Abstract
Streptococcus pneumoniae is a major cause of otitis media, pneumonia, meningitis, and septicemia in humans. The host defense against this pathogen largely depends on bacterial killing by neutrophils. A peculiar property of pneumococci is their tendency to undergo autolysis, i.e., autoinduced disruption of the bacterial cell wall mediated by activation of the enzyme LytA, under stationary growth conditions. LytA is a virulence factor, but the molecular background for this has not been fully clarified. Here we examine how bacterial compounds released upon autolysis affect the production of reactive oxygen species (ROS) in neutrophils. We found that the S. pneumoniae strains A17 and D39 induced activation of the NADPH oxidase and the production of ROS in human neutrophils and that this activation was blocked when LytA was inactivated. The ROS-inducing bacterial substance released from autolyzed bacteria was identified as the cytoplasmic toxin pneumolysin. Further screening of clinical pneumococcal strains of various sero- and genotypes revealed that selected strains expressing toxins with reduced pneumolysin-dependent hemolytic activity had decreased abilities to induce ROS in neutrophils. Furthermore, a mutated form of purified pneumolysin lacking hemolytic and complement binding functions (PdT) did not induce any oxygen radical production. The ROS produced in response to pneumolysin formed mainly intracellularly, which may explain why this production was not detected previously. ROS released intracellularly may function as signaling molecules, modifying the function of neutrophils in bacterial defense.
Streptococcus pneumoniae is a gram-positive bacterium that may be found as a commensal of the human upper respiratory tract. However, depending on host and bacterial factors not fully understood, pneumococci may spread to the middle ear, lung, or bloodstream and cause diseases such as otitis media, pneumonia, meningitis, and septicemia. Its significance as a pathogen can be illustrated by the fact that around 1 million children per year in developing countries die from pneumococcal diseases (47).
S. pneumoniae is regarded as a strictly extracellular pathogen whose elimination depends on ingestion and decomposition by phagocytes. These include alveolar and tissue-resident macrophages, as well as neutrophils recruited during the inflammatory response. Accordingly, an important determinant of pneumococcal pathogenicity is the thick and hydrophilic polysaccharide capsule, which impedes elimination by phagocytes in the absence of capsule-specific antibodies.
Another potent pneumococcal virulence factor is pneumolysin (PLY), which is an intracellular protein that exerts its effects when released to the environment. PLY is toxic to a range of cells and it has in addition various immunomodulatory effects, such as induction of cytokine production and activation of complement (12, 23, 32, 53). Other virulence factors include cell wall components (peptidoglycan), pneumococcal surface proteins A and C, and pneumococcal surface adhesin A (for a review, see reference 27).
A peculiar property of S. pneumoniae is its tendency to spontaneously undergo autolysis when reaching the stationary phase of growth. This is mediated by enzymes called autolysins (ALs), which, when activated, degrade the bacterial cell wall peptidoglycan. The major AL is an N-acetyl-muramyl-l-alanine amidase called LytA (31). Other ALs include LytB, involved mainly in daughter cell separation after cell division (25), and LytC, which has lysozyme activity at 30°C (26). Lysis of pneumococci releases cell wall degradation products and cytoplasmic components, including PLY, which may modulate the inflammatory reaction (8, 15, 16, 57). In several animal studies, LytA has been shown to play a role in virulence (5, 8, 14).
Neutrophils are regarded as the most important cell type for eliminating invading pneumococci. Central to the antibacterial activity of neutrophils is the oxidative burst that generates reactive oxygen species (ROS) through an NADPH oxidase system (55). ROS production starts when the NADPH oxidase components are assembled to a functional multicomponent electron transfer system on the cellular membrane surrounding the cytoplasm or the phagosome. Depending on the membrane onto which NADPH assembles, ROS may be produced intracellularly, extracellularly, or both. ROS production can be induced in response to interaction with microbial pathogen-associated molecular patterns and is also triggered by phagocytosis (24, 55). The crucial role of a functional NADPH system and ROS production in antimicrobial defense is evident from the observation that persons lacking functional NADPH oxidases are prone to bacterial and fungal infections (55). However, reactive oxygen products also damage the host's own cells and tissue components. Indeed, neutrophil-generated oxidants are at least partly responsible for the tissue destruction associated with many microbial infections and inflammatory diseases. A third, more recently discovered biological function of ROS is that they act as intracellular and extracellular signaling molecules, which may regulate the function of immune cells (30, 33, 49), including the induction of apoptosis/necrosis (42, 45).
The pathogenesis of pneumococcal diseases is characterized by extensive cellular and tissue damage. For example, pneumococci, together with beta-hemolytic streptococci, cause the most aggressive form of otitis media and are overrepresented in cases with rupture of the tympanic membranes (our unpublished data). We hypothesized that pneumococcal autolysis, by releasing microbial cell wall and cytosol components, would induce deleterious activation of neutrophils and thereby contribute to tissue damage. Thus, the capacities of pneumococci capable or not capable of undergoing spontaneous autolysis to cause neutrophil activation were determined, and compounds released during spontaneous autolysis causing such activation were identified. Our data show that PLY released from autolyzed bacteria activates the neutrophil NADPH oxidase in a manner targeting the release of ROS to an intracellular compartment. Thus, neutrophils are activated to release oxygen species into vesicular compartments devoid of microbes, possibly accelerating the decay of the neutrophils.
MATERIALS AND METHODS
Bacterial strains.
A list of the strains used in the present study can be found in Table 1. The virulent strain S. pneumoniae D39 (D39 wild type [WT]) (NCTC 7466), its PLY-deficient mutant (D39 PLY−), and its AL-deficient mutant (D39 AL−) have been described previously (8, 11). Five isolates of S. pneumoniae (A17, A6, A7, A22, and A24) were isolated at Lundby Hospital, Göteborg, Sweden, from children with aggressive acute otitis media causing spontaneous rupture of the tympanic membrane, and two pneumococcal strains were from the University of Göteborg culture collection (CCUG 23261 and CCUG 33774). Two clinical strains with low hemolytic activity, 56 (44) and 4496, were isolated at Women's and Children's Hospital, North Adelaide, Australia. Bifidobacterium dentium (CCUG 17360) was used as a control for the induction of oxygen radicals after the addition of choline to the growth medium.
TABLE 1.
Serotype, sequence type, and ROS-inducing abilities of nine clinical S. pneumoniae isolates, D39 WT, and D39 AL- and PLY-deficient mutants
| Strain | Serotype | STa | Reference or source | ROSmax (Mcpm)b |
|---|---|---|---|---|
| D39 WT | 2 | 128 | 59 | 17 ± 5.3 |
| D39 AL− | 2 | 128 | 8 | 3.2 ± 0.8 |
| D39 PLY− | 2 | 128 | 11 | 2.1 ± 1.4 |
| A17 | 14 | 124 | Otitis media patient | 20 ± 4.8 |
| A6 | 19F | ND | Otitis media patient | 13 ± 3.3 |
| A7 | 9V | ND | Otitis media patient | 11 ± 1.3 |
| A22 | 6A | 481 | Otitis media patient | 16 ± 5.8 |
| A24 | 14 | ND | Otitis media patient | 19 ± 4.0 |
| 23261 | 11A | 62 | CCUG | 17 ± 2.8 |
| 33774 | 5 | ND | CCUG | 12 ± 1.1 |
| 56 | 8 | ND | 44 | 1.8 ± 1.3 |
| 4496 | 1 | 3018 | Women's and Children's Hospital, North Adelaide | 4.0 ± 2.0 |
ST, sequence type. ND, not determined.
