Abstract
Cryptococcus neoformans is an encapsulated opportunistic organism that can undergo phenotypic switching. In this process, the parent smooth colony (SM) switches to a more virulent mucoid colony (MC) variant. The host responses mounted against the SM and MC variants differ, and lower tissue interleukin 10 (IL-10) levels are consistently observed in lungs of MC-infected C57BL/6 and BALB/c mice. This suggested different roles of this cytokine in SM and MC infections. The objective of this study was to compare survival rates and characterize the host responses of SM- and MC-infected IL-10-depleted (IL-10−/−) mice, which exhibit a Th1-polarized immune response and are considered resistant hosts. As expected, SM-infected IL-10−/− mice survived longer than wild-type mice, whereas MC-infected IL-10−/− mice did not exhibit a survival benefit. Consistent with this observation, we demonstrated marked differences in the inflammatory responses of SM- and MC-infected IL-10−/− and wild-type mice. This included a more Th1-polarized inflammatory response with enhanced recruitment of macrophages and natural killer and CD8 cells in MC- than in SM-infected IL-10−/− and wild-type mice. In contrast, both SM-infected IL-10−/− and wild-type mice exhibited higher recruitment of CD4 cells, consistent with enhanced survival and differences in recruitment and Th1/Th2 polarization. Lung tissue levels of IL-21, IL-6, IL-4, transforming growth factor beta, IL-12, and gamma interferon were higher in MC-infected IL-10−/− and wild-type mice than in SM-infected mice, whereas tumor necrosis factor alpha levels were higher in SM-infected IL-10−/− mice. In conclusion, the MC variant elicits an excessive inflammatory response in a Th1-polarized host environment, and therefore, the outcome is negatively affected by the absence of IL-10.
Cryptococcus neoformans is an encapsulated yeast that causes disease primarily in patients with impaired immunity (13, 50). C. neoformans can undergo microevolution during chronic cryptococcosis (16). One mechanism for rapid microevolution is phenotypic switching, a phenomenon that generates switch variants, which exhibit differences in virulence relative to the parent strain (19, 22). We previously described phenotypic switching in C. neoformans strain RC-2, a serotype D strain (20). In that strain, the parent smooth colony (SM) variant switches to a mucoid colony (MC) variant at a rate of 0.5 × 10−4. Phenotypic switching also occurs in vivo during chronic infection and is associated with a lethal outcome in mice (20). In addition, the MC variant is selected for in mice that are infected with a switching strain and treated with antifungal drugs or with a capsule-specific monoclonal antibody (MAb) (18). Hypervirulence of the MC variant has been consistently documented in different mouse strains, including the more resistant BALB/c mice, as well as the highly sensitive C57BL/6 mice, which can develop a chronic pulmonary infection. Although the host immune response mounted against the SM and MC switch variants has not been fully characterized, lower interleukin 10 (IL-10) levels were observed in MC-infected than in SM-infected mice (20).
IL-10 is a multifunctional cytokine, as reviewed in detail elsewhere (42), and is produced by many different cells, including alternatively and classically activated macrophages, dendritic cells, B lymphocytes, and CD4+ regulatory T cells. IL-10 is a major regulator of innate immunity and interferes with the production of inflammatory mediators by polymorphonuclear neutrophils, monocytes, and macrophages. IL-10-deficient (IL-10−/−) mice develop chronic inflammatory bowel disease as a result of an inappropriate innate immune response to intestinal bacterial antigens. IL-10 is also classified as a Th2-type cytokine that downregulates Th1-type-associated cytokines (42), like IL-12 and IL-18, in antigen-presenting cells, as well as reducing expression of major histocompatibility complex class II molecules and costimulatory molecules. Therefore, IL-10−/− mice are often more resistant to intracellular pathogens. IL-10 directly affects the function of CD4+ T cells by inhibiting the expression of IL-2, tumor necrosis factor (TNF), IL-5, and chemokine receptor CXCR4. IL-10 also affects the humoral response, because it regulates isotype switching in B cells (32).
Several investigators have examined the role of IL-10 in C. neoformans infection. Blackstock et al. demonstrated that IL-10−/− mice infected intratracheally (i.t.) with C. neoformans strain NU-2 survive longer than the wild-type control (3). Beenhouwer et al. showed similar findings in mice that were intravenously (i.v.) infected with C. neoformans strain 20467 (2). Hernandez et al. highlighted the complexity of the role of IL-10 in chronic cryptococcosis and showed a difference between IL-4−/− and IL-10−/− mice, with augmented levels of IL-12 and tumor necrosis factor alpha (TNF-α) in the lungs of IL-10−/− mice, as well as a reduction of Th2-type cytokines (24).
Because IL-10 is a multifunctional cytokine that could affect the pathogenesis of chronic cryptococcosis on different levels, we pursued the observation that SM- and MC-infected mice consistently exhibited differences in IL-10 in lung tissue. We hypothesized that phenotypic switch variants may be differently affected by IL-10 deficiency, as they elicit strikingly different inflammatory responses. Hence, the objective of this study was to compare the effects and outcomes of infection with the isogenic C. neoformans SM and MC switch variants in IL-10−/− mice. We concluded from our data that the contribution of IL-10 to the pathogenesis of cryptococcosis is complex and also dependent on the infecting strain.
MATERIALS AND METHODS
C. neoformans strain.
RC-2 is a variant of serotype D strain 24067, which was originally obtained from the American Type Culture Collection (Manassas, VA). The RC-2 strain was streaked to single colonies and maintained on Sabouraud dextrose agar (SDA) plates. The RC-2 strain can produce two colony morphologies in agar, known as SM and MC, both of which are characteristic of C. neoformans colonies (16, 20).
Animal studies.
IL-10−/− mice (female, 6 to 12 weeks old) and the corresponding wild-type (wt) controls were obtained from the Jackson Laboratory (Bar Harbor, ME). We used predominantly B6.129P2-IL-10<tm 1Cgn> mice, and in one i.t. infection experiment, also B10.129P2(B6)-IL-10<tm1Cgn> mice. The two C. neoformans variants were single streaked onto SDA plates, and a single colony was grown in broth overnight, diluted 1:50, and grown again overnight. Dilutions of the infecting suspension were plated onto SDA plates to ensure that comparable numbers of yeast cells were injected. Anesthetized mice were infected i.t. by inoculation of 106 (high dose) or 5 × 104 (low dose) C. neoformans cells in 50 μl sterile nonpyrogenic phosphate-buffered saline (PBS) using a 26-gauge needle as described previously (29) or by injection in 100 μl PBS i.v. into the tail vein. The mice were observed daily for signs of disease. Mice that were moribund and unable to reach water were killed in accordance with regulations. The mice were killed by cervical dislocation after anesthesia, and the organ fungal burden was determined by homogenizing lung and brain tissue in 10 ml PBS and plating 100 μl of different dilutions of the homogenate on SDA (Difco Laboratories, Detroit, MI). Colonies were counted after 72 to 96 h (1 colony = 1 CFU). Experiments were done with 5 to 10 mice per group and repeated at least once.
