Abstract
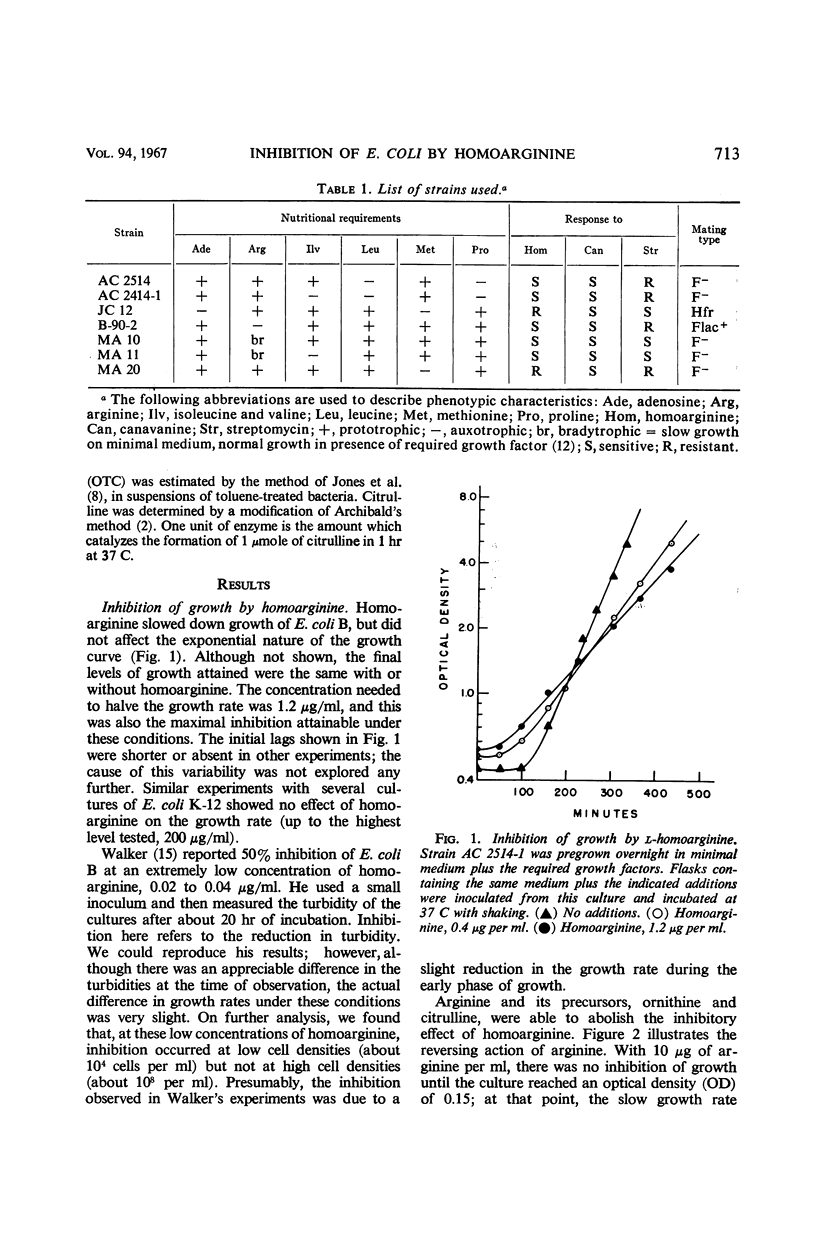
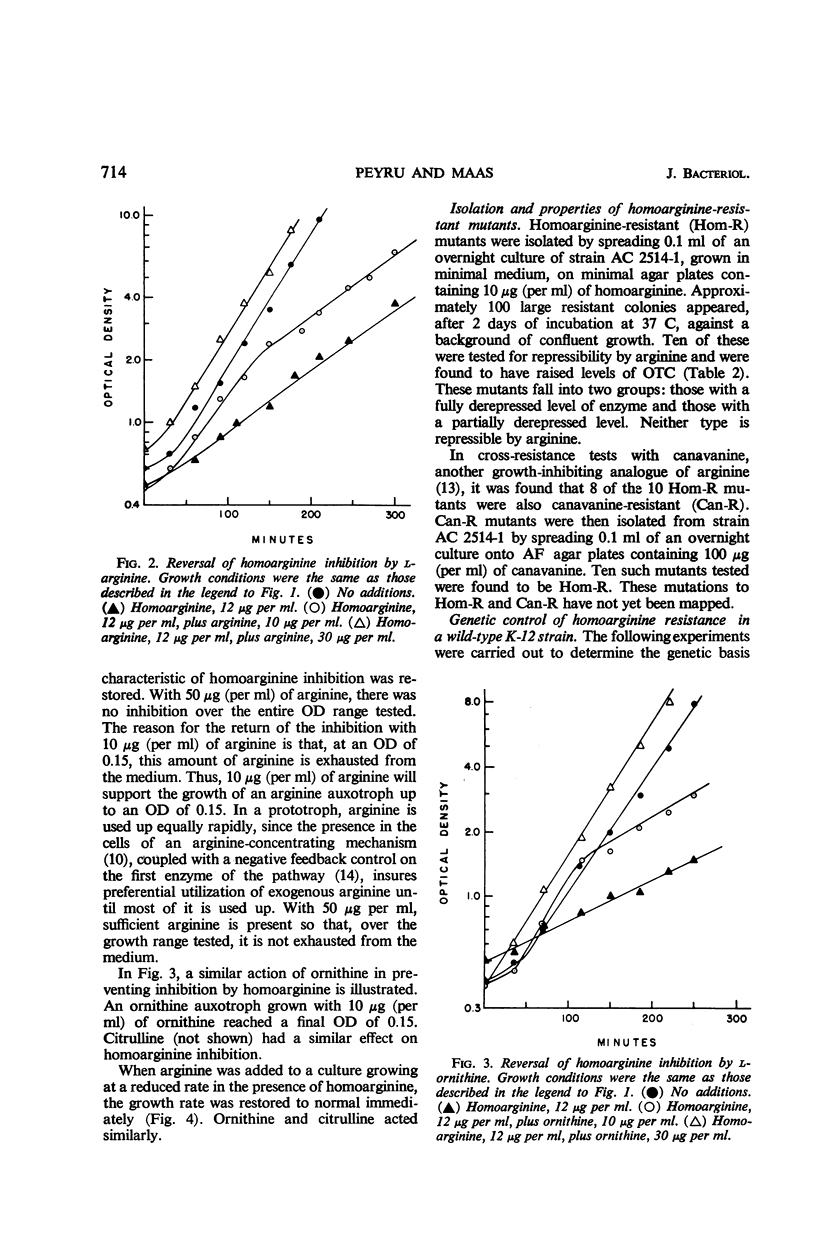
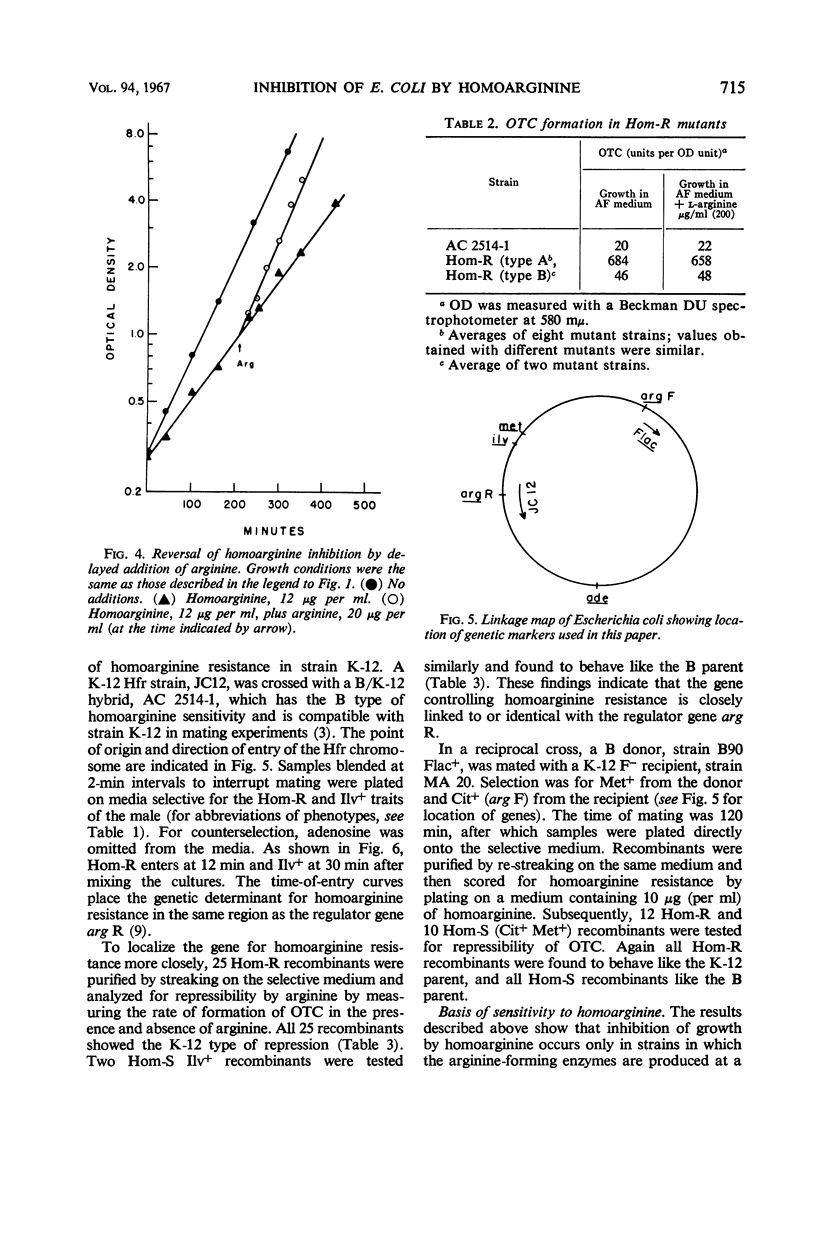
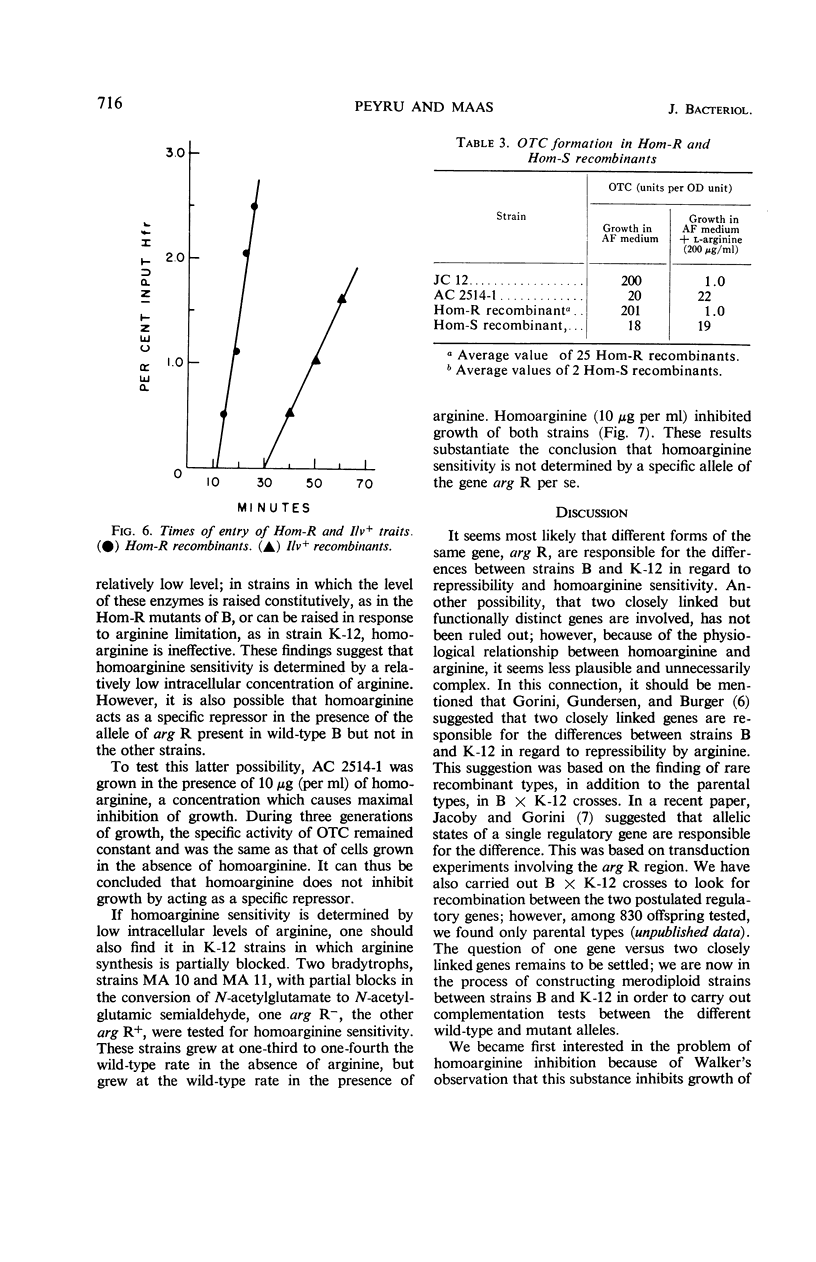
Homoarginine inhibits the growth of Escherichia coli B, but not of E. coli K-12. These two strains also differ in regard to repressibility of the arginine-forming enzymes. In K-12, arginine acts as a repressor whereas in B it does not. The latter difference is determined by different alleles of a regulator gene, arg R. In K-12 × B crosses, it was shown that the genetic determinant for