Abstract
Interleukin-17 (IL-17) is a proinflammatory cytokine secreted by the newly described CD4+ Th17 subset, which is distinct from classic Th1 and Th2 lineages. IL-17 contributes to bone destruction in rheumatoid arthritis but is essential in host defense against pathogens that are susceptible to neutrophils. Periodontal disease (PD) is a chronic inflammatory condition initiated by anaerobic oral pathogens such as Porphyromonas gingivalis, and it is characterized by host-mediated alveolar bone destruction due primarily to the immune response. The role of IL-17 in PD is controversial. Whereas elevated IL-17 levels have been found in humans with severe PD, we recently reported that female C57BL/6J mice lacking the IL-17 receptor (IL-17RAKO) are significantly more susceptible to PD bone loss due to defects in the chemokine-neutrophil axis (J. J. Yu, M. J. Ruddy, G. C. Wong, C. Sfintescu, P. J. Baker, J. B. Smith, R. T. Evans, and S. L. Gaffen, Blood 109:3794-3802, 2007). Since different mouse strains exhibit differences in susceptibility to PD as well as Th1/Th2 cell skewing, we crossed the IL-17RA gene knockout onto the BALB/c background and observed a similar enhancement in alveolar bone loss following P. gingivalis infection. Unexpectedly, in both strains IL-17RAKO female mice were much more susceptible to PD bone loss than males. Moreover, female BALB/c-IL-17RAKO mice were defective in producing anti-P. gingivalis immunoglobulin G and the chemokines KC/Groα and MIP-2. In contrast, male mice produced normal levels of chemokines and anti-P. gingivalis antibodies, but they were defective in granulocyte colony-stimulating factor upregulation. This study demonstrates a gender-dependent effect of IL-17 signaling and indicates that gender differences should be taken into account in the preclinical and clinical safety testing of anti-IL-17 biologic therapies.
Interleukin-17 (IL-17) is a proinflammatory cytokine secreted by activated T cells. Soon after its discovery (47), IL-17 was shown to be produced by CD4+ effector T cells that were not obviously Th1 or Th2 (1, 15, 25, 54). IL-17 also was found to contribute to inflammatory bone pathology in rheumatoid arthritis (RA) and is now known to be centrally involved in numerous autoimmune disorders (16, 32, 41). In contrast to the classic Th1 and Th2 cell populations, IL-17-secreting T cells arise as a distinct and novel T-helper subset, termed Th17. Moreover, gamma interferon and IL-4 derived from Th1 and Th2 cells inhibit Th17 differentiation (12). Mouse Th17 cells arise in the context of tumor growth factor beta in combination with IL-6 or IL-21 (10, 31, 37, 43, 59, 67). IL-23 stimulates the production of IL-17 (2) and is critical for the expansion of Th17 cells in vivo (61). Th17 cells also produce IL-22, IL-17F, IL-26, IL-10, and various chemokines (34). The discovery of Th17 cells has forced a major revision in our understanding of T-cell-mediated inflammation (56).
The receptor for IL-17, IL-17RA, is the founding member of a unique family of cytokine receptors (3, 64). Unlike its ligands IL-17 and IL-17F, IL-17RA is expressed ubiquitously, particularly on nonimmune cells such as fibroblasts, osteoblasts, and epithelial cells (18, 41). Signaling through IL-17RA results in the expression of inflammatory effectors such as IL-6, β-defensins, chemokines, PGE2, RANKL, and various growth factors (52, 53). In particular, studies of IL-17RA knockout (IL-17RAKO) mice have identified its essential role in mediating neutrophil responses. Indeed, IL-17RAKO mice are highly susceptible to bacterial, fungal, and parasitic infections and are linked to severe neutrophil defects (24, 28, 36, 65, 66).
Susceptibility to immune-mediated diseases is influenced by gender. Women are far more likely than men to succumb to autoimmune disorders, including rheumatoid arthritis (female to male ratio, 2:1), multiple sclerosis (2:1), and systemic lupus erythematosis (9:1) (62). Few studies with experimental models have attempted to discern gender-associated factors that contribute to disease (22, 46, 60), and none to date have linked the IL-23-IL-17 axis with gender and disease susceptibility.
Periodontal disease (PD) is a multifactorial inflammatory disease that is triggered by the colonization and invasion of periodontopathic organisms, particularly Porphyromonas gingivalis. Although infectious agents are required for disease initiation and progression, the resulting inflammation and alveolar (jaw) bone destruction requires an immune response. SCID or CD4KO mice are resistant to periodontal bone loss in experimental PD, implicating T cells in driving bone destruction (5, 8). Protection from PD also is associated with competent neutrophil activity, as patients with chronic granulomatous disease or leukocyte adhesion deficiency and mice with neutrophil defects experience severe PD (7, 9, 27, 42, 66). Accordingly, in our recent study we were not surprised to find that IL-17RAKO mice infected with P. gingivalis exhibit increased susceptibility to PD bone loss, which was due largely to neutrophil recruitment defects (33, 66).
PD susceptibility varies by genetic background (4, 6). Since Th subset skewing also is genetically influenced, we transferred the IL-17RA gene knockout from a C57BL/6 background to the PD-susceptible BALB/CJ background and assessed the contribution of IL-17 signaling. Here, we report that IL-17RAKO mice of both strains show increased susceptibility to PD bone loss. However, female IL-17RAKO mice showed more severe disease. This is the first report of a cytokine receptor knockout that is differentially affected by gender in the context of PD.