Lung leukocyte isolation and flow cytometry of leukocyte subsets.
Single-cell suspensions from infected lungs were obtained by mincing and enzymatically digesting the excised lungs of individual mice as previously described (24). After the lungs were minced, they were enzymatically digested for 30 min in 15 ml of digestion buffer (RPMI, 5% fetal calf serum, antibiotics, collagenase, and DNase). After a series of washings and further dispersion of undigested lung components, the erythrocytes were lysed with red blood cell lysing buffer (Sigma-Aldrich Co.). The total cell suspension was then pelleted before being counted in a hemocytometer. The percentages of macrophages and CD8, CD4, NK, T-cell immunoglobulin- and mucin domain-containing molecule (TIM3+), and TIM2+ cells were determined by flow cytometric analysis of the leukocyte suspension. Leukocytes were stained with various antibody combinations listed in Table 1. First, the cells were blocked with Fc blocker for 5 min and then stained with specific antibody (1 mg/106 cells) for 45 min at 4°C. Flow cytometry was analyzed with Flojow software version 8.5.
TABLE 1.
Antibody combinations for leukocyte staininga
| Reagentb | Catalog no. | Clone |
|---|---|---|
| APC anti-mouse F4/80 | 17-4801 | BM8 |
| PE-Cy7 anti-mouse CD4 (L3T4) | 25-0041 | GK1.5 |
| FITC anti-mouse CD8a (Ly-2) | 11-0081 | 53-6.7 |
| APC anti-mouse NK1.1 | 17-5941 | PK136 |
| PE anti-mouse TIM-3 | 12-5870 | RMT3-23 |
| Biotin anti-mouse TIM-2 | 13-5865 | RMT2-1 |
| Streptavidin-APC | 17-4317 | |
| Anti-mouse CD16/32 (Fc block) | 14-0161 | 93 |
All from eBioscience.
APC, allophycocyanin; PE, phycoerythrin; FITC, fluorescein isothiocyanate.
Measurement of cytokines in lung homogenates.
Groups of 10 mice were infected i.t. with 5 × 104 and 1 × 106 SM or MC cells in PBS. Mice were killed on days 7 and 14, and the left lungs were homogenized in 2 ml of PBS in the presence of protease inhibitors (Complete Mini; Boehringer Mannheim, Indianapolis IN). The homogenate was centrifuged at 6,000 × g for 10 min to remove cell debris, and the supernatant was frozen at −80°C until it was tested. The supernatants were assayed for transforming growth factor β (TGF-β), IL-21, IL-4, IL-6, IL-10, IL-12 p70, TNF-α, and gamma interferon (IFN-γ) concentrations using enzyme-linked immunosorbent assay kits (PharMingen, San Diego, CA; R&D Systems, Inc., Minneapolis, MN; and eBioscience, San Diego, CA).
Immunohistochemistry.
Formalin-fixed, paraffin-embedded lung sections were used for analysis. They were stained with a standard hematoxylin-eosin stain and with mucin carmine to detect polysaccharide. Localization of glucuronoxylomannan (GXM) in tissue was detected by staining it with a GXM-specific antibody, 18B7, and macrophages were identified by staining them with a macrophage-specific (Mac-3; PharMingen) antibody as previously described (20). Immunostaining of 18B7 and Mac-3 was performed with the M.O.M immunodetection kit (Vector Laboratories). All immunostaining was performed according to the manufacturer's protocol. Negative controls were done without the addition of the primary antibody. Finally, the immunostain was developed with the DAB substrate kit (Vector Laboratories). The stains were visualized at ×100 magnification with a Zeiss microscope linked to a digital camera.
Statistical analysis.
Standard statistical analysis, including Kaplan-Meyer and log rank regression analysis and t tests, were performed using the programs SPSS version 7.5.1 and Microsoft Excel version 11.3.6.
RESULTS
Effects of IL-10 deficiency on survival.
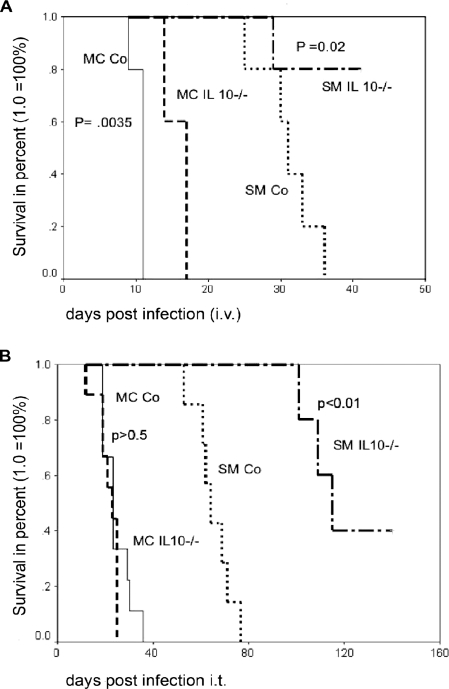
IL-10 is consistently downregulated in MC-infected BALB/c, C57BL/6, and even SCID mice relative to SM-infected mice (Table 2). This prompted us to hypothesize, that IL-10−/− mice might exhibit differences in their susceptibilities to infection with the SM and MC switch variants. As expected, the survival of IL-10−/− mice infected with SM was significantly (Fig. 1A and B) longer than the survival of wt controls both in an i.v. (median survival, 31 days versus 45 days; P < 0.02) and in an i.t. (median survival, 64 days versus 115 days; P < 0.01) infection model. In contrast, IL-10−/− mice infected i.v. with MC also survived longer than wt controls (median survival, 10 days versus 14 days; P = 0.03), although the survival benefit was not big. In contrast, there was no survival benefit in IL-10−/− mice relative to wt mice infected i.t. with MC (23 days for both; P > 0.5). This was documented in repeat experiments with different breeds of IL-10−/− mice, and also, this lack of survival difference was found in mice infected with low inoculum doses (data not shown). It was also noted that a certain IL-10−/− breed, namely, B10.129P2 (B6)-IL-10<tm1Cgn>, was slightly more susceptible than B6.129P2-IL-10<tm1Cgn> in pulmonary-infection models with MC. These mice died even earlier than the wt control (mean survival, 14 days for the IL-10−/− mice versus 24 days for the wt control).
