Abstract
The ScpC protease of Streptococcus pyogenes degrades interleukin-8 (IL-8), a chemokine that mediates neutrophil transmigration and activation. The ability to degrade IL-8 differs dramatically among clinical isolates of S. pyogenes. Bacteria expressing ScpC overcome immune clearance by preventing the recruitment of neutrophils in soft tissue infection of mice. To study the role of ScpC in streptococcal sepsis, we generated an ScpC mutant that did not degrade IL-8 and thus failed to prevent the recruitment of immune cells as well as to cause disease after soft tissue infection. In a murine model of sepsis, challenge with the ScpC mutant resulted in more severe systemic disease with higher bacteremia levels and mortality than did challenge with the wild-type strain. As expected, the blood level of KC, the murine IL-8 homologue, increased in mice infected with the ScpC mutant. However, the elevated KC levels did not influence neutrophil numbers in blood, as it did in soft tissue, indicating that additional factors contributed to neutrophil transmigration in blood. In addition, the absence of ScpC increased tumor necrosis factor, IL-6, and C5a levels in blood, which contributed to disease severity. Thus, the ScpC mutant triggers high neutrophil infiltration but not lethal outcome after soft tissue infection, whereas intravenous infection leads to highly aggressive systemic disease.
The human pathogen Streptococcus pyogenes (group A streptococcus [GAS]) can cause a wide range of diseases from mild infections of the throat to life-threatening deep tissue infections. The manifestations and outcome of the disease depend on a combination of bacterial factors and host susceptibility (1, 15, 20). S. pyogenes isolates of certain M serotypes are associated with particular clinical syndromes. For example, strains of M1 and M3 serotypes are frequently, although not exclusively, associated with outbreaks of severe invasive diseases such as streptococcal toxic shock syndrome and necrotizing fasciitis and are associated with high mortality (9, 24, 33). A major threat upon an invasive infection by S. pyogenes is the excessive cytokine production of the host in response to the bacterial infection (5, 28). The systemic effects seen in patients with severe invasive infections are largely triggered by inflammatory mediators induced in response to bacterial virulence factors, particularly the streptococcal superantigens that are by far the most potent inducers of inflammatory responses during infections (20, 22).
In addition, S. pyogenes is capable of immune evasion, mainly by binding of the M and M-related proteins to complement regulators (2, 4, 10). S. pyogenes can also interfere with chemotactic factors such as complement factor 5a (C5a) (7, 33) and degrade the antimicrobial peptides α-defensins and LL-37 (11, 23, 26). Furthermore, it was recently shown that S. pyogenes persists intracellularly in human phagocytic cells during acute soft tissue infections (32). Hence, S. pyogenes has developed numerous immunomodulatory properties that enhance survival in a hostile environment and thereby also increase their colonization and persistence in the human host; however, these properties may vary among isolates.
Necrotizing fasciitis caused by S. pyogenes is characterized by extensive local necrosis of subcutaneous soft tissues and skin. The rapid progression of necrosis often leads to medical treatment that typically includes extensive debridement of tissue and occasionally amputation of extremities. Neutrophils are the first line of defense against infection and are recruited to the site of infection primarily by the chemokine interleukin-8 (IL-8). It was recently proposed that a bacterial protease, ScpC (also called SpyCEP), causes the degradation of the chemokine IL-8 (8, 13). The degradation of IL-8 was shown to be the result of a single specific cleavage between 59glutamine and 60arginine within the IL-8 C-terminal alfa helix (8). In a recent study, the targeted mutagenesis of an M14 serotype strain was used to demonstrate that the ScpC protease degrades IL-8 as well as the murine homologues KC and macrophage inflammatory protein 2 and thereby facilitates local soft tissue infection in a murine model (14). Recently, it was shown that ScpC also cleaves granulocyte chemotactic protein 2 and growth-related oncogene alpha (29). In these studies, the ScpC mutant generated larger lesions than those formed following infection with the wild-type strain, suggesting increased inflammation due to the activation of neutrophils.
In this work, we studied the role of the ScpC protease in streptococcal sepsis. Using an M1 serotype strain, we generated an ScpC mutant that is unable to degrade IL-8 and that has the ability to recruit immune cells during soft tissue infection in mice, in contrast to the wild-type strain. Surprisingly, the ScpC mutant induced more severe sepsis with higher bacteremia and mortality rates than the wild-type strain. These data argue that ScpC contributes to different disease outcomes depending on the site of infection and host environment.
MATERIALS AND METHODS
Bacterial isolates and host cell culture.
Clinical S. pyogenes isolates of types emm1 (S291), emm3 (S40), and emm6 (S165) isolated from blood of patients with severe invasive streptococcal disease were kindly provided by Birgitta Henriques Normark, Swedish Institute for Infectious Disease Control. Bacteria were grown in Todd-Hewitt broth (Difco Laboratories) supplemented with 2% yeast extract (Oxoid) or on Todd-Hewitt yeast (THY) agar plates at 37°C in a 5% CO2 atmosphere. The human pharyngeal FaDu cell line (ATCC HTB-43) was maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 0.1 mM nonessential amino acids, and 1.0 mM sodium pyruvate. Unless stated otherwise, all experiments were performed using 100% confluent FaDu cells maintained in DMEM-FBS.
Cytokine induction and analysis of the IL-8 gene.
FaDu cells were cultivated in 24-well plates (Costar), and the cell culture medium was changed 24 h prior to infection. Cultures of bacteria grown overnight were washed with phosphate-buffered saline (PBS), followed by resuspension in DMEM-FBS. Cell culture medium in wells was replaced by bacterial suspensions at a multiplicity of infection (MOI) of 10 to 100. Infected cells were incubated at 37°C in 5% CO2. Supernatants were collected at 3 h and 6 h postinfection and filtered before analysis of cytokines by enzyme-linked immunosorbent assay (ELISA) (Diaclone).