MATERIALS AND METHODS
Mice.
Wild-type (WT) BALB/CJ and C57BL/6 (B6) mice (6 to 8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). IL-17RAKO mice (B6) were from Amgen (Seattle, WA). IL-17RAKO mice on a BALB/CJ background were generated by successive backcrossing for seven generations. Animals were housed in specific-pathogen-free conditions under protocols approved by the University at Buffalo IACUC.
Bacterial culture.
Porphyromonas gingivalis (strain A7A1-28) was stored at −70°C in brain heart infusion broth (BHI; BD Biosciences, San Jose, CA) with 15% glycerol, 5.0 μg/ml hemin, and 5.0 μg/ml menadione (Sigma, St. Louis, MO). Bacteria were plated on BHI agar supplemented with 5% defibrinated sheep blood (Bio Link Incorporated, Liverpool, NY), 5.0 μg/ml hemin, and 5.0 μg/ml menadione and were grown anaerobically in 5% CO2, 5% H2, and 90% N2 at 37°C.
Mouse model of PD.
Mice were infected with P. gingivalis as previously described (8, 21). Briefly, mice were given fulfatrim (2 mg/ml [wt/vol] sulfamethoxazle and 0.4 mg/ml [wt/vol] trimethoprim; Alpharma USPD Inc., Baltimore, MD) in drinking water for 10 days, followed by 5 days of water without antibiotics. Mice were infected with ∼1011 CFU P. gingivalis in 500 μl 2% carboxymethylcellulose (CMC) suspension via oral gavage three times at 2-day intervals. Sham-treated mice were given 500 μl CMC alone. Mice were sacrificed after 6 weeks, and serum was collected by cardiac puncture. Maxillary jaws were autoclaved, defleshed, and stained with 1% methylene blue to distinguish enamel from bone. Horizontal bone loss was assessed morphometrically by measuring the distance between the alveolar bone crest (ABC) and cementoenamel junction (CEJ) at 14 buccal sites on the maxillae under a dissecting microscope (Brook-Anco, Rochester, NY) fitted with an Aquinto imaging measurement system (a4i America, Rochester, NY). Analyses were performed by two investigators in a blinded fashion, as described previously (66).
ELISA.
P. gingivalis-specific enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (8, 66). Briefly, 96-well Immuno-Maxisorp plates (Nalgene Nunc International, Rochester, NY) were coated with formalin-fixed P. gingivalis. Sera were added in twofold serial dilutions, and anti-P. gingivalis immunoglobulin G (IgG) was detected using alkaline phosphatase-conjugated goat anti-mouse IgG (Zymed Technologies-Invitrogen, Carlsbad, CA). The titer was defined as the log2 of the highest dilution with a signal that was 0.1 optical density units above the level of the background signal. Cytokine ELISA kits were from R&D Systems (Minneapolis, MN) or eBioscience (San Diego, CA).
Cell culture.
Primary mouse spleen cells were stimulated with IL-17 or IL-17F (200 ng/ml), lipopolysaccharide (5 μg/ml), IL-1β (10 ng/ml), or tumor necrosis factor alpha (TNF-α) (2 ng/ml), and supernatants were assayed for IL-6 by ELISA. Cytokines were from R&D Systems or Peprotech.
Data analysis.
Data were analyzed on GraphPad Prism software (GraphPad, San Diego, CA). Net bone loss is defined as the ABC-CEJ distance of P. gingivalis-infected sites minus the mean ABC-CEJ distance of sham-treated sites. The net antibody level is the antibody titer of P. gingivalis-infected mice minus the mean titer of sham-treated mice. The (n-fold) increase in cytokine production is the concentration of cytokines in P. gingivalis-infected mice divided by the concentration in sham-treated mice, multiplied by 100. Comparisons between groups were made using a Student's t test or analysis of variance, as appropriate. Statistical significance is defined as P < 0.05.
RESULTS
Female IL-17RAKO mice exhibit increased bone loss.
Susceptibility to alveolar bone destruction in murine models of periodontal disease (PD) depends on environmental and genetic factors (reviewed in reference 21). Inbred strains of mice, including B6 mice, are resistant to alveolar bone loss, whereas BALB/CJ mice are susceptible (6). We previously reported that an IL-17RA deficiency on the B6 background increased susceptibility to alveolar bone loss induced by P. gingivalis (66). Intriguingly, however, only female IL-17RAKO mice exhibited this phenotype (Fig. 1A, left), whereas male IL-17RAKO mice were resistant, similarly to male and female WT controls. This finding was surprising, as this model has not been reported to favor one gender. Additionally, human PD is not known to affect women disproportionately over men (reviewed in reference 11; also see Discussion). Indeed, there was no gender difference in terms of PD in the WT mice (Fig. 1A). Therefore, we hypothesized that increased susceptibility in female IL-17RAKO mice was due to the differential regulation of IL-17RA-regulated gene products by gender.
FIG. 1.
