Abstract
Pathogenic Yersinia species utilize a type III secretion system (T3SS) to translocate effectors called Yersinia outer proteins (Yops) into infected host cells. Previous studies demonstrated a role for effector Yops in the inhibition of caspase-1-mediated cell death and secretion of interleukin-1β (IL-1β) in naïve macrophages infected with Yersinia enterocolitica. Naïve murine macrophages were infected with a panel of different Yersinia pestis and Yersinia pseudotuberculosis strains to determine whether Yops of these species inhibit caspase-1 activation. Cell death was measured by release of lactate dehydrogenase (LDH), and enzyme-linked immunosorbent assay for secreted IL-1β was used to measure caspase-1 activation. Surprisingly, isolates derived from the Y. pestis KIM strain (e.g., KIM5) displayed an unusual ability to activate caspase-1 and kill infected macrophages compared to other Y. pestis and Y. pseudotuberculosis strains tested. Secretion of IL-1β following KIM5 infection was reduced in caspase-1-deficient macrophages compared to wild-type macrophages. However, release of LDH was not reduced in caspase-1-deficient macrophages, indicating that cell death occurred independently of caspase-1. Analysis of KIM-derived strains defective for production of functional effector or translocator Yops indicated that translocation of catalytically active YopJ into macrophages was required for caspase-1 activation and cell death. Release of LDH and secretion of IL-1β were not reduced when actin polymerization was inhibited in KIM5-infected macrophages, indicating that extracellular bacteria translocating YopJ could trigger cell death and caspase-1 activation. This study uncovered a novel role for YopJ in the activation of caspase-1 in macrophages.
The genus Yersinia is composed of 11 species, of which three (Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica) are pathogenic to humans. Y. pestis is the etiological agent of plague, an acute and often fatal disease of both humans and animals. Infection by Y. pestis occurs through the bite of an infected flea or by inhalation, resulting in bubonic or pneumonic plague, respectively (39). Y. pseudotuberculosis and Y. enterocolitica are enteropathogens that are transmitted by the fecal-oral route and cause a self-limiting gastroenteritis (5, 54). All highly pathogenic yersiniae possess a ∼70-kb virulence plasmid (designated pCD1 in Y. pestis and pYV in Y. pseudotuberculosis and Y. enterocolitica), which encodes a type III secretion system (T3SS) and the secreted substrates known as Yersinia outer proteins (Yops) and LcrV (12). Expression of the Yersinia T3SS is upregulated at 37°C, and contact with a host cell leads to activation of secretion and translocation of effector Yops into the target cell (41). The translocation of effector Yops into the host cell is dependent on LcrV and the translocator Yops, YopB and YopD. Both YopB and YopD have hydrophobic domains, which are thought to insert into the host cell membrane to form a channel to allow the T3SS to translocate effector Yops (11, 18, 51). The six effector Yops (YopE, YopJ [YopP], YopH, YopT, YopM, and YopO [YpkA]) as well as the translocator Yops and LcrV are involved in disrupting or activating many host cellular response pathways, including those responsible for phagocytosis, cytokine production, or apoptosis (6, 10, 11, 23, 32, 51). For example, YopE and YopT target Rho GTPases that regulate a number of cellular functions, including actin cytoskeleton rearrangement and gene expression (51). YopE acts as a GTPase-activating protein, which can inactivate Rho GTPases. YopT is a cysteine protease that can cleave Rho GTPases at their membrane anchor, releasing them into the cytosol. YopJ (YopP in Y. enterocolitica) is an enzyme with acetyltransferase and or deubiquitinase activities that counteracts the proinflammatory response in the host cell by inhibiting the mitogen-activated protein kinase and NF-κB pathways (4, 34, 35, 59). YopJ can sensitize macrophages to die of apoptosis following activation of Toll-like receptor 4 (TLR4) by inhibiting the expression of survival factors regulated by the mitogen-activated protein kinase and NF-κB pathways (11, 43, 57, 58).
In this study, the activation of caspase-1 in macrophages infected with Y. pestis or Y. pseudotuberculosis was examined. Caspase-1 (also called interleukin-1β [IL-1β] converting enzyme) is a cysteine protease involved in the processing and release of the proinflammatory cytokines IL-1β and IL-18. Caspase-1 is a member of a family of inflammatory caspases, which includes human caspase-4 and caspase-5, as well as mouse caspase-11 and caspase-12 (30, 48). Caspase-1 is synthesized as a 45-kDa inactive zymogen that is cleaved at aspartic residues to generate the active heterotetramer composed of two p10 and two p20 subunits (7, 13, 28). Activation of caspase-1 occurs through its recruitment to the inflammasome complex (25, 28). Upon activation of caspase-1, cleavage of pro-IL-1β and pro-IL-18 occurs and the mature forms of these cytokines are secreted.
IL-1β is a proinflammatory cytokine produced mainly from monocytes, macrophages, and dendritic cells and is intimately involved in the innate immune response. It is a potent endogenous pyrogen, causing fever, hypotension, synthesis of adhesion molecules, and production of the acute-phase response (14, 15). Unregulated production of IL-1β can be deleterious to the host, leading to excessive inflammation and septic shock. The expression of IL-1β is tightly regulated, in that distinct signals are required for its transcription and its processing and release (7, 13, 15, 27, 33). The first signal can be transmitted via stimulation of a TLR signaling pathway (i.e., TLR4 recognition of lipopolysaccharide [LPS]), leading to activation of the transcription factor NF-κB and the expression of the 35-kDa precursor form of IL-1β. A secondary signal is required to activate the inflammasome complex, leading to the activation of caspase-1 and subsequent processing and secretion of mature IL-1β.
Previous studies have examined caspase-1 activation and IL-1β secretion in macrophages infected with Yersinia species (2, 47, 49). Schotte et al. (47) examined these responses after they infected murine Mf4/4 macrophage-like cells with Y. enterocolitica E40 as well as different single or multiple yop mutants of this strain. Their results suggested that YopP (YopJ) could inhibit expression of pro-IL-1β and that YopE could inhibit caspase-1 activation and IL-1β secretion in Y. enterocolitica-infected Mf4/4 cells (47). Inhibition of caspase-1 activation was also observed when either YopE or YopT was overexpressed by transfection in cultured HEK293T cells. Overall, these findings implicated YopP as an inhibitor of pro-IL-1β synthesis and YopE and YopT as inhibitors of caspase-1 activation. Shin and Cornelis (49) obtained evidence that pores generated by the T3SS translocation channel trigger activation of caspase-1 in Mf4/4 cells infected with a Y. enterocolitica E40 multi-yop effector mutant. Thus, in their model (49), YopE and YopT inhibit activation of caspase-1 by counteracting T3SS-dependent pore formation (51). More recently, Bergsbaken and Cookson obtained evidence that Y. pseudotuberculosis strain YPIII could induce caspase-1 activation in murine bone-marrow derived macrophages (BMDMs) that were activated by preexposure to LPS (2). They also observed that LPS-activated BMDMs infected with YPIII died of a caspase-1-mediated form of cell death termed pyroptosis (2, 16). It was hypothesized that the T3SS in YPIII transports an inflammasome-stimulating factor into the macrophage cytosol and that priming macrophages with LPS overcomes the ability of translocated effector proteins to inhibit the activation of caspase-1 (2).
Here, we aimed to determine whether effector Yops of Y. pestis and Y. pseudotuberculosis regulate caspase-1 activation and IL-1β secretion in naïve BMDMs. Among a panel of different strains tested, Y. pestis isolates derived from the KIM background displayed an unusual ability to stimulate caspase-1 activation, IL-1β secretion, and cell death in infected macrophages. IL-1β secretion from, but not death of, KIM5-infected macrophages required caspase-1 activity. Furthermore, caspase-1 activation, IL-1β secretion, and death of macrophages infected with KIM5 required the translocator YopB and the enzymatic activity of the effector YopJ. These results suggest a novel role for YopJ in the activation of caspase-1 following infection of macrophages by Y. pestis KIM strains.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Yersinia pestis and Yersinia pseudotuberculosis strains used in this study are listed in Table 1. The KIM5, KIM-D27, and KIM8 strains are related to a parental KIM strain, and the pCD1 plasmid is the same for KIM5, KIM-D27, and KIM8, with the exception of the ampicillin (Ap) marker when used. CO92 contains its own unique pCD1. All pCD1+ Y. pestis strains used in this study lack the pigmentation locus (pgm) and therefore are conditionally virulent. KIM-D27 was obtained from Stephen Smiley (Trudeau Institute). Derivatives of KIM8 containing mutant pCD1 plasmids (Tables 1 and 2) were obtained from Greg Plano (University of Miami). Derivatives of KIM6+ and CO92 in which the pigmentation (pgm) locus was spontaneously deleted (KIM6 and CO92 Δpgm, respectively) were isolated and the deletion verified as described previously (20). A pCD1 plasmid containing an ampicillin resistance cassette (pCD1Ap) was obtained from Robert Perry (University of Kentucky) and subsequently used to transform KIM6 as described previously (20), resulting in KIM5. The KIM5 yopH::kan and KIM5 ypkA::kan mutants were constructed using the lambda Red recombination method as described previously (46) to insert Kmr cassettes into the corresponding genes. For the yopH mutant the primers used were Y1013koF (5′-GTTCTAACTCAAGAAGATACCGCTAAGCTATTGCAAAGTACGGTAAAGCATATGTAGGCTGGA GCTGCTTCG-3′) and Y1013koR (5′-TGTATTACCGGCCGTAATTTGGAGTCATCTCCTACCGCTGAACTTCCTTTGATGGGAATTAGCCATGGTCC-3′), and for the ypkA mutant the primers used were Y1009koF (5′-ATCTTTTCGGGGAAGATGTTTAACTTTTCAATTGCTCGTAACCTTACTGACATGTAGGC TGGAGCTGCTTCG-3′) and Y1009koR (5′-CCGCCATCATCTGACGGTGAATTGCGGTATACTGCTCAGTGCCGAATTTCATGGGAATTAGCCATGGTCC-3′).
TABLE 1.