Total RNA of FaDu cells infected with S. pyogenes (MOI of 50) was isolated using an RNeasy Mini kit (Qiagen). RNA (1 μg) was reverse transcribed with oligo(dT) using a Superscript III first-strand cDNA synthesis kit (Invitrogen). Amplification of IL-8 cDNA was carried out with primers IL-8F (5′-AACATGACTTCCAAGCTGGCC-3′) and IL-8R (5′-TTATGAATTCTCAGCCCTCTTC-3′). The expression of IL-8 was normalized to the β-actin internal control, which was amplified with primers β-actinF (5′-GGCACCACACCTTCTACAATGAG-3′) and β-actinR (5′-CGTCATACTCCTGCTTGCTGATC-3′).
Generation of an ScpC-deficient S. pyogenes mutant.
The scpC gene was insertionally inactivated by the transformation of wild-type S. pyogenes isolate S291 with a plasmid containing the scpC gene disrupted with a spectinomycin resistance marker (Spcr). An internal fragment encompassing positions 181 to 1971 of the scpC gene was amplified by PCR using primers scpCF (5′-CAATACAGAATTCAGCTCAGCTGAATC-3′) and scpCR (5′-GGATTAACATGAATTCGAGCATTGCTC-3′). This fragment was cloned into the EcoRI site of pUC18 to produce pSE11. A 1.1-kb spectinomycin resistance gene was amplified from pLZ12Spec (15) with primers specF (5′-ATCTGAATCAATTGTCGTTCTGTAATACATG-3′) and specR (5′-ATCTGATTACCAATTGGAATGAATATTTCCC-3′) and was further inserted into a unique MunI site of the scpC fragment carried by pSE11 to produce the plasmid pSS2 linear fragment. The plasmid was digested with EcoRI, and the scpC/Spcr fragment was purified from the gel. S. pyogenes isolate S291 was transformed with the linear fragment as described previously (27), and transformants were selected on THY agar plates (100 μg/ml spectinomycin). The correct insertion of the spectinomycin resistance marker into the scpC gene was confirmed by PCR and sequencing (MWG Biotech) using primers scpSF (5′-ACGATGACACCAAATACGAG-3′) and scpSR (5′-ACAGACTCTGAATAGATGGC-3′).
Similar to what was reported previously by Sumby et al. (29), we were unable to complement the mutant despite extensive efforts. However, the mutant shows similar growth rates, similar attachment to host cells, and similar growth in blood in vitro.
IL-8 Western blotting and immunofluorescence.
Cultures of bacteria grown overnight were washed in PBS before being resuspended in DMEM-FBS. Bacterial suspensions with 108 CFU/ml were incubated for 6 h with 250 ng recombinant IL-8 (R&D Systems) at 37°C in 5% CO2, followed by the removal of bacteria by centrifugation. Degradation of IL-8 was detected by Western blotting with a goat anti-IL-8 antibody (0.2 μg/ml; R&D Systems). Cells were grown in 24-well microtiter plates to 100% confluence. Survival of cells were checked by staining with propidium iodine, and cell numbers were counted to ensure loading of identical numbers of cells into each well.
For immunofluorescence, FaDu cells were grown on coverslips (In Vitro Diagnostics) and infected with S291 or the ScpC mutant for 6 h at 37°C in 5% CO2. Control cells were incubated with lipopolysaccharide (100 ng/ml; R&D Systems) and polymyxin B (5 μg/ml; Sigma). The cells were fixed in 4% paraformaldehyde on ice for 30 min, treated with 0.5% Triton X-100 at room temperature for 10 min, blocked in 4% bovine serum albumin, and incubated for 1 h with a phycoerythrin-conjugated IL-8 monoclonal antibody (20 μg/ml; BD Biosciences) on ice. Coverslips were mounted onto glass slides with Vectashield mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories Inc.) for nuclear staining. Slides were analyzed in a Zeiss LSM510 Meta confocal microscope.
C5a peptidase and gene analysis.
Pellets of bacterial cultures grown overnight were resuspended in lysozyme buffer (2 mg/ml) to give 50 mg (wet weight) cells/ml, followed by 1 h of incubation at 37°C. Samples were centrifuged, and the supernatant was used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis with a rabbit anti-ScpA sera (1:500 dilution; a generous gift from P. Cleary, MN). The scpA gene was amplified from the genomic DNA of bacteria with primers scpAF (5′-AAGGTCCTCTTGATTTACTC-3′) and scpAR (5′-GCTGCTCTACAAATACCACG-3′). The PCR products were sequenced at MWG Biotech AG, Germany.
Animal infection assay.
Five- to eight-week-old C3H/HeN mice (n = 6 to 10) were infected intravenously (i.v.) with 3 × 107 CFU of bacteria. Survival was monitored, and blood samples were taken from the tail daily for 6 days after infection, diluted in PBS, and spread onto THY agar plates to determine the bacterial loads. Blood smears were made for analysis of neutrophil infiltration by light microscopy after Wright's staining according to the manufacturer's recommendations (Sigma). Serum was collected 2, 6, and 30 h postchallenge. Concentrations of murine IL-6, KC, and tumor necrosis factor (TNF) were measured by ELISA according to the manufacturer's recommendations (BioSource). For the detection of C5a, a rat anti-mouse C5a monoclonal antibody, I52-1486 (2 μg/ml), and a biotinylated rat anti-mouse C5a monoclonal antibody, I52-278 (1 μg/ml; BD Biosciences), were used as ELISA capture and detection antibodies, respectively. For histological analysis, mice were infected with 1 × 108 CFU of wild-type strain S291 or the ScpC mutant (n = 4). PBS was administered into the opposite flank and in control mice. Tissue samples were collected at 72 h postinfection, fixed in 10% formalin, paraffin embedded, and hematoxylin-eosin stained. Histological tissue sections were examined by a Carl Zeiss Axio Vision 2.05 image processing and analysis system (Zeiss). The experiments have been approved by the appropriate ethical committees of animal experiments. In addition, all animal procedures were performed in accordance with institutional protocol guidelines.
