Abstract
Mannoproteins are cell wall components of pathogenic fungi and play major virulence and immunogenic roles with both their mannan and protein moieties. The 65-kDa mannoprotein (MP65) of Candida albicans is a β-glucanase adhesin recognized as a major target of the human immune response against this fungus, and its recombinant product (rMP65; devoid of the mannan moiety) is presently under consideration as a vaccine candidate. Here we investigated cellular and molecular aspects of the interaction of rMP65 with human antigen-presenting cells. We also assessed the ability of rMP65 to initiate a T-cell response. Both the native mannosylated MP65 (nMP65) and the recombinant product were efficiently bound and taken up by macrophages and dendritic cells. However, contrarily to nMP65, rMP65 did not induce tumor necrosis factor alpha and interleukin-6 release from these cells. On the other hand, rMP65 was rapidly endocytosed by both macrophages and dendritic cells, in a process involving both clathrin-dependent and clathrin-independent mechanisms. Moreover, the RGD sequence inhibited rMP65 uptake to some extent. After internalization, rMP65 partially colocalized with lysosomal membrane-associated glycoproteins 1 and 2. This possibly resulted in efficient protein degradation and presentation to CD4+ T cells, which proliferated and produced gamma interferon. Collectively, these results demonstrate that the absence of the mannan moiety does not deprive MP65 of the capacity to initiate the pattern of cellular and molecular events leading to antigen presentation and T-cell activation, which are essential features for further consideration of MP65 as a potential vaccine candidate.
Disseminated systemic fungal infections frequently occur in immunocompromised patients (30). A critical role in pathogenic potential as well as in elicitation of the host immune response has been ascribed to fungal cell wall components. In this context, proteins and glycoproteins, with the latter being mostly mannoproteins (MP) (2), appear to have a prominent function (12). Because MP are common and abundant structural components of fungal cell walls, they have been studied extensively in different models of pathogenic fungi, such as Candida albicans, Cryptococcus neoformans, and Penicillium marneffei (3, 29, 32). Clinical and experimental studies have identified a heterogeneous family of MP that includes antigens which are responsible for stimulating the T-cell response and are potential vaccine candidates (19, 32).
The intrinsic capacity of MP isolated from Cryptococcus neoformans (CnMP) to stimulate an immune response was reported previously. In particular, CnMP were found to be responsible for inducing a delayed-type hypersensitivity response (22), T-cell proliferation of peripheral blood mononuclear cells isolated from patients who recovered from cryptococcosis, and cytokine production (18, 20). Furthermore, CnMP induce a protective Th1 response against both C. neoformans and C. albicans (29).
C. albicans is a common agent of mucosal, particularly vaginal, infections in immunocompetent subjects (8), and it represents the fourth cause of bloodstream infections in hospitalized immunocompromised hosts (26). A 65-kDa MP (MP65) from the cell wall of this fungus has been purified and biochemically characterized (11, 12). Some of its immunological properties have been elucidated. From in vitro and ex vivo studies, MP65 has emerged as a major target of the T-cell response in humans (25, 37). In a mouse model, MP65 is able to induce a delayed-type hypersensitivity reaction, production of Th1 profile cytokines, and partial protection against lethal Candida infection (21). Moreover, MP65 from C. albicans confers partial protection against C. neoformans, suggesting that CnMP and MP65 share some common antigenic determinants (29). A more recent report has qualified MP65 as a putative β-glucanase adhesin playing a critical role in hyphal morphogenesis and pathogenicity (32), thus justifying the importance of the host immune response against this MP.
In a previous study (28), we demonstrated that interaction of the native mannosylated MP65 with human dendritic cells (DC) leads to tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) release and to IL-12 gene expression. MP65 induced DC maturation by increasing the expression of costimulatory molecules, decreasing CD14 and Fcγ receptor (FcγR) molecule expression, and enabling DC to activate the T-cell response. Overall, the mannan moiety of MP is considered critical for interaction with pattern recognition receptors (PRRs), in particular Toll-like receptors (TLRs), which are believed to drive the cytokine response by antigen-presenting cells (APC) and plausibly govern subsequent T-cell activation (28). Therefore, we considered that a recombinant C. albicans MP65 (rMP65) deprived of mannosyl residues could present a partial or total inability to stimulate the above interaction with DC and the consequent immune activation, with all of these events being critical for a potential use of this recombinant product as a vaccine and/or immunomodulator. In this paper, our objective was therefore to determine whether the recombinant preparation of MP65 (16, 25) deprived of its glycosylated moiety interacts with APC and eventually with other immune cells and whether it is able to induce cellular immune responses in terms of T-cell activation and cytokine release.
MATERIALS AND METHODS
Reagents and media.
RPMI 1640 with glutamine and fetal calf serum were obtained from Gibco-BRL.
rMP65, nMP65, and rPLD purification.
rMP65 and native MP65 (nMP65) were generated as previously described (12, 016). Recombinant Chlamydia pneumoniae PLD protein (rCpPLD), cloned, generated, and purified exactly as rMP65, was used as a control for rMP65 (5). Preparations of the rMP65, nMP65, and rCpPLD components tested negative for endotoxin contamination in a Limulus assay (Coatest endotoxin; Kabi Diagnostica) with a sensitivity of 25 pg of Escherichia coli lipopolysaccharide (LPS). Nevertheless, all in vitro experiments were carried out at least once in the presence of 10 μg/ml of polymyxin B (Sigma), a polycationic antibiotic, to neutralize any undetected contamination with bacterial LPS (15). Results were not changed in the presence of polymyxin B.
Protein labeling.
An Alexa fluor 488 kit from Invitrogen was used for rMP65, nMP65, rCpPLD, albumin, and transferrin labeling. The labeling solution was prepared according to the manufacturer's instructions.
The degree of labeling of each protein was calculated with the following formula: degree of labeling = moles of dye/moles of protein = (A494 × dilution factor)/(71,000 × protein concentration [M]). The degree of labeling was 2.73 for rCpPLD, 2.92 for transferrin, 2.3 for albumin, 2.53 for rMP65, and 2.69 for nMP65.
