Abstract
The Dr family of Escherichia coli adhesins are virulence factors associated with diarrhea and urinary tract infections. Dr fimbriae are comprised of two subunits. DraE/AfaE represents the major structural, antigenic, and adhesive subunit, which recognizes decay-accelerating factor (DAF) and carcinoembryonic antigen (CEA)-related cell adhesion molecules (CEACAMs) CEA, CEACAM1, CEACAM3, and CEACAM6 as binding receptors. The DraD/AfaD subunit caps fimbriae and has been implicated in the entry of Dr-fimbriated E. coli into host cells. In this study, we demonstrate that DAF or CEACAM receptors independently promote DraE-mediated internalization of E. coli by CHO cell transfectants expressing these receptors. We also found that DraE-positive recombinant bacteria adhere to and are internalized by primary human bladder epithelial cells which express DAF and CEACAMs. DraE-mediated bacterial internalization by bladder cells was inhibited by agents which disrupt lipid rafts, microtubules, and phosphatidylinositol 3-kinase (PI3K) activity. Immunofluorescence confocal microscopic examination of epithelial cells detected considerable recruitment of caveolin, β1 integrin, phosphorylated ezrin, phosphorylated PI3K, and tubulin, but not F-actin, by cell-associated bacteria. Finally, we demonstrate that the DraD subunit, previously implicated as an “invasin,” is not required for β1 integrin recruitment or bacterial internalization.
Urinary tract infections (UTIs) represent the most common kidney and urologic disease, affecting more than 60% of women during their lifetime (17, 18). A common feature of UTI is its recurrence. About 25% of women with a first UTI have a second episode within 6 months (16). Escherichia coli is the most common etiologic agent, causing more than 80% of all UTIs. One E. coli virulence factor associated with UTI is the Dr family of adhesins. The expression of Dr adhesins has been associated with a twofold increased risk of a second episode of UTI (18). The Dr family of adhesins includes DraE, DaaE, AfaEI, AfaEIII, AfaEV, NfaE, and others (52). Dr adhesins are encoded by highly homologous gene clusters which include genes for transcriptional activators, a periplasmic chaperone (DraB/AfaB), an usher (DraC/AfaC), a surface protein with invasive properties (DraD/AfaD), a small protein associated with a novel translation-dependent mRNA cleavage mechanism (DraP) (35), and the major structural subunit which also serves as the adhesin (DraE/AfaE) (52). Recently, it was shown that Dr fimbriae are capped at the tip by the DraD/AfaD subunit joined to the terminal DraE/AfaE subunit via donor strand complementation (2). DraD/AfaD is not required for fimbrial synthesis or fimbria-mediated adherence (19, 55).
DraE and other related Dr adhesins recognize decay-accelerating factor (DAF) (46, 47) and carcinoembryonic antigen (CEA)-related cell adhesion molecules (CEACAMs) as binding receptors (3, 31). DAF is a glycosylphosphatidylinositol (GPI)-anchored membrane protein which contributes to the protection of host tissues from damage by autologous complement by inhibiting the formation of C3 and C5 convertases and accelerating their decay (45). DAF is present on a variety of epithelial surfaces, including the gastrointestinal mucosa, exocrine glands, renal pelvis, ureter, bladder, cervix, and uterine mucosa (39). The CEACAM family is a group of highly glycosylated cell surface intercellular adhesion molecules. CEA, CEACAM1, CEACAM3, and CEACAM6 have been shown to serve as receptors for the DraE adhesin (3, 30). CEACAM1 and CEACAM3 are inserted into the cellular membrane via a carboxy-terminal transmembrane and cytoplasmic domain, while CEA and CEACAM6 are anchored to the membrane via GPI (27). The attachment of Dr-expressing (Dr+) bacteria to epithelial cells induces clustering of DAF and CEACAMs at the sites of bacterial adherence (25). Recognition of CEA and CEACAM6 receptors by Dr adhesins is accompanied by activation of Cdc42 and phosphorylation of ezrin/radixin/moesin (ERM), which triggers the reorganization of the cellular actin cytoskeleton (3).
Dr+ E. coli organisms are able to enter and replicate within epithelial cells in vitro (22, 24, 51). This process has been proposed to play an important role in recurrent and chronic UTIs (52). Dr-fimbriated E. coli cells enter eukaryotic cells via a zipper-like mechanism which is dependent on dynamic microtubules, lipid rafts, and α5β1 integrin (22, 24, 29). AfaD, encoded by the afa-3 operon, has been implicated as an invasin capable of triggering the internalization of E. coli by eukaryotic cells (28, 49). DraD, encoded by the dra operon, is identical in sequence to AfaD. Adherent Dr-fimbriated bacteria trigger the recruitment of β1 integrins to the site of bacterial attachment, and AfaD binds directly to α5β1 and αvβ3 integrins with relatively low affinities (12, 49). It remains uncertain whether recruitment of β1 integrins by Dr-fimbriated E. coli requires the integrin-binding activity of DraD/AfaD or if integrin recruitment results from engagement of DAF and CEACAM receptors by the adhesin. Furthermore, conflicting data have been reported regarding the ability of laboratory strains expressing the Dr adhesin family operon afa-3 with inactivated afaD to invade HeLa cells (28, 49). An alternative model of internalization has proposed the involvement of the Dr adhesin in attachment and invasion of epithelial cells through its interaction with DAF (14, 22, 51). It has been demonstrated that the hydrophilic domain II of DraE and the complement control protein domain 3 and GPI anchor of DAF are critical for internalization of Dr+ E. coli (14, 22, 51). However, these studies have not directly addressed the role of adhesin recognition of CEACAM receptors in E. coli internalization. It has been demonstrated that CEACAM receptors are involved in Neisseria gonorrhoeae and Neisseria meningitidis internalization by epithelial cells (4, 23, 40), and Dr adhesins recognize the same CEACAM domains as the neisseriae (33).
Here we employ CHO cell transfectants, Caco-2 cells, and primary human bladder epithelial cells to address (i) the relative roles of DAF and CEACAMs in the internalization of Dr-fimbriated E. coli, (ii) the role of DraD in the recruitment of β1 integrins to the sites of adherent bacteria, and (iii) the role of DraD in the internalization of Dr-fimbriated E. coli.
Our results suggest that DraE interactions with both DAF and CEACAMs play key roles in pathogenesis of UTI caused by E. coli. We were unable to demonstrate such a role for DraD.
MATERIALS AND METHODS
Reagents and antibodies.
α5β1 integrin was obtained from Chemicon International. The polyclonal antiserum against fimbriae comprised of DraE-DraD was obtained previously in our laboratory. PP2 {4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine} and wortmannin were obtained from Calbiochem EMD Biosciences. Methyl-β-cyclodextrin (MβCD), nocodazole, and anti-tubulin mouse antibody were obtained from Sigma-Aldrich. Rabbit polyclonal antibodies against DAF, phosphorylated forms of phosphatidylinositol 3-kinase (PI3K), and ezrin (H-100) and a mouse monoclonal antibody against CEA were from Santa Cruz Biotechnology Inc. Polyclonal rabbit antibodies against caveolin, a mouse monoclonal antibody against DAF (IA10), and a rat monoclonal antibody against β1 integrin were obtained from BD Pharmingen, Transduction Labs. A rat monoclonal antibody against CEACAMs (YTH71.3) was obtained from Abcam Inc. Alexa Fluor 488-phalloidin and all secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 555 were purchased from Molecular Probes, Invitrogen, Inc. Enzymes were purchased from New England Biolabs (Beverly, MA) and used as recommended by the manufacturer.
Bacterial strains and eukaryotic cell lines.
Bacterial strains were grown in Luria-Bertani (LB) medium at 37°C. Derivatives of pET-21d and pCC90 were grown in 100 μg/ml ampicillin or carbenicillin. Derivatives of pUC-Cm were grown in the presence of 25 μg/ml chloramphenicol. E. coli AAEC191A was used as a host strain for the expression of Dr adhesin variants. Strain AAEC191A does not express type 1 fimbriae (5). Strain BL21(DE3) (Novagen, San Diego, CA) was the host for the pET-21d plasmid.