Yersinia strains used in this study
| Species and strain | Relevant characteristicsa | Reference or source |
|---|---|---|
| Y. pestis | ||
| KIM6+ | Biovar Medievalis, pCD1−, pMT1+, pPCP1+, pgm+ | 20 |
| KIM6 | Biovar Medievalis, pCD1−, pMT1+, pPCP1+, Δpgm | This study |
| KIM5 | Biovar Medievalis, KIM6/pCD1Ap, pMT1+, pPCP1+, Δpgm, Apr | This study |
| KIM5/GFP | KIM5/pMMB207gfp3.1, Apr, Cmr | 20 |
| KIM-D27 | pCD1+, pMT1+, pPCP1+, Δpgm | 38 |
| CO92 Δpgm | Biovar Orientalis, pCD1CO92+, pMT1+, pPCP1+, Δpgm | This study |
| KIM5 yopB | pCD1Ap yopB18 (in-frame deletion of nucleotides 496-774), Apr | This study |
| KIM8 Δ1 | pCD1-Δ1, pPCP1−, pMT1+, Δpgm, Kmr | 1 |
| KIM8 Δ2 | pCD1-Δ2, pPCP1−, pMT1+, Δpgm, Kmr | 1 |
| KIM8 Δ34 | pCD-Δ34, pPCP1−, pMT1+, Δpgm, Kmr | G. Plano, unpublished |
| KIM8 Δ4 | pCD1-Δ4, pPCP1−, pMT1+, Δpgm, Tmr | 1 |
| KIM8 Δ12 | pCD1-Δ12, pPCP1−, pMT1+, Δpgm, Kmr | 1 |
| KIM8 Δ123 | pCD1-Δ123, pPCP1−, pMT1+, Δpgm, Kmr, Cmr | 1 |
| KIM8 Δ1234 | pCD1-Δ1234, pPCP1−, pMT1+, Δpgm, Tmr, Kmr, Cmr | 1 |
| KIM5 yopJC172A | pCD1Ap yopJC172A (codon change of Cys172 to Ala172), Apr | This study |
| KIM5 yopH::kan | pCD1Ap (Kmr cassette inserted into yopH), Apr, Kmr | This study |
| KIM5 ypkA::kan | pCD1Ap (Kmr cassette inserted into ypkA), Apr, Kmr | This study |
| Y. pseudotuberculosis | ||
| IP2777 | Serogroup O1, pYV+ | 50 |
| IP2666 | Serogroup O3, pYV+ (naturally ΔyopTsycT) | 50 |
| IP2666 E− | pYV yopE::kan, alternative name is IP6, Kmr | 3 |
| IP2666 J− | pYV yopJΔ1-867, alternative name is IP26 | This study |
| IP2666 EJ− | pYV yopE::kan yopJΔ1-867, alternative name is IP31, Kmr | This study |
| IP2666 JEHMOK− | pYV yopJΔ1-867 yopE::kan yopH::cam yopM yopO yopK, alternative name is IP37, Kmr, Cmr | This study |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Tmr, trimethoprim resistance.
TABLE 2.
KIM8 pCD1 deletion mutant strains used in this study
| Strain | Deleted genes and ORFs |
|---|---|
| KIM8 Δ1 | ylpA, yopK, yopT, sycT, ORF61, ORF60, yopM, ORF54 |
| KIM8 Δ2 | ORF5, ORF7, ypkA, yopJ, yopH |
| KIM8 Δ34 | yadA, yadA′, ORF85, ORF84, sycH, sycE, yopE, ORF75, ORF74, ORF73 |
| KIM8 Δ4 | sycE, yopE, ORF75, ORF74, ORF73 |
| KIM8 Δ12 | ylpA, yopK, yopT, sycT, ORF61, ORF60, yopM, ORF54, ORF5, ORF7, ypkA, yopJ, yopH |
| KIM8 Δ123 | ylpA, yopK, yopT, sycT, ORF61, ORF60, yopM, ORF54, ORF5, ORF7, ypkA, yopJ, yopH, yadA, yadA′, ORF85, ORF84 |
| KIM8 Δ1234 | ylpA, yopK, yopT, sycT, ORF61, ORF60, yopM, ORF54, ORF5, ORF7, ypkA, yopJ, yopH, yadA, yadA′, ORF85, ORF84, sycE, yopE, ORF75, ORF74, ORF73 |
The KIM5 yopB mutant was constructed by allelic exchange using the plasmid pJB4 (37). The resulting mutation in pCD1Ap corresponds to the yopB18 allele, which contains an in-frame deletion of nucleotides 496 to 774 (37). The KIM5 yopJC172A mutant was constructed using allelic exchange, placing the C172A mutation into the yopJ gene in the pCD1Ap plasmid of KIM5, and verified as described previously (58). The C172A mutation corresponds to a change from TGTGGT, encoding Cys172 and Gly173, to GCCGGC, encoding Ala172 and Gly173. The Y. pseudotuberculosis yopJ and yopE yopJ mutants of IP2666 (Table 1) were constructed by allelic exchange, using the plasmid pLP13 (37) to delete the entire yopJ open reading frame (ORF) (nucleotides 1 to 867) and IP2666 and IP6 yopE (3) as the recipient strains, respectively. The yopJ yopE yopH yopM yopO yopK mutant of IP2666 (Table 1) was generated from the pYV-cured strain IP2666c (50). The pYV plasmid purified from YP37 (52) was used to transform IP2666c by electroporation; the kanamycin resistance gene inserted in yopE (yopE::kan) was used to select for transformants. The arabinose-inducible plasmid carrying the ORF of KIM YopJ, pYopJ-GSK, was obtained from Gregory Plano (University of Miami). The plasmid was used to transform KIM8 Δ2 and IP2666 J− by electroporation and selection on ampicillin-containing plates. Y. pestis and Y. pseudotuberculosis strains were cultivated on heart infusion (HI) (Difco) or Luria-Bertani (LB) agar plates, respectively, for 2 days at 28°C. Cultures were grown overnight with aeration in HI (Y. pestis) or LB (Y. pseudotuberculosis) broth at 28°C. The next day the cultures were diluted to an optical density (OD) at 600 nm of 0.1 in the same medium supplemented with 2.5 mM CaCl2 and incubated for 2 h at 37°C with aeration. Bacterial cultures were washed once with phosphate-buffered saline (PBS) then resuspended in PBS for measurements of OD at 600 nm. The bacterial growth medium was supplemented with ampicillin (25 μg/ml) and/or kanamycin (25 μg/ml) when appropriate. KIM5/green fluorescent protein (GFP) was cultured in the presence of 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) during growth at 37°C to induced expression of GFP. Where indicated, a final concentration of 0.2% l-arabinose was maintained in cell culture medium to induce expression of YopJ from the pYopJ-GSK plasmid during infection.
BMDM isolation and culture conditions.
BMDMs were isolated from the femurs of 6- to 8-week-old C57BL/6 female mice (Taconic Laboratories) or caspase-1-deficient (Casp1−/−) mice (55) (obtained from Richard Flavell and Craig Roy, Yale University) as previously described (9, 40). The Casp1−/− mice were backcrossed to C57BL/6 mice for seven generations.
Macrophage infections.
Twenty-four hours before infection, BMDMs were seeded into 24-well plates at a density of 1.5 × 105 cells/ml as described previously (40), with the exception that the tissue culture medium (infection medium) contained 10% fetal bovine serum, 15% L-cell-conditioned medium, and 1% 0.1 M sodium pyruvate. Bacteria were grown as described above and used to infect macrophages at a multiplicity of infection (MOI) of 10 bacteria per macrophage. After addition of bacteria, plates were centrifuged for 5 min at 95 × g to induce contact between bacteria and macrophages. After incubation at 37°C with 5% CO2 for 15 min, macrophages were washed once with prewarmed PBS to remove any bacteria that had not been taken up. Fresh infection medium containing 8 μg/ml of gentamicin was added and left for 1 h at 37°C with 5% CO2 to kill or reduce viability of extracellular bacteria (>99%) (36, 40). After 1 h, macrophages were washed once with PBS and a lower concentration of gentamicin (4.5 μg/ml) in fresh tissue culture medium was added and left for the remaining incubation times as indicated in the figure legends to inhibit growth of extracellular bacteria. To inhibit bacterial uptake, macrophages were exposed to 3.9 μM of cytochalasin D (CD) (Sigma) for 2 h prior to infection as well as during the 20 min of infection. To inhibit caspase-1 activity, macrophages were exposed to 100 μM of caspase-1 inhibitor Ac-YVAD-cmk (Calbiochem) for 1 h prior to infection. Dimethyl sulfoxide (DMSO) was used as the solvent for CD and Ac-YVAD-cmk. The final concentrations of DMSO used as a solvent control for CD and Ac-YVAD-cmk treatment conditions were 28 mM and 140 mM, respectively. In some experiments macrophages were exposed to 50 ng/ml of Escherichia coli 026:B6 LPS (Sigma-Aldrich, catalog no. L-2654) for 4 h prior to infection.
Cytokine measurements.
Levels of IL-1β and TNF-α secreted into tissue culture media during infection assays were measured with the Quantikine mouse IL-1β and tumor necrosis factor alpha (TNF-α) immunoassay kits, respectively, as per the manufacturer's instructions (R&D Systems). Levels of IL-18 secreted into tissue culture media were measured using the mouse IL-18 enzyme-linked immunosorbent assay (ELISA) kit as per the manufacturer's protocol (Medical & Biological Laboratories Co., Ltd.). Supernatants from two replicate wells per infection condition were collected, centrifuged to remove cellular debris, and transferred to new tubes. Supernatants were diluted appropriately, and 50 μl of each diluted sample was analyzed. Standard curves were used to estimate levels of cytokines in each sample. The replicate values for each infection condition were averaged.
LDH release.