In vitro generation of MDM and DC.
The generation of DC and monocyte-derived macrophages (MDM) from monocytes (MN) was performed as previously described, with minor modifications (31). Heparinized venous blood was obtained from healthy donors and diluted with RPMI 1640 (Gibco-BRL). Peripheral blood mononuclear cells were separated by density gradient centrifugation over Ficoll-Hypaque Plus (Pharmacia Biotech, Uppsala, Sweden), recovered, washed twice and suspended in RPMI 1640 supplemented with 10% fetal calf serum, 100 U penicillin/ml, and 100 μg streptomycin/ml, plated in cell culture flasks (Corning Incorporated), and incubated for 1 h at a density of 2 × 106 to 3 × 106/ml. Adherent cells recovered were >95% CD14+, as evaluated by flow cytometry. Immature DC were obtained by treating monocytes with 50 ng/ml of human recombinant granulocyte-macrophage colony-stimulating factor (Biosource) and 30 ng/ml of human recombinant IL-4 (Biosource) for 5 to 6 days. MDM were obtained by treatment with human recombinant macrophage colony-stimulating factor (50 ng/ml) for 5 days.
Lymphocyte and PMN purification.
Peripheral blood T lymphocytes (PBL) were recovered from nonadherent cells by E rosetting and washed twice with RPMI 1640 as previously described (27). Polymorphonuclear cells (PMN) were obtained from the pellet of a Ficoll-Hypaque gradient. Erythrocytes were hypotonically lysed, and cells were washed twice in RPMI 1640 by centrifugation at 4°C for 10 min at 250 × g. PMN were resuspended, counted, and used for the experiments.
Flow cytometry analysis.
The uptake of rMP65 or rCpPLD by human DC, MDM, PMN, and PBL was analyzed by flow cytometry. Cells were incubated with 5 or 25 μg/ml of Alexa fluor 488-conjugated rMP65 (rMP65 fluor) or Alexa fluor 488-conjugate rCpPLD (rCpPLD fluor) for 30 min or 6 h at 37°C in the presence of 5% CO2, and after incubation, cells were collected by centrifugation, fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS), washed twice and resuspended in PBS containing 0.5% bovine serum albumin and 0.1% sodium azide, and read using a FACScan flow cytometer (Becton Dickinson). In parallel experiments, the involvement of PRRs was analyzed by receptor blocking with 2 μg/ml of specific antibodies against human TLR2, TLR4 (HyCult Biotechnology), TLR6, and TLR9 (Alexis Biochemicals) for 30 min at 4°C before rMP65 fluor addition.
For RGD inhibition study (36), MDM were preincubated for 15 min at 37°C with the indicated concentrations of the peptide Arg-Gly-Asp-Ser (RGDS; Sigma) and a control peptide (Sigma). After RGDS pretreatment, rMP65 fluor (25 μg/ml) was added to each sample, and samples were incubated for 2 h.
Cytotoxicity assay.
MDM and DC (2.5 × 105) were treated with nystatin (2.5, 5, 25, 50, 100, and 200 μg/ml) (Sigma-Aldrich) for 30 min at 4°C or with chlorpromazine (7.5, 15, 30, 45, and 90 μM) for 2 h at 37°C, and cell viability was evaluated by the use of an ATP bioluminescence kit (Via Light kit; Cambrex). Briefly, 100 μl of each sample was added to a 96-well culture plate, 50 μl of lysis reagent was added to each well, and after 10 min of incubation, 100 μl of ATP monitoring reagent (AMR Plus) was added to each sample. After 2 min of incubation at room temperature, luminescence was measured by a luminometer (Infinite 200; Tecan).
Caveolin- and clathrin-dependent uptake of MP65 fluor.
MDM and DC (2.5 × 105) were treated with nystatin (5 and 2.5 μg/ml), an inhibitor of clathrin-independent uptake (Sigma-Aldrich), for 30 min at 4°C or with chlorpromazine (45 and 30 μM), an inhibitor of clathrin-dependent uptake (Sigma-Aldrich), for 30 min at 37°C (33, 40), followed by 2 h of incubation with rMP65 fluor (25 μg/ml). Transferrin protein-Alexa fluor 488 (transferrin fluor; 25 μg/ml) and albumin protein-Alexa fluor 488 (albumin fluor; 25 μg/ml), both from Invitrogen, were used as controls for chlorpromazine and nystatin, respectively (34).
In particular, for transferrin treatment, cells were preincubated in serum-free medium for 2 h at 37°C to upregulate the transferrin receptor. Samples were then incubated with 25 μg/ml of transferrin fluor for 45 min at 0°C and further incubated at 37°C for the indicated times. In selected experiments, cells were treated with a combination of 5 μg/ml of nystatin and 45 μM chlorpromazine.
Confocal microscopic examination.
DC (5 × 105) were incubated with 25 μg/ml of rMP65 fluor, rCpPLD fluor, or nMP65 fluor for 2 h at 37°C in PBS. To evaluate the colocalization of rMP65 fluor with lysosomal membrane-associated glycoprotein 1 (LAMP-1) and LAMP-2, cells were fixed, immediately after incubation with rMP65 fluor, in 3% paraformaldehyde in PBS (pH 7.4) for 30 min at room temperature and then washed and permeabilized with 0.05% saponin in PBS for 15 min. Cells were then incubated for 1 h in blocking solution (1% bovine serum albumin in PBS; Acros Organics) with primary mouse anti-human LAMP-1 and mouse anti-human LAMP-2 antibodies (both from Santa Cruz Biotechnology). After incubation, cells were washed with blocking solution and incubated for 30 min with a secondary Cy-conjugated anti-mouse immunoglobulin G antibody diluted in blocking solution (Sigma-Aldrich). After that, cells were washed three times with 2 ml of blocking solution, and a single drop of cell suspension was dispensed on a slide. Gel Mount Aqueous mounting medium (Sigma-Aldrich) was used to protect the fluorescence. Slides were mounted with coverslips and analyzed with a confocal microscope.