Values are means ± standard deviations (three to five samples per group) for peak values of ROS produced by neutrophils in response to 50 μl of bacterial suspensions. (The suspensions contained 7 × 108 bacteria/ml before autolysis.)
The pneumococcal isolates were serotyped by standard methods. Selected strains were also analyzed by multilocus sequence typing (MLST). MLST was performed as previously described (21). In brief, internal fragments of the aroE, gdh, gki, recP, spi, xpt, and ddl genes were amplified by PCR directly from the bacteria by use of the primer pairs indicated at the website http://spneumoniae.mlst.net/misc/info.asp#experimental. Sequences were obtained for both DNA strands by use of an ABI 3730xl DNA analyzer. Alleles from the MLST website (http://spneumoniae.mlst.net) were downloaded for alignment analyses and sequence type determination.
Culture and preparation of bacteria.
Bacteria were cultured for 12 to 14 h in Todd-Hewitt broth supplemented with 0.5% yeast extract in the presence or absence of 2% choline chloride (Substrate Department, Clinical Bacteriology Laboratory, Sahlgrenska University Hospital). Choline binds to the AL LytA, preventing its anchoring to choline residues within the cell wall. Thus, pneumococci that are cultivated with choline do not autolyze (13, 29). After growth to late log phase, the bacteria were washed twice and suspended in Dulbecco's endotoxin-free phosphate-buffered saline (PBS) (PAA Laboratories, Linz, Austria) with or without 1% sterile filtered choline chloride. The optical density at 580 nm was adjusted to 0.87, corresponding to 7 × 108 bacteria/ml, as determined by counting in the microscope. The bacteria were inactivated by exposure to UV light for 18 min, and inactivation was confirmed by a negative viable count. The UV-killed bacteria were frozen at −70°C until being used in the experiments.
The thawed bacterial preparations were analyzed for remaining intact bacteria by determination of optical density and examination in the microscope. The LytA-negative mutant D39 AL− and the strains grown in the presence of choline were only slightly reduced in numbers (≥90% bacteria remaining intact), whereas AL-positive strains grown in the absence of choline contained only 10 to 20% of the initial numbers of intact bacteria. In all stimulations, the bacterial concentrations used were based on the numbers of bacteria in the original preparation, before autolysis took place. Thus, the AL-positive strains contained approximately one-fifth of this concentration in the form of intact bacteria, while the rest was in the form of fragmented bacteria.
To separate and analyze the factors released by autolysis, bacteria were centrifuged at 13,000 × g for 10 min at 4°C, whereafter the supernatant was collected and the pellet resuspended in an equal volume of PBS. The supernatant was heated to 56°C for 1 h or treated for 1 h at 37°C with trypsin (0.2 to 20 μg/ml; Sigma) or lysozyme (15 mg/ml; Sigma).
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
The preparations of S. pneumoniae (prepared as described above) were centrifuged (13,000 × g, 10 min, 4°C) and the supernatant was collected. In some experiments, the bacterial cells in the pneumococcal preparations were disrupted by glass beads (106 μm and finer; G-4649; Sigma) as described by van de Guchte et al. (58). The cell-free supernatant was collected and the proteins were separated on 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto nitrocellulose membranes. Purified PLY was used as a size marker. The blots were blocked with 5% bovine serum albumin, stained with a 1:5,000 dilution of primary mouse anti-PLY antibody (NCL-SPNm; Novocastra, United Kingdom), and detected with a 1:7,500 dilution of alcalic phosphatase-conjugated anti-mouse secondary antibody (S372B; Promega, Wisconsin). The filters were developed by the Nitro Blue Tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) (Promega) color reaction.
PLY preparations.
Native PLY (59) and PdT, a modified PLY in which three amino acids have been substituted (Asp385 → Asn, Cys428 → Gly, and Trp433 → Phe), leading to the abolition of cytolytic and complement-activating abilities, were purified as described previously (1). The specific activity of purified native PLY was 500,000 hemolytic units (HU) per mg of protein (52). One HU/milliliter lyses 50% of a 1% suspension of human erythrocytes in 30 min at 37°C.
Isolation of neutrophils.
Neutrophils were isolated from blood donor buffy coats (blood bank, Sahlgrenska University Hospital). After dextran sedimentation and density gradient centrifugation (Lymphoprep; Axis-Shield, Norway) for 20 min at 400 × g at room temperature, the neutrophils were washed and remaining red blood cells were lysed in distilled H2O. Finally, the cells were resuspended in Krebs-Ringer phosphate buffer (KRG, pH 7.3) containing glucose (10 mM), Ca2+ (1 mM), and Mg2+ (1.5 mM); kept on ice; and used for experiments within 6 h.
Measurement of NADPH oxidase activity.
NADPH oxidase activity was determined using isoluminol/luminol and chemiluminescence systems that allow determination of both extracellularly and intracellularly released ROS (20). The chemiluminescence activity was measured in a six-channel Biolumat LB 9505 apparatus (Berthold Co., Wildbad, Germany) by use of disposable 4-ml polypropylene tubes with a 1-ml reaction mixture containing 5 × 105 neutrophils. For measuring total ROS, 2 U horseradish peroxidase (a cell-impermeable peroxidase) (Boehringer Mannheim, Mannheim, Germany) and luminol (2 × 10−5 M; a cell-permeable luminescence substrate) were used. The tubes were equilibrated in the Biolumat apparatus for 5 min at 37°C, after which time the stimulus (50 to 100 μl) was added. The light emission was recorded continuously.
For measurements of intracellular ROS alone, extracellular radicals were scavenged by superoxide dismutase (200 U/ml) and catalase (2000 U/ml), which were added to the reaction mixture before stimulation. To measure only extracellular radicals, the non-membrane-permeable luminescence amplifier isoluminol (2 × 10−5 M) was used instead of luminol.
Inhibition of PLY activity by cholesterol.
Cholesterol inactivation experiments were performed in a manner similar to that described by Iliev et al. (34). Supernatant from autolyzed bacteria (3.5 × 109 bacteria/ml before autolysis) was mixed with cholesterol (Sigma) at concentrations of up to 500 ng/ml at 37°C for 20 min. Purified PLY (5 μg/ml) and N-formyl-methionyl-leucyl (fMLF) (5 × 10−6 M) were also treated with 2.5 μg/ml of cholesterol at 37°C for 20 min. At the end of incubation, all samples were diluted 1/5 in PBS, and 50-μl portions were used directly to trigger neutrophils.