TABLE 2.
IL-10 levels in lung homogenates of SM- and MC-infected mice at day 14
| Mouse strain | SM (pg/ml) | MC (pg/ml) | Sham (pg/ml) | P valuea |
|---|---|---|---|---|
| BALB/c | 2,188 ± 1,063 | 861 ± 389 | 479 ± 140 | <0.001 |
| C57BL/6 | 121 ± 65.5 | 27.2 ± 13.4 | Below detection | 0.03 |
| SCID-BALB/c | 522 ± 218 | 278 ± 79 | 88 ± 65 | <0.001 |
The P value reflects the difference in IL-10 levels by t test in SM- and MC-infected mice (n = 5 to 8 mice per group).
FIG. 1.
Survival differences in wt and IL-10−/− mice after SM and MC infection. Mice (n = 7 to 10 per group) were infected both i.v (A) and i.t (B) with 1 × 106 cells. Survival rates were compared by a log rank test. MC Co and SM Co, MC- and SM-infected control (wt) mice, respectively.
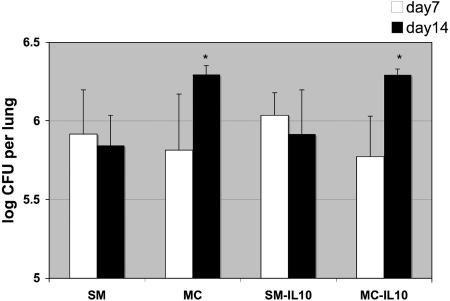
Consistent with survival differences in the pulmonary model, we found differences in fungal burdens between MC- and SM- infected mice. Higher CFU (P = 0.009 for wt mice; not significant for IL-10−/− mice) was detected on day 12 in the lungs of MC (high-dose)-infected mice (wt, 7.0 ± 0.6; IL-10−/−, 6.8 ± 0.6) compared to SM-infected mice (wt, 5.5 ± 0.9; IL-10−/−, 6.1 ± 0.6). Similarly, in low-dose-infected mice, the CFU increased from day 7 to day 14 in both MC-infected IL-10−/− and wt mice. In contrast, SM-infected IL-10−/− mice and wt mice demonstrated decrease and stabilization of fungal burdens (Fig. 2). At the time of death (or sacrifice), the brain CFU were lower (40% undetectable) in MC-infected mice (wt, 4.0 ± 1.7; IL-10−/−, 3.7 ± 1.5) than in SM-infected mice (wt, 5.7 ± 0.8; IL-10−/−, 6.8 ± 0.2). Therefore, we concluded that MC-infected mice died from uncontrolled pulmonary disease associated with inflammation, and accordingly, the mice were panting and clinically short of breath. In contrast, the SM-infected mice that died (40% survived and cleared the infection by day 160) died of pulmonary and concomitant central nervous system disease (log 5.7 to 6.8), with the expected neurological symptoms (hunched back).
FIG. 2.
Lung fungal burdens (CFU) in MC-infected wt and IL-10−/− mice infected i.t. with 5 × 104 cells are significantly higher than in corresponding SM-infected mice (n = 5 per group), which start to clear the infection from the lung by day 14. *, P = 0.03 and 0.04 for differences in CFU of SM versus MC and SM-infected IL-10−/− mice versus MC-infected IL-10−/− mice at day 14. The error bars indicate standard deviations.
Inflammatory responses in SM- and MC-infected wt and IL-10−/− mice.
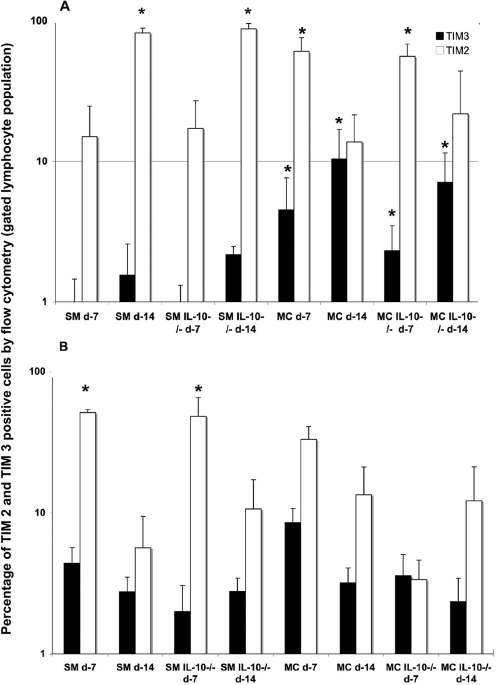
Next, we characterized the inflammatory responses of SM- and MC-infected IL-10−/− mice and wt controls with respect to Th1/Th2 polarization. Cellular recruitment in the lungs of SM- and MC-infected wt and IL-10−/− mice is summarized in Table 3 and was mostly comparable in the high-dose-infected mice and higher in MC-infected (low dose) mice at day 14. Flow cytometry was performed on lung leukocytes stained with TIM3/TIM2 antibodies, which were predominantly found on Th1 (TIM3)- and Th2 (TIM2)-type cells, respectively (10, 36, 38) (Fig. 3). These experiments demonstrated differences in Th1/Th2 polarization for SM- and MC-infected wt mice, which were also dependent on the inoculum doses. Differences between wt and IL-10−/− mice were more noticeable at a low dose of inoculum. Wt and IL-10−/− mice infected with high-dose SM exhibited predominantly a Th2-polarized inflammatory response, which increased from day 7 to day 14, and only a small number of Th1-type cells were present. In contrast, MC-infected (high dose) IL-10−/− and wt mice exhibited a downregulation of Th2-type cells between days 7 and 14 and had significantly (P < 0.05) more Th1-type cells than SM-infected mice. At a low dose, SM-infected wt mice downregulated Th2-type cells between days 7 and 14 and exhibited more Th1-type cells than high-dose SM-infected mice. In a low-dose MC infection, the recruitment of Th1- and Th2-type cells was similar to that in low-dose SM-infected wt mice. Differences in Th1/Th2 polarization between IL-10−/− and wt mice were noted at low doses, especially in MC-infected IL-10−/− mice, where Th1-type cells dominated early on.