Images were collected on a Nikon C1 laser scanning confocal unit (Nikon D-Eclipse C1) attached to an inverted fluorescence microscope (Nikon Eclipse TE 2000-U).
Determination of TNF-α and IL-6 production.
MDM (2 × 106/ml) were incubated with 5 μg/ml of rMP65 or nMP65 or 1 μg/ml of LPS for 18 h. MDM (2 × 106/ml) were activated with LPS and gamma interferon (IFN-γ), alone or in combination, before incubation with 5 μg/ml of rMP65 for 18 h. The presence of human TNF-α and IL-6 in culture supernatant fluids was measured with an enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences Pharmingen).
Determination of proliferation and IL-4 and IFN-γ production.
For proliferation assay, 2 × 104 MDM or immature DC were left unstimulated or stimulated with rMP65 or nMP65 (5 μg/ml), and autologous T cells (2 × 105) were added. As a positive control, cells were stimulated with 10 μg/ml of phytohemagglutinin (PHA).
After 4 days of incubation, the proliferation was calculated as previously described (28). Proliferation was expressed as mean values for the indicated triplicates ± standard errors of the means (SEM).
In parallel experiments for cytokine production, supernatant fluids were recovered after 4 days of incubation and tested for IL-4 and IFN-γ by ELISA (BD Biosciences Pharmingen).
Flow cytometry analysis of CD4 and CD8 T cells.
After proliferation, T cells were recovered and labeled with mouse anti-human CD4-fluorescein isothiocyanate and mouse anti-human CD8-phycoerythrin (Alexis) for 30 min at 4°C. Surface molecule expression was quantified by flow cytometry as previously described (38).
Statistical analysis.
Statistical significance was determined using analysis of variance (ANOVA), corrected for multiple comparisons by the Bonferroni test. Results are presented as means ± SEM.
RESULTS
MP65 uptake by immune cells.
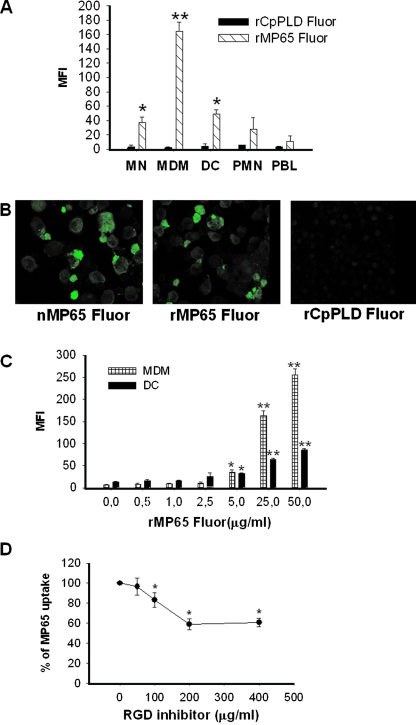
We first analyzed the capacity of recombinant MP65 fluor to interact with different types of immune cells from blood samples of human healthy donors. To this end, we added various concentrations of rMP65 fluor to purified MN, MDM, DC, PMN, or PBL. The cells were then incubated with rMP65 fluor or rCpPLD fluor as a control for 30 min at 37°C and analyzed for protein uptake. The results reported in Fig. 1A show that MDM, DC, and MN, in this quantitative ranking order, were able to take up rMP65. Conversely, the recombinant rCpPLD fluor made in the same E. coli vector and used to exclude a possible nonspecific uptake of rMP65 did not associate with any type of immune cells under the experimental conditions used. None of the cases displayed significant uptake with purified PMN and PBL (Fig. 1A).
FIG. 1.
Uptake of rMP65 fluor by different cell types. (A) MN, MDM, DC, PMN, and PBL treated for 30 min at 37°C with rMP65 fluor (5 μg/ml) or rCpPLD fluor (5 μg/ml) were analyzed by flow cytometry to assess rMP65 and rCpPLD uptake. The rMP65 fluor uptake by different cell types is expressed as the mean fluorescence intensity (MFI). Data are expressed as means ± SEM for three independent experiments. *, P < 0.05; **, P < 0.001 (rMP65 fluor-treated cells versus rCpPLD fluor-treated cells). (B) rMP65 fluor, nMP65 fluor, and rCpPLD fluor uptake by DC, tested using confocal microscopy. DC were treated with 25 μg/ml of rMP65 fluor, rCpPLD fluor, or nMP65 fluor for 2 h at 37°C. The cells were then fixed and analyzed with a confocal microscope. (C) MDM or DC were stimulated for 30 min at 37°C with different concentrations of rMP65 fluor (0.5, 1, 2.5, 5, 25, and 50 μg/ml). The rMP65 fluor uptake by different cell types is expressed as the MFI. Data are expressed as means ± SEM for three independent experiments. *, P < 0.05; **, P < 0.001 (rMP65-treated cells versus untreated cells). (D) MDM were pretreated with various concentrations of RGDS or control peptide for 15 min, and then rMP65 fluor (25 μg/ml) was added. Data are expressed as means ± SEM for three independent experiments. *, P < 0.05 (RGDS-treated cells versus untreated cells).
Parallel experiments were performed to directly compare the uptake by DC of rMP65 to that of nMP65. To this end, DC were incubated for 2 h with rMP65 fluor, nMP65 fluor, and rCpPLD fluor, and the cells were then analyzed by confocal microscopy. As shown in Fig. 1B, nMP65 and rMP65, but not rCpPLD, were taken up by DC to apparently similar extents.
Experiments to evaluate the dose dependency of rMP65 fluor uptake by MDM and DC were also carried out. Figure 1C shows a significant continuous uptake of rMP65 fluor associated with both kinds of cells over the concentration range of 0.5 to 50 μg/ml. Furthermore, macrophages appeared to have the highest uptake capacity.
Since MP65 possesses an RGD sequence, the possibility for the rMP65 motif to mediate its interaction with MDM was analyzed. Cells were preincubated with the inhibitor (RGDS) for 15 min, and then rMP65 fluor was added. As shown in Fig. 1D, the RGDS peptide inhibited the uptake of rMP65 in a dose-dependent manner, with a reduction of 40% at 200 μg/ml. The specificity of uptake inhibition by RGDS was tested by using an RGD control peptide not affecting the uptake of rMP65 at a concentration of 250 μg/ml. Inhibition of rMP65 uptake was 5% ± 1%.