Supernatants from infected macrophages were collected and analyzed for lactate dehydrogenase (LDH) release using the CytoTox-96 nonradioactive cytotoxicity assay (Promega), following the manufacturer's instructions. Supernatants from two replicate wells per infection condition were collected and centrifuged to remove cellular debris. LDH levels in each replicate sample were measured in triplicate. Spontaneous LDH release was measured from supernatants of uninfected cells, while total LDH release was measured from uninfected cells that were lysed by freezing and thawing. OD values of the six measurements for each infection condition were averaged, and the percentage of LDH per infection condition was calculated as follows: percent LDH release = [(infected-cell LDH release − spontaneous LDH release)/(total LDH release − spontaneous LDH release)] × 100.
Detection of active caspase-1 using FAM-YVAD-FMK.
To assess the presence of active caspase-1 by fluorescence microscopy, infected BMDM on coverslips were incubated with 6-carboxyfluorescein-YVAD-fluoromethylketone (FAM-YVAD-FMK) (Immunochemistry Technologies) as per the manufacturer's protocol. After 1 hour, macrophages were washed and fixed in 2.5% paraformaldehyde in PBS for 30 min at room temperature. The fixed cells were incubated in 0.1% Triton X-100 in PBS for 10 min to permeabilize the cell membrane and washed once with PBS and once with 3% bovine serum albumin (BSA) in PBS to prevent nonspecific antibody binding. Bacteria were immunolabeled by incubation for 30 min with rabbit anti-Yersinia antiserum SB349 (3) diluted 1:1,000 in 3% BSA in PBS. Cells were washed three times with PBS and incubated for 30 min with goat anti-rabbit secondary conjugated to AlexaFluor594 (Molecular Probes) diluted at 1:1,500. After washing, coverslips were mounted on glass microscope slides and visualized by fluorescence microscopy using a Nikon Eclipse E600 microscope equipped with a 40× objective. Images were captured using a Sony Progressive 3CCD camera and processed with Adobe Photoshop 6.0.
CFU assay.
At various time points postinfection, infected macrophages were washed three times with PBS and lysed with 0.5 ml of 0.1% Triton X-100. Lysates were then removed, and an additional 0.5 ml of 0.1% Triton X-100 was used to wash the wells. Lysates and washes were collected into 2-ml microcentrifuge tubes and used for serial 10-fold dilutions. Dilutions were spread on HI plates and incubated for 2 days at 28°C, after which output CFU were counted. Two replicates for each infection condition were analyzed in each experiment and the results averaged. The averages presented (log10 CFU per ml) are derived from three independent experiments.
Bacterial phagocytosis assay.
Macrophages on coverslips were infected with KIM5/GFP for 20 min and then washed and fixed in 2.5% paraformaldehyde in PBS for 30 min at room temperature. The fixed cells were incubated washed once with PBS, and once with 3% BSA in PBS to prevent nonspecific antibody binding. Bacteria were immunolabeled with rabbit anti-Yersinia antiserum SB349 and goat anti-rabbit secondary conjugated to AlexaFluor594 as described above. After washing, coverslips were mounted on glass microscope slides and visualized by fluorescence microscopy using a Zeiss Axioplan2 microscope equipped with a 40× objective. A Spot camera (Diagnostic Instruments) was used to sequentially capture the AlexaFluor594 (red) and GFP (green) signals in four or five random fields from each coverslip. The red and green images were overlaid using Adobe Photoshop 6.0, and the percentage of internalized bacteria was quantified by counting the number of intracellular bacteria (green) and dividing this number by the sum of internalized and extracellular bacteria (red). Between 1,200 and 2,500 bacteria were counted for each condition.
PI uptake assay.
Macrophages in 24 well dishes were infected for 4, 8, or 12 h; washed twice with PBS; and then incubated in 1 μg/ml of propidium iodide (PI) in Hanks balanced salt solution for 10 min at room temperature. Cells were washed once again with PBS and imaged through the bottom of the dishes using a Zeiss Axiovert S100 microscope equipped with a 40× objective. Phase-contrast and PI fluorescence images were captured using a Spot camera (Diagnostic Instruments, Inc.) and processed with Adobe Photoshop 7.0.
Statistical analysis.
In general, experiments analyzed for significance were performed three independent times. Probability (P) values were calculated by one-way analysis of variance and Tukey's multiple-comparison posttest and were considered significant if they were less than 0.05 (GraphPad Prism 4.0).
RESULTS
Yersinia pestis KIM isolates stimulate infected macrophages to secrete higher levels of IL-1β than other Yersinia strains.
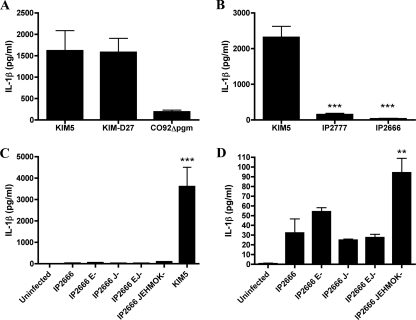
It has been previously shown that Yop effectors inhibit expression and secretion of IL-1β in the macrophage-like cell line Mf4/4 infected with Y. enterocolitica (47, 49). BMDMs were infected with different Y. pseudotuberculosis and Y. pestis strains to determine whether the T3SS and Yops of these species play a similar role in the inhibition of IL-1β release. Our standard infection protocol involved infection at an MOI of 10, followed by brief centrifugation to initiate contact, incubation for 15 min to allow infection, and then application of medium containing a low concentration of gentamicin to prevent growth of extracellular bacteria (see Materials and Methods). Initially, BMDMs were infected with the pCD1+ Y. pestis strain KIM5, KIM-D27, or CO92 Δpgm (Table 1) pregrown at 37°C to upregulate expression of the T3SS and Yops. The levels of IL-1β present in tissue culture supernatants were determined by ELISA after 24 h of infection (see Materials and Methods). As shown in Fig. 1A, macrophages infected with KIM5 or KIM-D27 secreted much higher levels of IL-1β than macrophages infected with CO92 Δpgm. This analysis was extended to the pYV+ Y. pseudotuberculosis strains IP2777 and IP2666 (Table 1). The results showed that KIM5-infected macrophages secreted substantially higher levels of IL-1β than macrophages infected with IP2777 or IP2666 (Fig. 1B). Thus, strains derived from Y. pestis KIM displayed an unusual ability to stimulate IL-1β secretion from macrophages compared to other strains of Y. pestis (CO92) or Y. pseudotuberculosis. To determine whether the phenotype observed for KIM5 would be similar to that of a Y. pseudotuberculosis multiple yop mutant, we next compared the response of BMDMs infected with KIM5 to that of macrophages infected with isogenic single (yopE or yopJ) or multiple (yopEJ or yopJEHMOK) yop mutants of IP2666 (Table 1) pregrown at 37°C. Surprisingly, the amount of IL-1β secreted from macrophages infected with KIM5 was ∼40-fold higher than that of IL-1β secreted from BMDMs infected with the Y. pseudotuberculosis yopJEHMOK mutant (Fig. 1C). The yopJEHMOK mutant did induce significantly larger amounts of IL-1β to be secreted from infected BMDMs compared to wild-type IP2666 (Fig. 1D), as expected from previous studies performed with Y. enterocolitica (47). Macrophages infected with the single yopE mutant reproducibly secreted slightly larger amounts of IL-1β than macrophages infected with the wild type, reflecting the proposed role of YopE as an inhibitor of caspase-1 activation (47), but this difference was not statistically significant (Fig. 1D). The other Y. pseudotuberculosis mutants tested (yopJ and yopEJ) did not induce higher levels of IL-1β secretion from BMDMs compared to the parental strain (Fig. 1D). Thus, although these results suggested that YopE and perhaps additional Yops inhibit IL-1β secretion from macrophages infected with Y. pseudotuberculosis IP2666 and are similar to those reported by Schotte et al. for Y. enterocolitica E40 (47), we have demonstrated that Y. pestis KIM strains have a novel capacity to stimulate high-level secretion of IL-1β from infected BMDMs.
FIG. 1.
Determination of levels of IL-1β secreted from macrophages infected with different Y. pestis or Y. pseudotuberculosis strains. BMDMs were left uninfected or infected with the indicated strains of Y. pestis or Y. pseudotuberculosis (Table 1). IL-1β levels in the supernatants of BMDMs at 24 h postinfection were measured by ELISA. Results shown are the averages from three independent experiments. Error bars represent standard deviations. Statistical significance compared to KIM5 (B) or IP2666 (C and D) was determined (P < 0.01, **; P < 0.001, ***).
Caspase-1 activity and the Yersinia translocator YopB are important for IL-1β secretion during KIM5 infection.