Real-time reverse transcription (RT)-PCR analysis of the scpC gene.
Wild-type strain S291 was grown overnight in THY medium and washed with PBS. Bacteria (1 × 107 CFU) were inoculated in THY medium or heparin-anticoagulated mouse whole blood. The mixture was incubated at 37°C with 5 rpm of rotation for 3 h. Total bacterial RNA was extracted using a RNeasy Mini kit (Qiagen) and reverse transcribed to cDNA with random hexamer primers using a Superscript III first-strand cDNA synthesis kit (Invitrogen). For real-time PCR amplification, Power Sybr green PCR master mix (ABI) was used with the ABI Prism 7300 sequence detector system (Applied Biosystems) according to the manufacturer's guidelines. The gyrA (gyrase subunit A) gene was chosen as an internal control (14). Primers specific for scpC were SpyCEPF (5′-GACCGTGGATTAGCTGGTGT-3′) and SpyCEPR (5′-TGTCGCTCCACAAATGTTTT-3′) and for gyrA were GyrAF (5′-CGACTTGCTTGAACGCCAAA-3′) and GyrAR (5′-TTATCACGTTCCAAACCAGTCAA-3′). The data were analyzed using the standard curve method and are presented as the abundance of mRNA levels normalized to gyrA. The experiment was performed in duplicate with three RNA samples prepared independently.
Statistical analysis.
A Student's t test was used to assess significance in cytokine studies and RT-PCR. Mouse survival studies were assessed using Fisher's exact test, and bacteremia levels were monitored with a nonparametric Mann-Whitney test.
RESULTS
IL-8 degradation by S. pyogenes clinical isolates.
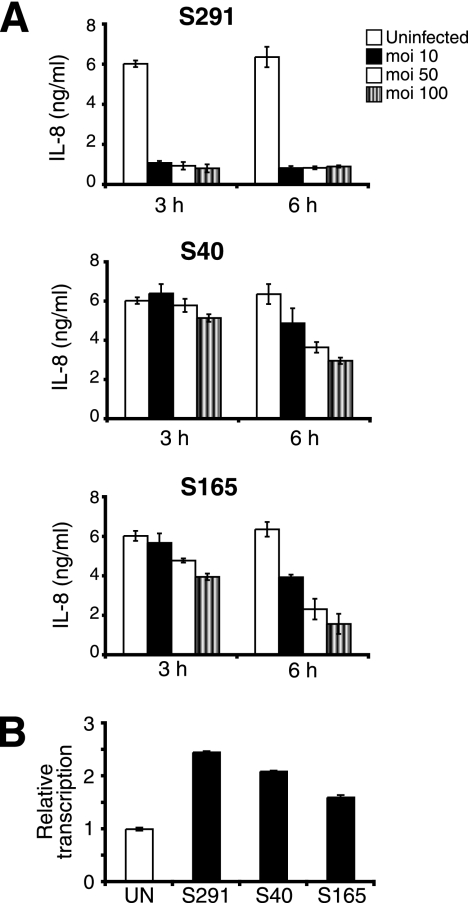
Three different S. pyogenes strains, S291 (emm1), S40 (emm3), and S165 (emm6), causing severe invasive streptococcal infections were screened for their abilities to degrade IL-8. After infection of the epithelial pharyngeal cell line FaDu, IL-8 decreased in a dose- and time-dependent manner. Strain S291 mediated the most rapid loss of IL-8 with low levels observed after only 3 h with an MOI of 10, indicating that S291 has an effective mechanism to degrade IL-8 (Fig. 1A). The two other strains, S40 and S165, showed only a moderate reduction in the levels of IL-8. These data support previous reports (8, 13) and further substantiate important differences in the manipulation of host defense among S. pyogenes strains. To assess if the loss of IL-8 was due to decreased transcription, we used real-time RT-PCR and showed that the transcription of the IL-8 gene increased up to twofold in infected cells compared to uninfected cells at 6 h postinfection (Fig. 1B). These data indicate that the ability to degrade IL-8 vary among S. pyogenes strains and that the loss of IL-8 is not the result of reduced IL-8 mRNA levels.
FIG. 1.
S. pyogenes degrades IL-8 upon infection of epithelial cells. (A) FaDu epithelial cells were infected with strain S291, strain S40, and strain S165 at different MOIs for 3 h and 6 h. Supernatants were collected and analyzed for IL-8 by ELISA. (B) Transcription of IL-8 in FaDu cells increases after infection with S. pyogenes. The data are presented as relative transcription normalized to the housekeeping β-actin gene. The experiments were performed in triplicate and repeated at least three times, with similar results. The results shown represent the means ± standard deviations of data from two independent experiments.
IL-8 cleavage by S. pyogenes envelope protease ScpC.