The RGD sequence is recognized by β2-integrins, such as CR3 (Cd11b/Cd18) and CR4 (Cd11c/Cd18); therefore, we determined whether rMP65 uptake could be related, at least in part, to the availability of CR3 expression. The analysis of CR3 on different cell types (MN, DC, MDM, and PMN) showed that MDM and DC expressed the highest concentrations of such molecules compared to MN and PMN. This was correlated with an increased amount of rMP65 uptake (data not shown). PBL did not show any CR3 expression or rMP65 uptake (not shown).
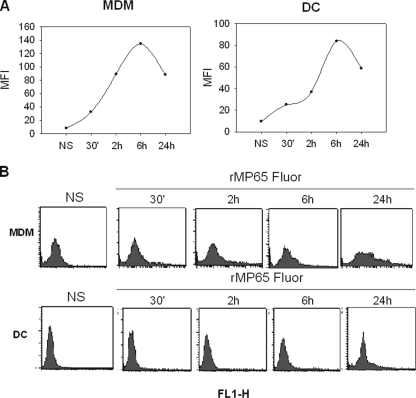
Given that MDM and DC, which have similar functions, including the capacity to process antigens and to present them to PBL, were both able to take up MP65 (1), these two types of cells were selected to further analyze their interaction with rMP65. The time course of rMP65 uptake was examined to evaluate whether the antigen was stored, degraded, or excreted by the cells. For this purpose, DC and MDM were treated for 30 min and 2, 6, and 24 h with 5 μg/ml of rMP65 fluor and then analyzed for protein uptake. As shown in Fig. 2, the largest amount of rMP65 taken up was found after 6 h of incubation for both macrophages and DC. However, appreciable rMP65 uptake was also evident after 30 min of incubation. A decline was observed within 24 h of incubation, suggesting partial MP degradation or excretion thereafter.
FIG. 2.
Kinetics of rMP65 fluor uptake by MDM and DC. MDM or DC left untreated (NS) or treated with 5 μg/ml of rMP65 fluor were incubated at 37°C for different times (30 min and 2, 6, and 24 h). (A) rMP65 fluor uptake by different cell types is expressed as the MFI. (B) Fluorescence-activated cell sorting histograms represent the background cell fluorescence (NS) and cell fluorescence after different times of incubation. Data shown are from one representative experiment out of four performed with similar results.
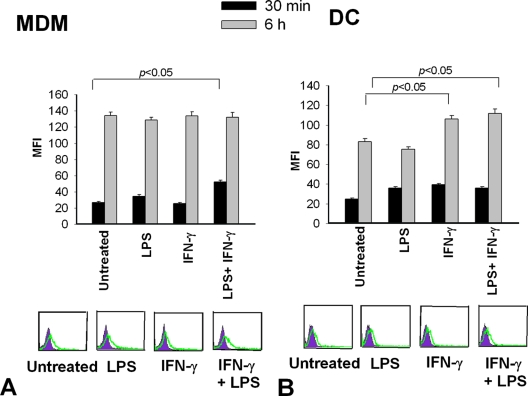
To verify whether the state of activation of DC or MDM could influence rMP65 uptake, we pretreated our cells with LPS and IFN-γ, considered to be canonical stimulators of innate immune cells (13, 14, 35). MDM and DC were treated with LPS (1 μg/ml) or IFN-γ (100 ng/ml) alone or in combination for 30 min, and then rMP65 fluor was added to the cells for 30 min or 6 h. As shown by fluorescence-activated cell sorting analysis (Fig. 3A), a significant increase in rMP65 fluor uptake occurred in MDM activated for 30 min with a combination of LPS and IFN-γ compared to that in nonactivated cells. Conversely, when rMP65 fluor was added to MDM for 6 h, regardless of the activation state, its uptake did not show any increase (Fig. 3A). No antigen uptake modulation was manifested after stimulation with either LPS or IFN-γ alone compared to the uptake in untreated cells (Fig. 3B). An increase in rMP65 uptake was observed in DC pretreated with IFN-γ or with IFN-γ plus LPS and stimulated for 6 h, but not for 30 min, with rMP65.
FIG. 3.
rMP65 fluor uptake by activated MDM and DC. MDM (A) or DC (B) were left untreated or activated with LPS (1 μg/ml) or IFN-γ (100 ng/ml), alone or in combination, for 30 min at 37°C. The cells were incubated with 5 μg/ml of rMP65 fluor for 30 min or 6 h at 37°C. The rMP65 fluor uptake by different cell types is expressed as the MFI. Data are expressed as means ± SEM for four independent experiments. Statistical analysis was performed with ANOVA and corrected by the Bonferroni test for multiple comparisons. In the lower part of the figure, filled histograms represent the autofluorescence of cells, while the green lines represent fluorescence of rMP65 fluor uptake by cells for a 30-min incubation. Results of cytofluorimetric analysis presented are from one representative experiment.
Endocytosis as the mechanism of rMP65 uptake by APC.
PRRs recognizing the polysaccharide moiety of MP have been implicated in the uptake of these molecules by cells of innate immunity (23). To evaluate whether PRRs could also be involved in the association of rMP65 with MDM and DC, cells were pretreated with antibodies to TLRs and mannose receptor (2 μg/ml for 30 min at 4°C), and then rMP65 fluor was added (5 μg/ml for 30 min). Cytofluorimetric analysis showed that blocking TLRs as well as mannose receptors did not affect the uptake of rMP65 into either type of cell (data not shown). These results suggested that endocytosis, involving clathrin-dependent or -independent pathways, such as caveolar uptake, could be a possible mechanism for rMP65 uptake.
