Abstract
Infectivity and persistence by Borrelia burgdorferi, the etiologic agent of Lyme disease, rely stringently on regulatory events. Among these is the downregulation of lipoprotein antigen expression, exemplified by outer surface protein C (OspC), at the advent of specific immunity in the mammalian host. B. burgdorferi spirochetes that lack the linear plasmid 28-1 (lp28-1) succumb to the host's immune response. We thus explored the notion that these two phenomena were related—that lp28-1− organisms fail to downregulate ospC and thus are cleared following the appearance of anti-OspC antibody in the murine host. The lp-28-1− isolate and a wild-type (wt) isolate bearing the complete set of plasmids were grown in dialysis membrane chambers that were implanted into rat peritoneal cavities. Analysis of mRNA and protein from these cultures showed that OspC expression levels by lp28-1− organisms are abnormally high in vivo. A time course analysis of ospC expression in tissues following infection indicates also that temporal diminution of the dominant antigen OspC is impaired in lp28-1− spirochetes. Finally, passive transfer of monoclonal OspC-specific antibody into SCID mice 8 days postinfection cleared lp28-1− spirochetes, yet the wt organisms persisted in a majority of animals. These findings indicate that incomplete repression of OspC by lp28-1− organisms renders them susceptible to immune-mediated clearance. The lp28-1 plasmid must harbor one or more genes involved in OspC downregulation.
Lyme disease spirochetes are transmitted to mammalian hosts by ticks of the Ixodes genus. The complex interplay of spirochetes with the tick milieu and the vertebrate host environment necessitates adaptation by Borrelia burgdorferi throughout the infection process. These bacteria possess up to 21 plasmids, both circular and linear. Several plasmid-encoded genes are known to be differentially expressed in response to changes in temperature, pH, density, and even stage of the tick life cycle (4, 11, 13, 29-31, 35, 41). Adaptation is thus associated with the presence of the large number of extrachromosomal elements in Borrelia spirochetes, a characteristic unique to this prokaryotic genus (36).
A necessary adaptation by B. burgdorferi is the ability to evade host immunity. While the pathology from infection with this organism results largely from the induction of inflammation (32-34), survival within the mammalian host by B. burgdorferi also involves multiple mechanisms of immune evasion (reviewed in reference 8). Among these survival tactics are direct and indirect suppression of host immune responses, the incursion into immune-privileged sites, and variation of antigens. Mammalian hosts generate a vigorous immune response to B. burgdorferi, yet most fail to clear the infection (25).
Spirochetal infectivity and persistence exhibit stringent dependence on regulatory events. As indicated by recent evidence, B. burgdorferi on the brink of infection, while still in the tick, initiates or accentuates the expression of proteins that are crucial for its survival in the mammal. The expression of outer surface protein C (OspC) has long been known to be upregulated in the feeding tick (31) and serves as an example of such a protein, in that spirochetes that do not express ospC are unable to initiate infection in mice (12, 38). Continuity of the infection process through confrontation with the host's immune response appears to further depend on the ability of the organisms to then downregulate expression of surface lipoproteins, including that of OspC. Spirochetes that express OspC, and likely other antigens as well (21), are selected against when specific antibodies accumulate in the host's circulation (20). The concept that OspC expression beyond initial infection is an antigenic liability is best supported by the demonstration that spirochetes genetically manipulated to constitutively express functional OspC are completely cleared from the immunocompetent host (40).
Genes that can affect infectivity in the vertebrate host are encoded by select plasmids (37). The infectivity phenotype of B. burgdorferi spirochetes that lack linear plasmid 28-1 (lp28-1−) has been described as intermediate (28). While these spirochetes are able to initiate infection and disseminate, they do not survive beyond 3 weeks in mice (17, 18). This inability to persist is a direct result of the host's adaptive immune response. The clearance of spirochetes coincides with the onset of the antibody response and does not occur in mice with severe combined immunodeficiency (SCID) (17). We thus hypothesized that the regulated expression of one or more antigens fails in lp28-1-deficient organisms. An inability of the organism to downregulate expression of a dominant antigen that elicits borreliacidal antibody responses, we surmised, could lead to clearance by the host.
We initially sought to determine if organisms that lack lp28-1 expressed unique antigens (any not expressed by those with the full complement of plasmids) that rendered them susceptible to host immunity. Second, with OspC as the quintessential lipoprotein whose repression is crucial for spirochetal persistence, we aimed to determine if its expression was altered in the immune-susceptible isolate. The results we present here provide evidence toward the understanding of why lp28-1-deficient B. burgdorferi spirochetes fail to evade host immunity. They incriminate, moreover, genes that are potentially involved in the downregulation of ospC, a crucial mechanism of spirochetal persistence.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Two B. burgdorferi strain B31 isolates were used for all experiments: 5A19, which contains the full complement of plasmids, designated as the wild type (wt); and 5A8, which lacks only lp28-1 (28), designated as lp28-1−. Low-passage (≤8) organisms were cultured in BSK-H medium (Sigma Chemical, St. Louis, MO) supplemented with rifampin (4.5 μg/ml), phosphomycin (180 μg/ml), and amphotericin B (0.25 μg/ml) (all from Sigma) at 34°C.