Female IL-17RAKO mice exhibit enhanced alveolar bone loss in response to P. gingivalis infection. (A) Net bone loss is increased in female IL-17RAKO mice. WT and IL-17RAKO mice (B6, left; BALB/c, right; n = 6 to 8) were infected with P. gingivalis or were sham treated. Alveolar bone loss was assessed 6 weeks after infection. Net bone loss was defined as the ABC-CEJ distance of sham-treated mice subtracted from that of P. gingivalis-infected mice. (Data representing net bone loss from female B6 WT and IL-17RAKO mice were modified from reference 66 with permission of the publisher.) (B) Baseline jaw measurements in BALB/c-IL-17RAKO and B6 mice. Baseline (i.e., uninfected) ABC-CEJ distances are shown for male (M) and female (F) B6 (left) and BALB/c (right) mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant. (C) Net bone loss is enhanced in female BALB/c-IL-17RAKO mice. Female WT (top) and IL-17RAKO mice (bottom) (n = 8) on the BALB/c background were infected as described for panel A. Bone loss at 14 buccal sites (R1 to R7, right side; L1 to L7, left side) was evaluated. Standard deviations are shown, and significance was assessed by an unpaired t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant. Pg, P. gingivalis. (D) Representative jaw phenotypes of female P. gingivalis-infected BALB/c WT and IL-17RAKO mice. Maxillary bones were stained with methylene blue. Images were acquired with a Nikon SMZ 1000 microscope (magnification, ×3).
To determine whether the increased susceptibility to alveolar bone loss was restricted to the B6 background, the IL-17RA gene deletion was backcrossed onto the BALB/c background for seven generations. IL-17RAKO mice on the BALB/c background did not exhibit gross abnormalities and produced litter sizes that are typical of this strain. There was a noticeable increase in the prevalence of genitourinary irritation, but this did not affect procreation or the elimination of bodily waste. Males needed to be housed separately, as there was an increase in the aggression characteristics of BALB/c mice (http://www.informatics.jax.org/external/festing/mouse/docs/BALB.shtml). With regard to alveolar bone phenotype, the baseline distance between the CEJ and the ABC was similar between male and female WT mice (Fig. 1B). BALB/c WT male mice exhibited a trend toward higher ABC-CEJ distances than females, but the findings were not statistically different. Mice with IL-17RAKO on the B6 background did not exhibit altered baseline ABC- CEJ distances. However, male, but not female, IL-17RAKO mice on the BALB/c background showed increased ABC-CEJ distances. The differential effect of the IL-17RA deficiency between genders illustrates a complex interaction between IL-17RA signaling, gender, and bone anatomy. These differences in baseline bone characteristics also highlight the necessity of normalizing changes seen in infection to the baseline levels that are characteristic of each gender and genotype.
When BALB/c WT mice were infected with P. gingivalis, increased PD bone loss was observed, as measured by the increased distances between the CEJ and ABC in all infected mice compared to those of sham-treated mice (Fig. 1A, C), which has been reported previously (6). There was considerably more bone loss in BALB/c-IL-17RAKO mice than in WT mice, reproducing findings made with B6 mice (66) (Fig. 1A, right). To account for the baseline bone distances unique to each gender and genotype, net bone loss was expressed as the CEJ-ABC distances of P. gingivalis-infected mice minus the mean CEJ-ABC distances of sham-treated matched mice. In both males and females, increased bone loss was observed in IL-17RAKO mice (Fig. 1A). However, in contrast to the B6-IL-17RAKO mice, both male and female BALB/c-IL-17RAKO mice exhibited significantly increased bone loss. Nonetheless, female IL-17RAKO mice in both strains were more susceptible than their male counterparts.
In our prior study, B6-IL-17RAKO mice exhibited most bone loss in the vicinity of the first molar (sites 1 to 3) on both the left and right maxillae (66). However, infection in both genders of BALB/c WT and IL-17RAKO mice resulted in increased bone loss in the left compared to the right maxillae (Fig. 1C). Asymmetry in bone loss has been observed by others for this strain, although the underlying mechanism is not understood (30). Additionally, increased bone loss was observed in the vicinity of the first molar, but the loss also was significant at the second and third molars of BALB/c-IL-17RAKO mice (Fig. 1C, D). Thus, BALB/c mice exhibit qualitatively similar but quantitatively greater PD bone loss due to an IL-17RA deficiency than do B6 mice.
P. gingivalis-specific antibody response is impaired in female BALB/c-IL-17RAKO mice.
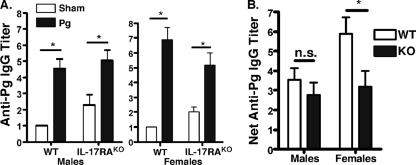
Many studies exploring murine models of PD have described a requirement for antigen-specific lymphocytes contributing to alveolar bone loss (58). Other studies have described the immunomodulatory functions of gender-specific factors regarding the activity of lymphocytes (62). To determine the role of IL-17RA in the regulation of antibody production as well as to assess the extent of antigen-specific responses, serum samples from sham- and P. gingivalis-infected mice were evaluated for P. gingivalis-specific IgG. Both BALB/c WT and BALB/c-IL-17RAKO male and female mice exhibited a robust anti-P. gingivalis IgG response (Fig. 2A). Interestingly, the sham antibody titers in BALB/c-IL-17RAKO mice were higher than sham titers in BALB/c WT mice. This phenotype is consistent with the findings of our previous study, in which sham titers in B6-IL-17RAKO mice were higher than sham titers in B6 WT mice, but both WT and IL-17RAKO mice responded vigorously to P. gingivalis, indicating successful infection (Fig. 2A) (66). To normalize the baseline differences between WT and IL-17RAKO mice, the mean titer of sham-treated mice was subtracted from the titer of P. gingivalis-infected mice (Fig. 2B). By this analysis, the net induction of P. gingivalis-specific IgG was impaired in female BALB/c-IL-17RAKO mice compared to that of WT mice (Fig. 2B, right). In contrast, the IL-17RA gene deletion in males did not significantly affect the levels of P. gingivalis-specific IgG (Fig. 2B, left). Serum anti-P. gingivalis IgA or IgM was not detected in any mouse (data not shown). Therefore, serum IgG responses reflected gender differences in PD bone loss.