Measurements of intracellular Ca2+ mobilization.
Neutrophils were suspended at 2 × 107 cells/ml in KRG without calcium containing 0.1% bovine serum albumin and loaded with 2 μM Fura-2/AM (Molecular Probes, Inc., Eugene, OR) for 30 min at room temperature. The cells were washed twice at ambient room temperature and resuspended in KRG containing calcium. Aliquots of neutrophils, at a final concentration of 2 × 106 cells/ml, were dispensed into cuvettes maintained at 37°C and equipped with a stirring device. After 5 min of equilibration at 37°C, stimulus (50 μl) was added and the Fura-2/AM fluorescence was monitored using a LC50 fluorescence spectrophotometer (LS50B; Perkin Elmer, Wellesley, MA). Fura-2/AM was excited with light of dual wavelengths (340 and 380 nm) and monitored for emission at 510 nm. Data are presented as the 340-nm/380-nm ratio. To calibrate the system, Triton X-100 (0.04% final concentration) was added to disrupt cells. Fluorescence was then determined in 1 mM free calcium, whereafter EGTA (5 mM final concentration) together with Tris-HCl (30 mM; pH 8.7) was added and fluorescence was determined at a low (<1 nM) level of free calcium. To determine if the increase in cytosolic Ca2+ was due to an influx over the plasma membrane or a release from intracellular stores, experiments were conducted in the presence of EGTA, which was added to a final concentration of 3 mM 20 s prior to the addition of stimulus.
Tests of viability and functional capacity of neutrophils.
To measure the viability of neutrophils after 30 min of incubation with purified PLY or pneumococcal components, the cells were washed once with PBS, whereafter the nucleic acid dye 7-amino-actinomycin D (BD Pharmingen) was added. The cells were analyzed by flow cytometry (Becton Dickinson FACScan equipped with FlowJo software).
To measure remaining functional capacity, neutrophils were incubated with native PLY or with PdT for 30 min at 37°C, after which the cells were stimulated with the chemotactic peptide fMLF (10−7 M) (Sigma) or the phorbol ester phorbol myristate acetate (PMA) (5 × 10−8 M) (Sigma), and ROS production was determined as described above.
RESULTS
The autolytic S. pneumoniae strain A17 produces soluble neutrophil-activating compounds in stationary phase.
The clinical S. pneumoniae isolate A17 was cultured in broth for 14 h to reach stationary phase, and the entire bacterial culture, containing intact as well as autolyzed cells, was added to human blood donor neutrophils. The bacterial suspension dose-dependently activated neutrophils to produce superoxide anions (Fig. 1). The response in neutrophils was not immediate but occurred after around 1 min and reached a maximum after about 5 to 10 min.
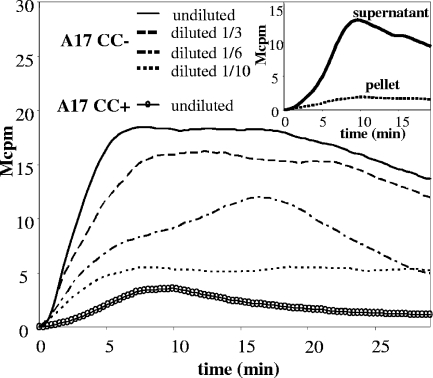
FIG. 1.
A soluble factor in a bacterial suspension of S. pneumoniae A17 induces activation of the neutrophil NADPH oxidase. Neutrophils (5 × 105/ml) were preincubated at 37°C for 5 min, and then, at time zero, 50-μl portions of diluted and undiluted suspensions of S. pneumoniae A17 (the undiluted suspension contained 7 × 108 bacteria/ml before autolysis took place; see Materials and Methods), grown in the absence (CC−) or presence (CC+) of the autolysis inhibitor choline, were added. ROS production was measured and the time course of the response determined. Data from a representative experiment are shown (n = 5). (Inset) The bacterial suspension of S. pneumoniae A17 grown in the absence of choline was separated into soluble and particulate fractions by centrifugation (13,000 × g, 10 min). Neutrophils were stimulated with the soluble and particulate fractions, and ROS production was measured. The curves are from a representative experiment (n = 5).
Following centrifugation (13,000 × g, 10 min), the ability to trigger the neutrophil NADPH oxidase remained in the supernatant (Fig. 1, inset), suggesting that the active material was soluble and released from bacteria.
A fraction of S. pneumoniae spontaneously autolyzes when reaching the stationary phase by activation of peptidoglycan-degrading enzymes called ALs, the dominant one being LytA (31). To determine the role of spontaneous autolysis for the release of the neutrophil-activating compound, S. pneumoniae A17 was cultured in the presence or absence of choline, an inhibitor of AL action (13, 29). When pneumococci were cultivated and prepared in the presence of choline, the bacterial suspension contained no neutrophil-activating activity (Fig. 1). As a control for an effect of choline on neutrophil viability or activation, Bifidobacterium dentium, which, like S. pneumoniae, is gram positive and a potent inducer of ROS in our system, was cultivated in the presence or absence of choline and used to stimulate neutrophils. The presence of choline did not affect the ability of B. dentium to induce NADPH oxidase activation (data not shown). Furthermore, the addition of choline to bacteria after culture did not impede their capacity to generate ROS (data not shown).
Ability to activate the neutrophil NADPH oxidase is diminished in a LytA-deficient strain of S. pneumoniae.
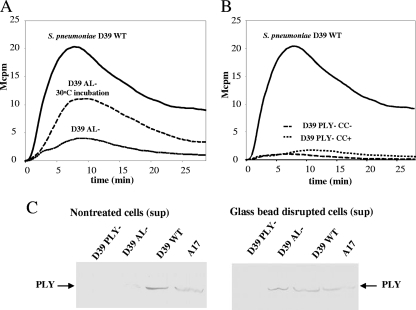
We next compared the NADPH oxidase-activating abilities of a LytA-deficient pneumococcal strain and its otherwise isogenic WT control. Whereas S. pneumoniae D39 WT induced a chemiluminescence response, its AL-deficient mutant, S. pneumoniae D39 AL−, was much less active (Fig. 2A). Although LytA has been knocked out, the D39 AL− strain contains a functional minor AL, LytC, which is enzymatically active at 30°C (26). We incubated LytA-deficient bacteria at 30°C for 1 h prior to the stimulation of neutrophils. This treatment increased the capacity of the AL− mutant to induce a chemiluminescence response (Fig. 2A).
FIG. 2.