Animals.
Animals were maintained as per the Guide for the Care and Use of Laboratory Animals (24) in an AAALAC-accredited animal facility. All animal research was reviewed and approved by the Tulane National Primate Research Center's Institutional Animal Care and Use Committee.
DMCs.
The growth of each isolate in dialysis membrane chambers (DMCs) was accomplished essentially as described previously (1). The initial quantity of organisms added to each bag was 5 × 105 in a 5-ml volume. Rats were anesthetized by isoflurane gas (1.5 to 2% in oxygen) via nose cone through the entire procedure and received analgesics (buprenorphine subcutaneously at 0.1 mg/kg of body weight) postoperatively. Following implantation of DMCs and suture of rat incisions, organisms were grown for 8 or 14 days. Only one chamber was implanted into each rat. Every result but the reverse transcription-PCR (RT-PCR) result was derived from the 14-day culture only, due to the suitable quantities of organisms obtained with the longer growth period. In order to control for cell density, bacterial samples collected from each DMC were counted by dark-field microscopy and the two samples with the closest concentrations (one from each isolate) were chosen for comparison. While the initial inocula were the same, growth rates varied between individual chambers. Replicates from DMCs, thus, are not true replicates because of the disparities in cell densities that led to variation. Therefore, representative samples are shown, though similar results were obtained.
Mouse infections and passive immunization.
For passive immunization with immune serum, eight mice per isolate (5A19 or 5A8) were infected via nymphal tick, so as to generate only immune (antibody) responses akin to those with natural infection. Briefly, ticks were fed with a capillary tube cultures of either 5A19 or 5A8, as described previously (14). After 3 weeks of storage at 22°C in a humidified chamber, ticks were placed on mice for feeding. For each isolate, 16 ketamine (10 mg/kg of body weight)-anesthetized mice were fed upon by placing 5 to 6 ticks on a preshaven area of the upper back. At least two ticks fed to repletion on all mice. Ticks dropped off animals and were collected 4, 5, or 6 days postplacement and then crushed to expel midgut contents. These were placed on microscope slides, dried, and fixed. The samples were stained with a fluorescein isothiocyanate-conjugated polyclonal goat anti-Borrelia species antibody (Kirkegaard & Perry, Gaithersburg, MD) and examined by fluorescence microscopy to verify the presence of spirochetes postfeeding. All samples were positive (data not shown). Blood was collected from each mouse on days 21, 28, and 34 post-tick placement. At the last time point (34 days), mice were euthanized and exsanguinated via heart puncture. The blood samples were centrifuged for 5 min at 6,050 × g; serum was removed from the blood cell pellet and stored at −20°C until it was used for passive immunization. Approximately 500 to 700 μl of serum was collected from each mouse; samples were pooled and sterilized for passive immunization by passage through 0.22-μm syringe filters. Seventeen C.B-17SCID mice (Charles River Laboratories, Wilmington, MA) were inoculated with 5A19 spirochetes, and 12 mice were inoculated with 5A8 via subcutaneous injection of a low dose (2 × 103) of organisms. After 7 days, half of the mice in each group received 100 μl immune serum from 5A8-infected mice and the other half received serum from 5A19-infected mice. Control animals (two mice per isolate) received preimmune serum instead. Eight days after passive transfer, mice were euthanized. The ear skin, heart, bladder, spleen, axillary lymph nodes, and tibiotarsal joints were removed and placed in saline. From each tissue, ∼2-mm segments were cut and placed into 5 ml of BSK-H medium at 34°C for the recovery of spirochetes, if present. All cultures were inspected for live spirochetes by dark-field microscopy after 8 to 10 days of incubation. Growth of spirochetes from any culture of tissue was scored as positive.
For passive transfer of anti-OspC antibodies, for two separate experiments, a total of 16 C.B-17SCID mice (Charles River) per spirochetal isolate were infected via subcutaneous injection of a low dose of organisms (2 × 103). At days 8 and 10 postinoculation, mice were passively immunized. On day 8 postinoculation, 11 mice per group were given an intraperitoneal injection of 100 μg (total protein) of sterile-filtered ascites fluid containing the anti-OspC immunoglobulin G2a (IgG2a) monoclonal antibody B5.1 (20, 23). On day 10, five mice in each experimental group received a second dose of anti-OspC ascites; six in each experimental group received 100 μg purified anti-OspC antibodies (a gift from Robert Gilmore, Centers for Disease Control and Prevention) that had been dialyzed against phosphate-buffered saline (PBS) and sterile filtered. As controls, five mice per group received either 100 μg of isotype control antibody (UPC-10; mouse IgG2a, κ [Sigma]) or 100 μg of control ascites at each immunization. At 14 days postinoculation, mice were euthanized and their organs were cultured for the presence of spirochetes (as described above). Each organ was also placed in RNAlater (Qiagen) for storage. If tissue culture was negative for spirochetes, RNA was isolated from tissues and examined for active infection by RT-PCR for FlaB (see “Quantitative PCR” section). All control animals were positive for infection by posteuthanasia tissue culture.