FIG. 2.
Anti-P. gingivalis IgG production is impaired in female BALB/c-IL-17RAKO mice. (A) WT and IL-17RAKO mice exhibit anti-P. gingivalis antibody responses. Serum anti-P. gingivalis IgG was evaluated in P. gingivalis-infected mice by ELISA, and means ± standard deviations are shown. *, P < 0.05. Pg, P. gingivalis. (B) Net antibody titer is impaired in female mice. The titer is defined as the antibody titer of sham-treated mice subtracted from that of P. gingivalis-infected mice. Standard deviations are shown, and statistical significance was assessed by an unpaired t test. *, P < 0.05; n.s., not statistically different.
Female and male BALB/c-IL-17RAKO mice show impairments in different IL-17RA target genes.
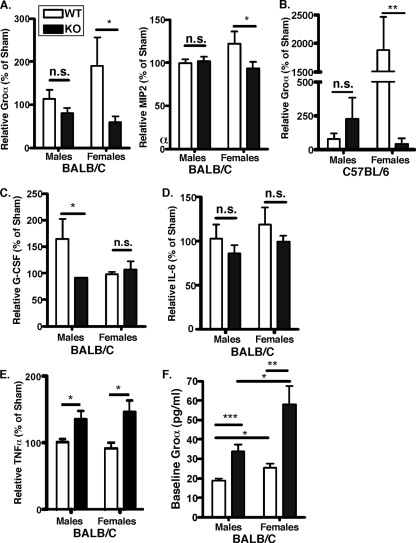
Neutrophil-attracting chemokine gene expression is controlled by IL-17 both in vivo and in vitro (48, 63), and many studies have demonstrated a requirement of IL-17RA for neutrophil-mediated host protection (24, 28, 65). Although both male and female BALB/c-IL-17RAKO mice exhibited increased bone loss compared to that of WT mice, we hypothesized that the magnitude of the IL-17RA defect would be greater in female BALB/c-IL-17RAKO mice. Indeed, the induction of Groα (also called KC or CXCL1) was significantly impaired in P. gingivalis-infected female BALB/c-IL-17RAKO mice compared to that of female WT mice. However, no changes in the levels of Groα were observed in P. gingivalis-infected IL-17RAKO male mice (Fig. 3A, left). Similarly, MIP-2 (also called CXCL2) was significantly impaired in infected female IL-17RAKO mice compared to the levels in WT mice, whereas MIP-2 levels did not differ between P. gingivalis-infected IL-17RAKO and WT male mice (Fig. 3A, right). Baseline levels of MIP-2 were similar across genders and genotypes and would not account for the differences seen in MIP-2 upregulation after infection (data not shown). A similar finding was found between the chemokine levels of Groα in males and females in the B6 background (Fig. 3B). This selective defect of neutrophil chemokine regulation likely contributes to the increased bone loss in female IL-17RAKO mice due to impaired neutrophil recruitment, as shown previously (66).
FIG. 3.
Differential impairment of IL-17-dependent gene expression in male and female BALB/c-IL-17RAKO mice. (A) Female, but not male, BALB/c-IL-17RAKO mice show the impaired expression of CXC chemokines. Chemokine levels in sera from male and female IL-17RAKO mice were determined by ELISA and are defined as the chemokine concentrations from P. gingivalis-infected mice as percentages of the levels of chemokines from sham-treated mice. (B) Female, but not male, B6-IL-17RAKO mice show the impaired expression of CXC chemokines. Sera from B6-IL-17RAKO mice were analyzed for Groα as described for panel A. (Groα data from the female B6 WT and IL-17RAKO mice were modified from reference 66 with permission of the publisher.) (C) G-CSF expression is impaired in male, but not female, IL-17RAKO mice. G-CSF serum levels in the indicated mice are shown, normalized as described for panel A. (D) Serum IL-6 production is not altered in IL-17RAKO mice. IL-6 levels in serum from the indicated mice are shown, normalized as for panel A. (E) Serum TNF-α production is enhanced in male and female IL-17RAKO mice. TNF-α levels in serum from the indicated mice are shown, normalized as for panel A. (F) Baseline levels of Groα are increased in IL-17RAKO mice. Serum Groα levels from uninfected mice are shown. Standard deviations are shown, and significance was assessed by an unpaired t test. *, P < 0.05; n.s., not statistically different.
Interestingly, the baseline or uninfected levels of Groα differed depending on gender and genotype. IL-17RAKO mice exhibited increased baseline Groα levels in both males and females. Additionally, females had higher Groα levels than males in WT and IL-17RAKO backgrounds (Fig. 3F). Surprisingly, the increased baseline levels of Groα in IL-17RAKO mice does not translate to increased protection from P. gingivalis-induced bone loss, since IL-17RAKO mice are more susceptible to bone loss than WT mice (Fig. 1). The Groα defect observed in P. gingivalis-infected female IL-17RAKO mice likely is not caused by inherent differences in chemokine levels but rather by defects in chemokine upregulation after infection. The additional differences in Groα from uninfected mice emphasize the importance of reporting the induction of chemokine levels due to P. gingivalis infection measured relative to baseline levels.