LytA-deficient and PLY-deficient strains of S. pneumoniae induce less activation of the neutrophil NADPH oxidase. (A) Neutrophils were stimulated with 50 μl of undiluted bacterial suspensions of S. pneumoniae D39 WT or its LytA-deficient mutant (D39 AL−), and the time course of ROS production was determined. One of the curves shows ROS production in response to 50 μl of a suspension of D39 AL− after preincubation at 30°C for 1 h, allowing LytC-dependent autolysis to take place. Data from a representative experiment are shown (n = 3). (B) The kinetics of ROS production from neutrophils stimulated with 50-μl bacterial suspensions of S. pneumoniae D39 WT and its PLY-deficient mutant (D39 PLY−) grown in the absence (CC−) or presence (CC+) of choline are shown. The curves are from a representative experiment (n = 6). (C) The proteins in supernatants of nontreated or glass-bead-disrupted bacterial suspensions of S. pneumoniae A17, D39 WT, D39 AL−, and D39 PLY− were separated by SDS-PAGE and blotted onto nitrocellulose membranes. The presence of PLY was detected as described in Materials and Methods. The experiment was repeated twice with similar results. Sup, supernant.
Basic characterization of the pneumococcal component responsible for activation of the neutrophil NADPH oxidase.
In order to characterize the NADPH oxidase-activating factor released during pneumococcal autolysis, we treated the supernatant of S. pneumoniae A17 with heat or degrading enzymes. Treatment of pneumococcal supernatant with heat (56°C for 30 min) or trypsin (20 μg/ml, 37°C, 1 h) completely abrogated its ability to activate neutrophils, whereas treatment with lysozyme (15 mg/ml, 37°C, 1 h), which degrades peptidoglycan components, had no effect on the chemiluminescence response (data not shown).
PLY as the activator of the neutrophil NADPH oxidase.
We hypothesized that PLY was the neutrophil-activating proteinaceous factor released during the growth of AL-positive pneumococci, since it is an intracellular toxin known to be released during autolysis (12). We tested the capacity of a PLY-deficient strain of S. pneumoniae, D39 PLY− (which still has an intact AL), to activate the neutrophil NADPH oxidase. Preparations of D39 PLY− bacteria lacked neutrophil-stimulating activity, regardless of whether they were grown in the presence or absence of choline (Fig. 2B).
To verify the presence of PLY in the NADPH oxidase-activating pneumococcal preparations, Western blotting was performed. The supernatants of S. pneumoniae A17, of D39 WT, and of the AL- and PLY-deficient variants of D39 were subjected to SDS-PAGE, blotted onto nitrocellulose membranes, and probed with a PLY-specific antibody. As shown in Fig. 2C, PLY was present in the supernatants of A17 and D39 WT, whereas only a weak band was visible for the blotted culture supernatant of D39 AL−, and, as expected, no PLY was detected in supernatant from D39 PLY−. After the disruption of bacterial membranes with glass beads (see Materials and Methods), PLY was also detected in the supernatant originating from D39 AL− bacteria (Fig. 2C).
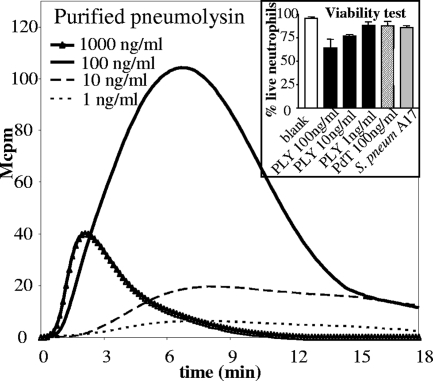
Next, highly purified PLY was added to neutrophils and the chemiluminescence response was monitored. Indeed, PLY was highly effective in inducing neutrophil NADPH oxidase activation, and concentrations down to 1 ng/ml (representing 0.5 HU/ml) induced measurable responses (Fig. 3). Optimal concentration was determined using twofold dilutions of purified PLY, starting with 1,000 ng/ml. A maximal response, of a magnitude comparable to that induced by the phorbol ester PMA, was seen at a concentration of 50 to 100 ng/ml of PLY, whereas the response was reduced at higher concentrations (Fig. 3). High concentrations of PLY also compromised cell viability (Fig. 3, inset).
FIG. 3.
Purified PLY is a strong activator of the NADPH oxidase. Neutrophils were stimulated with various concentrations of highly purified PLY and the kinetics of the NADPH oxidative response were measured. Data are from a representative experiment (n = 3). (Inset) Neutrophils (5 × 105/ml) were incubated at 37°C for 30 min with various concentrations of native PLY, PdT, or 50 μl of undiluted S. pneumoniae A17 suspension. After being washed, the cells were stained with 7-amino-actinomycin D and the numbers of viable neutrophils were determined by flow cytometry. The experiment was performed on neutrophils from three blood donors and data show the average numbers of viable cells (± standard deviations) after the various stimulations. S. pneum, S. pneumoniae.
Cholesterol binding and cytolytic activities of PLY are required for NADPH oxidase activation.
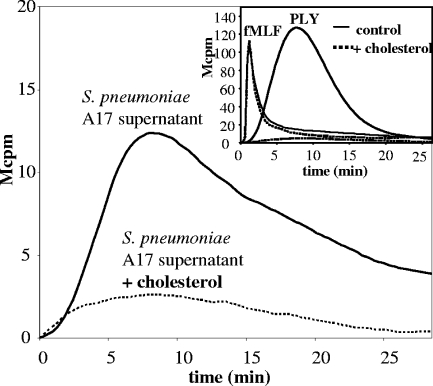
PLY binds to cholesterol in eukaryotic cell membranes and can, when present in high concentrations, form membrane pores. The addition of cholesterol to the neutrophil-activating supernatant of S. pneumoniae A17 dose-dependently inhibited the ability of the supernatant to activate the neutrophil oxidase. A concentration of 500 ng cholesterol/ml completely blocked the activity (Fig. 4). Purified PLY was also incubated with cholesterol, and, similarly, its NADPH oxidase-activating ability was completely abrogated (Fig. 4, inset). In control experiments, cholesterol was found to be without effect when the chemoattractant fMLF replaced PLY as the neutrophil-activating agonist (Fig. 4, inset).
FIG. 4.
Soluble cholesterol inhibits the pneumococcal component stimulating ROS production. The supernatant of S. pneumoniae A17 was treated with 500 ng/ml of soluble cholesterol at 37°C for 20 min (see Materials and Methods). Neutrophils were stimulated with the cholesterol-treated supernatants and the NADPH oxidase response was measured. Data show a representative experiment (n = 2). (Inset) Purified PLY was incubated with 2.5 μg/ml of cholesterol at 37°C for 20 min and used to stimulate neutrophils (the response to 50 ng/ml of PLY with or without cholesterol incubation is shown). In control experiments, NADPH oxidase activation in response to fMLF with or without preincubation with cholesterol was tested. Data show the time course of NADPH oxidase activation for a representative experiment (n = 2).
PdT, a modified PLY in which three amino acids have been substituted (Asp385 → Asn, Cys428 → Gly, and Trp433 → Phe), lacks both the cytolytic activity and the ability to activate the complement system (1, 6). We tested this mutated PLY in our system and found that it did not induce any NADPH oxidase activity at concentrations of up to 100 ng/ml when incubated with neutrophils (Fig. 6, inset).