For the temporal analysis of ospC expression in tissues, 10 immunocompetent C3H/HeN mice were given a subcutaneous injection of wt organisms and 11 mice were inoculated with lp28-1− organisms (1 × 105 organisms per inoculation). At 8, 10, and 12 days postinoculation, ear punch biopsy samples (both ears) were collected from half of the mice from each group, and at days 15, 17, and 19, ear punch biopsy samples were collected from the other half. On days 12 and 19, the mice from each respective group were euthanized; hearts and bladders were collected and placed in RNAlater (Qiagen, Valencia, CA).
Quantitative RT-PCR.
RNA was extracted from in vitro-cultured bacteria and tissue samples using the RNeasy minikit (Qiagen). For heart samples, the fibrous tissue RNeasy kit was used. Following elution, RNA was quantified on a Nanodrop-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). In order to determine relative expression of OspC in each sample, same-tube (multiplex) amplification and detection using separate fluorescently labeled probes for the constitutive FlaB transcript and the OspC transcript were employed. The primer/probe sets used (Biosearch Technologies, Novato, CA) were as follows: flaB forward primer (5′-ACAGCTGAAGAGCTTGGAAT-3′), flaB reverse primer (5′-TTGCTCCAACATGAACTCTT-3′), and flaB probe (5′-6-carboxyfluorescein-aminohexyl amidite [FAM]-AACACACCAGCATCACTTTCAGGGTC-black hole quencher-1 [BHQ]-3′); and ospC forward primer (5′-TTACGGATTCTAATGCGGTTT-3′), ospC reverse primer (5′-TTTACCAATAGCTTTAGCAGCAA-3′), and ospC probe (5′-N-hexachloro-fluorescein [HEX]-6-aminohexanol-TGTGAAAGAGGTTGAAGCGTTGCTG-BHQ-3′). The Qiagen QuantiTect probe RT-PCR kit was used to amplify transcripts via the following cycling on an ABI Prism 7700 sequence detector (Applied Biosystems, Foster City, CA): RT for 30 min at 50°C and 15 min at 95°C followed by 40 cycles of 15 s at 94°C and then 1 min at 60°C. For tissue samples, 250 ng of total RNA was added to each reaction mixture. All samples were run in triplicate, with RNA from late-log-phase in vitro-cultured organisms used to generate a standard curve and a baseline for comparison of ospC expression. Relative expression and significant differences were determined using relative expression software tool analysis (26, 27).
Confocal microscopy.
Upon syringe removal from the dialysis bags, organisms were counted and pelleted gently by centrifugation (15 min at 3,000 × g). Spirochetes were washed once with 10 ml of Gibco PBS (pH 7.4) (InVitrogen, Carlsbad, CA), pelleted, and resuspended in a volume of PBS to give 6 × 106 to 7 × 106/ml. A volume of 50 μl was added to each slide, and the slides were then air dried and fixed in acetone. Bacterial smears were blocked for 1 h with blocking buffer (PBS containing 10% normal-goat serum [Invitrogen], 0.2% fish skin gelatin [Sigma], and 0.02% sodium azide [Sigma]). Following a wash with PBS, slides were stained with the primary antibodies at 1:50 dilution in blocking buffer, either anti-OspC B5.1 monoclonal antibody (0.6 mg/ml) or the anti-FlaB monoclonal antibody H9724 (3). Following a 60-min incubation at 34°C, slides were washed three times with PBS and the Alexa 488-conjugated anti-IgG2a secondary antibody (Molecular Probes/InVitrogen) at 1:1,000 in PBS was added. After 60 min of incubation and three washes, the protein stain SyPro red (Molecular Probes/InVitrogen) was diluted to 5× and added. Slides were incubated for 20 min at 34°C and then washed three times before examination. Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Optical slices were collected at a pixel resolution of 512 by 512. The green and red channels were collected simultaneously.
Immunoblotting.
Bacterial samples were harvested from DMCs, counted, pelleted by centrifugation at 2,950 × g for 10 min, and then washed twice with PBS (as described above). After removal of PBS, bacterial pellets were stored at −20°C. For immunoblotting, pellets were lysed in 2× Laemmli buffer (31.25 mM Tris-Cl, 1% sodium dodecyl sulfate, 5% glycerol, 2.5% 2-mercaptoethanol) without dye, and the protein quantity per lysate was determined with the Pierce bicinchoninic acid assay kit (Pierce, Rockford, IL). Samples were combined with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) buffer and loaded onto precast 12% polyacrylamide gels (both from Bio-Rad Laboratories, Hercules, CA) in quantities of 20 μg (1× lysate) or 5 μg (0.25× lysate) and electrophoresed. Proteins were transferred to nitrocellulose and blocked for 2 h with 5% nonfat dry milk. Anti-OspC (ascitic fluid) and anti-FlaB monoclonal antibodies (described above) were diluted to 1:400 and 1:100, respectively, and incubated for 1.5 h with gentle rocking. Following three washes with PBS, the secondary antibody, horseradish peroxidase-conjugated anti-mouse IgG plus IgA plus IgM was diluted 1:1,000 and incubated with blots for 1 h. Following three washes, blots were developed with 4-chloro-1-naphthol. Immunoblot images were captured with the Gel Logic 200 imaging system, and densitometry was performed with 1D imaging analysis software (Eastman Kodak Co., Rochester, NY).