TABLE 3.
Summary of leukocyte recruitment to SM- and MC-infected lungs
| Cell recruitment and FACS analysis | Value (day 7/day 14)
|
|||||
|---|---|---|---|---|---|---|
| MC | SM | P value by t testa | IL-10−/− MC | IL-10−/− SM | P value by t test | |
| Recruitment of cells (low dose) | 7.1 ± 0.13/7.37 ± 0.07 | 7.2 ± 0.14/6.7 ± 0.44 | NS/.0002 | Not done/7.38 ± 0.15 | Not done/6.7 ± 0.32 | 0.01 |
| Recruitment of cells (high dose) | 5.8 ± 0.58/5.8 ± 0.37 | 5.9 ± 0.15/6.4 ± 0.25 | NS/0.03 | 6.14 ± 0.41/6.5 ± 0.18 | 6.2 ± 0.34/6.7 ± 0.29 | NS/NS |
| CD4+ | 4.1 ± 2.1/6.2 ± 2.8 | 5.9 ± 1.7/7.1 ± 1.8 | NS/NS | 11.8 ± 4.2/5.9 ± 0.39 | 3.6 ± 0.24/3.9 ± 2.5 | 0.002/NS |
| CD8+ | 5.9 ± 1.24/3.1 ± 1.0 | 2.68 ± 1.1/1.7 ± 1.3 | 0.004/NS | 3.9 ± 1.5/3.1 ± 0.9 | 1.6 ± 0.4/1.8 ± 1.6 | 0.01/NS |
| Macrophages (F4/80) | 23 ± 3.4/5.3 ± 3.5 | 9.2 ± 3.7/4.9 ± 2.6 | 0.0007/NS | 17.0 ± 0.01/5.2 ± 3.5 | 6.34 ± 1.4/5.2 ± 1.0 | 0.01/NS |
| NK cells | 6.7 ± 4.1/4.3 ± 2.4 | 4.0 ± 1.6/6.3 ± 5.3 | NS/NS | 8.0 ± 2.7/4.2 ± 2.4 | 2.8 ± 1.1/5.8 ± 2.1 | 0.004/NS |
n = 5 per group of mice. NS, not significant.
FIG. 3.
For analysis of Th1/Th2 polarization at day 7 and day 14, the percentages of TIM3- and TIM2-positive cells were determined by FACS analysis in wt and IL-10−/− mice, after both high-dose (A) and low-dose (B) infection (n = 5 mice per group and time point). The black bars represent the average percentages plus standard deviations of TIM3 (Th1)-positive cells, and the white bars represent the average percentages of TIM2 (Th2)-positive cells plus standard errors. *, significant P value (P < 0.05), which was calculated by a t test comparing percentages in SM- versus MC-infected wt and IL-10−/− mice, respectively.
To identify inflammatory cell types in infected lung tissue, Flow cytometry was performed with specific cell markers for macrophages (F4/80) and NK (Nk1.1), CD4, and CD8 cells was performed. These data demonstrated differences predominantly in high-dose-infected mice that are summarized in Table 3. Significantly more macrophages and CD8+ cells were detected in the lung tissue of MC (high-dose)-infected wt mice than in that of SM-infected wt and IL-10−/− mice at day 7. IL-10−/− mice also exhibited higher percentages of NK cells. Interestingly, more CD4 cells were found in the MC-infected lung tissue. The differences resolved by day 14. Similar trends of higher numbers of CD8 and NK cells in the lungs of MC-infected IL-10−/− mice were also documented in mice infected with a lower dose. At a lower infection dose, only CD4+ cell recruitment differed, and it was higher in SM-infected mice, which was consistent with prolonged survival (data not shown). In summary, these data confirm qualitative differences in the inflammatory cells in the lungs of wt and IL-10−/− mice that were infected with SM and MC. In addition, our data indicate that the inoculum dose greatly impacts cellular recruitment.
Cytokine profiles in the lung homogenates of SM- and MC-infected IL-10−/− mice.
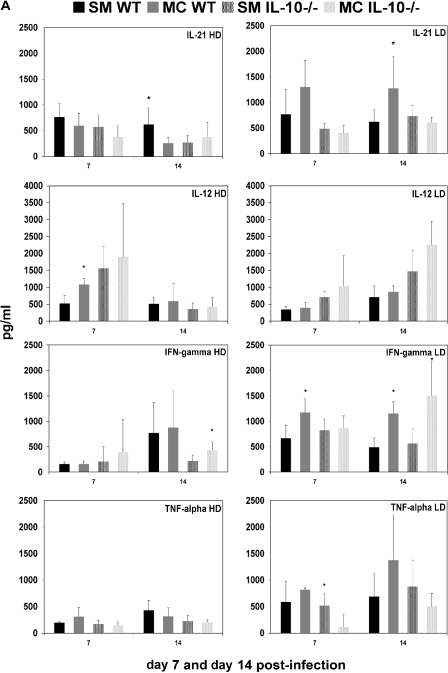
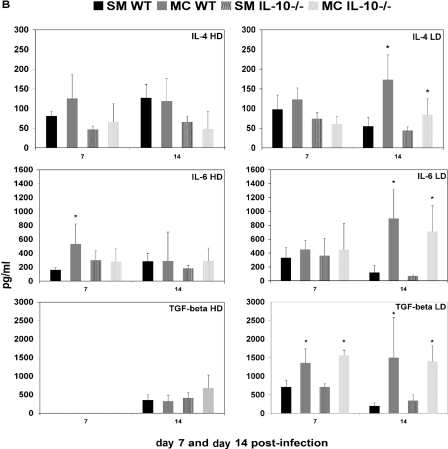
We determined the production of both Th1 (IFN-γ, IL-12, and TNF-α)- and Th2 (IL-6, IL-4, and TGF-β)-type cytokines and the Th17-associated cytokine IL-21 in the lung homogenates of SM- and MC-infected IL-10−/− mice (Fig. 4A and B). Consistent with altered Th1/Th2 polarization, we also established marked differences in cytokine levels in infected lung tissue. Interestingly, differences in cytokine levels in lung tissue were more pronounced in mice infected with the low-dose inoculum, whereas the Th1/Th2 polarization differed more in high-dose inoculum-infected mice at the time points examined. Cytokine profiles were remarkably similar in wt and IL-10−/− mice, despite a more similar recruitment of inflammatory cells (Table 3) in these mice. In wt mice infected with high-dose SM, we documented significantly higher IL-21 levels, whereas MC-infected lung tissue of wt mice exhibited higher levels of IL-12 and IL-6. In IL-10−/− mice, only IFN-γ levels were higher in MC-infected lung tissue.