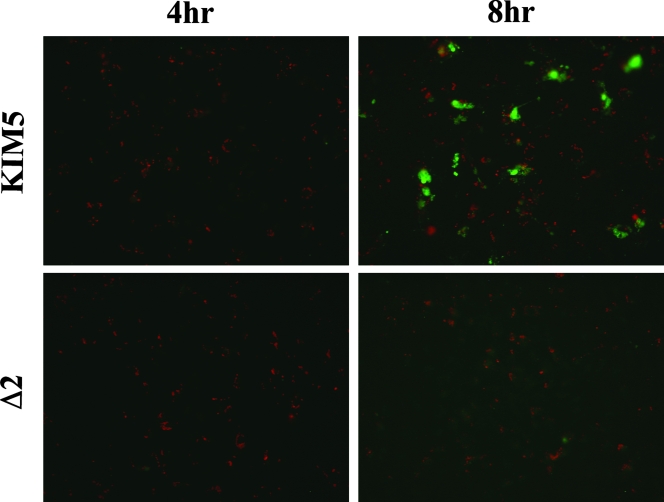
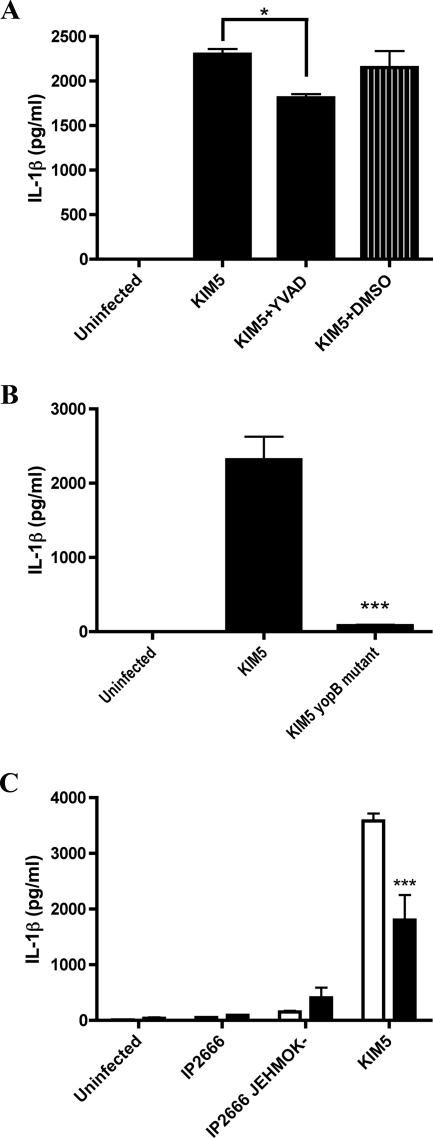
The importance of caspase-1 activation for IL-1β secretion from macrophages infected with various pathogenic bacteria has been previously demonstrated (24, 26, 29, 31, 42, 55). We obtained evidence that caspase-1 was activated in KIM5-infected macrophages by using a fluorochrome inhibitor of caspases (FLICA) reagent specific for active caspase-1 (FAM-YVAD-FMK) (see Materials and Methods). By microscopic analysis, the FAM-YVAD-FMK fluorescent signal was associated with KIM5-infected macrophages beginning at 4 h postinfection (see Fig. 6). To determine whether caspase-1 activity was required for IL-1β secretion from KIM5-infected macrophages, BMDMs were treated for 1 h before infection with 100 μM of the caspase-1 inhibitor Ac-YVAD-cmk (YVAD). As shown in Fig. 2A, a 1-h pretreatment with YVAD significantly inhibited IL-1β secretion from BMDMs infected with KIM5 for 24 h, suggesting that caspase-1 activity was important for IL-1β secretion under these infection conditions. Macrophages similarly treated with DMSO only as a solvent control showed no decrease in IL-1β secretion (Fig. 2A). Because YVAD treatment only partially reduced IL-1β secretion, it is possible that in the absence of caspase-1 activity (see also Fig. 10), other caspases can mediate processing and secretion of IL-1β in KIM5-infected BMDMs. Alternatively, because the ELISA used does not discriminate between pro and mature forms of IL-1β and KIM5-infected BMDMs are dying (see Fig. 4), it is possible that the IL-1β detected in the supernatant of KIM5-infected cells represents a mixture of mature IL-1β secreted via caspase-1 and pro-IL-1β released upon cell lysis.
FIG. 6.
Detection of active caspase-1 in macrophages infected with Y. pestis by fluorescence microscopy. BMDMs attached to coverslips were infected with Y. pestis KIM5 (upper panels) or with the KIM8 Δ2 mutant (lower panels) for 4 h (left panels) or 8 h (right panels). Infected macrophages were incubated with FLICA reagent (FAM-YVAD-FMK) to stain for active caspase-1 (green). The samples were then fixed, and bacteria were immunolabeled with a rabbit anti-Yersinia antibody (red). Coverslips were mounted on slides, and fluorescence microscopy was used to detect red and green signals. Representative images were captured by digital photomicroscopy. Images shown are overlays of the red and green signals and are representative of three independent experiments.
FIG. 2.
Determination of factors required for IL-1β secretion in macrophages infected with Y. pestis or Y. pseudotuberculosis. BMDMs were left uninfected or infected with the indicated strains of Y. pestis or Y. pseudotuberculosis (Table 1), and levels of IL-1β secreted at 24 h postinfection were measured by ELISA. (A and B) Macrophages were left untreated or exposed to 100 μM of the caspase-1 inhibitor Ac-YVAD-cmk (YVAD) or 140 mM DMSO as a solvent control for 1 h prior to infection. (C) Macrophages were left untreated (white bars) or exposed to 50 ng/ml of LPS for 4 h prior to infection (black bars). Results shown are the averages from three independent experiments (B and C) or from a single experiment with triplicate wells (A). Error bars represent standard deviations. Statistical significance (P < 0.05, *; P < 0.001, ***) compared to KIM5 (A and B) or untreated KIM5 (C) was determined.
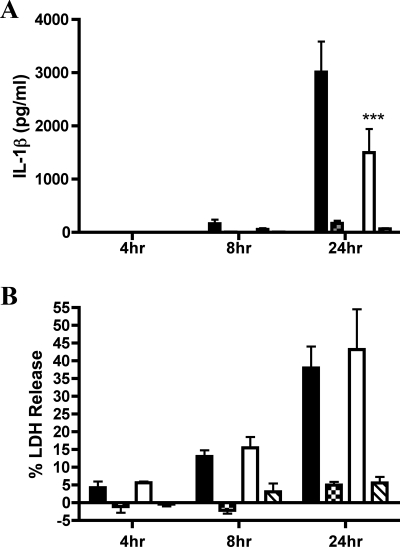
FIG. 10.
Determination of the importance of caspase-1 for IL-1β secretion and cell death by infection of Casp-1−/− macrophages with Y. pestis. BMDMs were left uninfected or infected with KIM5 (black bars, Casp-1+/+; white bars, Casp-1−/−) or KIM5 yopJC172A (crosshatched bars, Casp-1+/+; hatched bars, Casp-1−/−). Supernatants were collected at 4 h, 8 h, and 24 h postinfection and monitored for IL-1β secretion by ELISA (A) or for cell death by LDH assay (B). Results shown are the averages from three independent experiments. Error bars represent standard deviations. Statistical significance (P < 0.001, ***) compared to KIM5 was determined.
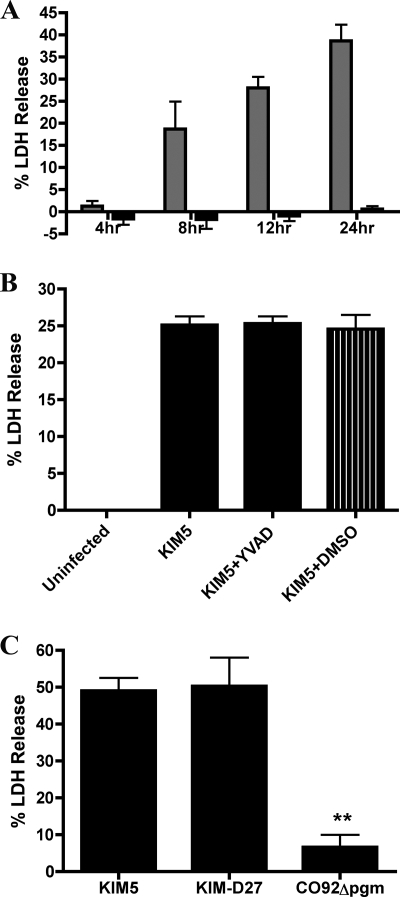
FIG. 4.
Determination of factors required for cell death in macrophages infected with Y. pestis. BMDMs were left uninfected or infected with Y. pestis KIM5, KIM5 yopB, CO92 Δpgm, or KIM-D27. Cell death was monitored by the percentage of LDH release. In panel A, supernatants from macrophages infected with KIM5 (gray bars) or KIM5 yopB (black bars) were collected at the time points indicated, and percent LDH was determined. In panels B and C, macrophages were left untreated or exposed to 100 μM YVAD or DMSO as a solvent control for 1 h prior to infection, and supernatants were collected at 24 h postinfection and analyzed for LDH release. Results shown are the averages from three independent experiments (A and C) or from one experiment with triplicate wells (B). Error bars represent standard deviations. Statistical significance (P < 0.01, **) compared to KIM5 (C) was determined.
YopB is required for translocation of effector Yops into host cells infected with Yersinia (18). A KIM5 yopB mutant (Table 1) was used to test whether effector translocation is required for IL-1β secretion during KIM5 infection of BMDMs. The yopB mutant stimulated significantly lower levels of IL-1β secretion from infected BMDMs than KIM5, suggesting a requirement for effector translocation in this response (Fig. 2B).
Macrophage activation with LPS decreases secretion of IL-1β from macrophages infected with Y. pestis KIM5.
Bergsbaken et al. (2) have shown that preactivation of BMDMs with LPS is required for activation of caspase-1 following infection with wild-type Y. pseudotuberculosis strain YPIII. BMDMs were exposed to 50 ng/ml LPS for 4 h prior to infection to investigate the role of macrophage activation in secretion of IL-1β from macrophages challenged with Y. pestis KIM5. Interestingly, activated BMDMs secreted significantly (twofold) less IL-1β after infection with KIM5 than naïve macrophages (Fig. 2C). We also observed little difference in the levels of IL-1β secreted from activated or naïve macrophages following infection with wild-type IP2666 (Fig. 2C). However, activated BMDMs infected with the Y. pseudotuberculosis yopJEHMOK mutant reproducibly secreted higher levels of IL-1β than naïve macrophages infected with the same strain, but this difference was not statistically significant (Fig. 2C).
Time course analysis of cytokine secretion from KIM5-infected macrophages.