RESULTS
lp28-1− spirochetes do not express unique antigens as targets for borreliacidal antibodies.
The clearance of lp28-1− spirochetes from the murine host coincides with the appearance of the anti-B. burgdorferi antibody response and does not occur in SCID mice (17). Thus, specific, borreliacidal antibodies must be the detriment of these organisms. Possibly, lp28-1− spirochetes express or expose distinct antigens in a way that induces and/or serves as a target for borreliacidal antibodies. If so, then the presentation of antigens that are not ordinarily expressed by organisms that have retained lp28-1 could explain the susceptibility of lp28-1− spirochetes to immune-mediated clearance. We used passive immunization with immune serum generated by infection with the same isolate (homologous) or an isolate carrying the differing plasmid complement (heterologous) to determine if the antigens were different by the specificities of antibodies each infection elicited. Here, mice from which serum was collected were previously infected using nymphal ticks that had been capillary fed the organisms so as not to induce artificial responses from antigens (e.g., OspA) that are expressed in vitro but not following a natural transmission. SCID mice were given a low dose (2 × 103) inoculum of B. burgdorferi. Seven days postinoculation, the mice were passively immunized with immune serum generated by infection with the homologous isolate or the heterologous isolate. The results are presented in Table 1. Each infection with the lp28-1− isolate was cleared by immune serum, irrespective of the isolate used to generate that serum, and hence, the specific antibodies. This was evident by the absence of spirochetes in cultures of mouse organs following the passive transfer of antibody. The wt isolate, which harbors the full complement of 21 plasmids, was more resistant to clearance by immune serum, as it survived challenge with homologous serum in two of five infections and with heterologous (lp28-1−) serum in four of five infections. This difference was not significant (P = 0.523, two-tailed Fisher's exact test). Thus, the antigens or epitopes that elicit specific antibodies by wt spirochetes must be shared targets for borreliacidal antibodies that kill lp28-1− organisms. Otherwise, serum from a wt infection would not kill lp28-1− organisms.
TABLE 1.
Passive immunization of infected SCID mice with homologous and heterologous immune sera
| Infection | Antiserum | No. of mice positive/total no. of micea |
|---|---|---|
| lp28-1+ (wt) | Anti-lp28-1− | 4/5 |
| Anti-wt | 2/5 | |
| Preimmune | 2/2 | |
| lp28-1− | Anti-lp28-1− | 0/7 |
| Anti-wt | 0/8 | |
| Preimmune | 2/2 |
By culture of organ tissue in B. burgdorferi growth medium.
This led us to reason that perhaps one or more antigens expressed upon initial infection are downregulated by the wt to evade the antibody response but not by the lp28-1 plasmid-deficient isolate. Among the B. burgdorferi antigens that dominate the early response to B. burgdorferi is OspC. OspC is an outer surface lipoprotein and could thus be a target for opsonizing and/or otherwise borreliacidal antibodies. Also, downregulation of ospC expression is critical for survival of the spirochetes (39). Thus, this antigen became the focus of exploration into this immune susceptibility phenomenon.
OspC expression levels by lp28-1− organisms are abnormally high in vivo.
Inspection of the expression levels of OspC by the two different isolates in the mammalian host requires growth and adaptation by the spirochetes inside the host and enough organisms to evaluate differences in specific RNA and protein levels. To accomplish this, the lp28-1− and wt isolates were grown for either 8 or 14 days in DMCs that were implanted into rat peritoneal cavities. RNA was extracted from the in vivo-cultured organisms, and the relative expression levels of ospC transcripts were determined by quantitative RT-PCR. Representative results, presented in Table 2, show changes in expression relative to values obtained from in vitro-cultured organisms. Expression levels of ospC, as expected, increased after 8 days in the mammalian environment. The extent of increase was significantly higher for lp28-1− organisms. By day 14, no significant difference in ospC expression in relation to in vitro-cultured organisms was apparent for the wt isolate (lp28-1+), but the lp28-1− isolate continued to express ospC at a level >3-fold higher than that of in vitro-cultured organisms (Table 2). This experiment was repeated and gave similar results, but as mentioned in Materials and Methods, only cell-density-controlled comparisons should be made.
TABLE 2.
Relative expression of ospC by spirochetes grown in rat DMCs
| Infection | Expression ata:
|
|||
|---|---|---|---|---|
| 8 days in vivo
|
14 days in vivo
|
|||
| Total amt of RNA (ng) | Relative increase | Total amt of RNA (ng) | Relative increase | |
| lp28-1+ (wt) | 24 | 1.44 | 25 | 1.097 |
| lp28-1− | 24 | 17.952* | 25 | 3.486* |
Relative expression was normalized by FlaB transcript compared to 20 ng of late-log-phase, in vitro-cultured B31 wt RNA. *, significant difference (P ≤ 0.05).