homoarginine sensitivity is closely linked to or identical with arg R. Homoarginine-resistant mutants of B were isolated. The biochemical mechanism of homoarginine inhibition is not known. However, whether or not a strain is sensitive to homoarginine seems to depend on the intracellular level of arginine. In B this level is relatively low and inflexible as a result of the action of a repressor whose formation is determined by the B-specific allele of arg R.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER H. GENETIC CONTROL OF RESTRICTION AND MODIFICATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1652–1660. doi: 10.1128/jb.88.6.1652-1660.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- Maas W. K. Genetic defects affecting an arginine permease and repression of arginine synthesis in Escherichia coli. Fed Proc. 1965 Sep-Oct;24(5):1239–1242. [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. Multiple pathways of putrescine biosynthesis in Escherichia coli. J Biol Chem. 1966 Jul 10;241(13):3129–3135. [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ J. H., MAAS W. K. Analysis of the inhibition of growth produced by canavanine in Escherichia coli. J Bacteriol. 1960 Jun;79:794–799. doi: 10.1128/jb.79.6.794-799.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VYAS S., MAAS W. K. Feedback inhibition of acetylglutamate synthetase by arginine in Escherichia coli. Arch Biochem Biophys. 1963 Mar;100:542–546. doi: 10.1016/0003-9861(63)90124-3. [DOI] [PubMed] [Google Scholar]
- WALKER J. B. Canavanine and homoarginine as antimetabolites of arginine and lysine in yeast and algae. J Biol Chem. 1955 Jan;212(1):207–215. [PubMed] [Google Scholar]


