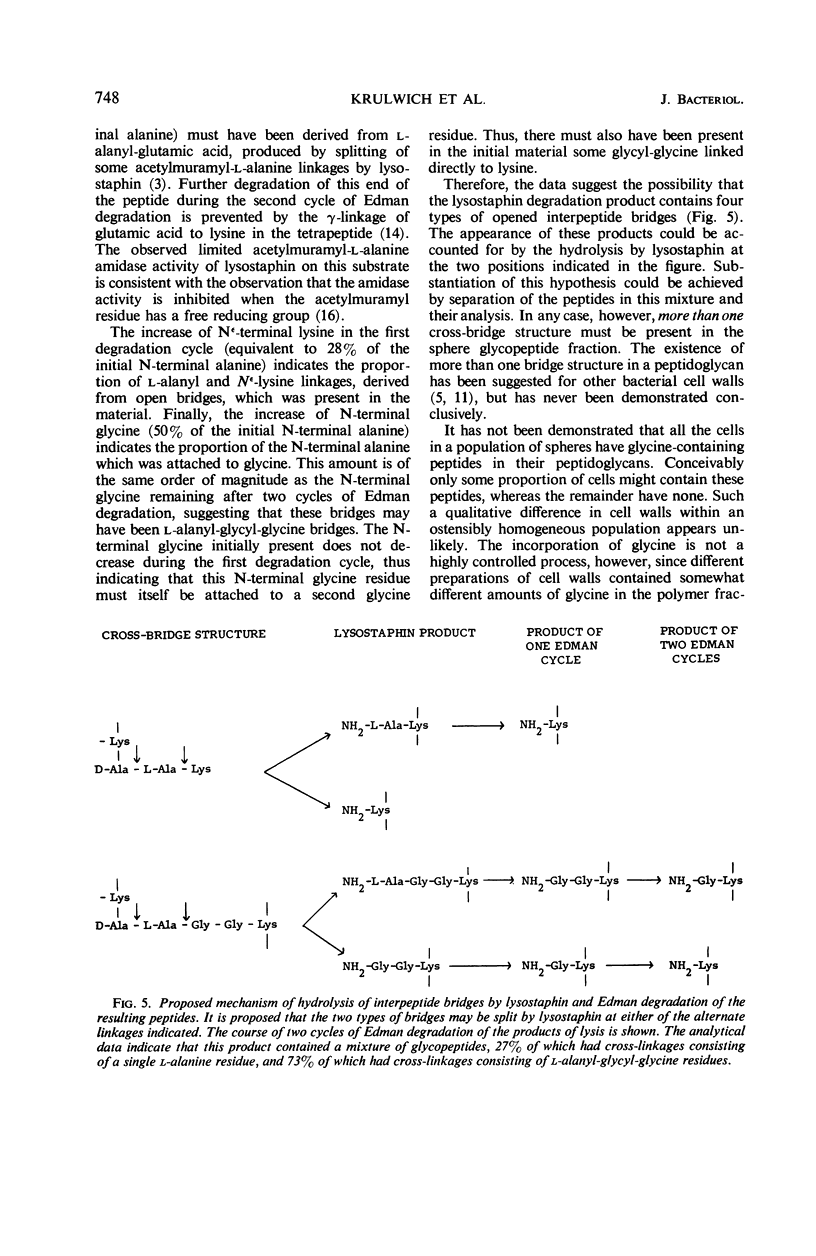
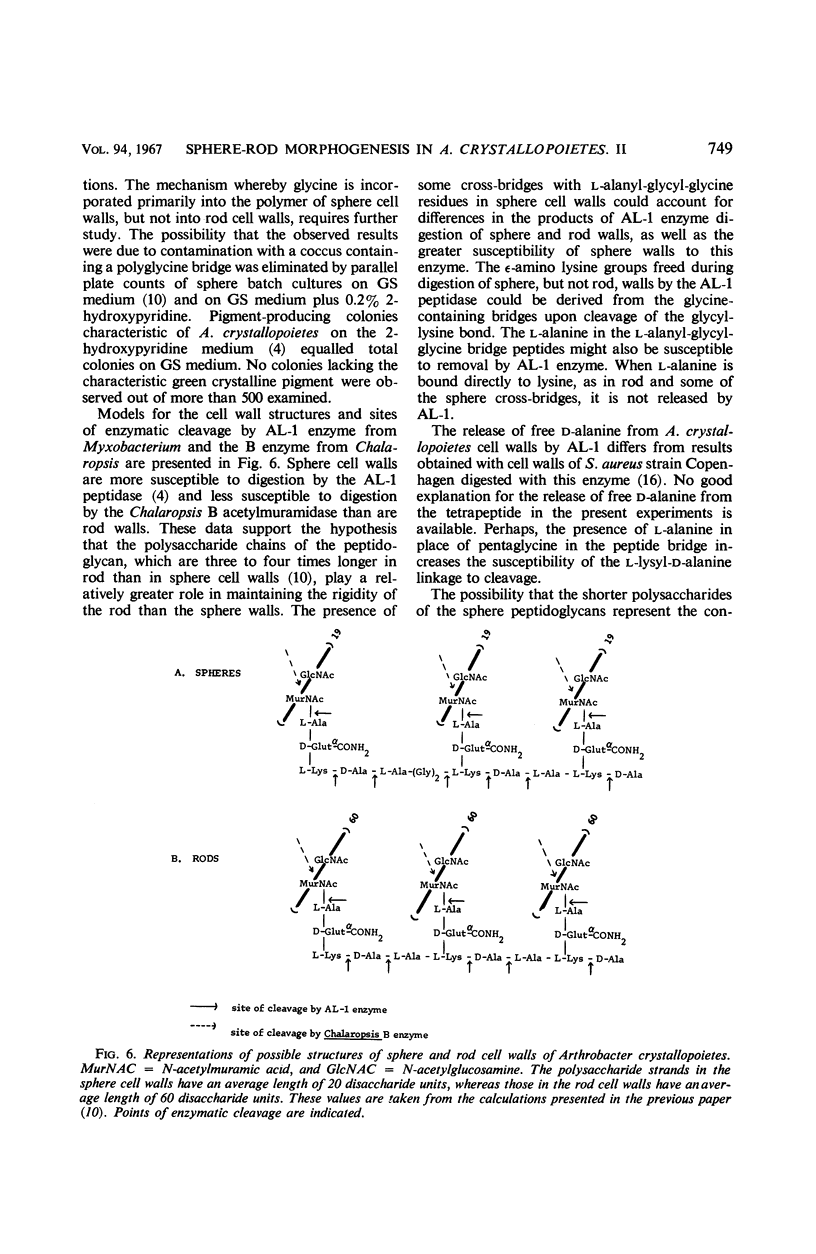
Abstract
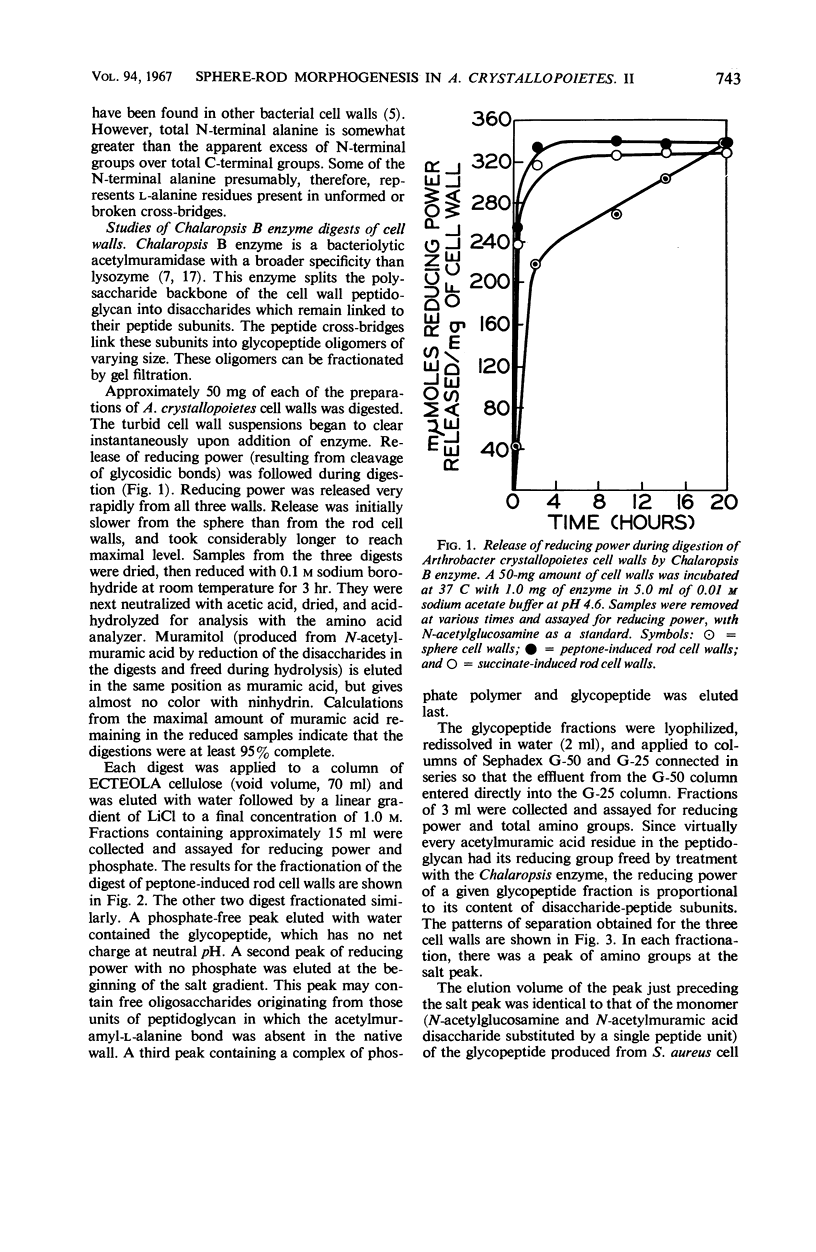
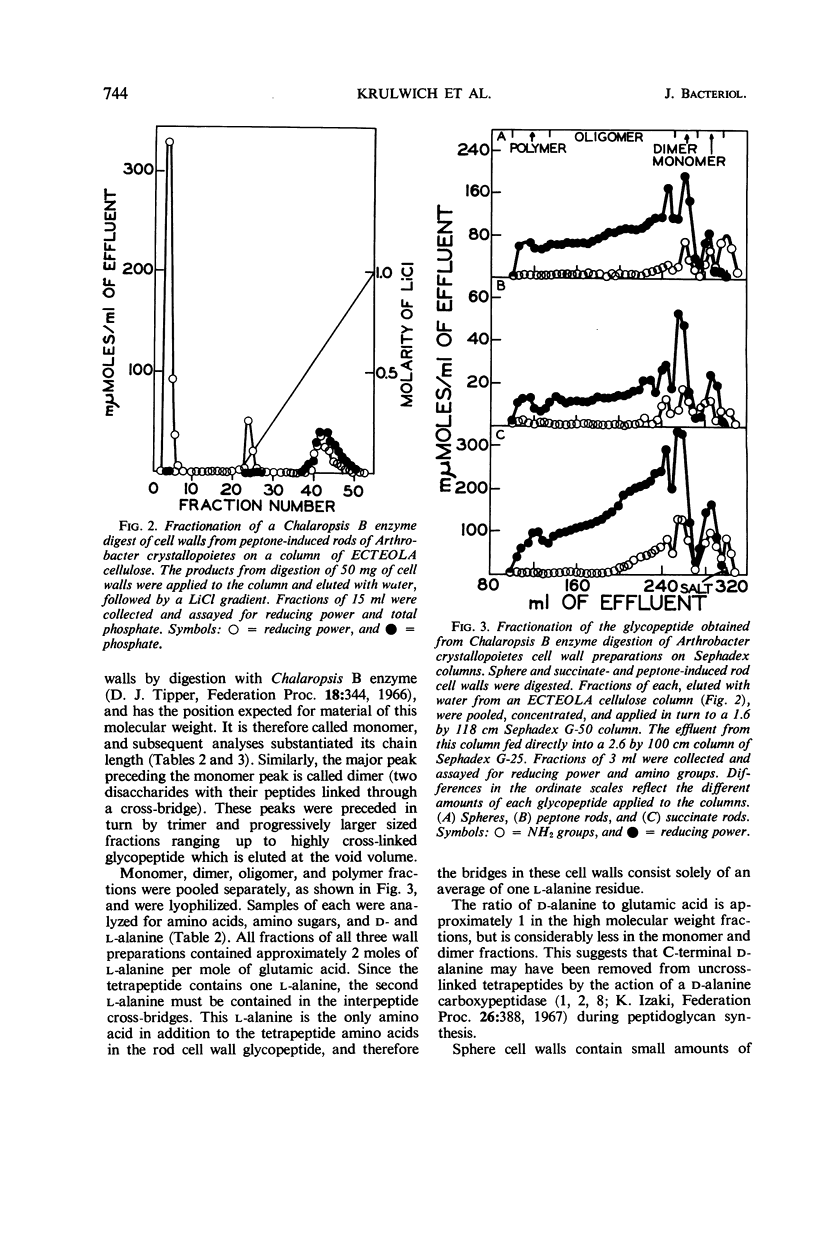
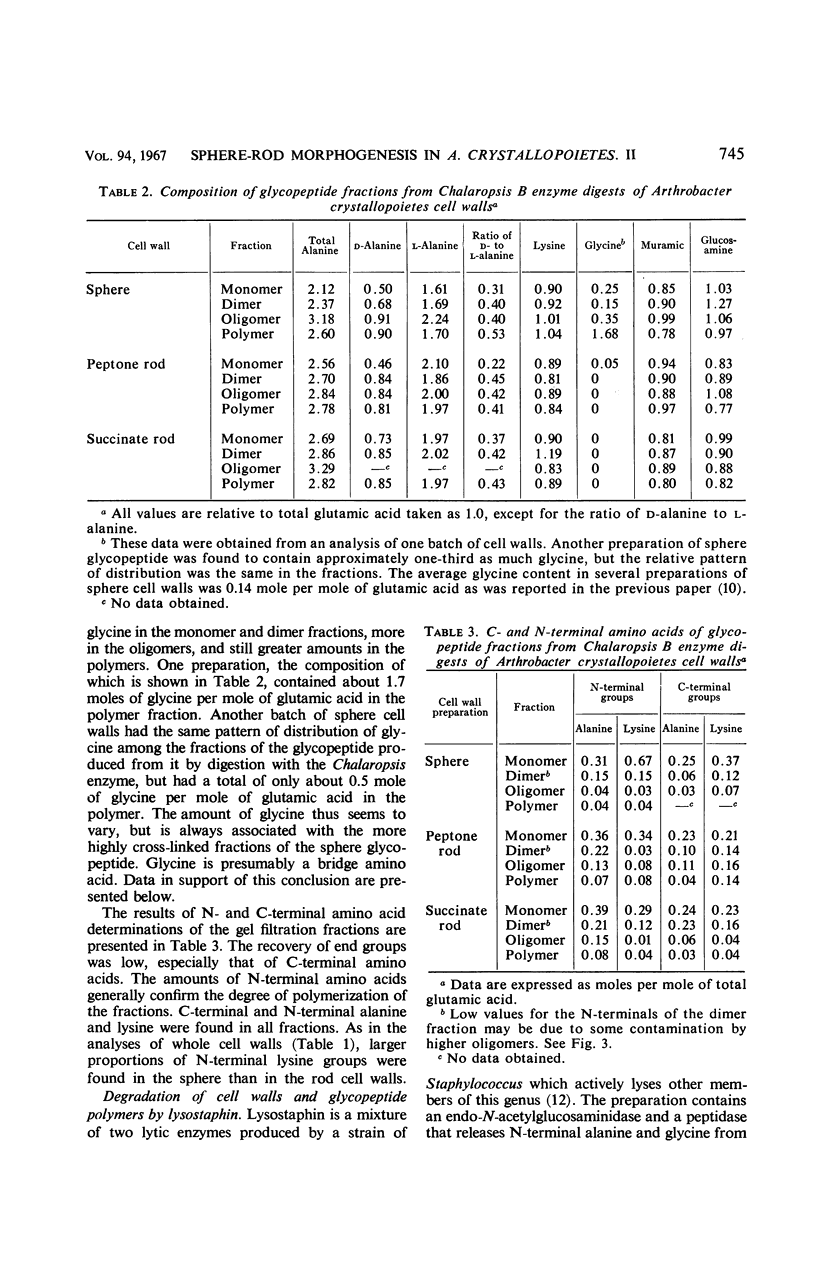
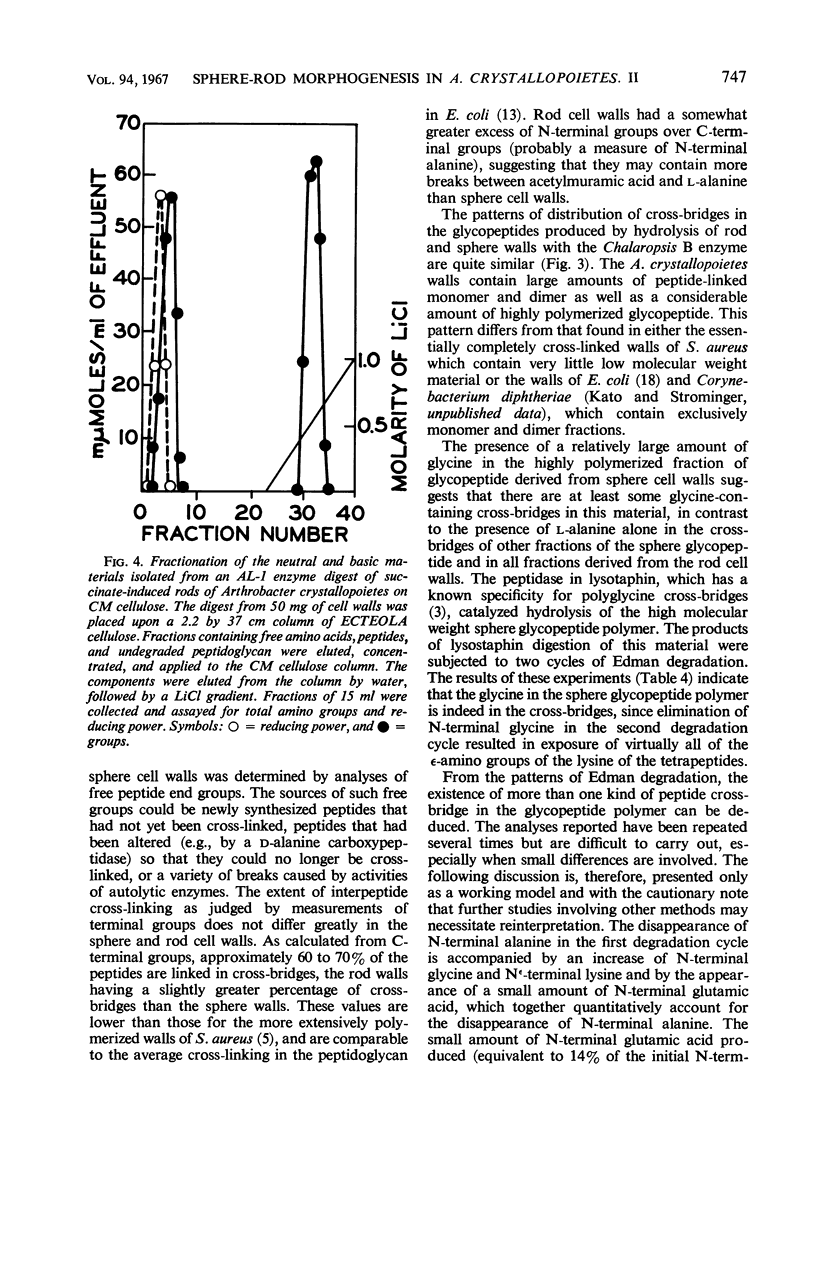
Cell walls of Arthrobacter crystallopoietes grown as spheres and as rods were solubilized by treatment with the B enzyme from Chalaropsis, an N-acetylmuramidase. The neutral glycopeptides were then isolated by chromatography on ECTEOLA cellulose. The glycopeptides, consisting of disaccharide-peptide units interlinked by peptide cross-bridges, were fractionated by gel filtration on Sephadex columns into oligomers of various sizes. The size