Chinese hamster ovary (CHO) cell transfectant clones that express human DAF, CEA, CEACAM1, CEACAM6, and CEACAM3 (CHO-DAF, CHO-CEA, CHO-CEACAM1, CHO-CEACAM6, and CHO-CEACAM3 cells, respectively) or the vector alone were used (3, 36). Clones expressing the receptors were selected by several rounds of sorting by flow cytometry with antibodies against DAF (IA10) or CEACAMs (YTH71.3). Cells were cultured in Ham's F12 medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 μg/ml of streptomycin. CHO-CEA, CHO-CEACAM1, and CHO-CEACAM6 cells were grown with 400 μg/ml of hygromycin B, while the CHO-CEACAM3 cells were grown with 1 mg/ml of hygromycin B. CHO-DAF cells were maintained in Geneticin at 25 μg/ml.
Primary cultures of human bladder epithelial cells were established from discarded surgical specimens obtained in accordance with human subject regulations at University of Washington, using standard tissue culture techniques (10). Bladder cells were maintained in keratinocyte-SFM medium with supplements (Invitrogen) and without antibiotics. Ten percent FBS was added to the medium 24 h before the experiment to stimulate cell differentiation (13).
The Caco-2 human colorectal carcinoma cell line was grown in Dulbecco's modified Eagle medium containing 10% FBS supplemented with 100 μg/ml streptomycin and 100 U/ml penicillin.
Plasmids.
The plasmid constructs utilized for this work are listed in Table 1. In preparing a construct for the purification of DraD protein, the sequence corresponding to the mature DraD amino acid sequence was PCR amplified using pCC90 as a template. To construct donor-strand-complemented DraD, we followed the strategy used for AfaE and DraD (2, 12) by moving the N-terminal 13 amino acids of the mature DraE protein to the C terminus of DraD to complete the structure formed by donor strand complementation.
TABLE 1.
Plasmids and corresponding protein products
| Plasmid | Expression vector | Description | Reference |
|---|---|---|---|
| pCC90 | pACYC177 | Expresses Dr operon (draBCDPE); expresses Dr fimbriae; DraE+ DraD+ phenotype | 9 |
| pCC90-DraEstop | pACYC177 | Expresses Dr operon (draBCDP); does not express DraE due to a stop codon at amino acid 54 in DraE; DraE− DraD+ phenotype | 14 |
| pCC90-DraDstop | pACYC177 | Expresses Dr operon (draBCPE); does not express DraD due to a stop codon at amino acid 31 in DraD; DraE+ DraD− phenotype | This study |
| pCC90-ΔSacI | pACYC177 | Expresses Dr operon (draBC); does not express DraD and DraE due to deletion of these genes; DraE− DraD− phenotype | This study |
| pUC-R | pUC-Cm | Expresses DraE adhesin | 54 |
| pUC-R-RFP | pUC-Cm | Expresses DraE adhesin and RFP | 33 |
| pUC-R-D61A | pUC-Cm | Expresses DraE adhesin with D61A mutation | 54 |
| pUC-R-R86G | pUC-Cm | Expresses DraE adhesin with R86G mutation | 31 |
| pUC-NfaE | pUC-Cm | Expresses NfaE adhesin | 32 |
| pUC-NfaE-RFP | pUC-Cm | Expresses NfaE adhesin and RFP | This study |
| pET-DraD-dsc | pET-21d | Expresses DraD self-complemented with C-terminal donor strand | This study |
To investigate the role of DraD in bacterial internalization, a DraD-deficient strain was constructed. Plasmid pCC90 was used as the template to introduce a stop codon at codon position 31 of DraD by site-directed mutagenesis, using a QuikChange kit as directed by the manufacturer (Stratagene). The resulting expressed peptide would include the signal peptide and only five amino acids of the mature protein. Constructs containing mutations were identified by sequence analysis.
The plasmid for expression of DraE and red fluorescent protein (RFP; pUC-R-RFP plasmid) was engineered previously (33). Recombinant E. coli expressing NfaE was also constructed in our previous study (32). To generate fluorescent NfaE-expressing (NfaE+) bacteria, the 0.77-kb fragment of the gene encoding monomeric RFP was inserted upstream of the NfaE gene on plasmid pUC-Cm. The resulting plasmid was transformed into E. coli AAEC 191A(pCC90-DraEstop). This strain contains genes of the dra operon necessary for fimbrial expression, with a premature stop codon at codon 54 within draE (no full-length DraE can be detected in this strain).
Plasmid pCC90-ΔSacI was constructed by deletion of the 1.1-kb SacI-SacI fragment of pCC90 (9), which includes the 3′ end of draD and the 5′ end of draE. The fluorescent DraE+ DraD− E. coli strain was generated by transformation of pUC-R-RFP into E. coli AAEC191A(pCC90-ΔSacI). Plasmid isolation, E. coli transformation, restriction enzyme digestion, and ligation were carried out as described previously (37). All constructs were confirmed by sequencing using the BigDye Terminator method and ABI sequencing (PE Applied Biosystems, Foster City, CA).
Purification of Dr fimbriae and DraD.
Dr fimbriae were purified from strain DH5α expressing pCC90 by mechanical shearing as previously described (30). For BIAcore analysis, the fimbriae were purified by gel filtration chromatography in HBS-E buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA), using a Superdex 200 column (Amersham Corporation, Piscataway, NJ). DraD was expressed in E. coli BL21(DE3) and purified from inclusion bodies as described previously (2). The refolded protein was purified by gel filtration chromatography, using a Superdex 75 column and HBS-E buffer.
Binding and internalization assay.
Recombinant CHO cells expressing DAF or CEACAMs and primary bladder cells were seeded in 24-well plates and allowed to grow to subconfluence. Bladder cells were differentiated with 10% FBS in the medium for 24 h prior to the experiment. The monolayers were first washed once with Hanks' balanced salt solution (HBSS), and then 1 ml of a bacterial strain (optical density at 540 nm, 0.03) resuspended in cell culture growth medium was added. After incubation for 2 h at 37°C under 5% CO2, the monolayers were washed six times with HBSS. To estimate the number of adherent bacteria, the monolayers were lysed in 1 ml of 0.1% Triton X-100. The bacterium-Triton solution was diluted 1,000 times, and then 10 μl was plated on LB agar with carbenicillin. Plates were incubated overnight at 37°C. For the parallel internalization assay, the monolayers were incubated in the cell culture growth medium plus gentamicin (150 μg/ml) for an additional 90 min to kill extracellular bacteria and to select for internalized bacteria. The monolayers were then washed three times with HBSS and lysed in 1 ml of 0.1% Triton. Aliquots were plated onto LB agar and incubated overnight at 37°C. The following day, the colonies were counted, and the results were expressed in CFU/well. Each cell line or bacterial strain was tested in triplicate.
To estimate the effects of inhibitors on the entry of bacteria, cell monolayers were preincubated with 20 μM PP2 (inhibitor of SRC kinase), 10 mM MβCD (cholesterol-depleting agent), or 10 μg/ml nocodazole (microtubule-depolymerizing drug) for 1 h prior to infection and with 100 μM wortmannin (inhibitor of PI3K) for 15 min prior to infection. Each inhibitor was tested in triplicate.
Immunofluorescence.
Cells were grown on glass coverslips (BD Biosciences) to subconfluence. The monolayers were incubated with bacterial strains for 2 h and washed six times with HBSS to remove unbound bacteria. For immunofluorescence detection, cells were fixed in 4% paraformaldehyde, permeabilized with 1% Triton, washed, and blocked with 20% goat serum-2% bovine serum albumin solution. Cells were incubated with specific primary antibodies for 1 h at room temperature, washed, and then incubated for 1 h with the respective secondary antibodies conjugated with fluorophores. Cells were examined by using a confocal Zeiss LSM510 Meta microscope (Carl Zeiss MicroImaging, Inc.) in multitrack configuration to avoid cross talk between fluorescence channels, and the appropriate controls, with and without primary antibodies, were performed. Images are representative of at least three independent experiments.
SPR studies.