IL-17 plays an important role in neutrophil homeostasis (55) as well as expanding neutrophil numbers by regulating granulocyte colony-stimulating factor (G-CSF) (15). Reduced levels of G-CSF have been observed in bacterially infected IL-17RAKO mice (65). Baseline levels of G-CSF were similar across both genders and genotypes (data not shown). In P. gingivalis-infected mice, no defect in G-CSF was observed in female BALB/c-IL-17RAKO mice (Fig. 3C). In contrast, male BALB/c-IL-17RAKO mice did not increase G-CSF levels to the same extent as male WT mice. This selective defect of G-CSF points to a mechanistic difference in neutrophil regulation in male compared to female mice. Nonetheless, defects in neutrophil regulation occur in both genders, which we have previously shown to be critical for controlling PD bone loss (66).
A major gene target of IL-17 is IL-6 (49, 64). IL-6 levels from uninfected mice were similar across genotypes and genders (not shown). In the context of experimental PD, the infected IL-17RAKO mice showed only slightly decreased production of IL-6, which was not statistically different from that for WT mice (Fig. 3D). Since the effect was similar in male and female mice, the gender-mediated effects of IL-17 on cytokine production appear to be specific for neutrophil growth factors and chemokines.
We and others have shown that IL-17 signals synergistically with TNF-α (40, 49). In this model, we found no gender difference in TNF-α levels for either strain (Fig. 3E). However, the induction of TNF-α was more pronounced in BALB/c mice than in B6 mice, which may partly explain the increased susceptibility to bone loss in the BALB/c background.
To determine whether there was an inherent signaling difference between genders in terms of IL-17 responsiveness, spleen cells from male or female WT B6 mice were stimulated with IL-17, IL-17F, and TNF-α for 24 h, and IL-6 secretion was assessed by ELISA. Strikingly, female mice showed reduced responses to several inflammatory cytokines, including IL-1β, TNF-α, IL-17, and IL-17F (Fig. 4).
FIG. 4.
Reduced IL-17 responses in female splenocytes. Splenocytes from three WT male (black bars) and three WT female mice (white bars) were incubated for 24 h with the indicated cytokines, and IL-6 in the supernatant was assessed in triplicate by ELISA. LPS, lipopolysaccharide.
DISCUSSION
The interaction between gender and immunity is intriguing. Although sex steroids are obvious targets of study, hormones such as prolactin, growth hormone, and insulin-like growth factor 1 are sexually dimorphic and have been implicated in the increased susceptibility of women to autoimmunity. Studies of multifactorial diseases that demonstrate differential susceptibilities between genders encounter confounding variables, such as lifestyle, socioeconomic status, environmental exposures, and genetic polymorphisms (reviewed in reference 62). Hence, experimental models with gender differences enable a more systematic study of the effects of gender on disease.
Several studies have addressed gender dimorphism using mouse models. For example, male mice with experimental autoimmune encephalomyelitis (a model of multiple sclerosis) exhibit a greater activity of Treg cells than females, which served to dampen the inflammatory response and protect males disproportionately from disease (46). T cells isolated from male mice with experimental autoimmune encephalomyelitis expressed elevated levels of peroxisome proliferator-activated receptor α mRNA, which inhibits the activity of NF-κB (13). Peroxisome proliferator-activated receptor α expression is sensitive to androgens, which may partly explain why males are resistant to certain forms of autoimmune inflammation. A model of Toxoplasma gondii infection in SCID mice showed that males were more resistant than females, perhaps implicating sexual dimorphism in innate immunity as well (60). In contrast, our data indicate that female splenocytes respond more poorly than male cells to inflammatory stimuli, including IL-17 (Fig. 4). Although somewhat unexpected, this finding may indicate that the enhanced sensitivity of IL-17RA-deficient female mice compared to that of males lies not in the inherent response to IL-17 but the immunoregulatory mechanisms involved in suppressing IL-17-mediated inflammation. Indeed, a myriad of immune-suppressive mechanisms are in place to limit Th17 development and IL-17 function, including IL-27, IL-2, and IL-10 (reviewed in references 17, 20, and 38). Related to this issue is the possible contribution of Treg cells to the limiting of inflammation, but to date there are no studies to our knowledge examining the status of Treg cells in IL-17RAKO mice.
To date, no study of PD has reported gender-specific effects related to a cytokine deficiency. This study shows that IL-17RA deficiency in the BALB/c background increased susceptibility to bone loss in both males and females, but the magnitude of bone loss is greater in females (Fig. 1). We previously reported a link between defective neutrophil recruitment and increased bone destruction in B6-IL-17RAKO mice (66), but only females were examined in that study. Similarly to the B6-IL-17RAKO mice (66), female BALB/c-IL-17RAKO mice are defective in the production of neutrophil chemokines such as Groα and MIP-2 (Fig. 3A), both of which are downstream of IL-17 (15, 51). However, impairment in chemokines could not be exclusively linked to increased bone destruction, as male IL-17RAKO mice of either strain exhibited no obvious reduction in chemokine production (Fig. 3A-B). Interestingly, male BALB/c-IL-17RAKO mice show a reduced production of G-CSF (Fig. 3C), providing an explanation in which the defective expansion of neutrophils in male IL-17RAKO mice contributes to increased bone loss.
One parameter that could contribute to susceptibility differences in PD is the baseline level of inflammatory factors. Of the cytokines and chemokines tested in uninfected mice, only Groα showed differences between genders and genotypes (Fig. 3F). Although IL-17RAKO mice exhibited increased baseline Groα levels, the induction of Groα in response to infection was defective and, thus, fits with our previously described model that increased neutrophil activity correlates with alveolar bone protection (66).
