition fed for 168 h than for ticks fed for a shorter time (P < 0.0001, Pearson chi-square test). Comparison of ticks infected with a single strain (Fig. 2) revealed that the EMΦ strain was predominant regardless of the duration of acquisition feeding (P < 0.0001).
FIG. 2.
Percentages of ticks infected with single A. marginale strains or coinfected following acquisition feeding on a superinfected reservoir host. The percentages of D. andersoni tick salivary gland pairs (SGP) in each infection category are indicated as follows: gray bars, coinfected ticks; open bars, EMΦ-infected ticks; black bars, St. Maries-infected ticks; cross-hatched bars, uninfected ticks. For calf 5854 two groups of ticks (60 ticks each) were used; one group was acquisition fed for 48 h, and the other group was acquisition fed 96 h. For calves 5877, 5882, 5890, and 5896 ticks (60 ticks/animal) were acquisition fed for 168 h.
A. marginale levels in coinfected ticks.
Pathogen levels within tick salivary glands were determined using quantitative real-time PCR. The coinfected ticks initially acquisition fed for 48 and 96 h had higher levels of the EMΦ strain (8.9 × 103 bacteria per salivary gland pair) than of the St. Maries strain, whose levels were below the lower limit (103 bacteria per salivary gland pair) of the linear range for the real-time PCR assay. For ticks that had acquisition fed for 168 h, there was no significant difference between the levels of the strains, and the levels of both strains consistently were greater than 103 bacteria per salivary gland pair (Fig. 3). The levels during coinfection were not significantly different from the levels previously reported for infections with a single strain (6, 16). Ticks acquisition fed on calves singly infected with the St. Maries strain using the same tick colony and a 168-h feeding period contained 103 to 107 organisms per salivary gland pair, a range similar to the range observed in the current experiment (Fig. 3).
FIG. 3.
Strain levels within coinfected ticks. Salivary gland pairs (SGP) from individual coinfected ticks that had acquisition and transmission fed for 168 h were analyzed by quantitative PCR in triplicate. The black bars indicate EMΦ strain levels, and the gray bars indicate St. Maries strain levels.
Transmission of A. marginale to naïve animals.
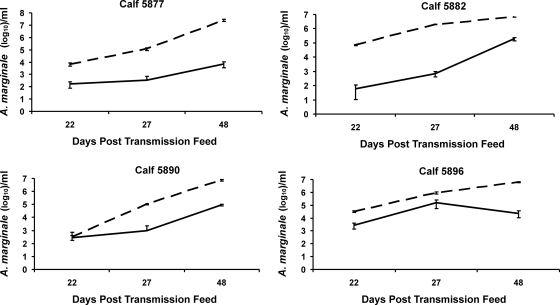
All calves (n = 4) on which ticks transmission fed for 168 h developed an infection with both A. marginale strains by 2 weeks after the transmission feeding. Tracking the bacteremia of each strain following transmission again revealed significantly higher (P < 0.0005, Wilcoxon matched-pair signed-rank test) levels of the St. Maries strain than of the EMΦ strain (Fig. 4). Ticks initially acquisition fed for 48 or 96 h were transmission fed for 48 h, and consistent with the requirement for a longer feeding time, the animal did not develop infection during the 75-day postfeeding observation period and did not seroconvert.
FIG. 4.
Strain levels within coinfected calves following tick transmission. Strain-specific bacteremia levels were determined during acute infection using quantitative PCR in triplicate. The data for the EMΦ strain are indicated by the solid lines, and the data for the St. Maries strain are indicated by the dashed lines.
DISCUSSION
The hypothesis that tick acquisition feeding on a reservoir host carrying two genetically distinct A. marginale strains results in competition between the strains, leading to exclusion of one strain to the advantage of the second strain, is rejected. This suggests that following the initial penetration of a new strain into a reservoir host population in an area of A. marginale endemicity by superinfection, both the new strain and the existing strain can be cotransmitted to naïve animals, thus expanding the representation of the new strain within the population. This is consistent with epidemiologic observations indicating that animals carrying two genetically distinct strains can be detected (12, 13). There is, however, a notable caveat to this conclusion: both strains used in the present study were highly transmissible strains that efficiently infect and replicate in adult male D. andersoni ticks and thus the analysis may not represent competition between strains with markedly different levels of transmission fitness. The evidence for strain-specific differences in transmission fitness includes evidence from field studies in which a specific strain was the predominant strain in the reservoir host population despite the presence of multiple circulating strains and evidence from experimental studies in which replication within the tick and subsequent transmission differ significantly for different strains (12, 16). However, none of the previous transmission fitness studies examined the competition between strains when two strains were acquired simultaneously.