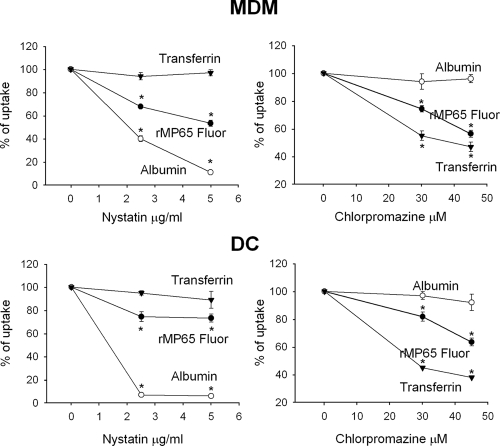
To clarify this issue, MDM or DC were incubated with chlorpromazine and nystatin, which are molecular inhibitors of the clathrin-dependent and clathrin-independent endocytosis pathways, respectively (33, 40). In order to evaluate the potential cytotoxicity of these compounds, DC and MDM were treated with different concentrations of nystatin (2.5, 5, 25, 50, and 100 μg/ml) for 30 min at 4°C or chlorpromazine (7.5, 15, 30, 45, and 90 μM) for 30 min at 37°C; the cells were then incubated for 2 h at 37°C, and cytotoxicity was analyzed. The results showed that nystatin at 5 μg/ml and chlorpromazine at 50 μM did not affect the viability of tested cells (data not shown). Therefore, MDM and DC were treated for 30 min with 5 or 2.5 μg/ml nystatin or with 30 or 45 μM chlorpromazine; rMP65 fluor was then added, and uptake was evaluated. The results reported in Fig. 4 show that rMP65 uptake was inhibited by both clathrin-dependent and -independent inhibitors. In particular, the uptake of rMP65 by DC was 73% and 63% with 5 μg/ml of nystatin and 45 μM of chlorpromazine, respectively, while in MDM the same doses induced uptake rates of 53% and 56%, respectively. The observed effects were dose dependent in both DC and MDM. A combination of 5 μg/ml of nystatin and 45 μM chlorpromazine did not result in a further decrease in rMP uptake by either MDM or DC. Appropriate controls for both nystatin and chlorpromazine activity were carried out (34) as follows: albumin was used as the positive control for nystatin and the negative control for chlorpromazine, and transferrin was used as the positive control for chlorpromazine and the negative control for nystatin. The results showed that albumin as well as transferrin uptake was significantly impaired by using nystatin and chlorpromazine (Fig. 4).
FIG. 4.
Inhibition of clathrin-independent and clathrin-dependent uptake of rMP65 fluor. MDM or DC were left untreated or treated with nystatin (2.5 or 5 μg/ml) for 30 min at 4°C or with chlorpromazine (30 or 45 μM) for 30 min at 37°C. The cells were then stimulated for 2 h with 25 μg/ml of rMP65 fluor, transferrin fluor, and albumin fluor. Results are expressed as percentages of rMP65 fluor, albumin fluor, and transferrin fluor uptake. Data are expressed as means ± SEM for three independent experiments. *, P < 0.05 (nystatin- or chlorpromazine-treated cells versus untreated cells).
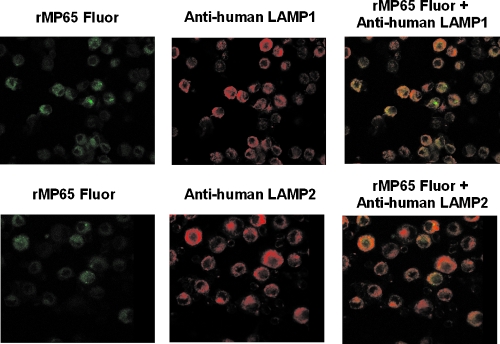
To better understand the fate of rMP65 in cells, the intracellular localization of rMP65 was analyzed in DC by using confocal microscopy. DC were incubated with rMP65 fluor, fixed, permeabilized, and treated with a monoclonal antibody to LAMP-1 or LAMP-2. Figure 5 shows that rMP65 is probably present in the LAMP-negative compartment and that it colocalizes in part with LAMP-1 and LAMP-2 in the lysosome compartment.
FIG. 5.
Colocalization of rMP65 with LAMP-1 and LAMP-2. DC were treated with rMP65 fluor (25 μg/ml) for 2 h at 37°C. The cells were then fixed, permeabilized, and incubated for 1 h with primary antibodies (mouse anti-human LAMP-1 and mouse anti-human LAMP-2). After incubation, the cells were treated for 30 min with secondary antibodies (anti-mouse immunoglobulin G conjugated with Cy). Cells were fixed and analyzed with a confocal microscope. The data reported are from one experiment out of three performed with similar results.
Cytokine production and T-cell activation.
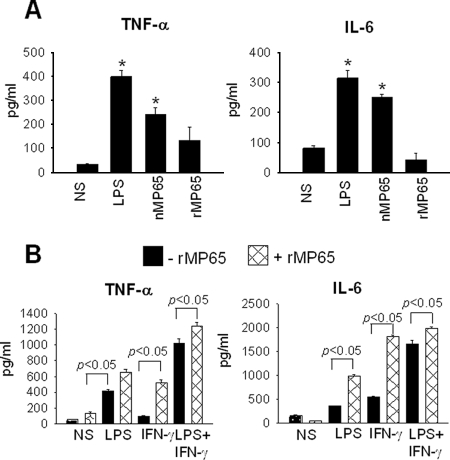
We previously demonstrated that nMP65 is able to stimulate production of proinflammatory cytokines, such as IL-6 and TNF-α, by macrophages and DC (28). Given that these cytokines are important in the protective response against human candidiasis (7), we examined whether lack of the mannan moiety affected production of the above cytokines. MDM left unstimulated or stimulated for 30 min with LPS (1 μg/ml) or IFN-γ (100 ng/ml), alone or in combination, were cocultured with rMP65 (5 μg/ml) for 18 h; subsequently, IL-6 and TNF-α production was evaluated in supernatant fluids. The results reported in Fig. 6A show that in contrast to the case for nMP65, rMP65 was unable to stimulate IL-6 and TNF-α. Moreover, we tested whether nMP65 stimulates TNF-α via the caveolin-dependent pathway. The results showed that the production of TNF-α was unchanged by adding nystatin to the culture (5 or 2.5 μg/ml) (not shown). Prolonged incubation (48 to 72 h) as well as higher doses of rMP65 (30 to 50 μg/ml) did not result in induction of the above cytokines. Nonetheless, rMP65 greatly increased cytokine production in response to LPS or IFN-γ, both alone and in combination (Fig. 6B).
