Abstract
Many cases of neonatal toxic shock syndrome (TSS)-like exanthematous disease but few cases of menstrual TSS (mTSS) have been reported in Japan. We determined the prevalence of mucosal colonization with Staphylococcus aureus and of positive antibodies to TSS toxin 1 (TSST-1) among 209 healthy Japanese women in Tokyo. S. aureus isolates from mucosal sites were characterized with respect to TSST-1 production and resistance genotype. Antibody titers were determined for test subjects and for 133 Japanese and 137 Caucasian control women living in the United States. S. aureus was isolated from at least one site in 108 of 209 women (52%) in Tokyo. Of the 159 S. aureus isolates recovered, 14 (9%) were TSST-1 positive (12 unique strains). Twelve of 209 women (6%) were colonized with a TSST-1-producing strain; two (<1%) had vaginal colonization. Only 2 of 12 unique toxigenic strains (14%) were methicillin resistant. Of the 12 TSST-1-positive strains isolated, 6 (50%) were pulsed-field gel electrophoresis type USA200, multilocus sequence type clonal complex 30. Fewer Japanese women in Tokyo (47%) than Caucasian and Japanese women in the United States (89% and 75%, respectively) had TSST-1 antibodies. The prevalences of colonization with TSST-1-producing S. aureus were comparable in Japan and the United States, despite low seropositivity to TSST-1 in Japan. Environmental factors appear to be important in promoting the development of anti-TSST-1 antibodies, as there was a significant difference in titers between Japanese women living in Tokyo and those living in the United States. Most colonizing TSST-1-producing S. aureus strains in Japan were genotypically similar to mTSS strains found in the United States.
Toxic shock syndrome (TSS) is an acute disease characterized by fever, rash, hypotension, multiple-organ-system dysfunction, and desquamation (41). The incidence of menstrual TSS (mTSS) in the United States has been calculated to be 1.05 per 100,000 menstruating women (14). The disease has been reported in many countries, but rigorously calculated incidences for countries other than the United States have not been reported; however, the impression has been that mTSS in other countries is rare. The basis for differences between countries is uncertain but may reflect reporting bias, failure to recognize TSS, or differences in a number of other factors, including rates of colonization by TSS toxin 1 (TSST-1)-producing strains, prevalence of antibody to TSST-1, health and hygiene practices, and genetic susceptibility to the superantigenic effects of the toxin.
mTSS among women in Japan appears to be rare, with approximately 12 cases reported in the literature; however, to the best of our knowledge, there have been no formal studies of the true incidence of mTSS in Japan. There have been numerous reports, however, of neonatal TSS-like exanthematous disease (NTED), which is typically characterized by fever, erythema, and thrombocytopenia (49). Most reported cases of NTED have been caused by TSST-1-producing, methicillin-resistant strains of Staphylococcus aureus (MRSA) (22, 29, 32, 33), and susceptibility to this toxin was indicated by low levels of antibody directed against TSST-1 (32).
The development of mTSS requires vaginal colonization or infection with a toxin-producing strain of S. aureus in the absence of positive antibody (titer of ≥1:32) against the toxin. Previous studies have reported vaginal colonization rates for toxigenic S. aureus ranging between 1 and 4% (4, 24, 36, 39). A positive titer of serum antibody to TSST-1 has been shown to be common, generally on the order of 80 to 90%, among healthy adults from multiple countries in North America, Europe, and Asia (8, 10, 25, 43; J. Seymour, presented at Unresolved Infectious Disease Issues in Obstetrics and Gynecology: an International Symposium, New York, NY, 2002). TSST-1-producing strains in the United States have generally been methicillin susceptible, but cases of mTSS caused by MRSA have been reported in the United States (27) and elsewhere (6, 13).
We undertook the current study to determine the prevalence of microbiologic and immunologic risk factors for mTSS among Japanese women, as well as the relationship between toxin production and other molecular characteristics, such as those coding for methicillin resistance.
MATERIALS AND METHODS
Study design and subjects. (i) Tokyo subjects.