The enhanced invasion and colonization of P. gingivalis could be another reason why female IL-17RAKO mice exhibited more bone loss than males. One limitation of this PD model is the difficulty in distinguishing between bacteria that have invaded gingival tissue and alveolar bone and the uninvaded bacteria that remain adsorbed to oral mucosa (66). Since male and female WT mice exhibited similar degrees of P. gingivalis-induced bone loss, it is unlikely that there is an inherent increased infectivity of P. gingivalis in normal female mice. Still, it is possible that the IL-17RA gene deficiency differentially increases infectivity in females compared to that in males.
Human PD is more prevalent in men than women (23). Some studies have attributed these differences to behavioral variations in hygiene, alcohol consumption, smoking habits, and the frequency of dental visits (11), but they have not ruled out gender-specific biological factors. Consistently with results for humans, this study reveals no significant difference in the bone loss between male and female PD-susceptible mice (Fig. 1A, right). Since we observe a gender bias only in the context of IL-17RA deficiency, our data argue that IL-17 plays a more profound role in host protection against PD in women than men. Presumably there are alternative factors that operate in males, including immunosuppressive cytokines such as IL-10, but at present these remain unknown.
These experiments support and extend our work that demonstrated a protective role for IL-17RA in PD. However, there is evidence that IL-17 contributes to established, chronic PD (57). For example, disease severity correlates with the increased levels of IL-17 and IL-23 in human gingival crevicular fluid (26, 35). These findings may reflect the possibility that IL-17, like many proinflammatory cytokines, can switch sides from host protection to destruction during conditions of chronic inflammation (45). Importantly, such findings indicate that the blockade of IL-17 could prove useful in treating active PD (33). Conversely, this work may provide a cautionary note with respect to anti-cytokine therapy and its potential side effects on PD. The inhibition of cytokines such as TNF-α and IL-1β is effective in autoimmune diseases (44), and anti-IL-17 and anti-IL-23 biologics are now in development (29, 33, 39). Since autoimmune diseases are more prevalent in women, inhibiting IL-17 may result in a greater susceptibility to PD in female patients (33). Although PD is not life threatening, it is a known risk factor for cardiovascular disease, diabetes, and chronic obstructive pulmonary disease (19, 50). This report illustrates the need for careful preclinical studies that explore the interaction between gender and immunity as they pertain to anti-cytokine therapies.
Acknowledgments
J.J.Y., H.R.C., and M.J.R. were supported by training grants to the SUNY Buffalo Department of Oral Biology (DE007034), the Witebsky Center for Microbial Pathogenesis & Immunology (AI07614), and the SUNY Buffalo Medical Scientist Training Program. S.L.G. was supported by the NIH (AR050458 and AR054389). K.B. was supported by the Thai government. B6-IL-17RAKO mice were kindly provided by Amgen.
We thank D. Graves, J. Kolls, and M. Russell for helpful discussions and A. Sharma and K. Kirkwood for the critical reading of the manuscript.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Aarvak, T., M. Chabaud, P. Miossec, and J. B. Natvig. 1999. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J. Immunol. 1621246-1251. [PubMed] [Google Scholar]
- 2.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. De Sauvage, and A. L. Gurney. 2002. Interleukin 23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin 17. J. Biol. Chem. 31910-1914. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal, S., and A. L. Gurney. 2002. IL-17: a prototype member of an emerging family. J. Leukoc. Biol. 711-8. [PubMed] [Google Scholar]
- 4.Baker, P. J. 2005. Genetic control of the immune response in pathogenesis. J. Periodontol. 762042-2046. [DOI] [PubMed] [Google Scholar]
- 5.Baker, P. J., M. Dixon, R. T. Evans, L. Dufour, E. Johnson, and D. C. Roopenian. 1999. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 672804-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker, P. J., M. Dixon, and D. C. Roopenian. 2000. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect. Immun. 685864-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker, P. J., L. DuFour, M. Dixon, and D. C. Roopenian. 2000. Adhesion molecule deficiencies increase Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect. Immun. 683103-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker, P. J., R. T. Evans, and D. C. Roopenian. 1994. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch. Oral Biol. 391035-1040. [DOI] [PubMed] [Google Scholar]
- 9.Beertsen, W., M. Willenborg, V. Everts, A. Zirogianni, R. Podschun, B. Schroder, E. L. Eskelinen, and P. Saftig. 2008. Impaired phagosomal maturation in neutrophils leads to periodontitis in lysosomal-associated membrane protein-2 knockout mice. J. Immunol. 180475-482. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector T(H)17 and regulatory T cells. Nature 441235-238. [DOI] [PubMed] [Google Scholar]