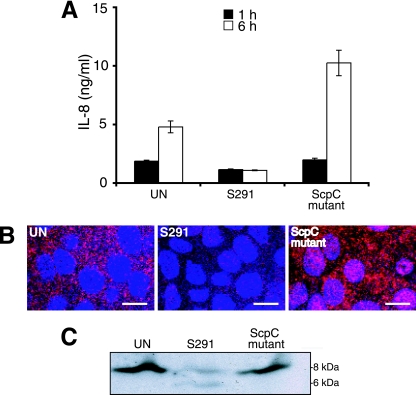
IL-8 is degraded by the ScpC protease that also cleaves the murine IL-8 homologues KC and macrophage inflammatory protein 2 (14). To further investigate the function of the ScpC protease, we constructed an ScpC mutant in emm1 strain S291, since this strain had the best ability to degrade IL-8 (Fig. 1A). In a previous report, using strain JS95 of the M14 serotype, it was impossible to inactivate scpC without inactivating scpA, encoding the C5a peptidase. In the M1 serotype strain used in this study, it was possible to generate a single scpC mutant. The intact function of the scpA gene was confirmed by gene sequence analysis and by the production of the C5a peptidase (data not shown). As shown in Fig. 2A, the ScpC mutant lost the ability to degrade IL-8 in supernatants of cells compared to the wild-type strain. Analysis of supernatants after 1 h and 6 h showed, as expected, that IL-8 accumulated over time, except in wells infected with wild-type strain S291. The infection of FaDu cells with the ScpC mutant generated levels of IL-8 similar to those with infection by lipopolysaccharide (data not shown). Immunofluorescence microscopy further confirmed that high levels of IL-8 were present in cells infected with the ScpC mutant, whereas IL-8 was barely detected in cells infected with wild-type strain S291 (Fig. 2B). The impaired ability of the ScpC mutant to degrade IL-8 was confirmed by immunoblotting using IL-8 antibodies. Strain S291 degraded the 8-kDa IL-8 protein, whereas the ScpC mutant failed to cleave IL-8 (Fig. 2C). Thus, ScpC in the investigated emm1-type strain is responsible for the reduction and cleavage of IL-8, similar to what was seen in the serotype M14 S. pyogenes strain (14). Furthermore, the wild-type strain and the ScpC mutant demonstrated equivalent growth levels in THY medium and similar proliferation rates in nonimmune whole human blood, as determined by a Lancefield assay (data not shown). These strains also had similar abilities to interact with host cells and to induce apoptosis (data not shown).
FIG. 2.
ScpC is responsible for IL-8 degradation. (A) IL-8 levels in supernatants of FaDu cells after infection with the S291 and the ScpC mutant (MOI of 50) for 1 h and 6 h as detected by ELISA. Two independent experiments were performed in duplicate. Data are expressed as means ± standard deviations of one representative experiment. (B) IL-8 detection by immunofluorescence after 6 h in uninfected cells (UN) or cells infected with S291 or the ScpC mutant (MOI of 50). The cells were fixed, blocked, and incubated for 1 h with a phycoerythrin-conjugated IL-8 monoclonal antibody (20 μg/ml; BD Biosciences) on ice. The bar represents 20 μM. (C) Western blot analysis of IL-8 cleavage. Bacterial suspensions (S291 or the ScpC mutant) were incubated with recombinant IL-8 (250 ng) for 6 h at 37°C. Samples were analyzed by Western blotting using a polyclonal goat anti-IL-8 antibody.
An ScpC mutant induces infiltration of cells in soft tissue infection.
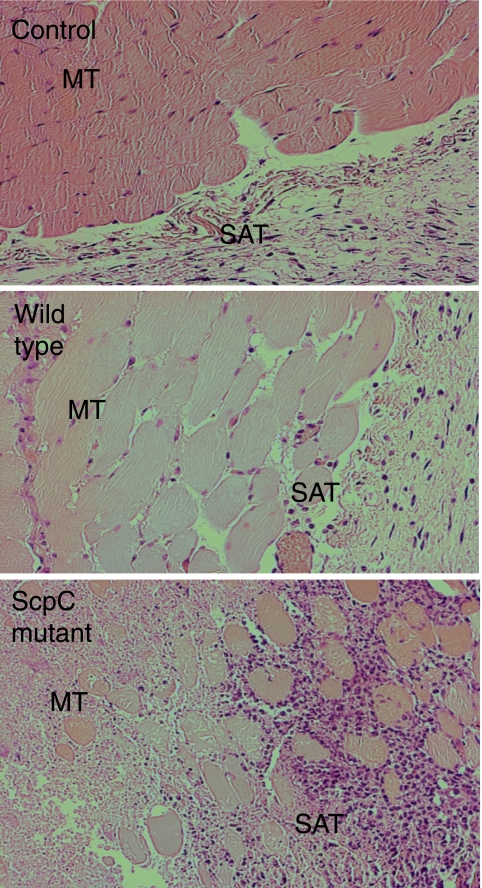
To evaluate the impact of the ScpC mutant to recruit immune cells in soft tissue infection, we infected mice subcutaneously with the mutant and the wild type. As shown in Fig. 3, tissue sections from mice infected with the ScpC mutant showed high numbers of infiltrating cells at 72 h postinfection, while tissue sections from mice infected with the wild type or with PBS (control) almost completely lacked infiltrating cells. These data demonstrate a direct role of ScpC in preventing the infiltration of immune cells in soft tissue and support the data reported previously by Hidalgo-Grass et al. (14), who showed that a double ScpC ScpA mutant but not an ScpA mutant or the wild type, mediated strong recruitment of immune cells and smaller skin lesions in soft tissue infections. It is likely that the strong recruitment of neutrophils mediates the elimination of bacteria that then results in smaller skin lesions.
FIG. 3.
Subcutaneous infection with the ScpC mutant leads to higher infiltration of cells into the infectious site than with the wild type. C3H/HeN mice (n = 4) were infected subcutaneously with 1 × 108 CFU of S291 or the ScpC mutant. PBS was injected in the opposite flank and in control mice. Tissue samples were collected 72 h postchallenge and analyzed for cell infiltration in the epidermis and underlying tissue. Shown are representative pictures of muscle tissue sections stained with hematoxylin-eosin. MT, muscle tissue; SAT, subcutaneous adipose tissue.
The ScpC mutant is more virulent in an experimental sepsis model.