Surface plasmon resonance (SPR) measurements were carried out in running buffer, i.e., HBS-EP buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, 0.005% P-20 surfactant [GE Healthcare, BIAcore]), using a BIAcore 2000 system (GE Healthcare, BIAcore). To analyze the interaction between DraD and α5β1 integrin, 800 response units of α5β1 integrin (Chemicon International) were immobilized on a CM5 research-grade sensor chip (GE Healthcare, BIAcore) by amine coupling chemistry, using the manufacturer's protocols. Different concentrations of Dr fimbriae (0.1 to 2 mg/ml) and DraD (2 to 200 μM) were injected over the integrin-immobilized surface at a flow rate of 20 μl/min. Average equilibrium responses were measured for six or seven concentrations of the analyte. Raw sensograms were corrected using the double-subtraction protocol (44), in which the experimental flow cell signal is corrected by the signal in the reference flow cell as well as by an average of signals from four corrected blank buffer injections. The resulting data were analyzed with BIAevaluation 3.0 software to globally fit the data and to derive equilibrium values.
Statistics.
The data are expressed as means ± standard deviations (SD) for at least three independent experiments. Statistical significance was assessed by Student's t test. Differences were considered significant if they had P values of <0.05.
RESULTS
Dr-fimbriated E. coli cells are internalized by CHO cells expressing CEACAMs.
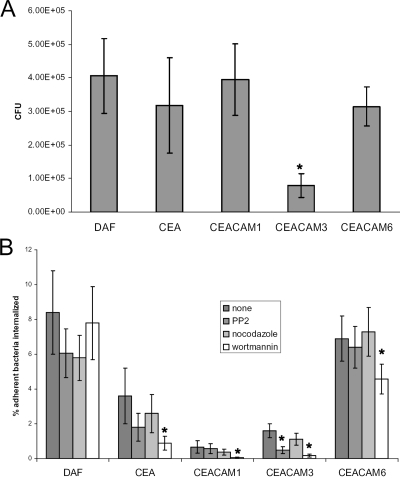
To determine the role of CEACAM receptors in Dr adhesin-mediated internalization, we employed transfected CHO cell lines expressing human CEA, CEACAM1, CEACAM3, and CEACAM6 (CHO-CEA, CHO-CEACAM1, CHO-CEACAM3, and CHO-CEACAM6 cells, respectively). Previously, it was shown that DraE adhesins promote invasion of CHO cells expressing DAF (14, 51). To compare the levels of CEACAM and DAF receptor-mediated internalization, we also employed transfected CHO cells expressing DAF. For enumeration of internalized bacteria, cells were treated with gentamicin prior to lysis to kill the extracellular bacteria. A recombinant strain expressing Dr fimbriae [AAEC191A(pCC90)] demonstrated similar levels of adherence to those of CHO cell transfectants expressing DAF, CEA, CEACAM1, or CEACAM6 and a lower level of adherence to CHO-CEACAM3 cells (Fig. 1A). No adherence was detected with a DraE-deficient mutant [AAEC191A(pCC90-DraEstop)] (data not shown). These data correlate with the previously observed low affinity of DraE for CEACAM3, as determined by SPR analysis (30).
FIG. 1.
Adherence and invasion of CHO cell transfectants expressing DAF, CEA, CEACAM1, CEACAM3, or CEACAM6 from a DraE+ recombinant strain [AAEC191A(pCC90)]. (A) Adherence of CHO cell transfectants by DraE+ E. coli. The results are expressed as CFU of cell-associated bacteria per well (n = 6) (data are means ± SD). (B) Invasion of CHO cell transfectants by DraE+ E. coli. Cells were preincubated with 20 μM PP2, 10 μg/ml nocodazole, and 100 μM wortmannin for 1 h (PP2 and nocodazole) or 15 min (wortmannin) prior to infection. The results are expressed as percentages of cell-associated bacteria internalized (means ± SD). All assays were conducted in triplicate. *, differences are statistically significant (P < 0.001).
Different levels of Dr fimbria-mediated internalization were observed for the various CHO cell transfectants. The GPI-anchored receptors (DAF, CEA, and CEACAM6) mediated the most efficient internalization of E. coli, whereas CEACAM1 promoted a significantly lower level of bacterial internalization than the other receptors did (Fig. 1B). Internalization is expressed as the percentage of internalized adherent bacteria, and thus the low internalization level supported by CEACAM3 is not merely a reflection of poor adherence. The DraE-deficient mutant was internalized by CHO cell transfectants at a significantly lower level than that of DraE+ E. coli (data not shown) and could not be expressed as a percentage of adherent bacteria, since no adherence was detectable. Nonspecific invasion of CHO cells by E. coli has been observed by other investigators (14).
The ability of CEACAMs to promote E. coli internalization prompted us to compare the mechanisms by which these receptors mediate bacterial internalization. We investigated the effects of inhibitors of Src and PI3K (PP2 and wortmannin, respectively) and of a tubulin-depolymerizing agent (nocodazole) on internalization of DraE+ fimbriated E. coli by CHO cells. In CHO cell transfectants expressing each of the CEACAM receptors, PI3K inhibition reduced the proportion of associated bacteria that became internalized (Fig. 1B) (P < 0.01). In addition, pretreatment of CHO cell transfectants with PP2 significantly reduced the internalization of E. coli in CHO-CEACAM3 cells (Fig. 1B) (P < 0.01). These results suggest that Src kinase is required only for internalization mediated by CEACAM3, the receptor uniquely expressed on human granulocytes (23). This is in agreement with published observations demonstrating that CEACAM3-mediated internalization of N. gonorrhoeae critically depends on Src family protein tyrosine kinase activity (50). These results indicate distinct mechanisms of internalization mediated by DAF and the various members of the CEACAM family.
Primary human bladder epithelial cells express CEACAM and DAF receptors and support the adherence and internalization of DraE+ E. coli.
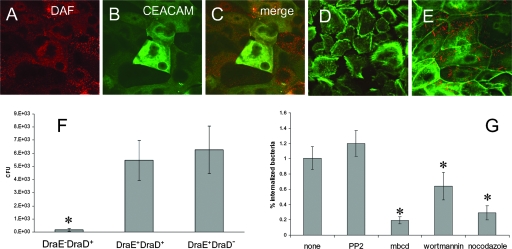
Study of the effects of Dr adhesin-CEACAM interactions on host cells is complicated by the lack of a proper eukaryotic cell line with relevant CEACAM expression. Because CEACAMs regulate cell proliferation and tumor growth, receptor expression in cancer-derived cells and commonly used transformed cell lines may not correlate with normal expression in the epithelium. Here, as cellular models, we employed human primary bladder epithelial cells (26), a cell type not previously exploited for the study of Dr adhesin function. These cells are directly relevant to the pathogenesis of UTI, as they represent both the form and function of the uroepithelium in vivo. Primary bladder epithelial cells proliferate in serum-free medium but do not display markers of terminal differentiation (10, 13). The addition of FBS induces stratification and differentiation (13). First, we examined nondifferentiated and differentiated primary bladder cells for the expression of DAF and CEACAM receptors by confocal microscopic detection of fluorescent anti-DAF and anti-CEACAM antibodies. Fluorescent signals corresponding to DAF and CEACAM expression were detected only in differentiated cells (Fig. 2A to C), although not all cells expressed one or both receptors and not all cells expressed the receptors at the same levels. No fluorescence was detected in cells not grown in FBS (undifferentiated cells) (data not shown). Next, we investigated the adherence of Dr+ E. coli to bladder cells. Nondifferentiated bladder cells demonstrated no adherent bacteria (Fig. 2D). The recombinant E. coli strain expressing Dr fimbriae was able to bind 60 to 80% of differentiated bladder cells (Fig. 2E). No adherence was observed with the DraE-deficient mutant (data not shown). Recombinant strains expressing DraE mutants impaired in DAF or CEACAM binding [AAEC191A(pCC90-DraEstop, pUC-R-D61A) and AAEC191A(pCC90-DraEstop, pUC-R-R86G)] (31, 54) demonstrated somewhat reduced binding to bladder cells (data not shown), suggesting that DraE-fimbriated E. coli cells use both DAF and CEACAM receptors for attachment to uroepithelium, consistent with our observations in CHO cell transfectants.