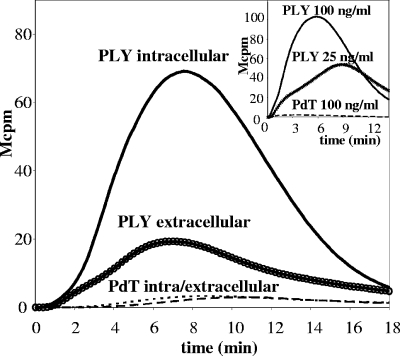
FIG. 6.
PLY induces intracellular production of ROS. Neutrophils were stimulated with PLY (50 ng/ml), and intra- and extracellularly generated superoxides were measured separately (see Materials and Methods). The curves show the kinetics of a representative experiment (n = 4). (Inset) Neutrophils were stimulated with native PLY or PdT in concentrations of up to 100 ng/ml. Data from a representative experiment are shown (n = 5).
Capacities to induce a neutrophil respiratory burst among clinical S. pneumoniae strains.
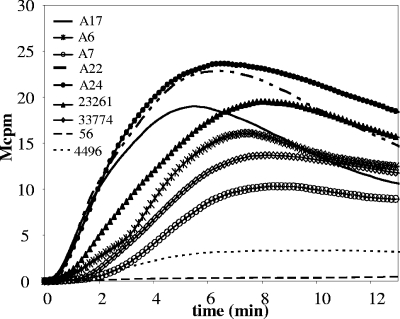
Though PLY is present in virtually all virulent strains of S. pneumoniae, its specific activity has been shown to vary (35, 41, 44). To study variations in oxidative responses to pneumococcal strains, neutrophils were stimulated with nine clinical isolates of various sero- and genotypes. The nine clinical isolates all expressed PLY, as verified by Western blotting (data not shown). Seven of the isolates induced significant levels of superoxide production when grown to allow autolysis (mean of ROSmax varying between 11 and 20 Mcpm) (Table 1 and Fig. 5). The presence of choline during bacterial growth strongly reduced the ROS production induced by the different isolates (data not shown), suggesting a role for the AL.
FIG. 5.
Capacities to induce a neutrophil respiratory burst among clinical pneumococcal strains. Neutrophils from three blood donors were stimulated with 50 μl of bacterial suspension from nine clinical isolates of S. pneumoniae (otitis media strains A17, A6, A7, A22, and A24; CCUG strains 23261 and 33774; and low-PLY strains 56 and 4496), and the NADPH oxidase response over 15 min was measured. The curves are for a neutrophil sample from a representative blood donor (n = 3).
Two of the clinical isolates, 56 and 4496, did not induce significant amounts of ROS from neutrophils (Table 1 and Fig. 5). S. pneumoniae 56 is of serotype 8 and has previously been shown to produce a PLY with low hemolytic activity (6.8 × 104 HU/mg versus 1.2 × 106 HU/mg for native PLY), due to an amino acid substitution (Thr172 → Ile) (44). The 4496 strain is of serotype 1 with the novel MLST type 3018, and it produces a low-hemolytic-activity PLY due to the presence of the same Thr172 → Ile substitution (J. C. Paton, unpublished data).
The NADPH oxidase activity induced by PLY is localized mainly in an intracellular compartment.
Activation of neutrophils may induce assembly of the NADPH oxidase in the plasma membrane and/or in membranes lining intracellular compartments, such as specific and azurophilic granules (for a review, see reference 38). To examine the localization of the oxidants generated in response to PLY, we employed the luminol/isoluminol method to discriminate between intra- and extracellularly generated superoxide (20). Stimulation of neutrophils revealed that PLY induced a low level of secreted superoxide but that the major fraction was generated intracellularly (Fig. 6).
PLY induces an influx in neutrophils of extracellular Ca2+ that is required for activation of the NADPH oxidase activity.
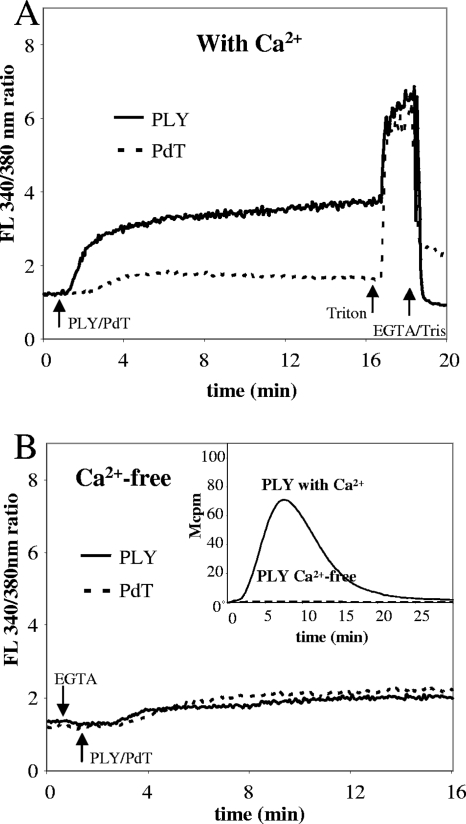
A large number of physiological, as well as synthetic, agonists induce a transient rise in the concentration of free calcium [Ca2+] in neutrophils. This rise occurs in two phases: a rapid increase in the cytosolic (intracellular) calcium concentration ([Ca2+]i), which is achieved when the intracellular storage organelles are emptied, and a slower but more sustained increase in [Ca2+]i, which comes about when ion channels in the plasma membrane are opened (4, 7). Whereas the presence of Ca2+ in the extracellular milieu is required for the increase linked to an influx over the membrane, the [Ca2+]i increase originating from the emptying of intracellular stores occurs independently of extracellular Ca2+. Stimulation of neutrophils with native PLY but not cytolytically deficient PLY (PdT) induced a rise in [Ca2+]i (Fig. 7A), and this rise was dependent on the presence of extracellular Ca2+ (Fig. 7B). Similar results were also obtained after stimulation with S. pneumoniae A17 and D39 supernatant (data not shown). Moreover, PLY-induced neutrophil NADPH oxidase activity was abolished in Ca2+-free medium (Fig. 7B, inset), whereas 30% of PMA-induced ROS production remained in Ca2+-free medium (data not shown).
FIG. 7.
PLY induces neutrophil influx of extracellular Ca2+, which is required to induce NADPH oxidase activation. Neutrophils (2 × 106/ml) loaded with the Ca2+-binding fluorescent dye Fura-2/AM were stimulated with native PLY or PdT (50 ng/ml) and the induced changes in [Ca2+]i were determined over 20 min. (A) [Ca2+]i changes in Ca2+-containing medium. (B) Results obtained for Ca2+-free medium (with EGTA added at the start of the experiment). The curves show the 340-nm/380-nm ratio and are from a representative experiment (n = 2). (Inset) Neutrophil NADPH oxidase activity in response to PLY (50 ng/ml) in Ca2+-free medium was measured. Data are from a representative experiment (n = 4). FL, fluorescence.