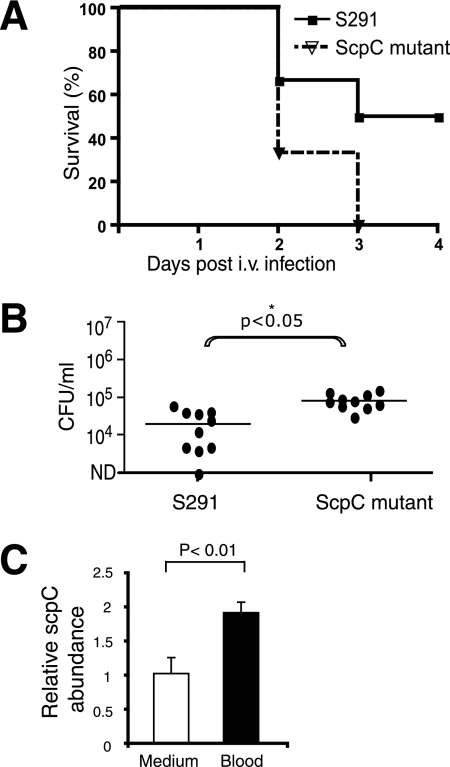
Sepsis and septic shock caused by gram-positive bacteria have become increasingly frequent clinical problems (16). These conditions are accompanied by an overwhelming inflammation and release of inflammatory mediators. The capacity of S. pyogenes to cause lethal disease has been studied in mouse model systems using i.v. inoculation doses of 2 × 108 to 4 × 108 cells per mouse (25). To establish the role of ScpC in severe sepsis, we infected C3H/HeN mice i.v. with 3 × 107 wild-type bacteria or the ScpC mutant. Surprisingly, the ScpC mutant showed increased virulence compared with that of the wild type. At 3 days postinfection, 50% of the mice infected with wild-type strain survived, whereas none of the mice infected with the ScpC mutant survived (Fig. 4A). At day 1, bacterial blood counts were significantly higher in mice infected with the ScpC mutant than in mice infected with the wild type, whereas there were no differences in bacteremia levels at day 2 (Fig. 4B). We were not able to collect material for quantitative real-time PCR analysis of scpC transcription in blood from mice after 1 or 2 days postinfection since there were too few bacteria left in the blood. To confirm that scpC gene expression actually occurred in blood, we isolated mRNA from bacteria incubated in blood and analyzed it by real-time RT-PCR. A twofold-higher scpC mRNA abundance (P < 0.01) was detected when bacteria replicated in the blood than in THY medium (Fig. 4C). These data demonstrate that the ScpC mutant is more virulent in a murine model of sepsis and that scpC is expressed in blood.
FIG. 4.
The ScpC mutant causes more severe sepsis than the wild-type strain. (A) Survival of C3H/HeN mice after i.v. challenge with 3 × 107 CFU of bacteria/mouse (n = 12). The results are shown as Kaplan-Meier survival plots, and the overall survival rates of the mice infected with the ScpC mutant (dotted line) were significantly lower (P = 0.0082 by the log rank test) than those of wild-type strain S291 (solid line). (B) Bacteremia levels in mice at day 1 and day 2 postchallenge with the wild type and the ScpC mutant. Horizontal lines mark the median value. The significant difference (P < 0.05) is marked with an asterisk. ND, not determined. (C) The abundance of the scpC mRNA normalized with gyrA was determined by real-time RT-PCR. RNA samples were isolated from wild-type bacteria grown in THY medium (white bar) or mouse whole blood (black bar). Growth in the blood upregulates the transcription of scpC (P < 0.01 by a Student's t test). The results shown represent the means ± standard deviations of data from two independent experiments.
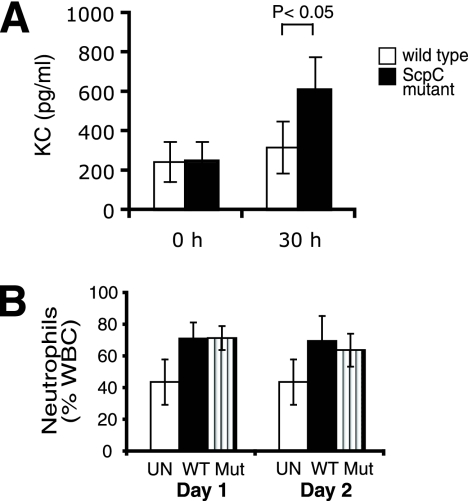
ScpC affects KC but not neutrophil numbers in blood after i.v. infection.
KC is a functional homologue of IL-8, which is degraded by ScpC (14). To assess the influence of ScpC on the KC level in blood, we collected serum after i.v. infection and analyzed the samples by ELISA. As shown in Fig. 5A, the KC level significantly increased after infection with the ScpC mutant, whereas the level remained low in mice infected with the wild type. These findings argue that ScpC promotes KC degradation in systemic S. pyogenes blood infection. In soft tissue infection, the degradation of KC led to the impairment of neutrophil infiltration (14) (Fig. 3). Consequently, we investigated the neutrophil numbers in blood at day 1 and day 2 postchallenge. The neutrophil levels were stable over time, with no significant differences between mice infected with the wild type or the ScpC mutant (Fig. 5B). Since the circulating system is constantly fed with immune cells, the effect of ScpC to inhibit neutrophil migration seems to be negligible.
FIG. 5.
ScpC is dispensable for neutrophil recruitment in blood. (A) KC levels in sera at 0 h and 30 h postinfection. The data were analyzed by a Student's t test. The results shown represent the means ± standard deviations of data from two independent experiments. (B) Number of neutrophils in blood of mice infected with 3 × 107 CFU of the wild type (WT) or the ScpC mutant (Mut). Uninfected (UN) mice were used as a control. The results shown represent the means ± standard deviations of data from two independent experiments (n = 6 to 10 mice/group).