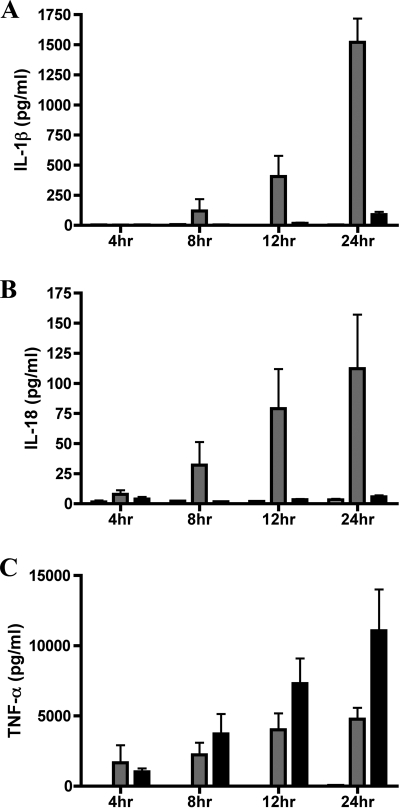
To begin to characterize the mechanism of caspase-1 activation in macrophages infected with KIM5, a time course analysis was performed. In addition to analyzing IL-1β, we also measured secretion of IL-18, which is dependent upon cleavage by active caspase-1 for its processing and release, and of TNF-α, which is not. A previous study has shown that Y. pestis strain EV76 can partially suppress secretion of TNF-α from infected RAW264.7 macrophage-like cells and that YopJ is required for this suppression (56). BMDMs were infected with KIM5 or KIM5 yopB, supernatants were collected at various time points, and ELISA was used to measure levels of secreted IL-1β, IL-18, and TNF-α. IL-18 was detected in the supernatants of KIM5-infected macrophages at 4 h postinfection, and IL-1β was detected at 8 h postinfection (Fig. 3A, B). Both cytokines continued to accumulate in the supernatants of KIM5-infected macrophages over time, and only background levels of these cytokines were secreted from BMDMs infected with KIM5 yopB. Quite different results were observed for TNF-α, compared to IL-1β and IL-18, under the same infection conditions (Fig. 3C). Both KIM5 and KIM5 yopB stimulated infected BMDMs to secrete TNF-α, which accumulated in the supernatants over time, although higher (∼2-fold) levels of TNF-α were secreted from macrophages infected with the yopB mutant (Fig. 3C). These results suggested that the T3SS of Y. pestis KIM5 was differentially regulating production of cytokines in infected BMDMs. The T3SS was partially inhibiting a pathway that regulates TNF-α production, likely via YopJ, while simultaneously activating a pathway required for IL-1β and IL-18 secretion.
FIG. 3.
Time course analysis of cytokine secretion from macrophages infected with Y. pestis. BMDMs were left uninfected (white bars) or were infected with either KIM5 (gray bars) or KIM5 yopB (black bars). Supernatants were collected at the indicated time points, and levels of IL-1β (A), IL-18 (B), and TNF-α (C) were quantified by ELISA. Results shown are the averages from three independent experiments. Error bars represent standard deviations.
KIM5 induces caspase-1-independent cell death in infected macrophages.
Previous studies have indicated that pathogenic bacteria such as Salmonella enterica serovar Typhimurium can induce macrophage cell death via a process mediated by caspase-1 and termed pyroptosis (17, 24, 26, 31). Since we obtained evidence that caspase-1 was activated in KIM5-infected macrophages, we wanted to determine whether these BMDMs were dying of pyroptosis. Microscopic analysis of membrane permeability by PI uptake assay (see Materials and Methods) showed that BMDMs infected with KIM5, but not KIM5 yopB, had compromised plasma membranes and that the percentage of KIM5-infected macrophages that were PI positive increased with time (data not shown). Supernatants from infected BMDMs were tested for LDH, a marker of cell lysis, to quantify cell death (see Materials and Methods). A time course analysis revealed that BMDMs infected with KIM5, but not KIM5 yopB, were dying as measured by LDH release (Fig. 4A). A marked increase in LDH in the supernatant of KIM5-infected macrophages was first detected at 8 h postinfection, and approximately 40% of macrophages underwent cell lysis by 24 h (Fig. 4A). To determine whether caspase-1 activity was required for cell death, macrophages were pretreated with the caspase-1 inhibitor YVAD and tested for both IL-1β secretion and LDH release. Surprisingly, under conditions in which pretreatment with 100 μM of YVAD significantly reduced secretion of IL-1β (Fig. 2A), LDH release from KIM5-infected macrophages was not significantly reduced (Fig. 4B). Additionally, LDH release was significantly lower in macrophages infected with CO92 Δpgm than in macrophages infected with either KIM5 or KIM-D27 (Fig. 4C). Thus, although Y. pestis KIM strains induced YopB-dependent caspase-1 activation and cell death in infected BMDMs, caspase-1 activity was not required for cell death. Therefore, KIM-infected macrophages did not appear to be dying of pyroptosis (16).
Identification of a region of pCD1 that is required for caspase-1 activation, IL-1β secretion, and LDH release from KIM5-infected macrophages.
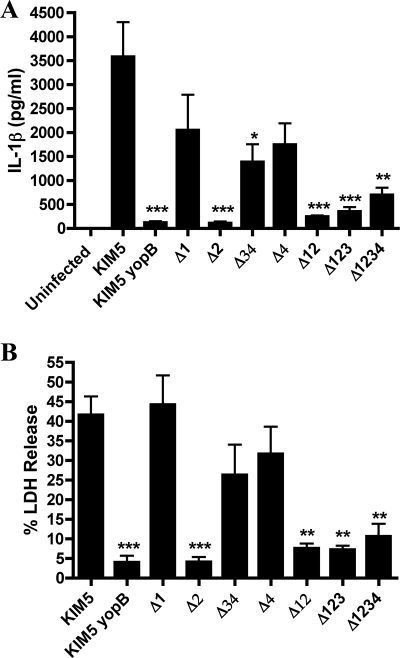
To determine whether one of the Yop effectors encoded on pCD1 was essential for inducing IL-1β secretion and LDH release from infected BMDMs, we obtained KIM8-derived strains that contained various deletions comprising ∼10-kb regions of pCD1, as listed in Tables 1 and 2 (1). The deletions were designed to remove genes encoding the six effector Yops, some of the chaperones, and all uncharacterized reading frames and transposable elements (1). In general, the deletions did not remove genes required for expression or assembly of the T3SS or for effector translocation, except for the deletion termed Δ34, in which sycH was deleted. SycH has been shown to play a role in T3SS expression (8). The KIM8-derived strains harboring the mutant pCD1 plasmids were tested along with KIM5 and KIM5 yopB in BMDM infection assays, and the results are shown in Fig. 5. All mutants containing the region 2 deletion (Δ2, Δ12, Δ123, and Δ1234), as well as the mutant with the region 3 and 4 deletion (Δ34), stimulated significantly lower levels of IL-1β secretion from infected BMDMs (Fig. 5A). Strains with deletions of region 1 (Δ1) or 4 (Δ4) showed a partial, but not statistically significant, reduction (∼2-fold) in IL-1β secretion-stimulating activity (Fig. 5A). Levels of LDH released from macrophages infected with the different strains were similar in trend to those seen for IL-1β secretion and pointed to a critical role for region 2 (Fig. 5B). A PI uptake assay confirmed that the KIM8 mutant missing region 2 (Δ2 mutant) did not permeabilize the plasma membrane of infected macrophages (data not shown). Furthermore, a factor encoded in region 2 was required for activation of caspase-1 activity, as shown by staining with FAM-YVAD-FMK (Fig. 6). BMDMs infected with KIM5 or the Δ2 mutant were incubated with FAM-YVAD-FMK and then washed, fixed and stained with anti-Yersinia antibody to detect macrophage-associated bacteria. Microscopic analysis showed the FAM-YVAD-FMK fluorescent signal associated with a percentage of KIM5-infected macrophages at 8 h (Fig. 6). Macrophages infected with the KIM8 Δ2 mutant showed only background staining with FAM-YVAD-FMK at either time point (Fig. 6). From these results we concluded that a factor(s) encoded in region 2 is essential for inducing activation of caspase-1 and cell death in KIM5-infected BMDMs. The apparent decrease in IL-1β secretion-stimulating activity observed for the Δ34 mutant (Fig. 5A) might be due to the absence of SycH in this strain, since deletion of sycH results in a reduction in the secretion of TTSS effectors (8).
FIG. 5.
Identification of a region of pCD1 required for IL-1β secretion and cell death in macrophages infected with Y. pestis. BMDMs were left uninfected or were infected with KIM5, KIM5 yopB, or the indicated KIM8-derived strains (Tables 1 and 2). Supernatants were collected at 24 h postinfection and analyzed for secreted IL-1β by ELISA (A) and for cell death by LDH release (B). Results shown are the averages from three independent experiments. Error bars represent standard deviations. Statistical significance (P < 0.05, *; P < 0.01, **; P < 0.001, ***) compared to KIM5 was determined.
Phagocytosis of KIM5 by macrophages is not required for IL-1β secretion or LDH release.
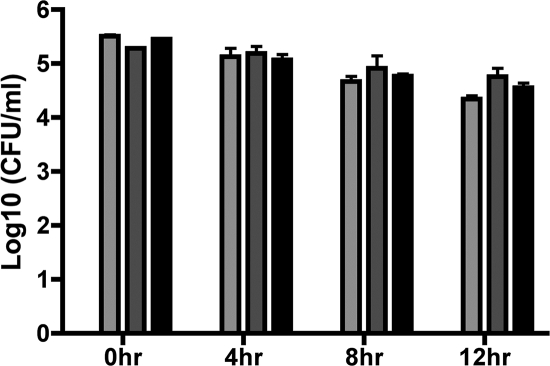
Two different assays were employed to investigate the role of bacterial internalization and intracellular survival in the response of BMDMs to Y. pestis KIM infection. First, a CFU assay was utilized to determine the number of KIM5, KIM5 yopB, and KIM8 Δ2 mutant bacteria associated with, and surviving within, macrophages at various time points (see Materials and Methods). The 0-h time point revealed the total number of viable bacteria associated with the BMDMs prior to application of gentamicin-containing medium. As shown in Fig. 7, similar numbers of KIM5, KIM5 yopB, and Δ2 mutant were associated with BMDMs at 0 h. The later time points reflected the abilities of the different strains to survive within the BMDMs, and as shown in Fig. 7, the numbers of viable intracellular bacteria remained constant over time for KIM5, the yopB mutant, and the Δ2 mutant and overall decreased only slightly. These results suggest that differences in bacterial association with macrophages or intracellular survival of Y. pestis were unlikely to explain the difference between KIM5 and the Δ2 mutant with respect to their ability to activate caspase-1 or induce macrophage cell death.
FIG. 7.