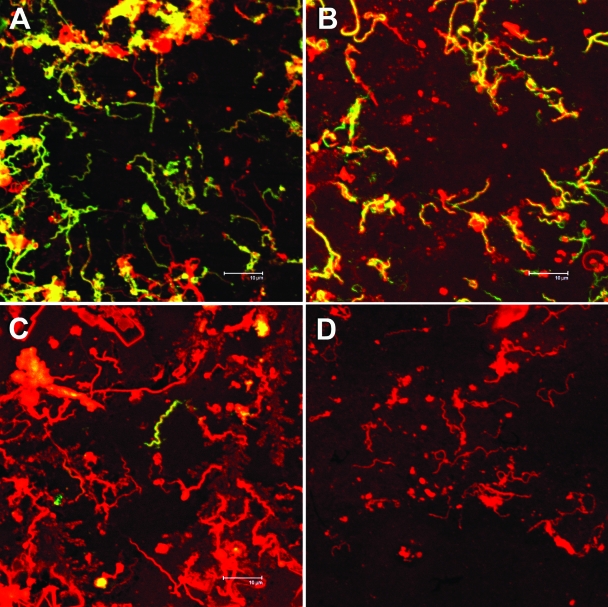
The results for ospC transcript levels were corroborated by examination of the OspC protein production by in vivo-cultured organisms using confocal microscopy and immunoblotting. For confocal imagery, organisms were collected from the dialysis bags, washed, and fixed with acetone for staining. Thus, proteins on the surface and interior of the spirochetes were exposed. The images in Fig. 1 show organisms grown for 14 days in vivo. The stain for total protein (to detect spirochetes), in red, and with an anti-OspC monoclonal antibody, in green, reveals differences in the levels of expressed OspC protein between the isolates. The OspC lipoprotein is manifest in a higher proportion of the lp28-1-deficient organisms (Fig. 1A). While OspC repression is incomplete for those organisms that harbor the plasmid, the levels are visibly lower (Fig. 1C) than in their counterpart, plasmid-deficient, isolate. Positive control staining with antiflagellin antibody and a negative control with secondary antibody alone are also shown (Fig. 1B and D, respectively). Aggregation of the organisms makes a quantitative determination difficult, so representative images from repeated experiments are shown (Fig. 1). This was performed with three different preparations of each isolate with similar results.
FIG. 1.
Confocal microscopy images of spirochetes grown in DMCs. Photographs show staining for total protein with SyPro red (red) and either anti-OspC (A and C), anti-FlaB (B), or the secondary antibody only (D) detected with Alexa-488 (green). Shown are 14-day cultures of the lp28-1− isolate 5A8 (A) and the wt isolate 5A19 (B, C, and D).
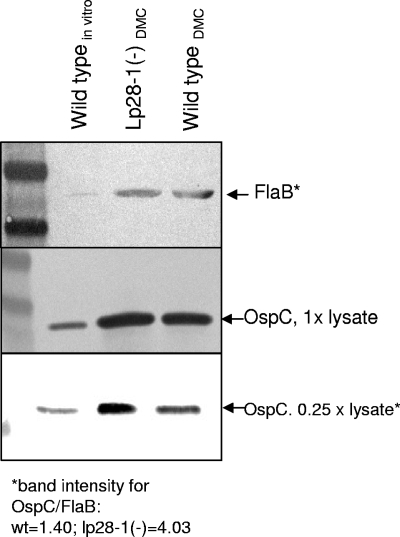
Figure 2 shows proteins detected by immunoblotting. OspC levels, shown by dilution of protein, appear to correspond well with the relative quantities of ospC mRNA, where the lp28-1− isolate shows levels ∼3-fold higher than those of the wt isolate. The confocal images show more dramatic differences, but these images present the proportion of OspC-expressing organisms and not the total levels of OspC expression across the isolate population. The densities of organisms in these in vivo cultures were nearly identical, suggesting that the disparity in OspC expression between the isolates is not simply a function of growth phase.
FIG. 2.
Immunoblot detection of OspC and FlaB (loading control) protein from isolates grown for 14 days in DMCs. Lane 1, in vitro-grown B31.5A19 (wt), early log phase; lane 2, lp28-1− cells from DMCs (1.88 × 107/ml); lane 3, wt from DMCs (1.93 × 107/ml). The band intensities shown were determined by densitometry for comparison of DMC samples only. The in vitro-grown sample was included as a positive control for the antigen.
Growth in the DMC provides the organism with a host-like environment with regard to temperature, pH, and small solutes. However, the organisms do not encounter host immunity and its associated chemical mediators, they do not engage in adherence, nor do they encounter possible tissue microenvironment-specific signals. Thus, examination of ospC expression levels in an immunocompetent host over time and in different tissues was conducted.
Temporal diminution of the dominant antigen OspC may be impaired in lp28-1− spirochetes.