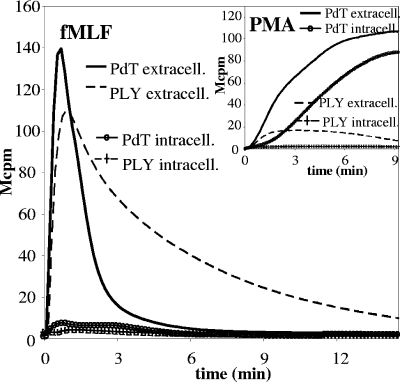
PLY pretreatment primes neutrophils for subsequent stimulation with fMLF and inhibits subsequent stimulation with PMA.
We asked whether interaction with PLY would alter the functional response of neutrophils to subsequent stimulation with unrelated stimuli. Neutrophils were preincubated (30 min at 37°C) with native PLY (50 ng/ml) or, as a control, with PdT; thereafter, they were stimulated with the chemotactic peptide fMLF or the phorbol ester PMA, and the induced oxidative responses were recorded. Figure 8 shows that the response to fMLF was sustained over a very long period of time in neutrophils that had been pretreated with PLY compared to what was found for PdT-treated neutrophils. In contrast, the subsequent response to PMA was strongly reduced in neutrophils pretreated with PLY (Fig. 8, inset).
FIG. 8.
Priming or inhibiting effects of PLY pretreatment depend on the nature of subsequent stimuli. Neutrophils were primed with native PLY or PdT (50 ng/ml) for 30 min at 37°C. The cells were then activated with fMLF (10−7 M) or PMA (5 × 10−7 M) (inset) and ROS production was determined. The figure shows the kinetics of representative experiments (n = 3). extracell., extracellular; intracell., intracellular.
DISCUSSION
PLY is an important virulence factor of S. pneumoniae, since mutants lacking PLY are strikingly less virulent than WT strains in mouse infection models (11, 54). Furthermore, the injection of purified PLY into laboratory animals causes many symptoms that mimic those of pneumococcal diseases (48, 54). PLY is a pore-forming toxin which, when present in high concentrations, can lyse any cell membrane provided that it contains cholesterol. This property makes it toxic to various cell types, but also in sublytic concentrations PLY has been shown to exert a number of effects on host systems, including inhibition of ciliary beating (22), induction of cytokine production (32), and activation of complement (53). We now add that PLY in sublytic concentrations and released by autolyzed pneumococci induces the activation of the NADPH oxidase in human neutrophils and that the ROS are produced into an intracellular compartment rather than being released extracellularly.
S. pneumoniae expressing PLY with defined mutations that affect cytolytic and complement-activating properties have reduced virulence compared to that of WT pneumococci (9, 10, 37). Though the amino acid sequence of PLY had been thought to be well conserved throughout pneumococcal serotypes (46), it has lately become clear that some clinical isolates bear mutations in their PLY genes (28, 35, 41, 44). Some of these mutations affect the hemolytic activity of PLY and are most commonly found in isolates of serotypes 1 and 8 (35, 41, 44). We tested the abilities of nine clinical strains, including one isolate each of serotype 1 and 8 with low hemolytic activity, to induce ROS production from neutrophils. Seven of the tested clinical strains of S. pneumoniae produced PLY in concentrations high enough to induce the activation of the NADPH oxidase without causing considerable toxicity to the neutrophils. Release of PLY from bacterial cells during autolysis was a prerequisite for the stimulation of the NADPH oxidase, since suspensions of autolyzed, but not of intact, pneumococci induced the production of ROS from neutrophils. This is in accordance with previous data demonstrating that PLY is expressed intracellularly and released mainly during bacterial autolysis mediated by breakdown of the peptidoglycan layer by activated ALs (12). Further, the two clinical strains producing PLYs with low hemolytic activity (isolates 4496 and 56, of serotypes 1 and 8, respectively) did not induce significant levels of ROS. Thus, hemolytic activity appeared to be required for the activation of the neutrophil NADPH oxidase. Accordingly, cholesterol binding (which is a prerequisite for pore formation) was required for the induction of oxygen radicals, and a mutant form of PLY lacking hemolytic and complement-activating abilities, PdT, did not induce ROS production.
Activation of the NADPH oxidase in neutrophils is tightly regulated. The dormant NADPH oxidase consists of separate components distributed in the cytosol and in the cellular membranes. Upon activation, the cytosolic proteins translocate to membrane-bound cytochrome b and form a functional electron transfer system which catalyzes the reduction of molecular oxygen to superoxide ions. If the NADPH oxidase is assembled at the plasma membrane, ROS are produced extracellularly, whereas assembly at the membrane of an intracellular organelle results in superoxide ions being released into that compartment, e.g., into a granule or a phagosome. Using a technique able to discriminate between superoxide produced intracellularly and that produced extracellularly, we could show that PLY mainly induced intracellular production of superoxide. Previous studies have reported that PLY does not induce activation of the NADPH oxidase (17, 51). However, the techniques used in these earlier investigations allowed measurements only of extracellular ROS and were also less sensitive, which could explain the discrepancy with the data presented here. In fact, our data show that the stimulatory capacity of optimal doses of PLY equaled that of PMA, one of the most potent inducers of ROS known.
Through the use of techniques allowing discrimination between intracellular and extracellular ROS (for a review, see references 20 and 40), it has become increasingly evident that different stimuli may favor either type of reaction. For example, signals triggered through the seven-transmembrane G-protein-linked chemoattractant receptors generate an assembly of the NADPH oxidase primarily in the plasma membrane, and, as a consequence, an extracellular release of superoxide anions (24). On the other hand, stimulation of neutrophils with lactose binding lectins, termed galectins, results in both extracellular release of ROS and activation of an intracellular (specific-granule-localized) pool of the oxidase, generating oxygen radicals retained by the cells (2, 3, 39). An intracellular localization of the formed oxygen metabolites without the formation of a classical phagosome/phagolysosome is apparent also when the oxidase is activated through the triggering of complement receptor 3 (56).
The intracellular signaling pathways leading to an assembly of the oxidase have not been clearly elucidated. It is clear, however, that an increase in intracellular calcium concentration is a signal sufficient to activate the granule-localized NADPH oxidase but not that of the plasma membrane (18, 19). We here demonstrate that PLY stimulation results in a flux of extracellular Ca2+ into neutrophils, a phenomenon which has been shown previously (17), but also that the production of ROS is completely dependent on this influx. Thus, our data suggest that the interaction of PLY with cholesterol in the plasma membrane of neutrophils allows the entry of calcium ions, leading to an intracellular assembly and activation of the NADPH oxidase. This occurred at concentrations (5 to 50 ng/ml) lower than the concentrations causing pore formation and cell lysis (approximately 10 μg/ml [50]).