Healthy, menstruating women between 18 and 45 years of age were recruited from Tokyo and the surrounding area by Sogo Clinical Pharmacology Co., Ltd., in Tokyo, with the intention that the subjects be evenly distributed among three age groups (18 to 25, 26 to 34, and 35 to 45 years). Women of Japanese descent were eligible for enrollment if they had a history of regular menstrual cycles for the past 2 years and agreed to refrain from the use of mouthwash, mouth rinse, medicated drops or sprays, douching substances, vaginal medications, suppositories, feminine sprays, genital wipes, and contraceptive spermicides and from sexual intercourse for 48 h prior to sample collection. Subjects were also required to refrain from bathing, showering, or swimming within 2 h prior to sample collection. Women were excluded if they worked in health care settings; had been hospitalized in the past 6 weeks; had experienced a genital or sexually transmitted infection within the past 6 weeks; were pregnant, actively trying to become pregnant, or suspected they were pregnant; had been diagnosed as having diabetes, kidney failure, hepatitis, human immunodeficiency virus infection, or TSS; were currently suffering from a sinus infection or pharyngitis (self-declared); or had taken immunosuppressive, chemotherapeutic, or systemic or topical antimicrobial drugs within the past 30 days.
(ii) U.S. control subjects.
Healthy, menstruating, and ethnically Japanese and Caucasian women in the same age range and with the same age distribution as the Tokyo subjects were recruited from Long Beach and Costa Mesa, CA, and the surrounding area by West Coast Clinical Trials, Inc. The Caucasian control subject group consisted of non-Hispanic women matched in age (± 2 years) to the Japanese study subjects. U.S. control subjects were eligible for enrollment if they were born in the United States, had regular menstrual cycles, had not recently used immunosuppressive drugs or had been diagnosed as having an immunosuppressive disease, and had not resided outside the continental United States or Canada for more than 5 years.
Written, informed consent was obtained from the subjects prior to the collection of any information or clinical samplings. Subjects were removed from the study if they failed to meet the inclusion criteria or satisfied any of the exclusion criteria at any time during the study.
Conduct of study.
In Japan, the study was conducted from 10 March 2005 through 28 March 2005. In the United States, the study was conducted from 30 December 2005 through 15 May 2006. The protocol and informed consent documents were reviewed and approved by an ethics committee or institutional review board, as appropriate, in both locations.
Demographics, habits and practices, and medical history questionnaires.
The subjects in both study groups were asked to answer questions regarding demographic characteristics, specific hygiene habits and practices, and medical history, including the use of feminine hygiene products, birth control methods, sexual activity, pregnancies, vaginal infections, vaginal discharge, and menstruation history. In addition, U.S. Japanese subjects were asked whether they lived a traditional Japanese lifestyle or a Western lifestyle.
Sample collection.
Samples for both microbiologic and immunologic analyses were collected from the subjects in Japan, while samples for immunologic analysis only were collected from the U.S. subjects. In both locations, blood samples for immunologic analysis were collected upon entry into the study. For Tokyo subjects only, the anterior nares were swabbed at a penetration of 1 to 2 cm. Throat samples were obtained by swabbing the area of the tonsillar fauces. Vaginal samples were obtained by inserting a swab into the vagina (without using a speculum) and swabbing the mid-upper vaginal walls approximately 5 cm past the introitus. The labia were spread during this procedure to minimize the potential for contamination by perineal flora.
Sample handling.
Serum samples from the Japanese subjects were frozen and shipped on dry ice within 96 h to Dartmouth-Hitchcock Medical Center (Lebanon, NH) for analysis of TSST-1 immunoglobulin G antibodies. The nasal, throat, and vaginal swabs collected in Japan were refrigerated upon collection and delivered within 24 h to SBS Laboratory (Kanagawa, Japan) for the isolation of S. aureus. Isolates of S. aureus were streaked onto Trypticase soy agar slants for shipment to Creighton University School of Medicine (Omaha, NE) and analyzed for the presence of tst, as well as the presence of mecA and the genetic relatedness of TSST-1-producing isolates. Isolates were also shipped to Dartmouth-Hitchcock Medical Center for analysis of the production of TSST-1 and for antibiotic susceptibility testing. Serum samples from the U.S. subjects were similarly stored, frozen, and shipped to Dartmouth-Hitchcock Medical Center for TSST-1 antibody analysis.