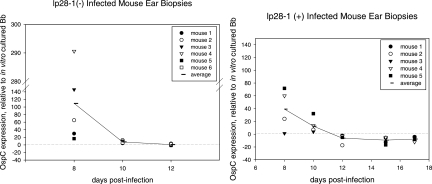
In this experiment, C3H/HeN mice were infected with either the wt or lp28-1− isolate. At various time points postinfection, tissues were collected and the quantities of ospC transcript expressed by spirochetes in the ears, hearts, and bladders were assessed. This method allowed for the assessment of transcript levels as a function of time and tissue environment. The results are presented as the expression ratio of ospC transcript normalized to the constitutively expressed FlaB transcript compared to transcript levels in in vitro-cultured organisms. Note that for days 8 to 12 and 15 to 19, each time point value was taken from the same mouse. As shown in Fig. 3, for spirochetes in ear tissue, the expression ratio of ospC transcript normalized by flaB initially (day 8) was markedly higher than the ospC transcript levels expressed in vitro and then declined substantially (days 8 to 10). In wt-infected mice, ospC mRNA levels dropped below baseline (the level expressed by late-log-phase, in vitro-cultured organisms) beginning on day 12. Conversely, the levels of ospC mRNA in the lp28-1− isolate fell to values closer to baseline levels but did not cease. At the time when ospC repression is evident for wild-type organisms, by negative values for the expression ratio, the lp28-1− organisms are no longer detectable, a result that indicates that they have been cleared by the host.
FIG. 3.
OspC transcript expression levels as a function of time in ear biopsy samples of mice infected with the lp28-1− isolate 5A8 (left panel) and the wt isolate 5A19 (right panel). ospC expression was normalized by FlaB transcript and compared to mRNA from 5A19 grown in vitro to the late log phase (represented by a dotted line equal to a value of 1). By day 15, no transcript was detected in the ears of 5A8-infected mice.
The expression ratios for ospC in heart and bladder tissues on days 12 and 19 are presented in Table 3. The discrepancy in ospC transcript between organisms that possess lp28-1 and those that do not is most evident in heart tissues (day 12). Here the average expression ratio by spirochetes with lp28-1 is −19.24, whereas those that do not have this plasmid are still overexpressing ospC with an average ratio of +10.99. By day 19, no lp28-1− organisms were detected in either tissue, whereas wt levels were significantly decreased, with averages of −47.75 and −14.29 in the heart and bladder, respectively.
TABLE 3.
Relative expression of ospC in heart and bladder tissue of infected mice
| Tissue and mouse no. | Relative expression at:
|
|||
|---|---|---|---|---|
| Day 12
|
Day 19
|
|||
| lp28-1− | lp28-1+ | lp28-1− | lp28-1+ | |
| Heart | ||||
| 1 | +12.70 | −5.22 | NDa | No OspCb |
| 2 | +9.72 | −73.54 | ND | −92.32 |
| 3 | ND | −8.27 | ND | −13.86 |
| 4 | ND | −5.41 | ND | −37.08 |
| 5 | +10.56 | −3.76 | ND | No OspC |
| 6 | ND | ND | ||
| Avg | +10.99 | −19.24 | ND | −47.75 |
| Bladder | ||||
| 1 | +3.47 | −1.43 | ND | −34.03 |
| 2 | +2.71 | −5.79 | ND | No OspC |
| 3 | +6.05 | -4.04 | ND | −7.33 |
| 4 | +4.87 | −3.21 | ND | −8.07 |
| 5 | ND | −2.42 | ND | −7.73 |
| 6 | ||||
| Avg | +4.27 | −3.38 | ND | −14.29 |
ND, no spirochetal RNA detected.
Only FlaB transcript detected.
These results suggest that lp28-1− organisms are competent for the induction of ospC expression initiated by transit into the host but do not cease expression entirely when this is required for survival. The positive regulation of ospC is RpoS mediated. Lysates prepared from the wt and lp28-1− isolates grown in vitro to different densities (early log, late log, and stationary) were examined by immunoblotting. RpoS was present, and its quantity increased for both isolates, as did OspC levels) as the density increased (data not shown).
This experiment demonstrates that ospC expression in lp28-1− and wt spirochetes is differentially regulated in an immunocompetent host and in different tissue environments. The question remained as to whether this difference was sufficient to determine the failure of lp28-1-deficient spirochetes to evade the immune response.
The absence of downregulated OspC expression in vivo by lp28-1− spirochetes results in specific antibody-mediated clearance.
To test whether lp28-1− spirochetes could be differentially cleared with anti-OspC antibody, SCID mice were inoculated with either the wt or the lp28-1− isolate. Following the initial host adaptation phase, where ospC expression is necessary, mice were subjected to passive immunization with OspC-specific monoclonal antibody. Based on previous findings (20), we reasoned that a proportion of wt organisms would be OspC nonexpressers and would survive the antibody assault. If, however, the organisms could not repress ospC expression, then they should be cleared from the host. The results, determined by organ culture or quantitative RT-PCR, are presented in Table 4.