In a situation optimal for the host, ROS should be delivered to the phagosome containing the invading microbe, aiding its eradication. However, S. pneumoniae clearly generated ROS production internally in neutrophils that had not phagocytosed any pneumococci, as cell-free supernatant induced ROS production but bacterium-containing pellet did not. Pneumococci are notorious for their ability to withstand phagocytosis, due to their thick and hydrophilic capsule. Our results suggest that PLY, released by autolysis, could help pneumococci to circumvent the phagocyte defense. After the PLY-induced respiratory burst, the functional response of the neutrophils to subsequent stimuli was altered. The response to PMA was strongly reduced, as reported previously (51). However, the neutrophils were not killed, as they were able to respond to fMLF. The ROS production to this second stimulus was as high as, and actually more sustained than, the response to fMLF seen for nontreated control neutrophils. This is also in accordance with results presented by others (17). The fMLF receptor signals via heterotrimeric G-proteins, and the signaling is terminated after association of the ligand-receptor complex to the cytoskeleton (36). The rise in intracellular Ca2+, triggered by PLY, may reduce the binding of the receptor-ligand complexes with the cytoskeleton (43) and thereby permit a prolonged oxidative response to fMLF. The fact that PLY, in concentrations as low as 1 ng/ml, inhibits both neutrophil chemotaxis and the killing of opsonized pneumococci (51) could also relate to its capacity to disturb cytoskeletal function.
As mentioned previously, pneumococcal diseases are often coupled to extensive cellular and tissue damage. Inflammatory cells are gradually accumulated in infected tissues, when neutrophils are recruited by chemoattractants generated by the invading microbe and stationary innate immune cells. The exudated inflammatory cells will meet increasing concentrations of pneumococcal components, including PLY, released from autolyzed bacteria in the infected tissue. Before reaching the viable bacteria that should be ingested and killed, the bactericidal mechanisms of the neutrophils may be diverted through activation of the neutrophil NADPH oxidase and the intracellular release of ROS. A sustained NADPH oxidase response to another bacterial component, fMLF, may further activate the neutrophils in vain, thereby leading to extracellularly released ROS, contributing to extensive tissue damage.
Acknowledgments
This work was supported by the Swedish Research Council (no. K2001-06GX-14072-01, K2007-58X-08298-20-3), the Medical Faculty of Göteborg, the Swedish government under the ALF agreement, the King Gustaf the Vth 80-Year Foundation, and the Adlerberth Research Foundation.
We are most grateful to Jessica Darenberg, Christina Johansson, and Birgitta Henriques-Normark at Smittskydds Institutet (SMI) in Stockholm, Sweden, for serotyping and MLST of the pneumococcal isolates.
Editor: A. Camilli
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24167-174. [DOI] [PubMed] [Google Scholar]
- 2.Almkvist, J., C. Dahlgren, H. Leffler, and A. Karlsson. 2002. Activation of the neutrophil nicotinamide adenine dinucleotide phosphate oxidase by galectin-1. J. Immunol. 1684034-4041. [DOI] [PubMed] [Google Scholar]
- 3.Almkvist, J., and A. Karlsson. 2004. Galectins as inflammatory mediators. Glycoconj. J. 19575-581. [DOI] [PubMed] [Google Scholar]
- 4.Andersson, T., C. Dahlgren, T. Pozzan, O. Stendahl, and P. D. Lew. 1986. Characterization of fMet-Leu-Phe receptor-mediated Ca2+ influx across the plasma membrane of human neutrophils. Mol. Pharmacol. 30437-443. [PubMed] [Google Scholar]
- 5.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 1833108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23201-209. [DOI] [PubMed] [Google Scholar]
- 7.Berridge, M. 2004. Conformational coupling: a physiological calcium entry mechanism. Sci. STKE 2004pe33. [DOI] [PubMed] [Google Scholar]
- 8.Berry, A. M., R. A. Lock, D. Hansman, and J. C. Paton. 1989. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 572324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry, A. M., A. D. Ogunniyi, D. C. Miller, and J. C. Paton. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry, A. M., J. C. Paton, and D. Hansman. 1992. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb. Pathog. 1287-93. [DOI] [PubMed] [Google Scholar]
- 11.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 572037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulnois, G. J., J. C. Paton, T. J. Mitchell, and P. W. Andrew. 1991. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol. Microbiol. 52611-2616. [DOI] [PubMed] [Google Scholar]
- 13.Briese, T., and R. Hakenbeck. 1985. Interaction of the pneumococcal amidase with lipoteichoic acid and choline. Eur. J. Biochem. 146417-427. [DOI] [PubMed] [Google Scholar]
- 14.Canvin, J. R., A. P. Marvin, M. Sivakumaran, J. C. Paton, G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 172119-123. [DOI] [PubMed] [Google Scholar]
- 15.Chetty, C., and A. Kreger. 1980. Characterization of pneumococcal purpura-producing principle. Infect. Immun. 29158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chetty, C., and A. Kreger. 1981. Role of autolysin in generating the pneumococcal purpura-producing principle. Infect. Immun. 31339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockeran, R., A. J. Theron, H. C. Steel, N. M. Matlola, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Proinflammatory interactions of pneumolysin with human neutrophils. J. Infect. Dis. 183604-611. [DOI] [PubMed] [Google Scholar]
- 18.Dahlgren, C. 1987. Difference in extracellular radical release after chemotactic factor and calcium ionophore activation of the oxygen radical-generating system in human neutrophils. Biochim. Biophys. Acta 93033-38. [DOI] [PubMed] [Google Scholar]
- 19.Dahlgren, C., P. Follin, A. Johansson, R. Lock, and K. Orselius. 1989. Localization of the luminol-dependent chemiluminescence reaction in human granulocytes. J. Biolumin. Chemilumin. 4263-266. [DOI] [PubMed] [Google Scholar]
- 20.Dahlgren, C., and A. Karlsson. 1999. Respiratory burst in human neutrophils. J. Immunol. Methods 2323-14. [DOI] [PubMed] [Google Scholar]
- 21.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 1443049-3060. [DOI] [PubMed] [Google Scholar]
- 22.Feldman, C., T. J. Mitchell, P. W. Andrew, G. J. Boulnois, R. C. Read, H. C. Todd, P. J. Cole, and R. Wilson. 1990. The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb. Pathog. 9275-284. [DOI] [PubMed] [Google Scholar]
- 23.Ferrante, A., B. Rowan-Kelly, and J. C. Paton. 1984. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect. Immun. 46585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu, H., J. Karlsson, J. Bylund, C. Movitz, A. Karlsson, and C. Dahlgren. 2006. Ligand recognition and activation of formyl peptide receptors in neutrophils. J. Leukoc. Biol. 79247-256. [DOI] [PubMed] [Google Scholar]
- 25.Garcia, P., M. P. Gonzalez, E. Garcia, R. Lopez, and J. L. Garcia. 1999. LytB, a novel pneumococcal murein hydrolase essential for cell separation. Mol. Microbiol. 311275-1281. [DOI] [PubMed] [Google Scholar]