FIG. 4.
Cytokine levels were measured in 2 ml of lung homogenates derived from SM- and MC-infected mice at day 7 and day 14. Shown are average values from wt and IL-10−/− mice at high-dose (HD) and low-dose (LD) infections (n = 5 mice per group). Cytokine production was measured by enzyme-linked immunosorbent assay and is charted as pg/ml lung homogenate. *, significant difference (P < 0.05) as determined by a t test. The error bars indicate standard deviations.
In low-dose infection, higher Th2-type cytokine levels (IL-6, IL-4, and TGF-β) and the Th17-associated IL-21 were detected in MC-infected lung tissue of wt mice, and except for IL-21, also In IL-10−/− mice. Only TNF-α production was higher in SM-infected IL-10−/− mice, which is consistent with the enhanced survival of these mice. Furthermore, along with higher levels of Th2-type cytokine production in MC-infected wt and IL-10−/− mice, we also documented higher levels of the Th1-type cytokine IFN-γ in both MC-infected wt and IL-10−/− mice than in SM-infected mice.
Histological analysis of SM- and MC-infected lung tissue.
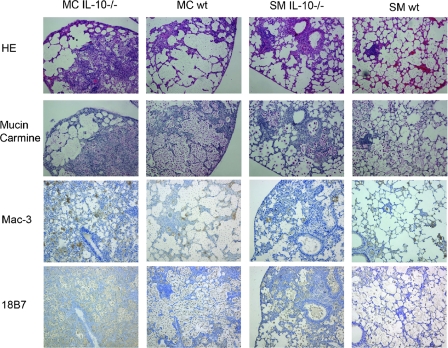
Next, we analyzed the inflammatory responses in SM- and MC-infected lungs of both IL-10−/− and wt mice. Consistent with marked differences in survival, Th1/Th2 polarization, cell recruitment, and cytokine production, histological analyses demonstrated extensive inflammation in MC-infected IL-10−/− mice, which were comparable to the inflammation observed in the wt mice and significantly enhanced compared to SM-infected wt and IL-10−/− mice. SM-infected mice exhibited signs of organized granuloma formation, and MC-infected mice failed to manifest organized granulomas. Immunohistochemistry with macrophage (Mac-3)- and GXM (18B7)-specific MAbs confirmed that MC-infected wt and IL-10−/− mice recruited more macrophages and exhibited extensive accumulation of capsular polysaccharide in tissue (Fig. 5). Thus, these data demonstrate that even when similar percentages of inflammatory cells are recruited, the organization of the inflammatory response can still exhibit marked differences.
FIG. 5.
Differences in inflammatory responses of MC- and SM-infected IL-10−/− and wt mice. Shown is the histology of lungs at day 14 after low-dose infection and staining with hematoxylin-eosin (HE), mucin carmine, and macrophage (Mac-3)- and GXM (18B7)-specific MAbs. Both MC-infected IL-10−/− and wt mice exhibited enhanced disorganized inflammation associated with failure to clear infection, whereas SM-infected IL-10−/− and wt mice exhibited signs of organized granuloma formation. Magnification, ×100.
DISCUSSION
In contrast to the observations of other investigators (64), we consistently found depression of IL-10 levels in lung tissue of mice infected with the hypervirulent MC switch variant relative to IL-10−/− levels in lungs infected with the parent SM variant. Furthermore, we now show that resistance in IL-10−/− mice is dependent on the infecting strain. Our data demonstrate that IL-10−/− mice infected with the MC switch variant are as susceptible as the wt mice, whereas SM-infected IL-10−/− mice are more resistant than wt mice. MC-infected IL-10−/− mice die earlier, or at the same time, with signs of an excessive overstimulated inflammatory response. In comparison to infection with the SM parent strain, the host response is characterized by enhanced recruitment of macrophages, NK cells, and CD8 cells. Furthermore, differences in Th1/Th2 polarization and cytokine production were noted. Consistent with these changes, histological analysis demonstrated failure to generate organized granuloma formation in MC-infected wt and IL-10−/− mice, accompanied by accumulation of capsular polysaccharide in the tissue and destruction of lung tissue.
Clearance of C. neoformans in resistant hosts involves the development of Th1 cell-mediated immune response (CMI) (6, 7, 26, 35, 46). T cells recruit and activate macrophages, inducing a granulomatous reaction (28). Many cytokines, including IFN-γ, IL-2, IL-12, IL-18, TNF-α, IL-13, and IL-15, and the CC chemokine ligands type 2 and type 3 (1, 12, 25, 30, 31, 33-35, 45, 47, 49, 54, 55, 61, 62), have been shown to be relevant for successful clearance. Previous studies have concluded that the susceptibility of C57BL/6 mice does not correlate with the level of inflammation at the site of infection, but rather with low levels of IFN-γ and IL-2 (25, 27). Taking the data together, the failure to eradicate the fungus is thought to be a function of a diminished CMI. Several observations, however, indicate that pathogen-specific factors affect the host response and impose a different challenge to the immune system. First, prolonged in vitro and in vivo passage of C. neoformans isolates can result in phenotypic changes associated with differences in virulence (20). Second, serial isolates from chronically infected patients can exhibit differences in virulence (17). Third, even immunocompromised patients exhibit variable inflammatory responses (39).