Analysis of bacterial association and intracellular survival in macrophages infected with Y. pestis. BMDMs were infected for 20 min with KIM5 (light gray bars), KIM5 yopB (dark gray bars), or the KIM8 Δ2 mutant (black bars). At the end of the 20-min infection period, some of the infected macrophages were washed and lysed, and serial dilutions of the lysates were plated for CFU determination (0-h time point). The infected macrophages remaining were exposed to gentamicin (see Materials and Methods), and at the time points indicated the BMDMs were processed for CFU determination as described above. Results shown are the averages from three independent experiments. Error bars represent standard deviations.
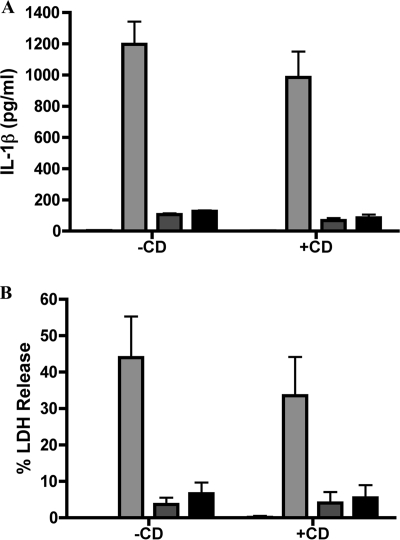
BMDMs were treated with CD, which blocks phagocytosis by inhibiting actin polymerization, to determine whether bacterial internalization was required for caspase-1 activation and cell death. Macrophages were left untreated or treated with CD as described in Materials and Methods and then infected, and ELISA was performed on supernatants collected after 24 h. Macrophages left untreated or treated with CD and infected with KIM5 showed no significant difference in IL-1β secretion (Fig. 8A) or LDH release (Fig. 8B). In addition, treatment of macrophages with CD did not alter the levels of IL-1β secretion or LDH release following infection with KIM5 yopB or the Δ2 mutant. To verify that CD was reducing bacterial internalization, a microscopic assay that measures levels of bacterial internalization was employed (see Materials and Methods). Macrophages pretreated or not with CD were infected with KIM5/GFP (Table 1). After 20 min, the macrophages were fixed and processed for immunofluorescence microscopy, and the percentage of intracellular bacteria was determined. We observed that approximately 70% of KIM5/GFP bacteria were intracellular in samples of untreated macrophages, while fewer than 1% of bacteria were intracellular in samples of CD-treated macrophages (data not shown). These results suggested that the factor(s) required for stimulating caspase-1 activation and cell death could be translocated into BMDMs by extracellular bacteria.
FIG. 8.
Determination of the role of bacterial phagocytosis in IL-1β secretion and cell death in macrophages infected with Y. pestis. BMDMs were left untreated (−CD) or treated with CD (+CD) and infected with KIM5 (light gray bars), KIM5 yopB (dark gray bars), or the KIM8 Δ2 mutant (black bars). At 24 hours postinfection, supernatants were collected and analyzed for secreted IL-1β by ELISA (A) or released LDH (B). Treatment of macrophages with DMSO as a solvent control under identical conditions used for CD exposure did not affect IL-1β secretion or LDH release (data not shown). Results shown are the averages from three independent experiments. Error bars represent standard deviations.
The activity of YopJ is required for caspase-1 activation and cell death in KIM5-infected macrophages.
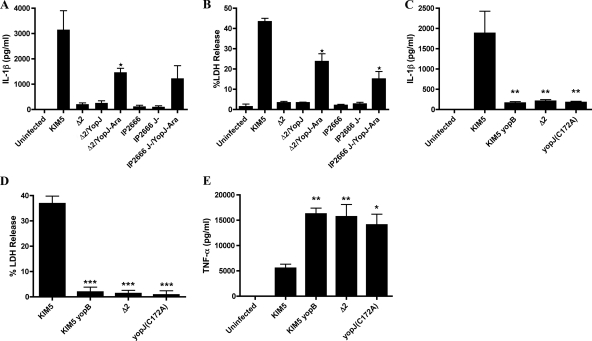
To determine whether any of the known effectors encoded in region 2 of pCD1 (yopH, ypkA, and yopJ) were required for caspase-1 activation and cell death, KIM5 strains with mutations in these genes were constructed (Table 1). There was no significant difference in the levels of IL-1β secreted or LDH released from BMDMs infected with yopH or ypkA mutants compared to KIM5 (data not shown). To determine whether YopJ was important for stimulating IL-1β secretion and LDH release from infected macrophages, a plasmid (pYopJ-GSK) (19) that expresses the KIM yopJ ORF under control of an arabinose-inducible promoter was used to transform KIM8 Δ2. When the resulting strain (Δ2/YopJ) was used to infect BMDMs, a significant increase in IL-1β secretion and LDH release from infected macrophages was observed in the presence of arabinose (Fig. 9A and B, respectively). The levels of IL-1β secreted and LDH released from macrophages infected with Δ2/YopJ in the presence of arabinose were lower than those observed in KIM5-infected BMDMs (Fig. 9A and B), suggesting that only partial complementation of phenotypes was obtained.
FIG. 9.
Determination of the role of YopJ in IL-1β secretion, TNF-α secretion, and cell death in macrophages infected with Y. pestis or Y. pseudotuberculosis. BMDMs were left uninfected or infected with KIM5, KIM5 yopB, KIM5 yopJC172A, KIM8 Δ2 IP2666, or IP2666 J−. Where indicated, KIM8 Δ2 and IP2666 J− carried the pYopJ-GSK plasmid (YopJ) and were cultured in the presence of arabinose (Ara). Supernatants were collected at 24 h postinfection and tested for IL-1β by ELISA (A and C), for cell death by LDH release (B and D), or for TNF-α by ELISA (E). Results shown are the averages from three independent experiments. Error bars represent standard deviation. Statistical significance (P < 0.05, *; P < 0.01, **; P < 0.001, ***) compared to KIM5 was determined (C, D, and E). In panel A, P < 0.05 (*), comparing Δ2 and Δ2/YopJ-Ara. In panel B, P < 0.05 (*), comparing Δ2 and Δ2/YopJ-Ara or comparing IP2666 and IP2666 J−/YopJ-Ara.
To determine whether the KIM YopJ protein is sufficient to stimulate IL-1β secretion and LDH release from infected macrophages, the pYopJ-GSK plasmid was used to transform the Y. pseudotuberculosis yopJ mutant IP2666 J−. The resulting strain (IP2666 J−/YopJ) exhibited an increased ability to stimulate IL-1β secretion and LDH release from infected macrophages when cultured in the presence of arabinose (Fig. 9A and B, respectively).
A requirement for YopJ catalytic activity in the elicitation of cell death and caspase-1 activation was examined by constructing a mutant of KIM5 that expresses enzymatically inactive YopJ (KIM5 yopJC172A). KIM5 yopJC172A was as defective as the yopB mutant or the Δ2 mutant for stimulating IL-1β secretion and LDH release from infected macrophages (Fig. 9C and D, respectively). KIM5 yopJC172A was also defective for inhibiting TNF-α secretion from infected BMDMs, as expected from previous results obtained with Y. pestis EV76 (Fig. 9E) (56). Taken together, our results demonstrate a novel role for the YopJ protein of Y. pestis KIM strains in activation of caspase-1 in macrophages.
Caspase-1-deficient macrophages have a reduced ability to secrete IL-1β during infection with KIM5 but undergo cell death at the same rate as wild-type macrophages.
We have shown that pretreatment with YVAD significantly reduced the amount of IL-1β secreted from macrophages infected with KIM5 (Fig. 2A), suggesting that caspase-1 is important for maximal secretion of this cytokine. To verify these results, we infected BMDMs deficient for caspase-1 (Casp-1−/−) with KIM5 or KIM5 yopJC172A for 4, 8, or 24 h and measured IL-1β secretion and LDH release. There was a significant decrease (∼2-fold) in IL-1β secretion at 24 h postinfection in the Casp-1−/− macrophages infected with KIM5 compared to Casp-1+/+ C57BL/6 BMDMs (Fig. 10A). In contrast, there was no significant difference in the rate of cell death in Casp-1−/− and Casp-1+/+ macrophages infected with KIM5 (Fig. 10B), confirming that although caspase-1 is important for maximal secretion of IL-1β, it is not required for death of BMDMs infected with Y. pestis KIM.
DISCUSSION
This study was undertaken to determine whether the T3SS and Yops of Y. pestis and Y. pseudotuberculosis inhibit caspase-1 activation and IL-1β secretion during infection of naïve macrophages. We unexpectedly found that Y. pestis KIM-derived strains stimulate infected murine BMDMs to secrete high concentrations of IL-1β. This phenotype was not seen in Y. pestis CO92 Δpgm or the Y. pseudotuberculosis strains IP2777 and IP2666 (Fig. 1), suggesting that KIM isolates differ genetically from these other Yersinia strains. Caspase-1 was activated in macrophages infected with KIM5 as shown by microscopic analysis of infected cells incubated with the fluorescent caspase-1 inhibitor Fam-YVAD-fmk (Fig. 6). IL-1β secretion from macrophages infected with KIM5 was significantly reduced when caspase-1 was inhibited (Fig. 2A) or absent (Fig. 10A) in BMDMs. KIM5 also stimulated a higher level of cell death in infected macrophages compared to CO92 Δpgm (Fig. 4C). However, cell death in KIM5-infected BMDMs did not require caspase-1 activity, since release of LDH was not significantly decreased in the presence of YVAD (Fig. 4B) or in the absence of caspase-1 (Fig. 10B). These results argued that although caspase-1 was being activated in KIM5-infected macrophages, the BMDMs were not dying of pyroptosis. Analysis of a KIM5 yopB mutant suggested that translocation of a T3SS effector into the macrophage cell was required for caspase-1 activation and cell death (Fig. 2B). Inhibition of actin polymerization with CD can suppress the induction of caspase-1 activation and cell death in macrophages infected with Salmonella and Francisella species (17, 29, 31). However, we found that caspase-1 activation and cell death in macrophages infected with KIM5 was not inhibited by CD treatment (Fig. 8). This result suggested that caspase-1 activation and cell death could result from the translocation of a T3SS effector by extracellular Y. pestis. Finally, analysis of Y. pestis KIM-derived mutants defective for expression of functional Yop effectors indicated that cell death and caspase-1 activation required T3SS-mediated delivery of active YopJ into macrophages (Fig. 5 and 9). All together, these results indicated that macrophages infected with Y. pestis KIM strains undergo a YopJ-dependent form of cell death that is coupled to activation of caspase-1. Although we had set out with the goal of learning how Yop effectors of Yersinia species inhibit activation of caspase-1, we instead discovered that the YopJ protein of Y. pestis KIM strains is required for activation of this proinflammatory caspase.