- 26.Garcia, P., M. Paz Gonzalez, E. Garcia, J. L. Garcia, and R. Lopez. 1999. The molecular characterization of the first autolytic lysozyme of Streptococcus pneumoniae reveals evolutionary mobile domains. Mol. Microbiol. 33128-138. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Suarez Mdel, M., F. Vazquez, and F. J. Mendez. 2006. Streptococcus pneumoniae virulence factors and their clinical impact: an update. Enferm. Infecc. Microbiol. Clin. 24512-517. [DOI] [PubMed] [Google Scholar]
- 28.Garnier, F., R. P. Janapatla, E. Charpentier, G. Masson, C. Grelaud, J. F. Stach, F. Denis, and M. C. Ploy. 2007. Insertion sequence 1515 in the ply gene of a type 1 clinical isolate of Streptococcus pneumoniae abolishes pneumolysin expression. J. Clin. Microbiol. 452296-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giudicelli, S., and A. Tomasz. 1984. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J. Bacteriol. 1581188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellstrand, K., A. Asea, C. Dahlgren, and S. Hermodsson. 1994. Histaminergic regulation of NK cells. Role of monocyte-derived reactive oxygen metabolites. J. Immunol. 1534940-4947. [PubMed] [Google Scholar]
- 31.Holtje, J. V., and A. Tomasz. 1976. Purification of the pneumococcal N-acetylmuramyl-l-alanine amidase to biochemical homogeneity. J. Biol. Chem. 2514199-4207. [PubMed] [Google Scholar]
- 32.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect. Immun. 621501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hultqvist, M., and R. Holmdahl. 2005. Ncf1 (p47phox) polymorphism determines oxidative burst and the severity of arthritis in rats and mice. Cell. Immunol. 23397-101. [DOI] [PubMed] [Google Scholar]
- 34.Iliev, A. I., J. R. Djannatian, R. Nau, T. J. Mitchell, and F. S. Wouters. 2007. Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc. Natl. Acad. Sci. USA 1042897-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jefferies, J. M., C. H. Johnston, L. A. Kirkham, G. J. Cowan, K. S. Ross, A. Smith, S. C. Clarke, A. B. Brueggemann, R. C. George, B. Pichon, G. Pluschke, V. Pfluger, and T. J. Mitchell. 2007. Presence of nonhemolytic pneumolysin in serotypes of Streptococcus pneumoniae associated with disease outbreaks. J. Infect. Dis. 196936-944. [DOI] [PubMed] [Google Scholar]
- 36.Jesaitis, A. J., and K. N. Klotz. 1993. Cytoskeletal regulation of chemotactic receptors: molecular complexation of N-formyl peptide receptors with G proteins and actin. Eur. J. Haematol. 51288-293. [DOI] [PubMed] [Google Scholar]
- 37.Jounblat, R., A. Kadioglu, T. J. Mitchell, and P. W. Andrew. 2003. Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin. Infect. Immun. 711813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karlsson, A., and C. Dahlgren. 2002. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid. Redox Signal. 449-60. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson, A., P. Follin, H. Leffler, and C. Dahlgren. 1998. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood 913430-3438. [PubMed] [Google Scholar]
- 40.Karlsson, H., C. Hessle, and A. Rudin. 2002. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 706688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkham, L. A., J. M. Jefferies, A. R. Kerr, Y. Jing, S. C. Clarke, A. Smith, and T. J. Mitchell. 2006. Identification of invasive serotype 1 pneumococcal isolates that express nonhemolytic pneumolysin. J. Clin. Microbiol. 44151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klebanoff, S. J. 2005. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77598-625. [DOI] [PubMed] [Google Scholar]
- 43.Liu, L., O. Harbecke, H. Elwing, P. Follin, A. Karlsson, and C. Dahlgren. 1998. Desensitization of formyl peptide receptors is abolished in calcium ionophore-primed neutrophils: an association of the ligand-receptor complex to the cytoskeleton is not required for a rapid termination of the NADPH-oxidase response. J. Immunol. 1602463-2468. [PubMed] [Google Scholar]
- 44.Lock, R. A., Q. Y. Zhang, A. M. Berry, and J. C. Paton. 1996. Sequence variation in the Streptococcus pneumoniae pneumolysin gene affecting haemolytic activity and electrophoretic mobility of the toxin. Microb. Pathog. 2171-83. [DOI] [PubMed] [Google Scholar]
- 45.Lundqvist-Gustafsson, H., and T. Bengtsson. 1999. Activation of the granule pool of the NADPH oxidase accelerates apoptosis in human neutrophils. J. Leukoc. Biol. 65196-204. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell, T. J., F. Mendez, J. C. Paton, P. W. Andrew, and G. J. Boulnois. 1990. Comparison of pneumolysin genes and proteins from Streptococcus pneumoniae types 1 and 2. Nucleic Acids Res. 184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulholland, K. A. 1997. A report prepared for the Scientific Advisory Group of Experts, Global Programme for Vaccines and Immunization. World Health Organization, Geneva, Switzerland.
- 48.Ng, E. W., N. Samiy, J. B. Rubins, F. V. Cousins, K. L. Ruoff, A. S. Baker, and D. J. D'Amico. 1997. Implication of pneumolysin as a virulence factor in Streptococcus pneumoniae endophthalmitis. Retina 17521-529. [PubMed] [Google Scholar]
- 49.Olofsson, P., J. Holmberg, U. Pettersson, and R. Holmdahl. 2003. Identification and isolation of dominant susceptibility loci for pristane-induced arthritis. J. Immunol. 171407-416. [DOI] [PubMed] [Google Scholar]
- 50.Paton, J. C. 1996. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 4103-106. [DOI] [PubMed] [Google Scholar]
- 51.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 411212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paton, J. C., R. A. Lock, and D. J. Hansman. 1983. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect. Immun. 40548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paton, J. C., B. Rowan-Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 431085-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubins, J. B., D. Charboneau, J. C. Paton, T. J. Mitchell, P. W. Andrew, and E. N. Janoff. 1995. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Investig. 95142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segal, A. W. 2005. How neutrophils kill microbes. Annu. Rev. Immunol. 23197-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serrander, L., J. Larsson, H. Lundqvist, M. Lindmark, M. Fallman, C. Dahlgren, and O. Stendahl. 1999. Particles binding beta(2)-integrins mediate intracellular production of oxidative metabolites in human neutrophils independently of phagocytosis. Biochim. Biophys. Acta 1452133-144. [DOI] [PubMed] [Google Scholar]
- 57.Tuomanen, E., H. Liu, B. Hengstler, O. Zak, and A. Tomasz. 1985. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 151859-868. [DOI] [PubMed] [Google Scholar]
- 58.van de Guchte, M., J. Kok, and G. Venema. 1991. Distance-dependent translational coupling and interference in Lactococcus lactis. Mol. Gen. Genet. 22765-71. [DOI] [PubMed] [Google Scholar]
- 59.Walker, J. A., R. L. Allen, P. Falmagne, M. K. Johnson, and G. J. Boulnois. 1987. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 551184-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]