In IL-10−/− mice, the T-cell response is Th1 polarized, although these mice still exhibit Th2-type cells and cytokines (24). To date, all data support the notion that IL-10−/− mice exhibit enhanced resistance. However, the data presented here qualify this conclusion. Survival, cytokine, and histology data demonstrate that in the absence of IL-10, excessive inflammation can occur in the setting of infection with the hypervirulent MC variant, resulting in an apparent increase in virulence relative to the SM. We hypothesize that this enhanced inflammatory response offsets the benefits of Th1 polarization and contributes to destruction of the lung tissue and early death. Both proinflammatory cytokines are upregulated, and anti-inflammatory cytokines, like TGF-β, are detected in MC-infected mice. In addition, recent observations that Th17 cells can contribute to enhanced inflammation during fungal infection (57) are of interest because IL-21 is a Th17 cytokine that has been associated with alternative macrophage activation and Th17 cell development (48). In a low-dose infection, IL-21 levels were higher in MC-infected wt mice. We hypothesize that the enhanced inflammation documented in MC-infected mice could also be the result of differences in Th17 cell regulation. As Korn et al. demonstrated, IL-6, IL-21, and TGF-β function together to produce Th17 cells (37) and all of these cytokines are upregulated in MC-infected lungs. Finally, Gu et al. have demonstrated that IL-10 negatively regulates the expression of Th17 cytokines by macrophages and T cells (23), which again would explain the original observation that IL-10 is consistently downregulated in MC-infected mice.
Both high-and low-dose MC infections yield similar outcomes in wt and IL-10−/− mice, although marked differences in recruitment of inflammatory cells and cytokine production were found. Future studies will have to clarify why we see such differences between inoculum doses, even though it does not affect the outcome. One possibility is that mice infected at a high dose are not able to completely phagocytose the initial inoculum, which then enhances the recruitment of phagocytic cells, and the pathogenesis is immediately altered, whereas in low-dose infection the macrophages initially succeed but then fail to contain the infection. The macrophage is a key effector cell in animal models and models of pulmonary cryptococcosis (15). The MC variant exhibits an enlarged polysaccharide capsule, which inhibits phagocytosis. Proliferation of lymphocytes in response to C. neoformans correlates with the magnitude of phagocytosis (59, 60) and thus links the innate immune response to CMI. In addition, the shed capsular polysaccharide GXM can be immunosuppressive in vivo (43, 44), and previous studies have shown that MC and SM GXMs are different and exhibit different effects on macrophages and lymphocyte proliferation (20, 51). Recent biophysical data suggest that distributions of charged residues on SM and MC GXMs are altered and affect phagocytosis (20), although by nuclear magnetic resonance the GXMs are not different (41). We hypothesize that enhanced recruitment of macrophages is a result of the inhibitory effects of the altered MC polysaccharide. Hence, analogous to other chronic inflammatory lung diseases, such as pneumoconiosis (63), cryptococcosis could result in overactivation of alveolar macrophages with the release of inflammatory mediators that promote destruction of lung tissue.
With respect to the relevance of IL-10 for chronic cryptococcosis, data from clinical specimens are not consistent. Studies of human immunodeficiency virus (HIV) patients have shown that baseline levels of IL-10 do not correlate with the severity of cryptococcosis (40), whereas in organ transplant patients, high IL-10 levels were documented in the cerebrospinal fluid (CSF) and blood of patients with central nervous system cryptococcosis and fungemia (58). One reason for the inconsistency may be that coinfection with HIV exacerbates the capability of C. neoformans to induce IL-10 via monocytes (52, 53). IL-10 levels in all clinical studies are drawn at different times and have a large range, and thus, it is difficult to know if they affect or predict outcomes. In addition, C. neoformans and the more mucoid Cryptococcus gattii strains elicit different IL-10 levels (5) in patients. We propose that IL-10 levels not only reflect Th2 responses, but also exhibit an important role in downregulation of excessive inflammation. Consistent with this finding, one case report documented initially undetectable and then high IL-10 levels during treatment of HIV patients with C. gattii meningitis and a paradoxical inflammatory reaction. In this case, IL-10 upregulation resulted in downregulation of excessive inflammation (a drop in the cell count in the CSF), which then permitted proper clearance (14).
Several studies have shown that most clinical strains have the ability to vary their phenotypes with respect to colony morphology, polysaccharide structure, and cellular morphology (4, 11, 21, 56), and different colony phenotypes can be observed in primary CSF isolates even before the start of therapy (18). Our data indicate that changes in phenotype translate into different host inflammatory responses and ultimately outcomes. We propose that certain phenotypes elicit proinflammatory host responses, and therefore, diseases caused by these strains or variants are differently affected when anti-inflammatory cytokines like IL-10 are absent.
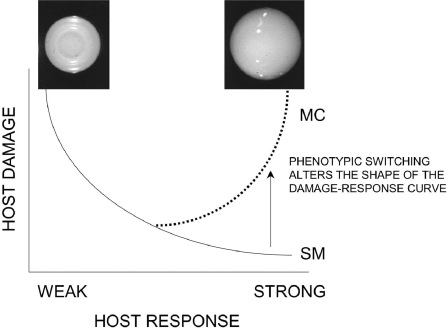
Finally, our data can be interpreted in the context of the “damage response” framework of microbial pathogenesis (8, 9). This framework classifies the virulence of pathogens with respect to their effects on host-pathogen interaction. According to that scheme, pathogens can be classified according to whether they cause host damage as a function of the strength of the immune response. Since C. neoformans usually causes disease in hosts with weakened immune systems, it was classified as a class II pathogen. The MC variant, however, appears to cause disease in association with eliciting exuberant, albeit histologically different, immune responses in immunocompetent and immunocompromised hosts. This would imply that the MC switch variant behaves as a class III pathogen as defined by the “damage response” framework classification, since damage occurs primarily at both extremes of the immune response (Fig. 6). In contrast, the SM switch behaves more like a class II pathogen, which is less virulent in a more resistant host.
FIG. 6.
Schemata outlining how phenotypic switching can change the damage response curve of a host infected with the SM and MC switch variants of C. neoformans. SM is classified as a class II pathogen and MC as a class III pathogen.
Acknowledgments
We thank Emily Cook and Neena Jain for technical help and Arturo Casadevall for careful review of the manuscript.