distribution ranged from monomers with no cross-bridges to polymers with a high degree of polymerization, but did not differ significantly between cell walls from cells grown as spheres or rods. Some small differences in the distribution of C- and N-terminal amino acids were found. Analyses revealed that all the peptide bridges in the glycopeptide fractions from rod cell walls were formed by one l-alanine residue. In sphere cell walls, l-alanine was also found, but, in addition, higher oligomers of the glycopeptide contained glycine in their cross-bridges. These results were confirmed by determinations of C- and N-terminal amino acids released after lysostaphin and AL-1 enzyme digestions and by Edman degradations. Models representing the structures of the sphere and rod cell walls are presented. These structures indicate that the sphere cell wall is probably a more loosely knit macromolecule than is the rod cell wall.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Shimada A., Ito E. Effect of penicillin on cell wall mucopeptide synthesis in a Escherichia coli particulate system. Biochem Biophys Res Commun. 1966 May 25;23(4):518–525. doi: 10.1016/0006-291x(66)90760-1. [DOI] [PubMed] [Google Scholar]
- Araki Y., Shirai R., Shimada A., Ishimoto N., Ito E. Enzymatic synthesis of cell wall mucopeptide in a particulate preparation of Escherichia coli. Biochem Biophys Res Commun. 1966 May 25;23(4):466–472. doi: 10.1016/0006-291x(66)90751-0. [DOI] [PubMed] [Google Scholar]