Analyses of sera for anti-TSST-1.
A sandwich enzyme-linked immunosorbent assay was used to measure human TSST-1 immunoglobulin G antibodies, as described previously (36). Nonimmune sera from healthy volunteers and commercially available human immunoglobulin (Sandoglobulin; Sandoz Pharmaceutical Company, East Hanover, NJ) served as negative and positive controls, respectively. Samples with titers of ≤1:4 were classified as negative for antibody, whereas those with titers of ≥1:32 were classified as positive; those with titers of 1:8 and 1:16 were classified as intermediate.
Analyses of S. aureus isolates.
The vaginal, nasal, and throat swabs from each Japanese subject were streaked for isolation onto mannitol salt agar plates (PML Microbiologicals, Mississauga, Ontario, Canada). The plates were incubated at 36°C for 48 h in 5% CO2. The vaginal swabs were also incubated overnight in brain heart infusion broth supplemented with polymyxin B (10 μg/ml) and nalidixic acid (10 μg/ml) for the enhancement of S. aureus, followed by streaking onto blood agar plates. After incubation, the plates were examined for the characteristic morphology of S. aureus, and suspicious colonies were subcultured onto tryptic soy agar plates with 5% sheep blood (PML Microbiologicals) and incubated for 24 h. Gram stain, catalase, and tube coagulase tests were performed to confirm the identification of S. aureus.
Cell supernatants from positively identified S. aureus isolates were analyzed in a competitive enzyme-linked immunosorbent assay for TSST-1 (38). S. aureus strains MN8 and ATCC 25923 were used as positive and negative controls, respectively.
The methicillin resistance of TSST-1-producing S. aureus isolates was determined by means of an oxacillin agar screening test (MRSA screening agar; Remel, Lenexa, KS) (5). The control strains of S. aureus included ATCC 25293, a penicillin-sensitive strain; ATCC 43300, a MRSA strain; and MN8, a penicillin-resistant, mTSS-associated strain.
Molecular analysis of S. aureus isolates.
Isolates were screened by Southern hybridization (46) for the presence of tst and mecA genes by PCR amplification of probes from S. aureus MN8 and S. aureus EMRSA16, respectively, using digoxigenin-labeled dideoxy-UTP (Roche, Indianapolis, IN). The primers and PCR conditions used to amplify both mecA and tst have been previously described (12, 28). In methicillin-resistant isolates, typing of the staphylococcal chromosomal cassette mec (SCCmec) was performed as described by Oliveira and de Lencastre (34). Multilocus sequence typing (MLST) was performed using the PCR primers and amplification conditions described by Enright et al. (11).
For S. aureus isolates producing TSST-1, molecular strain typing to assess genetic relatedness was performed by pulsed-field gel electrophoresis (PFGE) as previously described (15). The resulting pulsed-field types of isolates were categorized based on criteria established by the CDC (26).
Statistical analyses.
Data from the Tokyo subjects were analyzed by logistic regression using the GENMOD procedure in SAS release version 8 (47), both with and without age included as a continuous covariate, to examine the association of demographic, medical history, and habits and practices data with the presence of TSST-1-producing S. aureus and the prevalence of positive antibody titers. To compare groups (or subgroups) for the prevalence of positive antibody titers, a series of two-way logistic regression models was run with a subject characteristic variable included as a factor and age included as a covariate. A separate logistic model included only group (or subgroup) and age. To further examine the correlation between ordinal variables and the prevalence of positive antibody titers, Cochran-Mantel-Haenszel chi-square tests (47) were also conducted within groups (or subgroups) using the FREQ procedure in SAS. All data except for the data from one subject in the U.S. study who was inappropriately enrolled were included in the statistical analysis. Statistical significance was declared at the two-sided 0.05 significance level.
RESULTS
Demographic characteristics of subjects.
The age distribution and antibody status of the women enrolled in Tokyo and in the United States are summarized in Table 1.
TABLE 1.