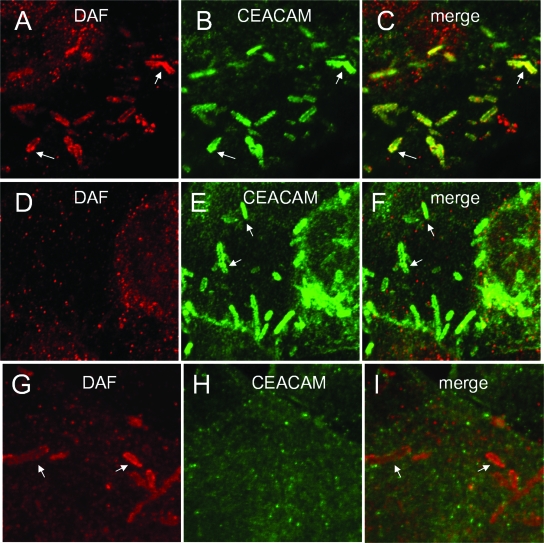
FIG. 2.
Differentiated primary epithelial bladder cells express DAF and CEACAM receptors and mediate adherence and internalization of DraE+ E. coli. (A and C) Expression of DAF by bladder cells. The cells were stained with anti-DAF antibodies and visualized by fluorescence microscopy. (B and C) Expression of CEACAM receptors by bladder cells. The cells were stained with anti-CEACAM antibodies and visualized by fluorescence microscopy. (D and E) Cells were infected with red fluorescent bacteria expressing DraE [AAEC191A(pCC90-DraEstop, pUC-R-RFP)] for 2 h. After infection, the samples were fixed and immunostained for actin expression (green fluorescence). (D) Nondifferentiated cells; (E) differentiated cells. (F) Invasion of primary epithelial bladder cells by DraE+ DraD+ E. coli [AAEC191A(pCC90)], DraE+ DraD− E. coli [AAEC191A(pCC90-DraDstop)], and DraE− DraD+ E. coli [AAEC191A(pCC90-DraEstop)]. The results are expressed as CFU of intracellular bacteria per well (n = 6) (data are means ± SD). All assays were conducted in triplicate. (G) Effects of inhibitors on invasion of primary epithelial bladder cells by DraE+ E. coli [AAEC191A(pCC90)]. Cells were preincubated with 20 μM PP2 (inhibitor of SRC kinase), 10 mM MβCD (cholesterol-depleting agent), or 10 μg/ml nocodazole (microtubule-depolymerizing agent) for 1 h prior to infection or with 100 μM wortmannin (inhibitor of PI3K) for 15 min prior to infection. The results are expressed as percentages of cell-associated bacteria internalized (means ± SD). All assays were conducted in triplicate. *, differences are statistically significant (P < 0.001).
We examined internalization of Dr-fimbriated E. coli by differentiated bladder epithelial cells in gentamicin protection experiments. The DraE+ recombinant was internalized by bladder cells, whereas the nonadherent DraE-deficient mutant was internalized at significantly lower levels (Fig. 2F). Bladder cells internalized Dr-fimbriated bacteria at significantly lower levels than did CHO cells expressing DAF, CEA, and CEACAM6 receptors (compare Fig. 1B and 2F).
E. coli internalization involves PI3K activation, lipid rafts, and microtubules.
Src and PI3K are involved in the regulation of the actin cytoskeleton, and roles for both kinases in the internalization of many bacterial pathogens by host cells have been demonstrated (11, 38). Previously, it was reported that Dr+ E. coli internalization by HeLa and Caco-2 cells is microtubule and lipid raft dependent (22, 24). These observations prompted us to examine the effects of inhibitors of Src and PI3K (PP2 and wortmannin, respectively) and of cholesterol-depleting (MβCD) and tubulin-depolymerizing (nocodazole) agents on internalization of Dr-fimbriated E. coli by primary bladder epithelial cells. None of these agents affected bacterial adherence. PP2 did not inhibit E. coli entry, suggesting no or a minor role of Src family kinases in modulation of bacterial internalization in these cells (Fig. 2G). Wortmannin, MβCD, and nocodazole inhibited E. coli internalization, implying the participation of PI3K, lipid rafts, and microtubules in the internalization of Dr+ bacteria by bladder cells (Fig. 2G).
Binding of Dr-fimbriated bacteria induces cytoskeletal redistribution in bladder cells.
The ability of Dr-fimbriated strains to trigger eukaryotic cellular responses has been demonstrated for HeLa, Caco-2, and CHO cells in previous reports (52). We investigated DraE adhesin-mediated recruitment of the cytoskeletal proteins actin, tubulin, and actin binding proteins in bladder epithelial cells.
The effects of DraE-mediated bacterial adherence on the host cell actin cytoskeletal network were visualized by phalloidin staining of bladder cells infected with a red fluorescent E. coli strain expressing Dr fimbriae [AAEC191A(pCC90-DraEstop, pUC-R-RFP)] (Table 1). In contrast to the results of previous reports employing transformed cell lines, binding of Dr-fimbriated bacteria to primary bladder epithelial cells did not promote detectable actin recruitment and remodeling (data not shown). However, we detected significant actin recruitment around Dr-fimbriated bacteria adherent to CHO transfectants (data not shown).
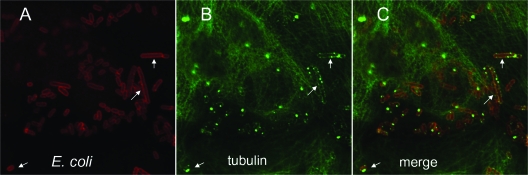
To determine whether interactions of Dr-fimbriated bacteria are associated with rearrangements of microtubules, bladder cells infected with bacteria were stained with a monoclonal antibody against α-tubulin. Aggregated tubulin could be seen at the sites of bacterial binding and formed profiles outlining individual bacterial cells (Fig. 3). Dr fimbria-associated redistribution of microtubules is consistent with our observation reported above that DraE-promoted internalization of E. coli by bladder cells is inhibited by the microtubule inhibitor nocodazole.
FIG. 3.
Binding of DraE+ E. coli [AAEC191A(pCC90)] to bladder cells elicits aggregation of tubulin around bacteria. After infection, the samples were fixed and processed for double immunofluorescence labeling with anti-Dr adhesin antibodies (red fluorescence) (A and C) and anti-α-tubulin antibody (green) (B and C). Arrows indicate examples of tubulin colocalized with bacteria.
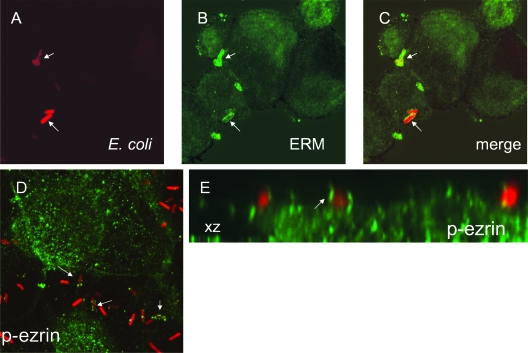
It has been reported that adherence of DraE-fimbriated E. coli to CHO-CEA transfectants triggers activation and recruitment of the actin binding proteins ERM (3). This phenomenon has been associated with elongated cell surface microvilli attaching to bacteria (3). We observed similar activation and recruitment of ERM in bladder epithelial cells. Confocal immunofluorescence analysis revealed colocalization of ERM with cell-associated bacteria (Fig. 4). Phosphorylated ezrin (p-ezrin) could also be observed in association with some adherent bacteria (Fig. 4D). Although p-ezrin association was not as consistently demonstrated as ERM association, x-z sections appeared to show specific close association of p-ezrin with adherent bacteria (Fig. 4E). To determine if ERM recruitment requires CEA-mediated bacterial adherence, the experiment was conducted with bladder cells infected with E. coli expressing the NfaE adhesin [AAEC191A(pCC90-DraEstop, pUC-NfaE-RFP)] (Table 1). NfaE is a member of the Dr family that recognizes DAF as a receptor but does not bind CEACAMs (31, 32). ERM and p-ezrin were also found in association with NfaE+ bacteria (data not shown), suggesting that CEACAM engagement is not essential for ERM recruitment.