FIG. 6.
TNF-α and IL-6 production from activated MDM after stimulation with rMP65. (A) MDM were left untreated (NS) or treated with rMP65, nMP65 (5 μg/ml), or LPS (1 μg/ml) for 18 h at 37°C. After incubation, supernatants were recovered and tested for the presence of TNF-α and IL-6. Data are expressed as means ± SEM for four independent experiments. *, P < 0.05 (rMP65-treated cells versus untreated cells). (B) MDM were left untreated (NS) or treated with LPS (1 μg/ml) or IFN-γ (100 ng/ml), alone or in combination, for 30 min at 37°C. After activation, cells were left untreated (−rMP65) or treated with 5 μg/ml rMP65 (+rMP65) for 18 h at 37°C. Data are expressed as means ± SEM for four independent experiments. Statistical analysis was performed with ANOVA and corrected by the Bonferroni test for multiple comparisons. *, P < 0.05 (rMP65-treated cells versus untreated cells).
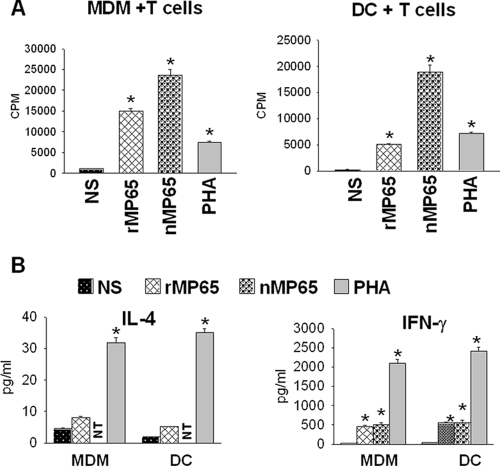
Finally, we tested whether rMP65-loaded DC or MDM were indeed able to stimulate T-cell activation. To this end, MDM or DC were treated with rMP65 or nMP65 and cocultured with purified PBL. The results showed that nMP65- as well as rMP65-loaded MDM or DC were able to stimulate PBL proliferation (Fig. 7A). The proliferating cells expressed mainly (68.3%) the CD4 phenotype (data not shown). Moreover, in parallel experiments, we tested the supernatant fluids of the above-mentioned cells for the presence of IFN-γ and IL-4. The results showed an appreciable production of IFN-γ but not of IL-4 by rMP65-treated PBL with respect to untreated cells (Fig. 7B). Positive controls for T-cell proliferation and cytokine production were performed using PHA-stimulated cells (Fig. 7).
FIG. 7.
Proliferation of T cells and IL-4 and IFN-γ secretion in supernatant fluids from MDM or DC treated with rMP65 and cocultured with autologous T cells. MDM or DC left untreated (NS) or treated with nMP65 (5 μg/ml) or rMP65 (5 μg/ml) were added to autologous T cells. PHA (10 μg/ml) was used as a positive control. (A) Proliferation was measured after 4 days of incubation. (B) The supernatant fluids were recovered after 5 days of incubation and tested for IL-4 and IFN-γ by ELISA. NT, not tested. Data are expressed as means ± SEM for four independent experiments. *, P < 0.05 (rMP65-, nMP65-, or PHA-treated cells versus untreated cells).
DISCUSSION
MP65 is a putative β-glucanase adhesin of C. albicans required for hyphal morphogenesis and pathogenicity (32). Among cell wall components, it represents a main target of the host immune response against C. albicans infection (11). In a previous paper, we demonstrated that nMP65 was immunogenic and protective against C. albicans (28). Collectively, the data generated so far indicate MP65 as a potential candidate vaccine against infections by this fungus (4, 6). However, there is little information on cellular and molecular events leading to MP65 recognition by innate immunity and the generation of a protective immune response. Particularly, it is not clear whether a recombinant mannan moiety-deprived MP65 protein, i.e., a preparation actually considered for a vaccine formulation, could replace the native immunogen. For instance, very little is known about the capacity of the recombinant product to induce production of cytokines by professional APC, a capacity largely possessed by nMP65 (28). Conversely, nonprofessional APC such as B cells do indeed have the capability to present rMP65, despite their incapacity to present nMP65 (25). This indicates that the mannan moiety of MP65, in spite of its prominent role in mediating molecular recognition by critical immune receptors such as TLRs (23), could be instrumental in limiting the antigen presentation process. MP65 also possesses an RGD signature putatively involved in adherence to tissue (2, 9). The RGD sequence is common to many extracellular ligands, and adherence to human MDM is mediated by an interaction between the RGD motif and integrin CD11b/CD18 (CR3) (36). In our experimental system, the RGD sequence partially inhibits rMP65 uptake by MDM and DC, suggesting that rMP65 is able to bind to the CR3 receptor through the RGD sequence. This is supported by a correlation between the amount of CR3 expression and rMP65 uptake. As a matter of fact, rMP65, although lacking the mannosylated moiety, is able to interact with cells of innate immunity; in particular, it is endocytosed and rapidly internalized by DC and MDM via different processes, including those involving clathrin-coated pits. A possible nonspecific uptake of rMP65 into cells of innate immunity, such as MDM, DC, and MN, was excluded by using a control consisting of rCpPLD generated in the same E. coli vector, endowed with the same genetic construction and purification as rMP65; in fact, rMP65, but not rCpPLD, was taken up by all of the above cells. While it was unable to induce proinflammatory cytokine release, rMP65 was able to induce T-cell activation. Further analysis performed with DC and MDM showed that there is a partial degradation or excretion of rMP65 within 24 h, suggesting that it is not fully accumulated inside these cells. This supports the hypothesis that, at least in part, rMP65 could be processed and presented, promoting the performance of APC, with subsequent induction of efficient adaptive immunity. In a previous paper, we demonstrated that TLR2 and TLR4 are key receptors in mediating proinflammatory MP65-induced cytokine release by immune cells (28). Therefore, the inability of rMP65 to stimulate proinflammatory cytokines should be greatly ascribable to its inability to bind to TLRs. Moreover, the caveolin pathway did not seem to be involved in mediating TNF-α secretion.