- 11.Burt, B. 2005. Position paper: epidemiology of periodontal diseases. J. Periodontol. 761406-1419. [DOI] [PubMed] [Google Scholar]
- 12.Dong, C. 2006. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. 6329-333. [DOI] [PubMed] [Google Scholar]
- 13.Dunn, S. E., S. S. Ousman, R. A. Sobel, L. Zuniga, S. E. Baranzini, S. Youssef, A. Crowell, J. Loh, J. Oksenberg, and L. Steinman. 2007. Peroxisome proliferator-activated receptor (PPAR) alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J. Exp. Med. 204321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, J. J. Pin, P. Garrone, E. Garcia, S. Saeland, D. Blanchard, C. Gaillard, B. Das Mahapatra, E. Rouvier, P. Golstein, J. Banchereau, and S. Lebecque. 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1832593-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaffen, S. L. 2004. Interleukin-17: a unique inflammatory cytokine with roles in bone biology and arthritis. Arth. Res. Ther. 6240-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffen, S. L., and G. Hajishengallis. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J. Dental Res., in press. [DOI] [PMC free article] [PubMed]
- 18.Gaffen, S. L., J. M. Kramer, J. J. Yu, and F. Shen. 2006. The IL-17 cytokine family. Vitamins Hormones 74255-282. [DOI] [PubMed] [Google Scholar]
- 19.Genco, R. J., S. G. Grossi, A. Ho, F. Nishimura, and Y. Murayama. 2005. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontol. 762075-2084. [DOI] [PubMed] [Google Scholar]
- 20.Ghilardi, N., and W. Ouyang. 2007. Targeting the development and effector functions of Th17 cells. Semin. Immunol. 19383-393. [DOI] [PubMed] [Google Scholar]
- 21.Graves, D. T., Y.-T. A. Teng, T. Van Dyke, and G. Hajishengallis. The use of rodent models of investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol., in press. [DOI] [PMC free article] [PubMed]
- 22.Gregory, M. S., D. E. Faunce, L. A. Duffner, and E. J. Kovacs. 2000. Gender difference in cell-mediated immunity after thermal injury is mediated, in part, by elevated levels of interleukin-6. J. Leukoc. Biol. 67319-326. [PubMed] [Google Scholar]
- 23.Heitz-Mayfield, L. J. 2005. Disease progression: identification of high-risk groups and individuals for periodontitis. J. Clin. Periodontol. 32(Suppl. 6)196-209. [DOI] [PubMed] [Google Scholar]
- 24.Huang, W., L. Na, P. L. Fidel, and P. Schwarzenberger. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190624-631. [DOI] [PubMed] [Google Scholar]
- 25.Infante-Duarte, C., H. F. Horton, M. C. Byrne, and T. Kamradt. 2000. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 1656107-6115. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, R. B., N. Wood, and F. G. Serio. 2004. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J. Periodontol. 7537-43. [DOI] [PubMed] [Google Scholar]
- 27.Kantarci, A., and T. E. Van Dyke. 2002. Neutrophil-mediated host response to Porphyromonas gingivalis. J. Int. Acad. Periodontol. 4119-125. [PubMed] [Google Scholar]
- 28.Kelly, M. N., J. K. Kolls, K. Happel, J. D. Schwartzman, P. Schwarzenberger, C. Combe, M. Moretto, and I. A. Khan. 2005. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect. Immun. 73617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikly, K., L. Liu, S. Na, and J. D. Sedgwick. 2006. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr. Opin. Immunol. 18670-675. [DOI] [PubMed] [Google Scholar]
- 30.Klausen, B., C. Sfintescu, and R. T. Evans. 1991. Asymmetry in periodontal bone loss of gnotobiotic Sprague-Dawley rats. Arch. Oral Biol. 36685-687. [DOI] [PubMed] [Google Scholar]
- 31.Korn, T., E. Bettelli, W. Gao, A. Awasthi, A. Jager, T. B. Strom, M. Oukka, and V. K. Kuchroo. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotake, S., N. Udagawa, N. Takahashi, K. Matsuzaki, K. Itoh, S. Ishiyama, S. Saito, K. Inoue, N. Kamatani, M. T. Gillespie, T. J. Martin, and T. Suda. 1999. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 1031345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer, J., and S. Gaffen. 2007. Interleukin-17: a new paradigm in inflammation, autoimmunity and therapy. J. Periodontol. 781083-1093. [DOI] [PubMed] [Google Scholar]
- 34.Laurence, A., and J. O'Shea. 2007. Th-17 differentiation: of mice and men. Nat. Immunol. 8903-905. [DOI] [PubMed] [Google Scholar]
- 35.Lester, S. R., J. L. Bain, R. B. Johnson, and F. G. Serio. 2007. Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J. Periodontol. 781545-1550. [DOI] [PubMed] [Google Scholar]
- 36.Lindén, A., M. Laan, and G. Anderson. 2005. Neutrophils, interleukin-17A and lung disease. Eur. Respir. J. 25159-172. [DOI] [PubMed] [Google Scholar]
- 37.Mangan, P. R., L. E. Harrington, B. O'Quinn, D. W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441231-234. [DOI] [PubMed] [Google Scholar]
- 38.McGeachy, M. J., K. S. Bak-Jensen, Y. Chen, C. M. Tato, W. Blumenschein, T. McClanahan, and D. J. Cua. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 81390-1397. [DOI] [PubMed] [Google Scholar]
- 39.McInnes, I. B., and G. Schett. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. 7429-442. [DOI] [PubMed] [Google Scholar]
- 40.Miossec, P. 2003. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 48594-601. [DOI] [PubMed] [Google Scholar]