FIG. 4.
Binding of DraE+ E. coli to bladder cells triggers recruitment and activation of ERM around bacteria. The cells were infected with red fluorescent bacteria (red fluorescence in panels A, C, D, and E) expressing DraE [AAEC191A(pCC90-DraEstop, pUC-R-RFP)] for 2 h. After infection, the samples were fixed and immunofluorescently labeled with anti-ERM antibodies (green fluorescence in panels B and C) or anti-p-ezrin antibodies (green fluorescence in panels D and E). (E) Confocal microscopy x-z section showing E. coli fluorescence (red) and p-ezrin (green) immunofluorescence in bladder cells. Arrows indicate examples of colocalization of ERM or p-ezrin with cell-associated bacteria.
Dr-fimbriated E. coli cells are associated with caveolae in bladder cells.
Lipid rafts and caveola-associated molecules have been observed in association with Dr-fimbriated bacteria attached to HeLa and Caco-2 cells (29). We observed a similar association in bladder epithelial cells. The bacterial recruitment of lipid rafts was analyzed by immunofluorescence confocal microscopy of bladder cells infected with DraE-fimbriated bacteria [AAEC191A(pCC90-DraEstop, pUC-R-RFP)]. The lipid raft marker caveolin was observed to be in close association with adherent bacteria (Fig. 5A to C). This process is likely linked to GPI receptor (CEA, CEACAM6, and DAF) clustering around bacteria detected in all cells expressing these receptors (52), including bladder epithelial cells.
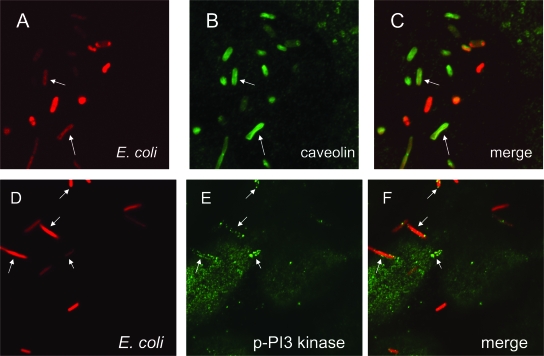
FIG. 5.
DraE+ E. coli is associated with caveolin and phosphorylated PI3K in bladder cells. The cells were infected with red fluorescent bacteria (red fluorescence in panels A, C, D, and F) expressing DraE [AAEC191A(pCC90-DraEstop, pUC-R-RFP)] for 2 h. After infection, the samples were fixed and immunofluorescently labeled with anti-caveolin antibodies (green fluorescence in panels B and C) or anti-p-PI3K antibodies (green fluorescence in panels E and F). Arrows indicate examples of bacteria colocalized with caveolin or p-PI3K.
Recruitment of PI3K.
The data presented above demonstrated the participation of PI3K in Dr fimbria-associated internalization of E. coli by bladder cells. In resting cells, the kinase is located in the cytoplasm, and upon activation, it is recruited to the plasma membrane (20). To determine whether activated PI3K is recruited to adherent bacteria, bladder cells were infected with fluorescent DraE-fimbriated E. coli and analyzed by confocal microscopy after staining of cell monolayers with anti-phosphorylated PI3K (p-PI3K) antibodies. We observed that Dr-fimbriated E. coli triggered the mobilization of activated PI3K around bacteria (Fig. 5D to F). In addition, p-PI3K was also associated with NfaE+ E. coli (data not shown). Thus, our results suggest that Dr adhesin binding to CEACAMs is not essential for ERM and PI3K activation and recruitment to bacteria.
Independent recruitment of DAF and CEACAMs.
Recruitment of GPI-anchored CEACAMs and DAF to the sites of adherent Dr-fimbriated bacteria has been reported (25). GPI-anchored molecules may colocate to lipid rafts and caveolae, and it is unclear whether recruitment of both of these receptor families requires simultaneous engagement by Dr adhesins. To address this question, we infected primary bladder epithelial cells with E. coli strains expressing Dr fimbriae [AAEC191A(pCC90-DraEstop, pUC-R)], Dr fimbriae with a DraE D61A mutant deficient in DAF binding (52) [AAEC191A(pCC90-DraEstop, pUC-R-D61A)], or Dr fimbriae with the NfaE subunit, which does not bind CEACAMs (28) [AAEC191A(pCC90-DraEstop, pUC-NfaE)]. We observed colocalization of DAF and CEACAMs only in bacteria expressing DraE (Fig. 6A to C). DraE-D61A-expressing bacteria recruited only CEACAMs (Fig. 6D to F), and NfaE-expressing bacteria recruited only DAF (Fig. 6G to I). These results indicate that DAF and CEACAMs are independently recruited by direct engagement of the adhesin with each receptor.
FIG. 6.
Independent recruitment of DAF and CEACAM to Dr-fimbriated E. coli adhered to bladder cells. Bladder cells were infected for 2 h with E. coli expressing Dr fimbriae comprised of DraE (A, B, and C), the DraE D61A mutant (D, E, and F), or NfaE (G, H, and I) [AAEC191A(pCC90-DraEstop, pUC-R), AAEC191A(pCC90-DraEstop, pUC-R-D61A), or AAEC191A(pCC90-DraEstop, pUC-NfaE), respectively]. After infection, the samples were fixed and processed for double immunofluorescence labeling with anti-DAF (red) (A, C, D, F, G, and I) and anti-CEACAM (green) (B, C, E, F, H, and I). Arrows denote bacteria.
Recruitment of β1 integrin is associated with DraE-promoted E. coli adherence.
Several published observations suggested that β1 integrins mediate cell entry of Dr+ E. coli by association with AfaD/DraD (12, 49). α5β1 integrin is recruited around DraE+ bacteria and a complex of AfaD-AfaE-coated particles (12, 49). We examined by immunofluorescence confocal microscopy the role of DraD in recruitment of β1 integrin to adherent Dr-fimbriated E. coli. DraE+ bacteria elicited recruitment of β1 integrin around bladder cell-associated bacteria, but the response was weak (data not shown). Therefore, we utilized the Caco-2 intestinal cell line. A DraD-deficient strain was constructed by introducing a premature stop codon in draD by site-directed mutagenesis. We observed the mobilization of β1 integrin around cell-associated DraD+ DraE+ [AAEC191A(pCC90-DraEstop, pUC-R)] and DraD− DraE+ [AAEC191A(pCC90-ΔSacI, pUC-R)] bacteria, indicating that DraD is not required for integrin recruitment (Fig. 7A to F). To identify receptors associated with integrin signaling, we analyzed β1 integrin recruitment in Caco-2 cells infected with E. coli expressing NfaE (does not bind CEACAMs) [AAEC191A(pCC90-DraEstop, pUC-NfaE)] (31) and a DraE D61A mutant defective in DAF recognition [AAEC191A-(pCC90-DraEstop, pUC-R-D61A)] (54). We found that integrin and DAF were colocalized around NfaE+ E. coli in Caco-2 cells (Fig. 7G to I) and that integrin and CEACAMs were colocalized around the DraE D61A mutant strain (data not shown). The results imply that integrin recruitment is associated with Dr adhesin-mediated binding to either CEACAMs or DAF. These data are in agreement with published observations demonstrating that CEA clustering by antibodies leads to colocalization of α5β1 integrin in CEA patches on the cell surface (8).
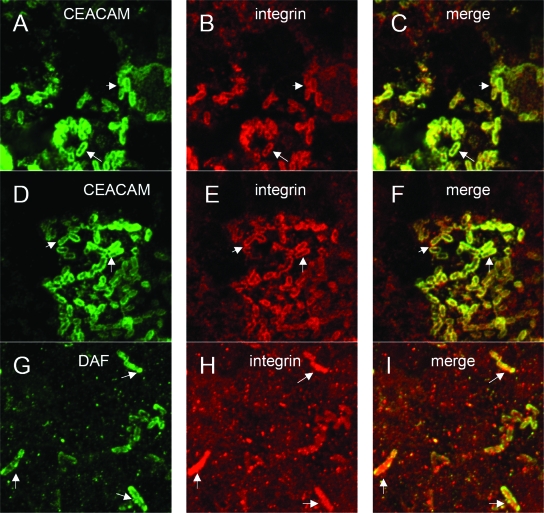
FIG. 7.