Professional and nonprofessional APC, i.e., DC and MDM, respectively, took up different amounts of rMP65, as demonstrated by the lesser uptake by DC than that by MDM. Although a significant increase in rMP65 uptake was observed when both types of cells were preactivated with IFN-γ and LPS alone or in combination, uptake differences between DC and MDM persisted. Specifically, MDM took up a significant amount of rMP65 within 30 min, while DC showed a greater rMP65 uptake within 6 h, suggesting that MDM are more prompt and more efficient than DC in taking up rMP65, even during the activation state. This could reflect, at least in part, the different capacities of MDM and DC to exploit the endocytic pathway, which is considered a constitutive process inherent to macrophages (10) but not to DC; in fact, these cells are believed to be less equipped than MDM for this function (17). At any rate, the above differences did not seem to affect the capacity of DC to induce a T-cell response: in fact, both cell types were able to stimulate efficient T-cell activation in response to rMP65.
Compelling evidence exists for the key role of endocytic machinery in bacterial internalization (39), and different proteins are endocytosed via clathrin-dependent and/or -independent pathways (24). Clathrin-dependent endocytic machinery is involved in rMP65 uptake by MDM and DC. However, differently from MDM, caveolae are less involved in DC endocytosis. This may be ascribable to intrinsic differences in cellular uptake mechanisms previously observed for these cells (41). It is worth noticing that the blockade of clathrin and caveolin rMP65-mediated uptake did not completely preclude the ingestion of rMP65, suggesting that it is transported into cells through additional mechanisms. A partial colocalization of rMP65 with LAMPs suggests that partial degradation of protein may occur inside lysosomes. The observed activation of CD4 T cells with production of IFN-γ may indeed be a consequence of presentation on APC of peptide-major histocompatibility complex class II complexes, formed in part in the lysosomal compartment.
nMP65 induces DC maturation and activation and proinflammatory cytokine production, as well as driving a protective T-cell response against C. albicans (28). In our study, in contrast to nMP65, rMP65 failed to stimulate TNF-α production from MDM and DC, suggesting that the polysaccharide moiety of the native protein plays a critical role in cytokine induction in APC. This is consistent with recent results published by Netea et al. showing that mannan as well as glucan is involved in cytokine stimulation induced by C. albicans (23).
In summary, our study reports for the first time a systematic investigation of rMP uptake by different human immune cells to demonstrate that rMP65 uptake by DC and MDM also occurs through the endocytic pathway, with involvement of clathrin-coated pits. This could imply that rMP65 is transported into endosome and lysosome compartments, where it is degraded and loaded on major histocompatibility complex molecules. The observation that rMP65 stimulates T-cell proliferation and drives the T-helper response is in line with this hypothesis. Overall, our data also suggest that the mannan moiety of MP65 is not essential in inducing the T-cell response and that the lack of it in the recombinant product may not be an obstacle to its potential use as a vaccine.
Acknowledgments
This work was supported by a grant from the National Research Program on AIDS (contract 50G.38) and by a donation from the Fondazione della Cassa di Risparmio di Perugia (project code 2006.020.0275).
We thank Antonio Cassone for critical support and Carla Proietti for confocal microscopy assistance.
Editor: A. Casadevall
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 2.Calderone, R., S. Suzuki, R. Cannon, T. Cho, D. Boyd, J. Calera, H. Chibana, D. Herman, A. Holmes, H. W. Jeng, H. Kaminishi, T. Matsumoto, T. Mikami, J. M. O'Sullivan, M. Sudoh, M. Suzuki, Y. Nakashima, T. Tanaka, G. R. Tompkins, and T. Watanabe. 2000. Candida albicans: adherence, signaling and virulence. Med. Mycol. 38(Suppl. 1)125-137. [PubMed] [Google Scholar]
- 3.Cao, L., K. M. Chan, D. Chen, N. Vanittanakom, C. Lee, C. M. Chan, T. Sirisanthana, D. N. Tsang, and K. Y. Yuen. 1999. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J. Clin. Microbiol. 37981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassone, A. 2008. Fungal vaccines: real progress from real challenges. Lancet Infect. Dis. 8114-124. [DOI] [PubMed] [Google Scholar]
- 5.Ciervo, A., F. Mancini, and A. Cassone. 2007. Transcription, expression, localization and immunoreactivity of Chlamydophila pneumoniae phospholipase D protein. Microb. Pathol. 4396-105. [DOI] [PubMed] [Google Scholar]
- 6.Cutler, J. E., G. S. Deepe, Jr., and B. S. Klein. 2007. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 513-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dongari-Bagtzoglou, A., and P. L. Fidel, Jr. 2005. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J. Dent. Res. 84966-977. [DOI] [PubMed] [Google Scholar]
- 8.Fidel, P. L., Jr., and J. D. Sobel. 1996. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin. Microbiol. Rev. 9335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gale, C. A., C. M. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. K. Hostetter. 1998. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 2791355-1358. [DOI] [PubMed] [Google Scholar]
- 10.Garin, J., R. Diez, S. Kieffer, J. F. Dermine, S. Duclos, E. Gagnon, R. Sadoul, C. Rondeau, and M. Desjardins. 2001. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152165-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez, M. J., B. Maras, A. Barca, R. La Valle, D. Barra, and A. Cassone. 2000. Biochemical and immunological characterization of MP65, a major mannoprotein antigen of the opportunistic human pathogen Candida albicans. Infect. Immun. 68694-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez, M. J., A. Torosantucci, S. Arancia, B. Maras, L. Parisi, and A. Cassone. 1996. Purification and biochemical characterization of a 65-kilodalton mannoprotein (MP65), a main target of anti-Candida cell-mediated immune responses in humans. Infect. Immun. 642577-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilkens, C. M., P. Kalinski, M. de Boer, and M. L. Kapsenberg. 1997. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood 901920-1926. [PubMed] [Google Scholar]