Although both the St. Maries and EMΦ strains are highly transmission-fit strains, quantitative analysis of infection in both the mammalian reservoir host and the tick vector revealed significant fitness differences. The St. Maries strain consistently maintains a higher level of bacteremia during persistent infection than the EMΦ strain. The difference was originally noted at early time points following strain superinfection, regardless of which strain was the primary strain and which strain was the superinfecting strain, and it was observed in the present study both in the persistently infected animal (15 months after superinfection) used for tick acquisition feeding and in the naïve animals following transmission. This consistent observation for a total of 13 calves, which was based on data for both strain superinfections and simultaneous strain transmission resulting in coinfection, indicates that the St. Maries strain has a competitive advantage within the reservoir host (5). Nonetheless, the higher level of bacteremia did not result in either a significantly higher percentage of ticks singly infected with the St. Maries strain than with the EMΦ strain or significantly higher numbers of the St. Maries strain within ticks. In contrast, there is evidence that the EMΦ strain has an early advantage within the tick, as indicated by the higher percentage of ticks singly infected with the EMΦ strain than with the St. Maries strain following the shorter acquisition feeding times (48 and 96 h). However, there was no difference following the longer 168-h acquisition feeding period, and both strains were efficiently transmitted. Whether more pronounced strain-specific differences in the bacteremia level in the reservoir host or the infection prevalence and level in the tick vector result in strain-specific differences in transmission remains unanswered.
Transmission of A. marginale requires initial invasion and a first round of replication in the tick midgut epithelium, followed by a second round of invasion, replication, and development of infectivity in the salivary gland (7, 9-11). The mechanisms underlying these events are poorly understood, as is the minimum time needed for replication to an infectious dose and development of a fully infectious phenotype in the salivary gland. The timing required for this development is critical, as shown by the lack of transmission when ticks were acquisition fed for up to 96 h and then transmission fed for 48 h. In contrast, longer periods of tick feeding are associated with consistent transmission, and there are data correlating increased efficiency of transmission with increased duration of tick transmission feeding (8, 9). The temporal requirement for tick feeding is epidemiologically important as adult male ticks are the primary vector, initially feeding on an infected reservoir host and then, following spermatogenesis, transferring among additional individual hosts in search of female ticks, resulting in interrupted feeding and transmission (8-10, 17; D. Stiller, M. E. Coan, W. Goff, L. W. Johnson, and T. C. McGuire, presented at the Eighth National Veterinary Hemoparasite Disease Conference, St. Louis, MO, 10 to 12 April 1989). Consequently, both tick behavior and the reservoir host population structure can affect transmission efficiency.
In summary, there is clear evidence for simultaneous acquisition and transmission of genetically distinct A. marginale strains following superinfection in the reservoir host. This supports the importance of superinfection for establishment of a new strain within an infected reservoir host population in an area of A. marginale endemicity. Whether the introduced strain is maintained at a lower or higher prevalence within the population than the original strain depends on the relative transmission fitness of each strain. The present study did not resolve whether simultaneous transmission of two strains was due to coinfected ticks that transmitted both strains or to a population of ticks individually infected with either the St. Maries or EMΦ strain; however, both of these transmission scenarios have the same epidemiological impact. While both scenarios seem plausible, resolution of this question awaits further transmission studies using only coinfected ticks. Similarly, this study did not directly address the “infection exclusion” hypothesis that ticks that acquire a first strain by feeding on an infected reservoir are prevented from acquiring a second strain and subsequently transmitting the two strains (3). Determining the mechanism and relative efficiency of these events is essential for obtaining a better understanding of how strains emerge and compete under natural vector-borne transmission conditions.
Acknowledgments
We thank Ralph Horn, James Futse, James Allison, Debbie Alperin, Bev Hunter, and Melissa Flatt for technical assistance and Marc Evans for assistance with statistics.
This work was supported by National Institutes of Health grant AI44005 and by U.S. Department of Agriculture Agricultural Research Service grant 5348-32000-027-00D/-01S.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 23 June 2008.