The mechanism of caspase-1 activation in BMDMs infected with Y. pestis KIM appears to be different from what has previously been observed by other groups studying Yersinia-infected macrophages (2, 47, 49). Bergsbaken et al. (2) found that caspase-1 was activated in BMDMs infected with wild-type Y. pseudotuberculosis YPIII, but only when the macrophages were pretreated with LPS, and that YopJ was dispensable for activation of caspase-1 under their infection conditions. In contrast, YopJ activity was required for caspase-1 activation (Fig. 9), and IL-1β secretion decreased when BMDMs were pretreated with LPS (Fig. 2C) in KIM5-infected macrophages. In Y. enterocolitica E40-infected Mf4/4 cells, activation of caspase-1 was observed only in strains deficient for production of YopE and YopT (47, 49), because the activities of these effectors normally prevent the formation of pores (51) that can apparently stimulate the inflammasome to activate caspase-1 (49). However, we found that activation of caspase-1 in KIM-infected BMDMs did not require the absence of YopE and YopT, and in fact the absence of these effectors appeared to result in decreased IL-1β secretion by macrophages (Fig. 5 [the Δ1 mutant is missing yopT and the Δ4 mutant is missing yopE]). Genetic differences that exist between the strains used in the studies discussed above and in our study may partially explain the different results that have been observed with respect to mechanisms of caspase-1 activation in Yersinia-infected macrophages. In addition, differences in experimental variables such as type of macrophage (primary, cell line, or activated versus nonactivated) and MOI may result in different conclusions being reached as to the role of Yops in modulating caspase-1 activation. For example, activation of caspase-1 as a result of T3SS-dependent pore formation requires a high MOI (≥50) and extended time of contact (≥2 h) between live extracellular bacteria and macrophages (49). We used a relatively low MOI (10) and a short period of contact with live extracellular bacteria (20 min), and as a result activation of caspase-1 via pore formation was minimal, as shown by the low levels of IL-1β secreted from naïve or LPS-stimulated BMDMs infected with multieffector mutants of Y. pseudotuberculosis IP2666 (Fig. 1C) or Y. pestis KIM8 (Fig. 5A).
Previous studies have shown that Y. pestis strains are limited in their ability to induce YopJ-dependent apoptosis in macrophages, unless high MOIs are used and the bacteria are centrifuged onto the host cells to force contact (53, 56). Similar to what has been reported for EV76 and Kimberly53 (56), CO92 Δpgm was limited in its ability to kill macrophages under our infection conditions (Fig. 4D). In contrast, O:8 serogroup strains of Y. enterocolitica, such as WA-314, have been shown to induce high levels of YopP-dependent apoptosis in macrophages at low MOI (45, 56). The limited ability of some Y. pestis strains to induce macrophage apoptosis, compared to WA-314, has been correlated with decreased translocation of Y. pestis YopJ compared to YopP of WA-314 (56). As discussed further below, it is possible that the YopJ protein of KIM has unique features or activities that result in high levels of cell death in macrophages infected at low MOI. We hypothesize that Y. pestis KIM strains induce a novel form of YopJ-dependent cell death in macrophages, which is coupled to activation of caspase-1. A previous study reported that murine bone marrow-derived dendritic cells infected with Y. enterocolitica strain WA-314 died of a necrotic form of cell death that required YopP activity (22). Although activation of caspase-1 or secretion of IL-1β was not investigated in that study, dendritic cells infected with Y. enterocolitica WA-314 released HMGB1 (22), which is a potent proinflammatory molecule (60). Thus, there is precedence for the idea that Yersinia infection can stimulate YopJ/P-dependent proinflammatory host cell death, although our study is the first to demonstrate YopJ-dependent activation of caspase-1 in infected host cells.
The ability of different Y. enterocolitica strains to effectively induce apoptosis in infected macrophages has been linked to variations at position 143 of the YopP sequence (45). Serogroup O8 strains such as WA-314 that effectively induce apoptosis in infected macrophages contain an Arg at position 143 (45). Y. enterocolitica strains that express YopP proteins containing the Arg at position 143 also strongly inhibit activation of NF-κB in macrophages (45). However, the YopJ proteins encoded by KIM, CO92, and Y. pseudotuberculosis all contain an Arg at position 143 (45), so variations at this residue are not responsible for the phenotypic differences seen in the present study. The sequence of the KIM YopJ protein (NP_857908) differs by two amino acids from the sequence of CO92 YopJ (NP_395205.1), corresponding to L177F and E206K substitutions. The sequence of the KIM YopJ protein differs by one amino acid from the sequence of Y. pseudotuberculosis IP32953 YopJ (NP_395205.1), corresponding to an L177F substitution. The amino acid differences that exist between the YopJ proteins of KIM, CO92, and Y. pseudotuberculosis are likely responsible for the phenotypic differences observed in this study, and in the future it will be important to determine how these substitutions alter YopJ protein function. Several possibilities exist, including differences in translocation efficiency, protein stability, or substrate specificity. In addition, we note that the sequence of a YopJ protein from Y. pestis Medievalis strain K1973002 (ZP_02318615) is identical to the sequence of YopJ from KIM, suggesting that the phenotype we are observing is not an artifact resulting from a mutation acquired during laboratory passage but rather is due to a unique yopJ genotype associated with Medievalis strains.
Another key issue that remains to be addressed is the connection between cell death and caspase-1 activation in macrophages infected with Y. pestis KIM. Caspase-1 activity is not required for cell death (Fig. 4C and 10B), which indicates that caspase-1 activation is a downstream effect of cell death or that separate pathways regulate cell death and caspase-1 activation. The kinetics of IL-1β/IL-18 secretion and LDH release from KIM5-infected macrophages was similar (compare Fig. 3AB with 4A), which is suggestive of a mechanistic connection between the two processes. LPS activation of macrophages decreases YopP-dependent apoptosis in response to Y. enterocolitica infection (44), and we observed that LPS pretreatment decreased secretion of IL-1β from KIM5-infected BMDMs (Fig. 2C), which is an indication that caspase-1 activation occurs downstream of the cell death program. One possibility is that YopJ-mediated inhibition of NF-κB activation in KIM5-infected macrophages triggers cell death and caspase-1 activation. Greten et al. have shown that gene products under control of NF-κB negatively regulate caspase-1 activation in macrophages and that inhibition of NF-κB activation before stimulating macrophages with LPS results in enhanced secretion of IL-1β (21). It may seem counterintuitive that strong inhibition of NF-κB activation in KIM5-infected macrophages could result in enhanced secretion of mature IL-1β, since expression of the pro form of IL-1β is positively regulated by NF-κB. In fact, Schotte et al. (47) reported that YopP inhibits expression of the pro form of IL-1β in Mf4/4 cells infected with Y. enterocolitica E40. We observed inhibition of TNF-α secretion from BMDMs infected with KIM5 (Fig. 9E), indicating that the YopJ protein of KIM is reducing expression of NF-κB target genes. However, we note that macrophages infected with KIM5 secrete more TNF-α than uninfected BMDMs (Fig. 9E), which suggests that activation of NF-κB and expression of NF-κB target genes was occurring at a low level in even in the presence of YopJKIM. Low levels of activated NF-κB may be sufficient for small amounts of pro-IL-1β to be made but not sufficient to prevent activation of caspase-1, resulting in measurable secretion of the mature form of IL-1β.
Greten et al. proposed that activation of caspase-1 in response to inhibition of NF-κB represents a mechanism of host defense against pathogens that target this transcription factor (21). It is possible that the differential ability of Y. pestis KIM and CO92 strains to activate caspase-1 in BMDMs is a consequence of a differential ability of these strains to inhibit, via YopJ, activation of NF-κB. Caspase-1 is known to play a protective role against several pathogens, including Salmonella and Francisella species, in murine infection models (26, 28, 42). If KIM and CO92 strains differentially activate caspase-1 in vivo, it could manifest in a virulence difference between these strains. This will be an important issue to address in future studies.
Acknowledgments
We thank Gregory Plano for the KIM8 deletion strains and the pYopJ-GSK plasmid, Hana Fukuto for construction of KIM5, Kathryn Klein for construction of KIM5 yopB, Selina Myrczek for constructing the KIM5 ypkA and yopH mutants, Yue Zhang and Shirou Wu for construction of the KIM5 yopJC172A mutant, Céline Pujol for construction of CO92 Δpgm, Stephen Smiley for the gift of KIM-D27, Craig Roy and Richard Flavell for Casp-1−/− mice, and Galina Romanov for preparation of BMDMs. We thank Céline Pujol and Timothy LaRocca for making the initial observation that KIM5 has an unusually high cytotoxic activity for BMDMs. We also thank Hana Fukuto, Céline Pujol, Kathryn Klein, Joseph McPhee, Gloria Viboud, and Yue Zhang for constructive comments on the manuscript.