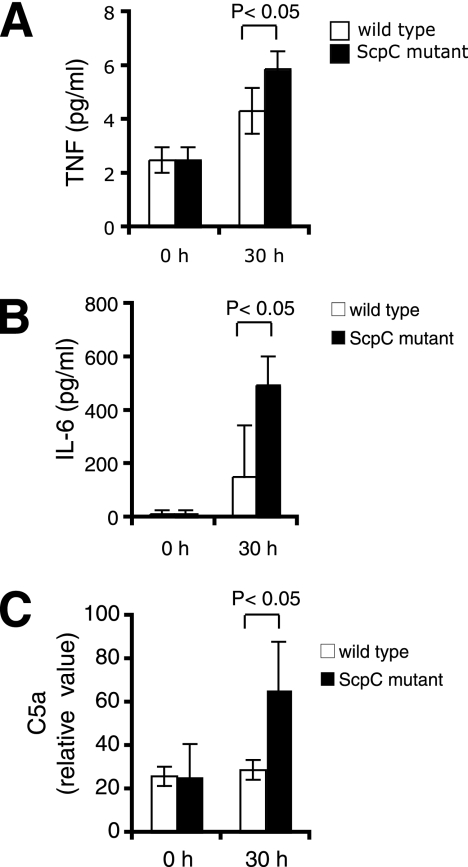
Increased inflammatory response in mice infected with the ScpC mutant.
Lethal sepsis not only is due to the infection itself but also results from hypotension and organ failure characteristic of septic shock. These symptoms are the result of the uncontrolled release of proinflammatory cytokines. We hypothesized that the more severe infection induced by the ScpC mutant could come from host cytokine responses. Host TNF and IL-6 responses were analyzed by measuring cytokine production in mouse serum at different time points postinfection. As shown in Fig. 6A, TNF levels were raised after challenge with both the wild type and the ScpC mutant compared to those in uninfected mice. However, stimulation was significantly higher in mice challenged with the ScpC mutant than with the wild-type strain at 30 h postinfection. IL-6 induction was also significantly increased in mice infected with the ScpC mutant, peaking at 451 ± 108 pg/ml at 30 h postinfection (Fig. 6B).
FIG. 6.
Infection with the ScpC mutant induces higher TNF, IL-6, and C5a levels than does infection with the wild-type strain. Mice (n = 6) were i.v. challenged with 3 × 107 CFU of the wild type or the ScpC mutant. Serum levels of TNF (A), IL-6 (B), and C5a (C) were determined by ELISA at 0 h and 30 h postinfection. The results shown represent the means ± standard deviations. The data were analyzed by a Student's t test.
C5a is generated upon the activation of the complement cascade. It has both anaphylatoxin and chemotactic activity and can trigger the degranulation of granulocytes. The excessive production of C5a in humans correlates with increased cytokine release and severe sepsis (19). As shown in Fig. 6C, serum C5a levels were significantly raised in mice challenged with the ScpC mutant at 30 h postinfection, but no activation could be detected after challenge with the wild-type strain. The C5a peptidase can have an important impact on disease development (31, 33, 34). Since we showed that the ScpC mutant expressed the C5a protease similarly to the wild type, the observed differences were not due to an altered ability to inactivate C5a among the strains. These data argue that infection with the ScpC mutant triggers an elevated proinflammatory response and complement activation, which are likely to contribute to the lethal outcome of disease.
DISCUSSION
S. pyogenes has developed several intrinsic mechanisms to evade the immune response of the human host, including, among others, host mimicry, modulation of host factors, and intracellular persistence. One of these mechanisms includes the streptococcal degradation of the important chemotactic factor IL-8 and the consequent impairment of neutrophil recruitment (13). In this work, we show that an ScpC mutant that is unable to degrade IL-8/KC is attenuated in virulence during soft tissue infection but is more virulent in systemic infection, suggesting that ScpC mediates different effects depending on the site of infection.
The ScpC mutant was constructed in strain S291 (emm1), collected from a patient with severe invasive streptococcal infection. The inactivation of scpC in another strain, JS95, of the M14 serotype required the coinactivation of the scpA gene, encoding the C5a peptidase (14). In this work, the ScpC mutant of strain S291 (emm1) still expressed the C5a peptidase, as evaluated by protein expression in immunoblots and by gene sequencing (data not shown). It is likely that the linkage between genes varies among strains and affects the genetic manipulation of bacteria. Importantly, the mutant and wild type differed in their abilities to degrade the chemokines IL-8 (Fig. 2) and KC (data not shown) but had similar growth rates and demonstrated similar levels of attachment and invasion of host epithelial cell in vitro (data not shown), supporting that no other major virulence factors were accidentally inactivated.
Life-threatening complications from bacterial infections such as sepsis and septic shock are the second leading cause of death among patients in noncoronary intensive care units and the 10th leading cause of death overall in the United States (17). Despite an improved intensive care system, the number of patients who die from bacterial infections is still high (12, 17). The pathogenesis of GAS infections is still poorly understood despite the fact that group A streptococci remain a major cause of human disease. Specifically, very little is known about the molecular mechanisms underlying the different clinical manifestations of GAS infections (18). The identification of mouse strains with susceptibility to GAS, such as C3H/HeN, are instrumental to the detailed characterization of cellular mechanisms associated with the development of sepsis in the mouse during GAS infection. To investigate the function of the ScpC protease in streptococcal sepsis, we infected C3H/HeN mice i.v. and monitored disease development. Surprisingly, infection with the ScpC mutant led to higher bacterial blood counts and mortality than does wild-type infection. As expected, the KC level increased in serum after infection of mice with the ScpC mutant, in contrast to challenge with the wild type, which kept the KC level down (Fig. 5), indicating the degradation of KC in blood. However, the neutrophil counts in peripheral blood were not affected. It is possible that high levels of circulating immune cells in the bloodstream lead to the recruitment of immune cells independent of KC levels or that other factors contribute to neutrophil recruitment. The recruitment and activation of neutrophils are considered to be one of the major defense mechanisms of innate immunity. We cannot exclude that differences in the numbers of activated versus nonactivated neutrophils affected the outcome of disease. Furthermore, KC is one of the major CXC chemokines required for neutrophil recruitment; however, it cannot be excluded that other CXC chemokines contribute to the entry of neutrophils into circulation.