Age distribution and antibody status among subjects
| Age group (yr)a | Tokyo Japanese
|
U.S. Japanese
|
U.S. Caucasians
|
|||
|---|---|---|---|---|---|---|
| No. of subjects | No. (%) of antibody-positive subjectsb | No. of subjects | No. (%) of antibody-positive subjectsb | No. of subjects | No. (%) of antibody-positive subjectsb | |
| 18-25 | 69 | 27 (39) | 45 | 30 (67) | 45 | 41 (91) |
| 26-34 | 70 | 28 (40) | 43 | 37 (86) | 44 | 39 (90) |
| 35-45 | 70 | 43 (61) | 45 | 33 (73) | 48 | 42 (86) |
| Total (avg %) | 209 | 98 (47) | 133 | 100 (75) | 137 | 122 (89) |
Specific age groups were targeted to ensure an even distribution across the age range of 18 to 45 years.
Antibody positivity is defined by an anti-TSST-1 titer of ≥1:32.
Antibody prevalence among Japanese subjects and U.S. control groups. (i) Japanese (Tokyo) subjects.
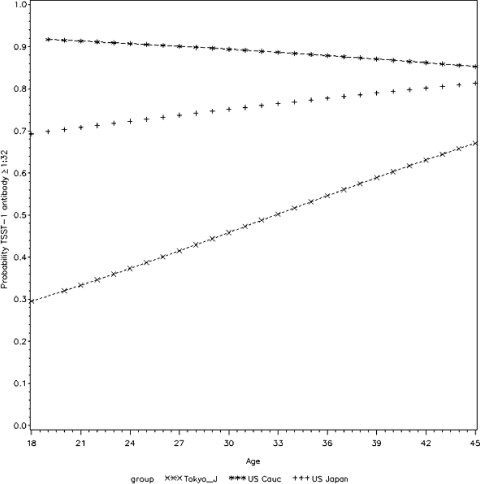
The prevalence of positive titers of antibody to TSST-1 (titer ≥ 1:32) among the Japanese (Tokyo) subjects was 47% (98 of 209 subjects). The prevalence of positive titers of antibody to TSST-1 exhibited a statistically significant association with older age (P = 0.002) (Fig. 1). Subjects colonized in the nose and throat with toxigenic S. aureus had higher antibody titers than those colonized in the vagina (Table 2). Out of the 12 subjects colonized with the TSST-1-positive strain, only one subject lacked a positive antibody titer. This one subject was colonized vaginally.
FIG. 1.
Logistic regression results: the probability of being seropositive for anti-TSST-1 antibodies versus the age of Japanese women in Tokyo and control women of Caucasian and Japanese ancestry in the United States. P values for age were 0.002 (Japanese women in Tokyo), 0.315 (Japanese women in the United States), and 0.465 (Caucasian women in the United States).
TABLE 2.
Characterization of Japanese women with TSST-1-producing S. aureus colonizationa
| Age (yr) | Ab titer | Colonization site | Oxacillin susceptible or resistant | Married | Ever pregnant | Sexual intercourse | Ever used tampon |
|---|---|---|---|---|---|---|---|
| 20 | >1,024 | Nose | S | N | N | N | N |
| 21 | 256 | Nose | S | N | Y | Y | Y |
| 21 | >1,024 | Throat | S | N | N | Y | Y |
| 21 | 32 | Vagina | R | N | N | Y | N |
| 22 | 64 | Throat | S | N | N | Y | Y |
| 22 | >1,024 | Throat | S | Y | Y | Y | N |
| 25 | <4 | Vagina | R | N | N | Y | Y |
| 28 | >1,024 | Nose | S | Y | Y | Y | N |
| 39 | >1,024 | Nose and throat | S | Y | Y | Y | Y |
| 40 | 64 | Throat | S | Y | Y | Y | Y |
| 42 | 64 | Nose | S | Y | N | Y | N |
| 44 | 512 | Nose and throat | S | Y | Y | Y | Y |
Ab, antibody; S, susceptible; R, resistant; N, no; Y, yes.
(ii) U.S. control groups.