This work was supported by Public Health Service grants AI043389 and AI055621 to J.B.B.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Bartra, S. S., M. W. Jackson, J. A. Ross, and G. V. Plano. 2006. Calcium-regulated type III secretion of Yop proteins by an Escherichia coli hha mutant carrying a Yersinia pestis pCD1 virulence plasmid. Infect. Immun. 741381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsbaken, T., and B. T. Cookson. 2007. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 3e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37515-527. [DOI] [PubMed] [Google Scholar]
- 4.Bliska, J. B. 2006. Yersinia inhibits host signaling by acetylating MAPK kinases. ACS Chem. Biol. 1349-351. [DOI] [PubMed] [Google Scholar]
- 5.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 713673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, K., F. Martinon, and J. Tschopp. 2003. New insights into the mechanism of IL-1beta maturation. Curr. Opin. Immunol. 1526-30. [DOI] [PubMed] [Google Scholar]
- 8.Cambronne, E. D., L. W. Cheng, and O. Schneewind. 2000. LcrQ/YscM1, regulators of the Yersinia yop virulon, are injected into host cells by a chaperone-dependent mechanism. Mol. Microbiol. 37263-273. [DOI] [PubMed] [Google Scholar]
- 9.Celada, A., P. W. Gray, E. Rinderknecht, and R. D. Schreiber. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 16055-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 978778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 621315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creagh, E. M., H. Conroy, and S. J. Martin. 2003. Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 19310-21. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello, C. A. 2006. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 83447S-455S. [DOI] [PubMed] [Google Scholar]
- 15.Ferrero-Miliani, L., O. H. Nielsen, P. S. Andersen, and S. E. Girardin. 2007. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 147227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink, S. L., and B. T. Cookson. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 731907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fink, S. L., and B. T. Cookson. 2006. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 81812-1825. [DOI] [PubMed] [Google Scholar]
- 18.Galan, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444567-573. [DOI] [PubMed] [Google Scholar]
- 19.Garcia, J. T., F. Ferracci, M. W. Jackson, S. S. Joseph, I. Pattis, L. R. Plano, W. Fischer, and G. V. Plano. 2006. Measurement of effector protein injection by type III and type IV secretion systems by using a 13-residue phosphorylatable glycogen synthase kinase tag. Infect. Immun. 745645-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabenstein, J. P., H. S. Fukuto, L. E. Palmer, and J. B. Bliska. 2006. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect. Immun. 743727-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greten, F. R., M. C. Arkan, J. Bollrath, L. C. Hsu, J. Goode, C. Miething, S. I. Goktuna, M. Neuenhahn, J. Fierer, S. Paxian, N. Van Rooijen, Y. Xu, T. O'Cain, B. B. Jaffee, D. H. Busch, J. Duyster, R. M. Schmid, L. Eckmann, and M. Karin. 2007. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130918-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobner, S., S. E. Autenrieth, I. Soldanova, D. S. Gunst, M. Schaller, E. Bohn, S. Muller, M. Leverkus, S. Wesselborg, I. B. Autenrieth, and S. Borgmann. 2006. Yersinia YopP-induced apoptotic cell death in murine dendritic cells is partially independent from action of caspases and exhibits necrosis-like features. Apoptosis 111959-1968. [DOI] [PubMed] [Google Scholar]
- 23.Heesemann, J., A. Sing, and K. Trulzsch. 2006. Yersinia's stratagem: targeting innate and adaptive immune defense. Curr. Opin. Microbiol. 955-61. [DOI] [PubMed] [Google Scholar]
- 24.Jarvelainen, H. A., A. Galmiche, and A. Zychlinsky. 2003. Caspase-1 activation by Salmonella. Trends Cell. Biol. 13204-209. [DOI] [PubMed] [Google Scholar]
- 25.Lamkanfi, M., T. D. Kanneganti, L. Franchi, and G. Nunez. 2007. Caspase-1 inflammasomes in infection and inflammation. J. Leukoc. Biol. 82220-225. [DOI] [PubMed] [Google Scholar]
- 26.Lara-Tejero, M., F. S. Sutterwala, Y. Ogura, E. P. Grant, J. Bertin, A. J. Coyle, R. A. Flavell, and J. E. Galan. 2006. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 2031407-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariathasan, S. 2007. ASC, Ipaf and cryopyrin/Nalp3: bona fide intracellular adapters of the caspase-1 inflammasome. Microbes Infect. 9664-671. [DOI] [PubMed] [Google Scholar]
- 28.Mariathasan, S., and D. M. Monack. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 731-40. [DOI] [PubMed] [Google Scholar]
- 29.Mariathasan, S., D. S. Weiss, V. M. Dixit, and D. M. Monack. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 2021043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinon, F., and J. Tschopp. 2004. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117561-574. [DOI] [PubMed] [Google Scholar]
- 31.Monack, D. M., W. W. Navarre, and S. Falkow. 2001. Salmonella-induced macrophage death: the role of caspase-1 in death and inflammation. Microbes Infect. 31201-1212. [DOI] [PubMed] [Google Scholar]
- 32.Navarro, L., N. M. Alto, and J. E. Dixon. 2005. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 821-27. [DOI] [PubMed] [Google Scholar]
- 33.Ogura, Y., F. S. Sutterwala, and R. A. Flavell. 2006. The inflammasome: first line of the immune response to cell stress. Cell 126659-662. [DOI] [PubMed] [Google Scholar]
- 34.Orth, K. 2002. Function of the Yersinia effector YopJ. Curr. Opin. Microbiol. 538-43. [DOI] [PubMed] [Google Scholar]
- 35.Orth, K., Z. Xu, M. B. Mudgett, Z. Q. Bao, L. E. Palmer, J. B. Bliska, W. F. Mangel, B. Staskawicz, and J. E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 2901594-1597. [DOI] [PubMed] [Google Scholar]
- 36.Oyston, P. C., N. Dorrell, K. Williams, S. R. Li, M. Green, R. W. Titball, and B. W. Wren. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 683419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27953-965. [DOI] [PubMed] [Google Scholar]
- 38.Parent, M. A., L. B. Wilhelm, L. W. Kummer, F. M. Szaba, I. K. Mullarky, and S. T. Smiley. 2006. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect. Immun. 743381-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis: etiologic agent of plague. Clin. Microbiol. Rev. 1035-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pujol, C., and J. B. Bliska. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 715892-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramamurthi, K. S., and O. Schneewind. 2002. Type III protein secretion in Yersinia species. Annu. Rev. Cell Dev. Biol. 18107-133. [DOI] [PubMed] [Google Scholar]
- 42.Raupach, B., S. K. Peuschel, D. M. Monack, and A. Zychlinsky. 2006. Caspase-1-mediated activation of interleukin-1β (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 744922-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruckdeschel, K. 2002. Immunomodulation of macrophages by pathogenic Yersinia species. Arch. Immunol. Ther. Exp. (Warsz) 50131-137. [PubMed] [Google Scholar]
- 44.Ruckdeschel, K., and K. Richter. 2002. Lipopolysaccharide desensitization of macrophages provides protection against Yersinia enterocolitica-induced apoptosis. Infect. Immun. 705259-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruckdeschel, K., K. Richter, O. Mannel, and J. Heesemann. 2001. Arginine-143 of Yersinia enterocolitica YopP crucially determines isotype-related NF-κB suppression and apoptosis induction in macrophages. Infect. Immun. 697652-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Runco, L. M., S. Myrczek, J. B. Bliska, and D. G. Thanassi. 2008. Biogenesis of the fraction 1 capsule and analysis of the ultrastructure of Yersinia pestis. J. Bacteriol. 1903381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schotte, P., G. Denecker, A. Van Den Broeke, P. Vandenabeele, G. R. Cornelis, and R. Beyaert. 2004. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1beta. J. Biol. Chem. 27925134-25142. [DOI] [PubMed] [Google Scholar]
- 48.Scott, A. M., and M. Saleh. 2007. The inflammatory caspases: guardians against infections and sepsis. Cell. Death Differ. 1423-31. [DOI] [PubMed] [Google Scholar]
- 49.Shin, H., and G. R. Cornelis. 2007. Type III secretion translocation pores of Yersinia enterocolitica trigger maturation and release of pro-inflammatory IL-1beta. Cell. Microbiol. 92893-2902. [DOI] [PubMed] [Google Scholar]
- 50.Simonet, M., and S. Falkow. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 604414-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 5969-89. [DOI] [PubMed] [Google Scholar]
- 52.Viboud, G. I., S. S. So, M. B. Ryndak, and J. B. Bliska. 2003. Proinflammatory signalling stimulated by the type III translocation factor YopB is counteracted by multiple effectors in epithelial cells infected with Yersinia pseudotuberculosis. Mol. Microbiol. 471305-1315. [DOI] [PubMed] [Google Scholar]
- 53.Weeks, S., J. Hill, A. Friedlander, and S. Welkos. 2002. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb. Pathog. 32227-237. [DOI] [PubMed] [Google Scholar]
- 54.Wren, B. W. 2003. The yersiniae—a model genus to study the rapid evolution of bacterial pathogens. Nat. Rev. Microbiol. 155-64. [DOI] [PubMed] [Google Scholar]
- 55.Zamboni, D. S., K. S. Kobayashi, T. Kohlsdorf, Y. Ogura, E. M. Long, R. E. Vance, K. Kuida, S. Mariathasan, V. M. Dixit, R. A. Flavell, W. F. Dietrich, and C. R. Roy. 2006. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7318-325. [DOI] [PubMed] [Google Scholar]
- 56.Zauberman, A., S. Cohen, E. Mamroud, Y. Flashner, A. Tidhar, R. Ber, E. Elhanany, A. Shafferman, and B. Velan. 2006. Interaction of Yersinia pestis with macrophages: limitations in YopJ-dependent apoptosis. Infect. Immun. 743239-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, Y., and J. B. Bliska. 2005. Role of macrophage apoptosis in the pathogenesis of Yersinia. Curr. Top. Microbiol. Immunol. 289151-173. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, Y., A. T. Ting, K. B. Marcu, and J. B. Bliska. 2005. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J. Immunol. 1747939-7949. [DOI] [PubMed] [Google Scholar]
- 59.Zhou, H., D. M. Monack, N. Kayagaki, I. Wertz, J. Yin, B. Wolf, and V. M. Dixit. 2005. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappa B activation. J. Exp. Med. 2021327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zong, W. X., and C. B. Thompson. 2006. Necrotic death as a cell fate. Genes Dev. 201-15. [DOI] [PubMed] [Google Scholar]