It is currently generally believed that the pathophysiology of severe infectious diseases is characterized by a massive and inappropriate systemic inflammatory response elicited by the invading microorganism (6, 30). It was recognized that patients with streptococcal toxic shock syndrome have elevated TNF and IL-6 levels in sera (20, 21). High levels of proinflammatory cytokines can be correlated with disease severity. In this study, using a murine model of sepsis, the induction of TNF and IL-6 was stronger in mice infected with the ScpC mutant than in the wild type, providing an explanation for increased disease development and mortality. It is likely that the induced levels of cytokines due to the ScpC mutant infection led to an increased level of activation of immune cells and subsequently led to a lethal outcome. C5a is an important immune mediator involved in stimulating mononuclear cells and the release of proinflammatory cytokines. A higher level of C5a was observed after challenge with the ScpC mutant but not with the wild-type strain. Furthermore, C5a activation was in accordance with IL-6 release. C5a induction and the consequent activation of immune cells are known to mediate cytokine release and may contribute to the more severe disease manifestation seen with the ScpC mutant. Notably, the increased levels of C5a are not likely due to an impaired ability of the mutant to produce the C5a peptidase since the scpA gene was intact and expressed in the ScpC mutant.
In soft tissue infection experiments using an M1 strain, we observed that ScpC prevented the infiltration of immune cells, similar to findings reported previously by Hidalgo-Grass et al. (14), which used an M14 strain. Our finding that the ScpC mutant induced more severe disease outcome is consistent with a recent study by Sumby et al. (29). They observed that the ScpC mutant of an S. pyogenes M1 strain generated lesions with larger sizes than those formed following infection with the parent strain (opposite results to those reported previously by Hidalgo-Grass). This was suggested to be a result of increased levels of inflammation and neutrophil infiltration (29).
The inflammatory cascades in response to infection are overlapping and complex. There are several possibilities that could explain the higher inflammatory response after infection with ScpC-deficient bacteria. It might be that KC/IL-8 itself stimulated inflammatory responses. It is known that IL-8 possesses several biological functions in addition to acting as a chemotactic factor; it can act as a more general proinflammatory factor in response to tissue stress and necrosis, a proangiogenic factor that promotes new vessel growth, or an autocrine growth factor secreted by tumor cells (3). In addition, it is tempting to speculate that ScpC may not only inactivate IL-8 but also inhibit other inflammatory mediators such as TNF, IL-6, or C5a, which would lead to the lighter inflammatory responses, as observed with the wild-type strain.
Taken together, these results demonstrate that bacterial virulence factors may trigger different disease symptoms and outcomes depending on the site of infection. Thus, in an experimental model of local severe soft tissue infection, ScpC protease augments disease severity, whereas in a systemic infection such as sepsis, the ScpC protease has a seemingly protective effect against disease. It has been proposed that the identification of factors involved in susceptibility to infection in mice will provide a basis for finding similar pathogenic mechanisms associated with invasive GAS infections in humans (18). In future studies, it would be interesting to analyze strains from patients with severe sepsis and streptococcal toxic shock versus other S. pyogenes strains to evaluate defects in scpC expression or protease function as well as to assess the expression of ScpC in human biopsies.
Acknowledgments
We thank Pat Cleary (Department of Microbiology, University of Minnesota, Minneapolis, MN) for kindly providing us with the anti-ScpA antibody.
This work was supported by grants from the Swedish Research Council (Dnr 2004-4831, 2002-6340, 2005-5701, 2006-5073, 2006-4112, and 2007-3369), Swedish Cancer Society, Torsten och Ragnar Söderbergs Stiftelse, Knut och Alice Wallenbergs Stiftelse, Tore Nilsons Stiftelse för Medicinsk Forskning, Stiftelsen Goljes Minne, Stiftelsen Lars Hiertas Minne, Magnus Bergvalls Stiftelse, Seda och Signe Hermanssons Stiftelse, Laerdal Foundation, and Uppsala University.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 23 June 2008.