DraE binding to GPI-anchored receptors is required for β1 integrin clustering around bacteria. Caco-2 cells were infected with DraE+ DraD+ [AAEC191A(pCC90-DraEstop, pUC-R)] (A, B, and C), DraE+ DraD− [AAEC191A(pCC90-ΔSacI, pUC-R)] (D, E, and F), or NfaE+ DraD+ [AAEC191A(pCC90-DraEstop, pUC-NfaE)] (G, H, and I) E. coli for 2 h. After infection, the samples were fixed and processed for double immunofluorescence labeling with anti-CEACAM (green) (A, C, D, and F), anti-DAF (green) (G and I), or anti-β1 integrin (red) (B, C, E, F, H, and I). Arrows indicate colocalization of CEACAM or DAF with β1 integrin at the site of bacterial adherence.
To examine the potential role of DraD association with β1 integrins in recruitment of actin binding proteins, tubulin, and PI3K, we infected bladder cells with the DraD− DraE+ E. coli strain [AAEC191A(pCC90-ΔSacI, pUC-R-RFP)]. Tubulin, ERM, p-ezrin, and PI3K recruitment was promoted by the DraD− strain in a manner indistinguishable from that of the wild type (data not shown).
DraE is sufficient to mediate bacterial internalization of bladder epithelial cells.
It has been reported that DraD is involved in E. coli internalization by HeLa and undifferentiated Caco-2 cells (28, 49). The DraD-deficient strain AAEC191A(pCC90-DraDstop) promoted levels of adherence (data not shown) and internalization (Fig. 2F) similar to those of the wild type, indicating that DraD is not required for Dr fimbria-induced internalization of E. coli by bladder cells.
Binding of DraD and Dr fimbriae to α5β1 integrin.
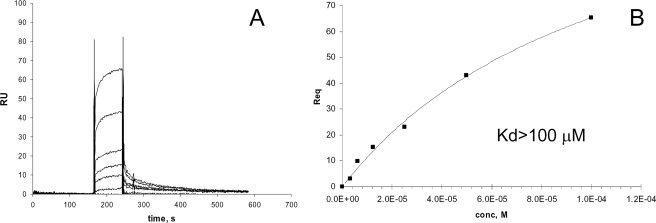
We purified a self-complemented DraD donor strand fusion protein and investigated its association with α5β1 integrin by SPR analysis. DraD-integrin interactions were studied by flowing soluble DraD or whole Dr fimbriae over integrin immobilized on the sensor surface. This method was successfully employed recently to examine α5β1 integrin interactions with CagL from Helicobacter pylori (34). Figure 8 presents an integrin-DraD sensogram (panel A) and a fit of these data (panel B). The low affinity (Kd [dissociation constant] of >100 μM) of DraD binding to integrin is in agreement with previously reported affinities of DraD-integrin interactions (12). It is possible that the self-complemented DraD construct does not optimally reproduce the binding characteristics of the native protein in complex with fimbriae. Therefore, we examined the binding of whole fimbriae to immobilized integrin. While the concentration of DraD might be expected to be low in the whole fimbria preparation, a single binding event would involve association of an entire fimbrial polymer of high molecular weight with the surface. Therefore, SPR should be very sensitive to DraD-integrin interactions of whole fimbriae. However, binding to integrin of whole fimbriae comprised of DraE and DraD was not detectable (data not shown). Together, these observations indicate that Dr fimbria-mediated recruitment of β1 integrins and internalization of adherent E. coli are due to interactions of the DraE adhesin with CEACAMs and DAF and that DraD does not play a major role in these processes.
FIG. 8.
SPR analysis of interactions between DraD and α5β1 integrin. (A) Sensogram depicting the binding of DraD to immobilized α5β1 integrin (800 response units). (B) Equilibrium measurements shown in panel A were analyzed with BIAevaluation 3.0 software to globally fit the data and to derive the dissociation constants (Kd). A fit of these data is shown. The injection time was 120 s, and the flow rate was 20 μl/ml. RU, response units; Req, response units at equilibrium; conc, M, molar concentration of DraD.
DISCUSSION
Previous studies of the Dr family of adhesins have revealed the remarkable ability of this bacterial virulence factor to simultaneously target multiple receptors on human cells. Dr adhesins display an exceptional degree of structural divergence among the adhesins (30). They are under strong positive selection, resulting in preservation of some binding properties of adhesins, in particular Dr adhesin recognition of DAF, and modification of others, in particular adhesin exploitation of CEACAMs (30). Functional diversification of Dr adhesins is the result of an evolutionary process of niche adaptation to E. coli for host recognition, attachment, tissue tropism, and persistence. This study was initiated to investigate the relative roles of DAF and CEACAM receptor binding by Dr adhesins in the pathogenesis of UTI to better understand the evolutionary forces involved in shaping these interactions.
Previous studies have indicated that DAF is capable of promoting internalization of Dr-fimbriated E. coli (14, 22, 51). We have found that CEACAM receptors also mediate internalization of DraE+ bacteria into host cells. CEACAM recruitment by Opa-expressing N. gonorrhoeae has also been demonstrated to induce internalization of bacteria (23). Interestingly, the GPI-anchored receptors DAF, CEA, and CEACAM6 promote more efficient internalization of bacteria than do transmembrane-anchored CEACAM1 and CEACAM3, suggesting that lipid rafts associated with GPI-anchored receptors play an important role in bacterial entry. Consistent with this interpretation, we observed that bacterial internalization by bladder cells was inhibited by the lipid raft-depleting agent MβCD, as previously shown in HeLa cells (24, 51). Lipid rafts are signaling domains implicated in internalization of a number of viruses, bacteria, and parasites into host cells (1, 53). Thus, functional adaptation of E. coli to recognize GPI-anchored receptors such as DAF and CEACAMs may be important for pathogen association with lipid rafts in the uroepithelium, providing bacteria with an effective vehicle to circumvent the mucosal barriers of the host. Colocalization of CEACAMs and DAF to Dr-fimbriated bacteria has been reported previously (25). In that report, mutant Dr fimbriae with reduced affinity for DAF, as reflected by reduced binding to DAF-expressing CHO cell transfectants, demonstrated reduced recruitment of both DAF and CEACAMs. These results suggested that DraE binding to DAF may indirectly recruit other GPI-anchored proteins, such as CEACAMs, as a result of colocalization of these protein to lipid rafts and caveolae, which might be recruited to the sites of adherent bacteria. We reexamined this question by utilizing new Dr mutants and variants with defined binding affinities for DAF and CEACAMs. Our results strongly indicate that colocalization of DAF and CEACAMs to adherent Dr-fimbriated bacteria requires binding affinity for both receptors (Fig. 6). Thus, DAF and CEACAM recruitment is independent, and colocalization requires simultaneous engagement of both receptors by the bacterial adhesin. Dr-fimbriated E. coli with multiple simultaneous receptor binding activities is therefore capable of modeling a specific receptor-associated complex of signaling molecules, including GPI-anchored receptors, associated kinases, and cytoskeletal elements associated with bacterial internalization and pathogenesis.
We observed that DraE+ E. coli cells do not adhere to undifferentiated bladder cells because these cells lack surface DAF and CEACAM expression. These results suggest that in contrast to type 1 fimbria-mediated attachment of uropathogenic E. coli to surface glycoproteins present in various urinary tract tissues (42), Dr fimbriae can bind to and stimulate DAF- and CEACAM-associated signaling networks only on superficial facet cells lining the luminal walls of the bladder, ureter, and renal pelvis. Stratified epithelial cells infected with bacteria rapidly exfoliate, leaving underlying nondifferentiated immature cells (7). This process would impair Dr adhesin-receptor cross talk. Interestingly, attachment of N. gonorrhoeae to CEACAM receptors present on cervix-derived epithelial cells failed to induce cell exfoliation and instead promoted host cell adhesion to the extracellular matrix by triggering CD105 expression (41). Type 1 fimbriated uropathogenic E. coli alters urothelial gene expression to boost proliferation, differentiation, and wound healing of infected host cells (43). It will be interesting to learn if Dr-fimbriated E. coli also exploit similar mechanisms to counteract the tissue-disrupting effects of bacterial uroepithelium infection and to stay attached to DAF and CEACAMs present on superficial cells.