This work was supported by grant R0-1 AI 59681 to B.C.F.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Bauman, S. K., G. B. Huffnagle, and J. W. Murphy. 2003. Effects of tumor necrosis factor alpha on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response. Infect. Immun. 7168-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beenhouwer, D. O., S. Shapiro, M. Feldmesser, A. Casadevall, and M. D. Scharff. 2001. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect Immun 696445-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackstock, R., K. L. Buchanan, A. M. Adesina, and J. W. Murphy. 1999. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun 673601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasi, E., A. Brozzetti, D. Francisci, R. Neglia, G. Cardinali, F. Bistoni, V. Vidotto, and F. Baldelli. 2001. Evidence of microevolution in a clinical case of recurrent Cryptococcus neoformans meningoencephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 20535-543. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer, A. E., A. A. Siddiqui, M. I. Kester, K. C. Sigaloff, A. Rajanuwong, S. Wannapasni, W. Chierakul, and T. S. Harrison. 2007. Immune dysfunction in HIV-seronegative, Cryptococcus gattii meningitis. J. Infect. 54e165-e168. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan, K. L., and J. W. Murphy. 1993. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect. Immun. 612854-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan, K. L., and J. W. Murphy. 1994. Regulation of cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect. Immun. 622930-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall, A., and L. Pirofski. 2001. Host-pathogen interactions: the attributes of virulence. J. Infect. Dis. 184337-344. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall, A., and L. A. Pirofski. 2003. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 117-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarti, S., C. A. Sabatos, S. Xiao, Z. Illes, E. K. Cha, R. A. Sobel, X. X. Zheng, T. B. Strom, and V. K. Kuchroo. 2005. Tim-2 regulates T helper type 2 responses and autoimmunity. J. Exp. Med. 202437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherniak, R., L. C. Morris, T. Belay, E. D. Spitzer, and A. Casadevall. 1995. Variation in the structure of glucuronoxylomannan in isolates from patients with recurrent cryptococcal meningitis. Infect. Immun. 631899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 664994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dromer, F., S. Mathoulin, B. Dupont, and A. Laporte. 1996. Epidemiology of cryptococcosis in France: a 9-year survey (1985-1993). French Cryptococcosis Study Group. Clin. Infect Dis 2382-90. [DOI] [PubMed] [Google Scholar]
- 14.Einsiedel, L., D. L. Gordon, and J. R. Dyer. 2004. Paradoxical inflammatory reaction during treatment of Cryptococcus neoformans var. gattii meningitis in an HIV-seronegative woman. Clin. Infect. Dis. 39e78-e82. [DOI] [PubMed] [Google Scholar]
- 15.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 684225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franzot, S. P., J. Mukherjee, R. Cherniak, L. C. Chen, J. S. Hamdan, and A. Casadevall. 1998. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect. Immun. 6689-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fries, B. C., and A. Casadevall. 1998. Serial isolates of Cryptococcus neoformans from patients with AIDS differ in virulence for mice. J. Infect. Dis. 1781761-1766. [DOI] [PubMed] [Google Scholar]
- 18.Fries, B. C., E. Cook, X. Wang, and A. Casadevall. 2005. Effects of antifungal interventions on the outcome of experimental infections with phenotypic switch variants of Cryptococcus neoformans. Antimicrob. Agents Chemother. 49350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fries, B. C., D. L. Goldman, R. Cherniak, R. Ju, and A. Casadevall. 1999. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect Immun 676076-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fries, B. C., C. P. Taborda, E. Serfass, and A. Casadevall. 2001. Phenotypic switching of Cryptococcus neoformans occurs in vivo and influences the outcome of infection. J. Clin. Investig. 1081639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Hermoso, D., F. Dromer, and G. Janbon. 2004. Cryptococcus neoformans capsule structure evolution in vitro and during murine infection. Infect. Immun. 723359-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman, D. L., B. C. Fries, S. P. Franzot, L. Montella, and A. Casadevall. 1998. Phenotypic switching in the human pathogenic fungus Cryptococcus neoformans is associated with changes in virulence and pulmonary inflammatory response in rodents. Proc Natl Acad Sci U S A 9514967-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu, Y., J. Yang, X. Ouyang, W. Liu, H. Li, J. Yang, J. Bromberg, S. H. Chen, L. Mayer, J. C. Unkeless, and H. Xiong. 2008. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur. J. Immunol. 381807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez, Y., S. Arora, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 1741027-1036. [DOI] [PubMed] [Google Scholar]
- 25.Hoag, K. A., N. E. Street, G. B. Huffnagle, and M. F. Lipscomb. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 13487-495. [DOI] [PubMed] [Google Scholar]
- 26.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 1602393-2400. [PubMed] [Google Scholar]
- 27.Huffnagle, G. B., G. H. Chen, J. L. Curtis, R. A. McDonald, R. M. Strieter, and G. B. Toews. 1995. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 1553507-3516. [PubMed] [Google Scholar]
- 28.Huffnagle, G. B., and M. F. Lipscomb. 1998. Cells and cytokines in pulmonary cryptococcosis. Res. Immunol. 149387-396, 512-514. [DOI] [PubMed] [Google Scholar]
- 29.Huffnagle, G. B., M. F. Lipscomb, J. A. Lovchik, K. A. Hoag, and N. E. Street. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 5535-42. [DOI] [PubMed] [Google Scholar]
- 30.Huffnagle, G. B., R. M. Strieter, L. K. McNeil, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1997. Macrophage inflammatory protein-1α (MIP-1α) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J. Immunol. 159318-327. [PubMed] [Google Scholar]
- 31.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 1574529-4536. [PubMed] [Google Scholar]
- 32.Jeannin, P., S. Lecoanet, Y. Delneste, J. F. Gauchat, and J. Y. Bonnefoy. 1998. IgE versus IgG4 production can be differentially regulated by IL-10. J. Immunol. 1603555-61. [PubMed] [Google Scholar]