REFERENCES
- 1.Allred, D. R., T. C. McGuire, G. H. Palmer, S. R. Leib, T. M. Harkins, T. F. McElwain, and A. F. Barbet. 1990. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc. Natl. Acad. Sci. USA 873220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brayton, K. A., L. S. Kappmeyer, D. R. Herndon, M. J. Dark, D. L. Tibbals, G. H. Palmer, T. C. McGuire, and D. P. Knowles, Jr. 2005. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc. Natl. Acad. Sci. USA 102844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Fuente, J., E. F. Blouin, and K. M. Kocan. 2003. Infection exclusion of the rickettsial pathogen Anaplasma marginale in the tick vector Dermacentor variabilis. Clin. Diagn. Lab. Immunol. 10182-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriks, I. S., D. Stiller, W. L. Goff, M. Panton, S. M. Parish, T. F. McElwain, and G. H. Palmer. 1994. Molecular and biological characterization of a newly isolated Anaplasma marginale strain. J. Vet. Diagn. Investig. 6435-441. [DOI] [PubMed] [Google Scholar]
- 5.Futse, J. E., K. A. Brayton, M. J. Dark, D. P. Knowles, Jr., and G. H. Palmer. 2008. Superinfection as a driver of genomic diversification in antigenically variant pathogens. Proc. Natl. Acad. Sci. USA 1052123-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Futse, J. E., M. W. Ueti, D. P. Knowles, Jr., and G. H. Palmer. 2003. Transmission of Anaplasma marginale by Boophilus microplus: retention of vector competence in the absence of vector-pathogen interaction. J. Clin. Microbiol. 413829-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kocan, K. M. 1986. Development of Anaplasma marginale in ixodid ticks: coordinated development of a rickettsial organism and its tick host, p. 472-505. In J. R. Sauer and J. A. Hair (ed.), Morphology, physiology, and behavioral ecology of ticks. Ellis Horwood, Ltd., Chichester, United Kingdom.
- 8.Kocan, K. M., W. L. Goff, D. Stiller, P. L. Claypool, W. Edwards, S. A. Ewing, J. A. Hair, and S. J. Barron. 1992. Persistence of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) transferred successively from infected to susceptible calves. J. Med. Entomol. 29657-668. [DOI] [PubMed] [Google Scholar]
- 9.Kocan, K. M., W. L. Goff, D. Stiller, W. Edwards, S. A. Ewing, P. L. Claypool, T. C. McGuire, J. A. Hair, and S. J. Barron. 1993. Development of Anaplasma marginale in salivary glands of male Dermacentor andersoni. Am. J. Vet. Res. 54107-112. [PubMed] [Google Scholar]
- 10.Kocan, K. M., D. Stiller, W. L. Goff, P. L. Claypool, W. Edwards, S. A. Ewing, T. C. McGuire, J. A. Hair, and S. J. Barron. 1992. Development of Anaplasma marginale in male Dermacentor andersoni transferred from parasitemic to susceptible cattle. Am. J. Vet. Res. 53499-507. [PubMed] [Google Scholar]
- 11.Lohr, C. V., F. R. Rurangirwa, T. F. McElwain, D. Stiller, and G. H. Palmer. 2002. Specific expression of Anaplasma marginale major surface protein 2 salivary gland variants occurs in the midgut and is an early event during tick transmission. Infect. Immun. 70114-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer, G. H., D. P. Knowles, Jr., J. L. Rodriguez, D. P. Gnad, L. C. Hollis, T. Marston, and K. A. Brayton. 2004. Stochastic transmission of multiple genotypically distinct Anaplasma marginale strains in a herd with high prevalence of Anaplasma infection. J. Clin. Microbiol. 425381-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez, J. L., G. H. Palmer, D. P. Knowles, Jr., and K. A. Brayton. 2005. Distinctly different msp2 pseudogene repertoires in Anaplasma marginale strains that are capable of superinfection. Gene 361127-132. [DOI] [PubMed] [Google Scholar]
- 14.Scoles, G. A., M. W. Ueti, S. M. Noh, D. P. Knowles, and G. H. Palmer. 2007. Conservation of transmission phenotype of Anaplasma marginale (Rickettsiales: Anaplasmataceae) strains among Dermacentor and Rhipicephalus ticks (Acari: Ixodidae). J. Med. Entomol. 44484-491. [DOI] [PubMed] [Google Scholar]
- 15.Torioni de Echaide, S., D. P. Knowles, T. C. McGuire, G. H. Palmer, C. E. Suarez, and T. F. McElwain. 1998. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5. J. Clin. Microbiol. 36777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueti, M. W., J. O. Reagan, Jr., D. P. Knowles, Jr., G. A. Scoles, V. Shkap, and G. H. Palmer. 2007. Identification of midgut and salivary glands as specific and distinct barriers to efficient tick-borne transmission of Anaplasma marginale. Infect. Immun. 752959-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaugg, J. L., D. Stiller, M. E. Coan, and S. D. Lincoln. 1986. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am. J. Vet. Res. 472269-2271. [PubMed] [Google Scholar]