REFERENCES
- 1.Basma, H., A. Norrby-Teglund, Y. Guedez, A. McGeer, D. E. Low, O. El-Ahmedy, B. Schwartz, and M. Kotb. 1999. Risk factors in the pathogenesis of invasive group A streptococcal infections: role of protective humoral immunity. Infect. Immun. 671871-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berggard, K., E. Johnsson, E. Morfeldt, J. Persson, M. Stalhammar-Carlemalm, and G. Lindahl. 2001. Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Mol. Microbiol. 42539-551. [DOI] [PubMed] [Google Scholar]
- 3.Brat, D. J., A. C. Bellail, and E. G. Van Meir. 2005. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-Oncology 7122-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson, F., K. Berggard, M. Stalhammar-Carlemalm, and G. Lindahl. 2003. Evasion of phagocytosis through cooperation between two ligand-binding regions in Streptococcus pyogenes M protein. J. Exp. Med. 1981057-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavaillon, J. M., M. Adib-Conquy, C. Fitting, C. Adrie, and D. Payen. 2003. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 35535-544. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420885-891. [DOI] [PubMed] [Google Scholar]
- 7.Collin, M., and A. Olsen. 2003. Extracellular enzymes with immunomodulating activities: variations on a theme in Streptococcus pyogenes. Infect. Immun. 712983-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards, R. J., G. W. Taylor, M. Ferguson, S. Murray, N. Rendell, A. Wrigley, Z. Bai, J. Boyle, S. J. Finney, A. Jones, H. H. Russell, C. Turner, J. Cohen, L. Faulkner, and S. Sriskandan. 2005. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J. Infect. Dis. 192783-790. [DOI] [PubMed] [Google Scholar]
- 9.Efstratiou, A. 2000. Group A streptococci in the 1990s. J. Antimicrob. Chemother. 45(Suppl.)3-12. [DOI] [PubMed] [Google Scholar]
- 10.Fischetti, V. A. 1989. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frick, I. M., P. Akesson, M. Rasmussen, A. Schmidtchen, and L. Bjorck. 2003. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J. Biol. Chem. 27816561-16566. [DOI] [PubMed] [Google Scholar]
- 12.Hack, C. E. 2003. Derangements of coagulation and fibrinolysis in infectious diseases. Contrib. Microbiol. 1018-37. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo-Grass, C., M. Dan-Goor, A. Maly, Y. Eran, L. A. Kwinn, V. Nizet, M. Ravins, J. Jaffe, A. Peyser, A. E. Moses, and E. Hanski. 2004. Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet 363696-703. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo-Grass, C., I. Mishalian, M. Dan-Goor, I. Belotserkovsky, Y. Eran, V. Nizet, A. Peled, and E. Hanski. 2006. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. EMBO J. 254628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotb, M., A. Norrby-Teglund, A. McGeer, H. El-Sherbini, M. T. Dorak, A. Khurshid, K. Green, J. Peeples, J. Wade, G. Thomson, B. Schwartz, and D. E. Low. 2002. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat. Med. 81398-1404. [DOI] [PubMed] [Google Scholar]
- 16.Low, D. E., B. Schwartz, and A. McGeer. 1998. The re-emergence of severe group A streptococcal disease: an evolutionary perspective. Emerg. Pathog. 793-112. [Google Scholar]
- 17.Martin, G. S., D. M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 3481546-1554. [DOI] [PubMed] [Google Scholar]
- 18.Medina, E., and A. Lengeling. 2005. Genetic regulation of host responses to group A streptococcus in mice. Brief. Funct. Genom. Proteom. 4248-257. [DOI] [PubMed] [Google Scholar]
- 19.Nakae, H., S. Endo, K. Inada, T. Takakuwa, T. Kasai, and M. Yoshida. 1994. Serum complement levels and severity of sepsis. Res. Commun. Chem. Pathol. Pharmacol. 84189-195. [PubMed] [Google Scholar]
- 20.Norrby-Teglund, A., S. Chatellier, D. E. Low, A. McGeer, K. Green, and M. Kotb. 2000. Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur. J. Immunol. 303247-3255. [DOI] [PubMed] [Google Scholar]
- 21.Norrby-Teglund, A., K. Pauksens, M. Norgren, and S. E. Holm. 1995. Correlation between serum TNF alpha and IL6 levels and severity of group A streptococcal infections. Scand. J. Infect. Dis. 27125-130. [DOI] [PubMed] [Google Scholar]
- 22.Norrby-Teglund, A., P. Thulin, B. S. Gan, M. Kotb, A. McGeer, J. Andersson, and D. E. Low. 2001. Evidence for superantigen involvement in severe group A streptococcal tissue infections. J. Infect. Dis. 184853-860. [DOI] [PubMed] [Google Scholar]
- 23.Nyberg, P., M. Rasmussen, and L. Bjorck. 2004. Alpha2-macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J. Biol. Chem. 27952820-52823. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien, K. L., B. Beall, N. L. Barrett, P. R. Cieslak, A. Reingold, M. M. Farley, R. Danila, E. R. Zell, R. Facklam, B. Schwartz, and A. Schuchat. 2002. Epidemiology of invasive group A streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35268-276. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai, A., N. Okahashi, I. Nakagawa, S. Kawabata, A. Amano, T. Ooshima, and S. Hamada. 2003. Streptococcus pyogenes infection induces septic arthritis with increased production of the receptor activator of the NF-κB ligand. Infect. Immun. 716019-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidtchen, A., I. M. Frick, and L. Bjorck. 2001. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol. Microbiol. 39708-713. [DOI] [PubMed] [Google Scholar]
- 27.Simon, D., and J. J. Ferretti. 1991. Electrotransformation of Streptococcus pyogenes with plasmid and linear DNA. FEMS Microbiol. Lett. 66219-224. [DOI] [PubMed] [Google Scholar]
- 28.Stevens, D. L. 2003. Group A streptococcal sepsis. Curr. Infect. Dis. Rep. 5379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumby, P., S. Zhang, A. R. Whitney, F. Falugi, G. Grandi, E. A. Graviss, F. R. Deleo, and J. M. Musser. 2008. A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect. Immun. 76978-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takala, A., I. Nupponen, M. L. Kylanpaa-Back, and H. Repo. 2002. Markers of inflammation in sepsis. Ann. Med. 34614-623. [DOI] [PubMed] [Google Scholar]
- 31.Terao, Y., M. Yamaguchi, S. Hamada, and S. Kawabata. 2006. Multifunctional glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pyogenes is essential for evasion from neutrophils. J. Biol. Chem. 28114215-14223. [DOI] [PubMed] [Google Scholar]
- 32.Thulin, P., L. Johansson, D. E. Low, B. S. Gan, M. Kotb, A. McGeer, and A. Norrby-Teglund. 2006. Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med. 3e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wexler, D. E., D. E. Chenoweth, and P. P. Cleary. 1985. Mechanism of action of the group A streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 828144-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wexler, D. E., and P. P. Cleary. 1985. Purification and characteristics of the streptococcal chemotactic factor inactivator. Infect. Immun. 50757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]