- 33.Kawakami, K., M. Hossain Qureshi, T. Zhang, Y. Koguchi, Q. Xie, M. Kurimoto, and A. Saito. 1999. Interleukin-4 weakens host resistance to pulmonary and disseminated cryptococcal infection caused by combined treatment with interferon-gamma-inducing cytokines. Cell Immunol. 19755-61. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami, K., M. H. Qureshi, Y. Koguchi, T. Zhang, H. Okamura, M. Kurimoto, and A. Saito. 1999. Role of TNF-alpha in the induction of fungicidal activity of mouse peritoneal exudate cells against Cryptococcus neoformans by IL-12 and IL-18. Cell Immunol. 1939-16. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami, K., M. Tohyama, X. Qifeng, and A. Saito. 1997. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect. Immun. 651307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khademi, M., Z. Illes, A. W. Gielen, M. Marta, N. Takazawa, C. Baecher-Allan, L. Brundin, J. Hannerz, C. Martin, R. A. Harris, D. A. Hafler, V. K. Kuchroo, T. Olsson, F. Piehl, and E. Wallstrom. 2004. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J. Immunol. 1727169-7176. [DOI] [PubMed] [Google Scholar]
- 37.Korn, T., E. Bettelli, W. Gao, A. Awasthi, A. Jager, T. B. Strom, M. Oukka, and V. K. Kuchroo. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchroo, V. K., D. T. Umetsu, R. H. DeKruyff, and G. J. Freeman. 2003. The TIM gene family: emerging roles in immunity and disease. Nat. Rev. Immunol. 3454-462. [DOI] [PubMed] [Google Scholar]
- 39.Lee, S. C., D. W. Dickson, and A. Casadevall. 1996. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum. Pathol. 27839-847. [DOI] [PubMed] [Google Scholar]
- 40.Lortholary, O., K. Sitbon, and F. Dromer. 2005. Evidence for human immunodeficiency virus and Cryptococcus neoformans interactions in the pro-inflammatory and anti-inflammatory responses in blood during AIDS-associated cryptococcosis. Clin. Microbiol. Infect. 11296-300. [DOI] [PubMed] [Google Scholar]
- 41.McFadden, D. C., B. C. Fries, F. Wang, and A. Casadevall. 2007. Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans. Eukaryot. Cell 61464-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mege, J. L., S. Meghari, A. Honstettre, C. Capo, and D. Raoult. 2006. The two faces of interleukin 10 in human infectious diseases. Lancet Infect. Dis. 6557-569. [DOI] [PubMed] [Google Scholar]
- 43.Mody, C. H., and R. M. Syme. 1993. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect. Immun. 61464-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monari, C., F. Bistoni, and A. Vecchiarelli. 2006. Glucuronoxylomannan exhibits potent immunosuppressive properties. FEMS Yeast Res. 6537-542. [DOI] [PubMed] [Google Scholar]
- 45.Muller, U., W. Stenzel, G. Kohler, C. Werner, T. Polte, G. Hansen, N. Schutze, R. K. Straubinger, M. Blessing, A. N. McKenzie, F. Brombacher, and G. Alber. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 1795367-5377. [DOI] [PubMed] [Google Scholar]
- 46.Murphy, J. W. 1993. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect. Immun. 614750-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olszewski, M. A., G. B. Huffnagle, R. A. McDonald, D. M. Lindell, B. B. Moore, D. N. Cook, and G. B. Toews. 2000. The role of macrophage inflammatory protein-1 alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J. Immunol. 1656429-6436. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang, W., J. K. Kolls, and Y. Zheng. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28454-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parayath, K. E., T. S. Harrison, and S. M. Levitz. 2000. Effect of interleukin (IL)-15 priming on IL-12 and interferon-gamma production by pathogen-stimulated peripheral blood mononuclear cells from human immunodeficiency virus-seropositive and -seronegative donors. J. Infect. Dis. 181733-736. [DOI] [PubMed] [Google Scholar]
- 50.Perfect, J. R., and A. Casadevall. 2002. Cryptococcosis. Infect. Dis. Clin. North Am. 16837-874. [DOI] [PubMed] [Google Scholar]
- 51.Pietrella, D., B. Fries, P. Lupo, F. Bistoni, A. Casadevall, and A. Vecchiarelli. 2003. Phenotypic switching of Cryptococcus neoformans can influence the outcome of the human immune response. Cell Microbiol. 5513-522. [DOI] [PubMed] [Google Scholar]
- 52.Pietrella, D., T. R. Kozel, C. Monari, F. Bistoni, and A. Vecchiarelli. 2001. Interleukin-12 counterbalances the deleterious effect of human immunodeficiency virus type 1 envelope glycoprotein gp120 on the immune response to Cryptococcus neoformans. J. Infect. Dis. 18351-58. [DOI] [PubMed] [Google Scholar]
- 53.Pietrella, D., C. Monari, C. Retini, B. Palazzetti, T. R. Kozel, and A. Vecchiarelli. 1999. HIV type 1 envelope glycoprotein gp120 induces development of a T helper type 2 response to Cryptococcus neoformans. AIDS 132197-2207. [DOI] [PubMed] [Google Scholar]
- 54.Qureshi, M. H., T. Zhang, Y. Koguchi, K. Nakashima, H. Okamura, M. Kurimoto, and K. Kawakami. 1999. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur. J. Immunol. 29643-649. [DOI] [PubMed] [Google Scholar]
- 55.Rayhane, N., O. Lortholary, C. Fitting, J. Callebert, M. Huerre, F. Dromer, and J. M. Cavaillon. 1999. Enhanced sensitivity of tumor necrosis factor/lymphotoxin-alpha-deficient mice to Cryptococcus neoformans infection despite increased levels of nitrite/nitrate, interferon-gamma, and interleukin-12. J. Infect. Dis. 1801637-1647. [DOI] [PubMed] [Google Scholar]
- 56.Rivera, J., M. Feldmesser, M. Cammer, and A. Casadevall. 1998. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect. Immun. 665027-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romani, L., T. Zelante, A. De Luca, F. Fallarino, and P. Puccetti. 2008. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J. Immunol. 1805157-5162. [DOI] [PubMed] [Google Scholar]
- 58.Singh, N., S. Husain, A. P. Limaye, K. Pursell, G. B. Klintmalm, T. L. Pruett, J. Somani, V. Stosor, R. del Busto, M. M. Wagener, and C. Steele. 2006. Systemic and cerebrospinal fluid T-helper cytokine responses in organ transplant recipients with Cryptococcus neoformans infection. Transpl. Immunol. 1669-72. [DOI] [PubMed] [Google Scholar]
- 59.Syme, R. M., T. F. Bruno, T. R. Kozel, and C. H. Mody. 1999. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect. Immun. 674620-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syme, R. M., J. C. Spurrell, L. L. Ma, F. H. Green, and C. H. Mody. 2000. Phagocytosis and protein processing are required for presentation of Cryptococcus neoformans mitogen to T lymphocytes. Infect. Immun. 686147-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Traynor, T. R., A. C. Herring, M. E. Dorf, W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2002. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 1684659-4666. [DOI] [PubMed] [Google Scholar]
- 62.Traynor, T. R., W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2000. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 1642021-2027. [DOI] [PubMed] [Google Scholar]
- 63.Vanhee, D., P. Gosset, A. Boitelle, B. Wallaert, and A. B. Tonnel. 1995. Cytokines and cytokine network in silicosis and coal workers’ pneumoconiosis. Eur. Respir. J. 8834-842. [PubMed] [Google Scholar]
- 64.Zaragoza, O., M. Alvarez, A. Telzak, J. Rivera, and A. Casadevall. 2007. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect. Immun. 752729-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]