We were unable to detect modification of the actin cytoskeleton of bladder epithelial cells associated with adherence of Dr-fimbriated E. coli. However, DraE-expressing E. coli triggered the recruitment and activation of ERM, proteins linking actin to transmembrane receptors. It has been suggested that activation of ERM is associated with the observed cell surface microvillus-like extensions wrapped around Dr+ bacteria attached to CEA receptors (3). We demonstrated that the adherence of E. coli cells expressing fimbriae without DraD or fimbriae capable of binding only DAF and not CEACAMs had the same effect on ERM mobilization, suggesting that ERM proteins are also regulated by DAF and lipid raft signaling.
The importance of microtubules for DraE-mediated internalization that we observed in bladder epithelial cells has been reported previously for other cell lines (22, 24). We observed that DraE+ bacteria binding to bladder cells alter the microtubule network by aggregating tubulin around adherent bacteria. Interestingly, some human pathogens, such as Mycoplasma penetrans (21) and Salmonella (15), also aggregate tubulin around attaching bacteria in host cells. The role of this response in bacterial internalization is not clear, and future studies will assess the phenomenon in more detail.
To gain entry into host cells, invasive pathogens subvert the host signal transduction cascade. PI3K has been shown to be involved in internalization of several pathogens, including type 1 fimbriated uropathogenic E. coli (38). Our observation that DraE adhesin-mediated internalization of E. coli by bladder cells could be inhibited by a PI3K inhibitor indicates a role for the kinase in Dr-mediated bacterial entry into uroepithelium. We also demonstrated the activation and recruitment of the kinase to bacterial attachment sites. Once activated, PI3K may influence many events, including endocytosis and transcriptional control of host cell survival, that would benefit bacterial persistence in the host (48). Studies with N. gonorrhoeae demonstrated that gonococcal binding to CEACAM3, but not CEACAM1, was associated with recruitment of PI3K to the site of bacterial attachment and internalization (6). CEACAM3 expression is restricted to neutrophils and therefore would not explain the PI3K recruitment observed in association with Dr-fimbriated E. coli adherence to primary bladder epithelial cells. Furthermore, Dr adhesin-associated PI3K recruitment did not require CEACAM binding, as the non-CEACAM-binding NfaE adhesin also effectively promoted recruitment. Mobilization of the kinase may be associated with DAF and/or lipid raft signaling or with other factors expressed by E. coli, such as, for example, endotoxin. Future studies will reveal the host-pathogen interactions involved in recruitment of this important kinase.
A role for the DraD subunit, located at the tip of Dr fimbriae, in Dr adhesin-mediated infection could not be established in our studies. While some previous studies have proposed a role for this protein in β1 integrin clustering and bacterial internalization (12, 49), our results clearly indicate that this protein is not essential for E. coli integrin recruitment or internalization in uroepithelium. Moreover, the low affinity of DraD for α5β1 integrin and the lack of detectable affinity of whole fimbriae for integrin cast doubt on the importance of these interactions in vivo. However, our results do not rule out more subtle effects of DraD-integrin interactions in concert with the major DraE-receptor interactions.
In summary, the results presented here demonstrate that E. coli utilizes Dr adhesins for attachment, recruitment of signaling and cytoskeletal molecules, and invasion of the uroepithelium. Our results allow a better understanding of the role of cell internalization in Dr adhesin-mediated pathogenesis and provide a framework for future investigation of mechanisms of Dr adhesin-promoted disease.
Acknowledgments
We are grateful to Diane Capps, Mandy Robinson, and Florentina Perianu for technical assistance. We thank Alain L. Servin (Institut National de la Santé et de la Recherche Médicale) and Douglas Lublin (Washington University) for providing CHO cells transfectants.
This work was supported by grant DK-064229 from the NIH (to S.L.M.) and by grant 3830 from the University of Washington's Royalty Research Fund (to N.K.).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Abraham, S. N., M. J. Duncan, G. Li, and D. Zaas. 2005. Bacterial penetration of the mucosal barrier by targeting lipid rafts. J. Investig. Med. 53318-321. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. L., J. Billington, D. Pettigrew, E. Cota, P. Simpson, P. Roversi, H. A. Chen, P. Urvil, L. du Merle, P. N. Barlow, M. E. Medof, R. A. Smith, B. Nowicki, C. Le Bouguenec, S. M. Lea, and S. Matthews. 2004. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol. Cell 15647-657. [DOI] [PubMed] [Google Scholar]
- 3.Berger, C. N., O. Billker, T. F. Meyer, A. L. Servin, and I. Kansau. 2004. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC). Mol. Microbiol. 52963-983. [DOI] [PubMed] [Google Scholar]
- 4.Billker, O., A. Popp, V. Brinkmann, G. Wenig, J. Schneider, E. Caron, and T. F. Meyer. 2002. Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1- and Cdc42-dependent and -independent pathways. EMBO J. 21560-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomfield, I. C., M. S. McClain, and B. I. Eisenstein. 1991. Type 1 fimbriae mutants of Escherichia coli K12: characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol. Microbiol. 51439-1445. [DOI] [PubMed] [Google Scholar]
- 6.Booth, J. W., D. Telio, E. H. Liao, S. E. McCaw, T. Matsuo, S. Grinstein, and S. D. Gray-Owen. 2003. Phosphatidylinositol 3-kinases in carcinoembryonic antigen-related cellular adhesion molecule-mediated internalization of Neisseria gonorrhoeae. J. Biol. Chem. 27814037-14045. [DOI] [PubMed] [Google Scholar]
- 7.Bower, J. M., D. S. Eto, and M. A. Mulvey. 2005. Covert operations of uropathogenic Escherichia coli within the urinary tract. Traffic 618-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camacho-Leal, P., A. B. Zhai, and C. P. Stanners. 2007. A co-clustering model involving alpha5beta1 integrin for the biological effects of GPI-anchored human carcinoembryonic antigen (CEA). J. Cell Physiol. 211791-802. [DOI] [PubMed] [Google Scholar]
- 9.Carnoy, C., and S. L. Moseley. 1997. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol. Microbiol. 23365-379. [DOI] [PubMed] [Google Scholar]
- 10.Cilento, B. G., M. R. Freeman, F. X. Schneck, A. B. Retik, and A. Atala. 1994. Phenotypic and cytogenetic characterization of human bladder urothelia expanded in vitro. J. Urol. 152665-670. [DOI] [PubMed] [Google Scholar]
- 11.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304242-248. [DOI] [PubMed] [Google Scholar]
- 12.Cota, E., C. Jones, P. Simpson, H. Altroff, K. L. Anderson, L. du Merle, J. Guignot, A. Servin, C. Le Bouguenec, H. Mardon, and S. Matthews. 2006. The solution structure of the invasive tip complex from Afa/Dr fibrils. Mol. Microbiol. 62356-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross, W. R., I. Eardley, H. J. Leese, and J. Southgate. 2005. A biomimetic tissue from cultured normal human urothelial cells: analysis of physiological function. Am. J. Physiol. Renal Physiol. 289F459-F468. [DOI] [PubMed] [Google Scholar]
- 14.Das, M., A. Hart-Van Tassell, P. T. Urvil, S. Lea, D. Pettigrew, K. L. Anderson, A. Samet, J. Kur, S. Matthews, S. Nowicki, V. Popov, P. Goluszko, and B. J. Nowicki. 2005. Hydrophilic domain II of Escherichia coli Dr fimbriae facilitates cell invasion. Infect. Immun. 736119-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlay, B. B., S. Ruschkowski, and S. Dedhar. 1991. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J. Cell Sci. 99283-296. [DOI] [PubMed] [Google Scholar]
- 16.Foxman, B. 1990. Recurring urinary tract infection: incidence and risk factors. Am. J. Public Health 80331-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foxman, B., R. Barlow, H. D'Arcy, B. Gillespie, and J. D. Sobel. 2000. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 10509-515. [DOI] [PubMed] [Google Scholar]