- 14.Kalinski, P., C. M. Hilkens, A. Snijders, F. G. Snijdewint, and M. L. Kapsenberg. 1997. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 15928-35. [PubMed] [Google Scholar]
- 15.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1398-401. [DOI] [PubMed] [Google Scholar]
- 16.La Valle, R., S. Sandini, M. J. Gomez, F. Mondello, G. Romagnoli, R. Nisini, and A. Cassone. 2000. Generation of a recombinant 65-kilodalton mannoprotein, a major antigen target of cell-mediated immune response to Candida albicans. Infect. Immun. 686777-6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennon-Dumenil, A. M., A. H. Bakker, R. Maehr, E. Fiebiger, H. S. Overkleeft, M. Rosemblatt, H. L. Ploegh, and C. Lagaudriere-Gesbert. 2002. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J. Exp. Med. 196529-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitz, S. M., and E. A. North. 1997. Lymphoproliferation and cytokine profiles in human peripheral blood mononuclear cells stimulated by Cryptococcus neoformans. J. Med. Vet. Mycol. 35229-236. [DOI] [PubMed] [Google Scholar]
- 19.Levitz, S. M., and C. A. Specht. 2006. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 6513-524. [DOI] [PubMed] [Google Scholar]
- 20.Mansour, M. K., L. S. Schlesinger, and S. M. Levitz. 2002. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J. Immunol. 1682872-2879. [DOI] [PubMed] [Google Scholar]
- 21.Mencacci, A., A. Torosantucci, R. Spaccapelo, L. Romani, F. Bistoni, and A. Cassone. 1994. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect. Immun. 625353-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy, J. W., F. Schafer, A. Casadevall, and A. Adesina. 1998. Antigen-induced protective and nonprotective cell-mediated immune components against Cryptococcus neoformans. Infect. Immun. 662632-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netea, M. G., N. A. Gow, C. A. Munro, S. Bates, C. Collins, G. Ferwerda, R. P. Hobson, G. Bertram, H. B. Hughes, T. Jansen, L. Jacobs, E. T. Buurman, K. Gijzen, D. L. Williams, R. Torensma, A. McKinnon, D. M. MacCallum, F. C. Odds, J. W. Van der Meer, A. J. Brown, and B. J. Kullberg. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 1161642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols, B. 2003. Caveosomes and endocytosis of lipid rafts. J. Cell Sci. 1164707-4714. [DOI] [PubMed] [Google Scholar]
- 25.Nisini, R., G. Romagnoli, M. J. Gomez, R. La Valle, A. Torosantucci, S. Mariotti, R. Teloni, and A. Cassone. 2001. Antigenic properties and processing requirements of 65-kilodalton mannoprotein, a major antigen target of anti-Candida human T-cell response, as disclosed by specific human T-cell clones. Infect. Immun. 693728-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., S. R. Lockhart, C. Pujol, J. A. Swails-Wenger, S. A. Messer, M. B. Edmond, R. N. Jones, R. P. Wenzel, and D. R. Soll. 1998. Hospital specificity, region specificity, and fluconazole resistance of Candida albicans bloodstream isolates. J. Clin. Microbiol. 361518-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer, K., B. Schoel, H. Gulle, S. H. Kaufmann, and H. Wagner. 1990. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur. J. Immunol. 201175-1179. [DOI] [PubMed] [Google Scholar]
- 28.Pietrella, D., G. Bistoni, C. Corbucci, S. Perito, and A. Vecchiarelli. 2006. Candida albicans mannoprotein influences the biological function of dendritic cells. Cell. Microbiol. 8602-612. [DOI] [PubMed] [Google Scholar]
- 29.Pietrella, D., R. Mazzolla, P. Lupo, L. Pitzurra, M. J. Gomez, R. Cherniak, and A. Vecchiarelli. 2002. Mannoprotein from Cryptococcus neoformans promotes T-helper type 1 anticandidal responses in mice. Infect. Immun. 706621-6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin, R. H., and L. S. Young (ed.). 1994. Clinical approach to infection in the compromised host. Plenum Press, New York, NY.
- 31.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1791109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandini, S., R. La Valle, F. De Bernardis, C. Macri, and A. Cassone. 2007. The 65 kDa mannoprotein gene of Candida albicans encodes a putative beta-glucanase adhesin required for hyphal morphogenesis and experimental pathogenicity. Cell. Microbiol. 91223-1238. [DOI] [PubMed] [Google Scholar]
- 33.Schnitzer, J. E., P. Oh, E. Pinney, and J. Allard. 1994. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 1271217-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh, R. D., V. Puri, J. T. Valiyaveettil, D. L. Marks, R. Bittman, and R. E. Pagano. 2003. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 143254-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snijders, A., P. Kalinski, C. M. Hilkens, and M. L. Kapsenberg. 1998. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 101593-1598. [DOI] [PubMed] [Google Scholar]
- 36.Sussmuth, S. D., A. Muscholl-Silberhorn, R. Wirth, M. Susa, R. Marre, and E. Rozdzinski. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 684900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torosantucci, A., C. Bromuro, M. J. Gomez, C. M. Ausiello, F. Urbani, and A. Cassone. 1993. Identification of a 65-kDa mannoprotein as a main target of human cell-mediated immune response to Candida albicans. J. Infect. Dis. 168427-435. [DOI] [PubMed] [Google Scholar]
- 38.Vecchiarelli, A., D. Pietrella, M. Dottorini, C. Monari, C. Retini, T. Todisco, and F. Bistoni. 1994. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin. Exp. Immunol. 98217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veiga, E., and P. Cossart. 2005. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 7894-900. [DOI] [PubMed] [Google Scholar]
- 40.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1231107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang, S. D., A. Scholzen, G. Minigo, C. David, V. Apostolopoulos, P. L. Mottram, and M. Plebanski. 2006. Pathogen recognition and development of particulate vaccines: does size matter? Methods 401-9. [DOI] [PubMed] [Google Scholar]