TABLE 4.
Passive immunization of infected SCID mice with anti-OspC antibodies
| Isolate | Antibodya | No. of mice positive/total no. of miceb | P valuec |
|---|---|---|---|
| wt | Control | 5/5 | |
| Anti-OspC | 7/11 | 0.2445* | |
| lp28-1− | Control | 5/5 | |
| Anti-OspC | 0/11 | 0.0038†§ |
For the control antibody, three of five mice received unrelated ascites and two of five received an isotype control. The OspC antibody was monoclonal antibody B5.1.
By organ culture or quantitative RT-PCR.
*, P value for wt isolates with anti-OspC versus wt isolates with control antibody; †, P value for wt isolates with anti-OspC versus lp28-1− isolates with anti-OspC; §, significantly different.
The mice received a low-dose spirochete inoculum (2 × 103) and were passively immunized with the monoclonal anti-OspC antibody or control antibody on days 8 and 10. Four days later, mice were euthanized; organs were cultured and tested by quantitative RT-PCR for the flaB transcript to assess survival. In 7 of 11 mice (64%), the wt isolate survived challenge with anti-OspC antibodies and could be recovered from mice. On the contrary, lp28-1− organisms could not be recovered from or detected in any of the mice that received anti-OspC antibodies. Using a two-tailed Fisher's exact test for comparison of the two proportions, this difference was determined to be significant (P = 0.0038). The difference in survivability by wt organisms challenged with either control or anti-OspC antibody was not significant (P = 0.2445).
DISCUSSION
In this communication, we have provided evidence to explain, in part, the immune susceptibility phenotype of B. burgdorferi spirochetes that have lost lp28-1. The OspC lipoprotein must not be expressed in vivo long after the onset of humoral immunity if spirochetes are to survive. While the expression of ospC may not be completely terminated throughout the spirochete population in all mouse tissues at this time (days 15 to 19 postinfection), its expression is substantially downregulated. Low-level expression may yet occur in immune-privileged sites or in aggregates of the spirochetes, but failure to terminate OspC expression by individual spirochetes is an antigenic liability by most convincing experimental results (39, 40).
The lp28-1 plasmid is comprised of 32 open reading frames designated BBF01 through BBF32. Many of these open reading frames are pseudogenes, frameshifted genes, or genes that include sequences duplicated on other plasmids (5, 10). The vlsE (BBF32) gene product is a lipoprotein that engages in antigenic variation (42, 43); the gene is retained among infectious isolates (15, 16). However, the specific function of VlsE is as yet unknown. Notably, a recent report (2) demonstrated by targeted deletion that VlsE is necessary for spirochete survival in an immunocompetent host, prompting a possible notion that loss of VlsE alone explains the lp28-1− phenotype. VlsE is an antigen that varies throughout the course of infection: lp28-1− organisms are killed by the antibody response. Were VlsE not present, one less target of the immune response would exist. VlsE could be directly involved in immune evasion by providing a “cloak” of variable antigen that restricts humoral immunity or by binding host serum proteins to avoid recognition. In light of reports that VlsE is the predominant antigen produced by host-adapted spirochetes in infected rabbits and SCID mice, these possibilities remain tenable (6, 7). Alternatively, if membrane topology were altered by loss of VlsE, thereby exposing “new” epitopes or antigens, its absence could be related to the known mechanism of clearance by immune susceptibility in lp28-1− organisms. Because OspC is already a dominant antigen that is downregulated at the advent of specific immunity as VlsE expression is upregulated (22), this is an unlikely scenario insofar as OspC is involved. VlsE may well displace OspC in the spirochetal membrane, but transcription of the ospC gene must be repressed (39).
Another explanation for the immune susceptibility of lp28-1− organisms would be that the VlsE protein itself affects the expression of the ospC gene. Because VlsE is a lipoprotein, the probability that it would act directly as a DNA-binding protein is low. Another lp28-1 gene that encodes a lipoprotein is BBF01. The expression of the BBF01 gene appears to parallel that of vlsE (22), and antibody to its gene product, arthritis-related protein (Arp), has been shown to reduce arthritis severity (9). While the function of BBF01/Arp remains largely unknown, consideration of this gene product in immune resistance is not warranted. The infectious B. burgdorferi strain 297, as well as other infectious human isolates (16), possesses only portions of lp28-1 that exclude BBF01. Additionally, the authors of the VlsE deletion report (2) demonstrated that infectivity was retained when a region encompassing only the genes BBF20 through BBF32 (vlsE) of lp28-1 was present; this infectivity was not demonstrated by complementation with a vlsE gene capable of antigenic variation alone. In fact, when an lp28-1-deficient isolate was complemented with the vlsE gene alone, infectivity was not restored (19). This construct lacked sequence variation, so either an intact vls antigenic variation locus or another determinant on lp28-1 is required for infectivity. Thus, a different lp28-1 gene may affect ospC expression.