- 41.Moseley, T. A., D. R. Haudenschild, L. Rose, and A. H. Reddi. 2003. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14155-174. [DOI] [PubMed] [Google Scholar]
- 42.Niederman, R., T. Westernoff, C. Lee, L. L. Mark, N. Kawashima, M. Ullman-Culler, F. E. Dewhirst, B. J. Paster, D. D. Wagner, T. Mayadas, R. O. Hynes, and P. Stashenko. 2001. Infection-mediated early-onset periodontal disease in P/E-selectin-deficient mice. J. Clin. Periodontol. 28569-575. [DOI] [PubMed] [Google Scholar]
- 43.Nurieva, R., X. O. Yang, G. Martinez, Y. Zhang, A. D. Panopoulos, L. Ma, K. Schluns, Q. Tian, S. S. Watowich, A. M. Jetten, and C. Dong. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448480-483. [DOI] [PubMed] [Google Scholar]
- 44.O'Dell, J. R. 2004. Therapeutic strategies for rheumatoid arthritis. N. Engl. J. Med. 3502591-2602. [DOI] [PubMed] [Google Scholar]
- 45.O'Shea, J. J., A. Ma, and P. Lipsky. 2002. Cytokines and autoimmunity. Nat. Rev. 237-45. [DOI] [PubMed] [Google Scholar]
- 46.Reddy, J., H. Waldner, X. Zhang, Z. Illes, K. W. Wucherpfennig, R. A. Sobel, and V. K. Kuchroo. 2005. Cutting edge: CD4+ CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 1755591-5595. [DOI] [PubMed] [Google Scholar]
- 47.Rouvier, E., M. F. Luciani, M. G. Mattei, F. Denizot, and P. Golstein. 1993. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1505445-5456. [PubMed] [Google Scholar]
- 48.Ruddy, M. J., F. Shen, J. B. Smith, A. Sharma, and S. L. Gaffen. 2004. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J. Leukoc. Biol. 76135-144. [DOI] [PubMed] [Google Scholar]
- 49.Ruddy, M. J., G. C. Wong, X. K. Liu, H. Yamamoto, S. Kasayama, K. L. Kirkwood, and S. L. Gaffen. 2004. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer binding protein family members. J. Biol. Chem. 2792559-2567. [DOI] [PubMed] [Google Scholar]
- 50.Scannapieco, F. A., and R. J. Genco. 1999. Association of periodontal infections with atherosclerotic and pulmonary diseases. J. Periodontal Res. 34340-345. [DOI] [PubMed] [Google Scholar]
- 51.Schwarzenberger, P., V. La Russa, A. Miller, P. Ye, W. Huang, A. Zieske, S. Nelson, G. J. Bagby, D. Stoltz, R. L. Mynatt, M. Spriggs, and J. K. Kolls. 1998. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J. Immunol. 1616383-6389. [PubMed] [Google Scholar]
- 52.Shen, F., and S. L. Gaffen. 2008. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine 4192-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen, F., M. J. Ruddy, P. Plamondon, and S. L. Gaffen. 2005. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J. Leukoc. Biol. 77388-399. [DOI] [PubMed] [Google Scholar]
- 54.Shin, H. C., N. Benbernou, S. Esnault, and M. Guenounou. 1999. Expression of IL-17 in human memory CD45RO+ T lymphocytes and its regulation by protein kinase A pathway. Cytokine 11257-266. [DOI] [PubMed] [Google Scholar]
- 55.Stark, M. A., Y. Huo, T. L. Burcin, M. A. Morris, T. S. Olson, and K. Ley. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22285-294. [DOI] [PubMed] [Google Scholar]
- 56.Steinman, L. 2007. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 13139-145. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi, K., T. Azuma, H. Motohira, D. F. Kinane, and S. Kitetsu. 2005. The potential role of interleukin-17 in the immunopathology of periodontal disease. J. Clin. Periodontol. 32369-374. [DOI] [PubMed] [Google Scholar]
- 58.Teng, Y. T. 2003. The role of acquired immunity and periodontal disease progression. Crit. Rev. Oral Biol. Med. 14237-252. [DOI] [PubMed] [Google Scholar]
- 59.Veldhoen, M., R. J. Hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24179-189. [DOI] [PubMed] [Google Scholar]
- 60.Walker, W., C. W. Roberts, D. J. Ferguson, H. Jebbari, and J. Alexander. 1997. Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect. Immun. 651119-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver, C. T., R. D. Hatton, P. R. Mangan, and L. E. Harrington. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25821-852. [DOI] [PubMed] [Google Scholar]
- 62.Whitacre, C. C., S. C. Reingold, and P. A. O'Looney. 1999. A gender gap in autoimmunity. Science 2831277-1278. [DOI] [PubMed] [Google Scholar]
- 63.Witowski, J., K. Pawlaczyk, A. Breborowicz, A. Scheuren, M. Kuzlan-Pawlaczyk, J. Wisniewska, A. Polubinska, H. Friess, G. M. Gahl, U. Frei, and A. Jorres. 2000. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J. Immunol. 1655814-5821. [DOI] [PubMed] [Google Scholar]
- 64.Yao, Z., W. C. Fanslow, M. F. Seldin, A. M. Rousseau, S. L. Painter, M. R. Comeau, J. I. Cohen, and M. K. Spriggs. 1995. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3811-821. [DOI] [PubMed] [Google Scholar]
- 65.Ye, P., F. H. Rodriguez, S. Kanaly, K. L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, J. E. Shellito, G. J. Bagby, S. Nelson, K. Charrier, J. J. Peschon, and J. K. Kolls. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu, J. J., M. J. Ruddy, G. C. Wong, C. Sfintescu, P. J. Baker, J. B. Smith, R. T. Evans, and S. L. Gaffen. 2007. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 1093794-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou, L., I. I. Ivanov, R. Spolski, R. Min, K. Shenderov, T. Egawa, D. E. Levy, W. J. Leonard, and D. R. Littman. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 9967-974. [DOI] [PubMed] [Google Scholar]