- 18.Foxman, B., L. Zhang, P. Tallman, K. Palin, C. Rode, C. Bloch, B. Gillespie, and C. F. Marrs. 1995. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J. Infect. Dis. 1721536-1541. [DOI] [PubMed] [Google Scholar]
- 19.Garcia, M. I., P. Gounon, P. Courcoux, A. Labigne, and C. Le Bouguenec. 1996. The afimbrial adhesive sheath encoded by the afa-3 gene cluster of pathogenic Escherichia coli is composed of two adhesins. Mol. Microbiol. 19683-693. [DOI] [PubMed] [Google Scholar]
- 20.Gillham, H., M. C. Golding, R. Pepperkok, and W. J. Gullick. 1999. Intracellular movement of green fluorescent protein-tagged phosphatidylinositol 3-kinase in response to growth factor receptor signaling. J. Cell Biol. 146869-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giron, J. A., M. Lange, and J. B. Baseman. 1996. Adherence, fibronectin binding, and induction of cytoskeleton reorganization in cultured human cells by Mycoplasma penetrans. Infect. Immun. 64197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goluszko, P., V. Popov, R. Selvarangan, S. Nowicki, T. Pham, and B. J. Nowicki. 1997. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J. Infect. Dis. 176158-167. [DOI] [PubMed] [Google Scholar]
- 23.Gray-Owen, S. D., and R. S. Blumberg. 2006. CEACAM1: contact-dependent control of immunity. Nat. Rev. Immunol. 6433-446. [DOI] [PubMed] [Google Scholar]
- 24.Guignot, J., M. F. Bernet-Camard, C. Pous, L. Plancon, C. Le Bouguenec, and A. L. Servin. 2001. Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: evidence for alpha5beta1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun. 691856-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guignot, J., I. Peiffer, M. F. Bernet-Camard, D. M. Lublin, C. Carnoy, S. L. Moseley, and A. L. Servin. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect. Immun. 683554-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta, K., M. Y. Chou, A. Howell, C. Wobbe, R. Grady, and A. E. Stapleton. 2007. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J. Urol. 1772357-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammarstrom, S. 1999. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 967-81. [DOI] [PubMed] [Google Scholar]
- 28.Jouve, M., M. I. Garcia, P. Courcoux, A. Labigne, P. Gounon, and C. Le Bouguenec. 1997. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect. Immun. 654082-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kansau, I., C. Berger, M. Hospital, R. Amsellem, V. Nicolas, A. L. Servin, and M. F. Bernet-Camard. 2004. Zipper-like internalization of Dr-positive Escherichia coli by epithelial cells is preceded by an adhesin-induced mobilization of raft-associated molecules in the initial step of adhesion. Infect. Immun. 723733-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korotkova, N., S. Chattopadhyay, T. A. Tabata, V. Beskhlebnaya, V. Vigdorovich, B. K. Kaiser, R. K. Strong, D. E. Dykhuizen, E. V. Sokurenko, and S. L. Moseley. 2007. Selection for functional diversity drives accumulation of point mutations in Dr adhesins of Escherichia coli. Mol. Microbiol. 64180-194. [DOI] [PubMed] [Google Scholar]
- 31.Korotkova, N., E. Cota, Y. Lebedin, S. Monpouet, J. Guignot, A. L. Servin, S. Matthews, and S. L. Moseley. 2006. A subfamily of Dr adhesins of Escherichia coli bind independently to decay-accelerating factor and the N-domain of carcinoembryonic antigen. J. Biol. Chem. 28129120-29130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korotkova, N., I. Le Trong, R. Samudrala, K. Korotkov, C. P. Van Loy, A. L. Bui, S. L. Moseley, and R. E. Stenkamp. 2006. Crystal structure and mutational analysis of the DaaE adhesin of Escherichia coli. J. Biol. Chem. 28122367-22377. [DOI] [PubMed] [Google Scholar]
- 33.Korotkova, N., Y. Yang, I. Le Trong, E. Cota, B. Demeler, J. Marchant, W. E. Thomas, R. E. Stenkamp, S. L. Moseley, and S. Matthews. 2008. Binding of Dr adhesins of Escherichia coli to carcinoembryonic antigen triggers receptor dissociation. Mol. Microbiol. 67420-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwok, T., D. Zabler, S. Urman, M. Rohde, R. Hartig, S. Wessler, R. Misselwitz, J. Berger, N. Sewald, W. Konig, and S. Backert. 2007. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 449862-866. [DOI] [PubMed] [Google Scholar]
- 35.Loomis, W. P., and S. L. Moseley. 1998. Translational control of mRNA processing in the F1845 fimbrial operon of Escherichia coli. Mol. Microbiol. 30843-853. [DOI] [PubMed] [Google Scholar]
- 36.Lublin, D. M., and K. E. Coyne. 1991. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J. Exp. Med. 17435-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 192803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medof, M. E., E. I. Walter, J. L. Rutgers, D. M. Knowles, and V. Nussenzweig. 1987. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J. Exp. Med. 165848-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muenzner, P., C. Dehio, T. Fujiwara, M. Achtman, T. F. Meyer, and S. D. Gray-Owen. 2000. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect. Immun. 683601-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muenzner, P., M. Rohde, S. Kneitz, and C. R. Hauck. 2005. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J. Cell Biol. 170825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 694572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mysorekar, I. U., M. A. Mulvey, S. J. Hultgren, and J. I. Gordon. 2002. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 2777412-7419. [DOI] [PubMed] [Google Scholar]
- 44.Myszka, D. G. 1999. Improving biosensor analysis. J. Mol. Recognit. 12279-284. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson-Weller, A., and C. E. Wang. 1994. Structure and function of decay accelerating factor CD55. J. Lab. Clin. Med. 123485-491. [PubMed] [Google Scholar]
- 46.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 1782115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowicki, B., J. Moulds, R. Hull, and S. Hull. 1988. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect. Immun. 561057-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer, L. G. 2007. The 60th symposium of the Society of General Physiologists. Chemotaxis, invasion, and phagocytosis: from bacteria to humans. J. Gen. Physiol. 12995-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plancon, L., L. Du Merle, S. Le Friec, P. Gounon, M. Jouve, J. Guignot, A. Servin, and C. Le Bouguenec. 2003. Recognition of the cellular beta1-chain integrin by the bacterial AfaD invasin is implicated in the internalization of afa-expressing pathogenic Escherichia coli strains. Cell. Microbiol. 5681-693. [DOI] [PubMed] [Google Scholar]
- 50.Schmitter, T., S. Pils, S. Weibel, F. Agerer, L. Peterson, A. Buntru, K. Kopp, and C. R. Hauck. 2007. Opa proteins of pathogenic neisseriae initiate Src kinase-dependent or lipid raft-mediated uptake via distinct human carcinoembryonic antigen-related cell adhesion molecule isoforms. Infect. Immun. 754116-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selvarangan, R., P. Goluszko, V. Popov, J. Singhal, T. Pham, D. M. Lublin, S. Nowicki, and B. Nowicki. 2000. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect. Immun. 681391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Servin, A. L. 2005. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin. Microbiol. Rev. 18264-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Goot, F. G., and T. Harder. 2001. Raft membrane domains: from a liquid-ordered membrane phase to a site of pathogen attack. Semin. Immunol. 1389-97. [DOI] [PubMed] [Google Scholar]
- 54.Van Loy, C. P., E. V. Sokurenko, R. Samudrala, and S. L. Moseley. 2002. Identification of amino acids in the Dr adhesin required for binding to decay-accelerating factor. Mol. Microbiol. 45439-452. [DOI] [PubMed] [Google Scholar]
- 55.Zalewska, B., R. Piatek, K. Bury, A. Samet, B. Nowicki, S. Nowicki, and J. Kur. 2005. A surface-exposed DraD protein of uropathogenic Escherichia coli bearing Dr fimbriae may be expressed and secreted independently from DraC usher and DraE adhesin. Microbiology 1512477-2486. [DOI] [PubMed] [Google Scholar]