Because OspC is a primary target of the antibody response in mammalian infection, and we found that no unique antigens were presented by lp28-1− organisms, we used this lipoprotein as a basis for our immune susceptibility experiments. The possibility that other lipoproteins are not appropriately regulated by loss of lp28-1 remains tenable. However, the significance of ospC regulation to immune evasion may overshadow the effect of other antigens on the demise of lp28-1− organisms within a mammalian host.
The results presented here do not delineate the mechanism whereby ospC would be overexpressed by spirochetes that lack lp28-1, but they shed light on the role of this plasmid in immune evasion. Whether vlsE/VlsE is directly, indirectly, or not at all involved in the regulation of OspC, one or more elements between BBF20 and BBF32 likely influence ospC expression. Hence, our results may lead to a better understanding of gene regulation critical for the ability of B. burgdorferi spirochetes to engage in persistent infection.
Acknowledgments
We are grateful to Steve Norris for the 5A8 and 5A19 isolates, Robert Gilmore for the gift of anti-OspC monoclonal antibody, Mary B. Jacobs for technical help, and Lara Doyle for veterinary assistance.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 1012240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bankhead, T., and G. Chaconas. 2007. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol. Microbiol. 651547-1558. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, A. G., S. F. Hayes, R. A. Heiland, M. E. Schrumpf, and S. L. Tessier. 1986. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect. Immun. 52549-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 673181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35490-516. [DOI] [PubMed] [Google Scholar]
- 6.Crother, T. R., C. I. Champion, J. P. Whitelegge, R. Aguilera, X.-Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 725063-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crother, T. R., C. I. Champion, X.-Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 713419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Embers, M. E., R. Ramamoorthy, and M. T. Philipp. 2004. Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease. Microbes Infect. 6312-316. [DOI] [PubMed] [Google Scholar]
- 9.Feng, S., E. Hodzic, and S. W. Barthold. 2000. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect. Immun. 684169-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390580-586. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3799-808. [DOI] [PubMed] [Google Scholar]
- 12.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 1013142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, and D. R. Akins. 2002. Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 703468-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indest, K. J., J. K. Howell, M. B. Jacobs, D. Scholl-Meeker, S. J. Norris, and M. T. Philipp. 2001. Analysis of Borrelia burgdorferi vlsE gene expression and recombination in the tick vector. Infect. Immun. 697083-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer, R., J. M. Hardham, G. P. Wormser, I. Schwartz, and S. J. Norris. 2000. Conservation and heterogeneity of vlsE among human and tick isolates of Borrelia burgdorferi. Infect. Immun. 681714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer, R., O. Kalu, J. Purser, S. Norris, B. Stevenson, and I. Schwartz. 2003. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect. Immun. 713699-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 714608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrenz, M. B., R. M. Wooten, and S. J. Norris. 2004. Effects of vlsE complementation on the infectivity of Borrelia burgdorferi lacking the linear plasmid lp28-1. Infect. Immun. 726577-6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang, F. T., J. Yan, M. L. Mbow, S. L. Sviat, R. D. Gilmore, M. Mamula, and E. Fikrig. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 725759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mbow, M. L., R. D. Gilmore, Jr., and R. G. Titus. 1999. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect. Immun. 675470-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 25.Nocton, J. J., and A. C. Steere. 1995. Lyme disease. Adv. Intern. Med. 4069-117. [PubMed] [Google Scholar]
- 26.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 9713865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramamoorthy, R., and M. T. Philipp. 1998. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect. Immun. 665119-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramamoorthy, R., and D. Scholl-Meeker. 2001. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect. Immun. 692739-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seiler, K. P., and J. J. Weis. 1996. Immunity to Lyme disease: protection, pathology and persistence. Curr. Opin. Immunol. 8503-509. [DOI] [PubMed] [Google Scholar]
- 33.Sigal, L. H. 1997. Lyme disease:a review of aspects of its immunology and immunopathogenesis. Annu. Rev. Immunol. 1563-92. [DOI] [PubMed] [Google Scholar]
- 34.Steere, A. C., and L. Glickstein. 2004. Elucidation of Lyme arthritis. Nat. Rev. Immunol. 4143-152. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 634535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart, P. E., R. Byram, D. Grimm, K. Tilly, and P. A. Rosa. 2005. The plasmids of Borrelia burgdorferi: essential genetic elements of a pathogen. Plasmid 531-13. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, V., J. Anguita, S. Samanta, P. A. Rosa, P. Stewart, S. W. Barthold, and E. Fikrig. 2001. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect. Immun. 693507-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilly, K., A. Bestor, M. W. Jewett, and P. Rosa. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 751517-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, Q., K. McShan, and F. T. Liang. 2007. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol. Microbiol. 64220-231. [DOI] [PubMed] [Google Scholar]
- 40.Xu, Q., S. V. Seemanapalli, K. McShan, and F. T. Liang. 2006. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect. Immun. 745177-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 371470-1479. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89275-285. (Erratum, 96:447, 1999.) [DOI] [PubMed] [Google Scholar]
- 43.Zhang, J.-R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 663689-3697. (Erratum, 67:468, 1999.) [DOI] [PMC free article] [PubMed] [Google Scholar]