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., RITTENBERG S. C. A CRYSTALLINE PIGMENT PRODUCED FROM 2-HYDROXYPYRIDINE BY ARTHROBACTER CRYSTALLOPOIETES N.SP. Arch Mikrobiol. 1963 Dec 10;47:137–153. doi: 10.1007/BF00422519. [DOI] [PubMed] [Google Scholar]
- HASH J. H. PURIFICATION AND PROPERTIES OF STAPHYLOLYTIC ENZYMES FROM CHALAROPSIS SP. Arch Biochem Biophys. 1963 Sep;102:379–388. doi: 10.1016/0003-9861(63)90245-5. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG W., HILL R. J. The structure of human hemoglobin. III. The sequence of amino acids in the tryptic peptides of the alpha chain. J Biol Chem. 1962 Aug;237:2547–2561. [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. F., Munoz E., Ghuysen J. M. Peptide cross-links in bacterial cell wall peptidoglycans studied with specific endopeptidases from Streptomyces albus G. Biochemistry. 1966 Aug;5(8):2764–2776. doi: 10.1021/bi00872a037. [DOI] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- TAKEBE I. EXTENT OF CROSS LINKAGE IN THE MUREIN SACCULUS OF ESCHERICHIA COLI B CELL WALL. Biochim Biophys Acta. 1965 Mar 1;101:124–126. doi: 10.1016/0926-6534(65)90038-2. [DOI] [PubMed] [Google Scholar]
- TIPPER D. J., STROMINGER J. L., GHUYSEN J. M. STAPHYLOLYTIC ENZYME FROM CHALAROPSIS: MECHANISM OF ACTION. Science. 1964 Nov 6;146(3645):781–782. doi: 10.1126/science.146.3645.781. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Katz W., Strominger J. L., Ghuysen J. M. Substituents on the alpha-carboxyl group of D-glutamic acid in the peptidoglycan of several bacterial cell walls. Biochemistry. 1967 Mar;6(3):921–929. doi: 10.1021/bi00855a036. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L., Ensign J. C. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. VII. Mode of action of the bacteriolytic peptidase from Myxobacter and the isolation of intact cell wall polysaccharides. Biochemistry. 1967 Mar;6(3):906–920. doi: 10.1021/bi00855a035. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]