Overall, the prevalence of positive antibody to TSST-1 in the U.S. control groups was 82% (222 of 270 subjects). Caucasians had a high prevalence of positive antibody titers, 89% (122 of 137 subjects), but the percentage of Japanese controls with positive antibody tests was found to be significantly lower (100 of 133 subjects; 75%) (P = 0.004 versus Caucasian controls). The statistical difference in seropositivity between Japanese and Caucasian control subjects was driven by the low level of positive antibody among Japanese women in the youngest age group (30 of 45 subjects; 67%) (Table 1).
(iii) Japanese subjects and U.S. control groups.
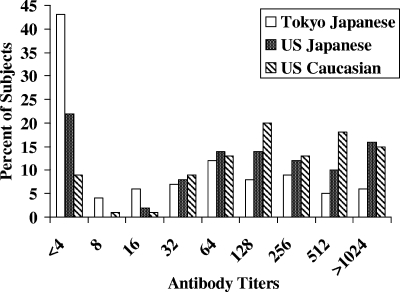
Caucasian subjects had a significantly higher prevalence of positive antibody titers (P ≤ 0.004) than Japanese subjects in Tokyo or the United States. U.S. Japanese subjects also had a significantly higher prevalence (P < 0.001) of positive antibody titers than Tokyo Japanese subjects. Figure 2 depicts the antibody titer distribution for all subjects. Only 12 of 137 Caucasian subjects (9%) had little or no detectable antibody (titer ≤ 1:4), compared with 90 of 209 Tokyo Japanese subjects (43%) and 30 of 133 U.S. Japanese subjects (23%).
FIG. 2.
Titers of anti-TSST-1 antibodies in Japanese women in Tokyo and in control women of Caucasian and Japanese ancestry living in the United States.
S. aureus colonization of Japanese women in Tokyo and characterization of isolates.
Table 3 summarizes the results of cultures for S. aureus colonization among the study subjects in Tokyo. Fourteen of 159 isolates (9%) were positive for TSST-1, the main cause of mTSS. The presence of S. aureus at any body site was documented in approximately half of all subjects (108 of 209). Only 12 (6%) were colonized with a toxigenic (TSST-1-positive) staphylococcal strain. The throat was the most frequent site of S. aureus colonization (74 subjects). Seven subjects were colonized with S. aureus at all three body sites (nares, throat, and vagina); 25 were culture positive for S. aureus in both the throat and the nares, and 4 were colonized in both the vagina and the throat.
TABLE 3.
S. aureus and TSST-1-producing S. aureus colonization results for Tokyo Japanese subjectsa
| Organism and colonization site | No. (%) of subjects |
|---|---|
| S. aureus | |
| Any site | 108 (52) |
| Nose only | 22 (10) |
| Throat only | 38 (18) |
| Vagina only | 12 (6) |
| Vagina and throat | 4 (2) |
| Nose and throat | 25 (12) |
| Nose, throat, and vagina | 7 (3) |
| TSST-1-producing S. aureus | |
| Any site | 12 (6) |
| Nose only | 4 (2) |
| Throat only | 4 (2) |
| Vagina only | 2 (1) |
| Nose and throat | 2 (1) |
Total number of subjects, 209.
Characterization of TSST-1-positive isolates.
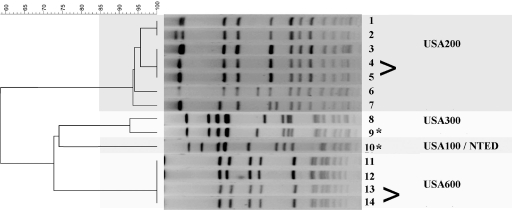
The 14 isolates found to produce TSST-1 were further evaluated for methicillin resistance and genetic relatedness. The TSST-1 isolates were of diverse genotypes (Fig. 3). Analysis by PFGE revealed that two isolates from two subjects represented the same strains. Thus, 12 unique TSST-1 isolates were recovered in this study. Of these 12 unique isolates, only two (17%) were found to be methicillin resistant. Methicillin resistance was not found in any of the 145 TSST-1-negative isolates. Molecular analysis of all 12 TSST-1-positive isolates showed 6 to be similar to the predominant TSST-1-positive S. aureus U.S. isolates of PFGE type USA200 and MLST clonal complex (CC) 30. The genotype for three of the TSST-1-positive isolates was USA600 and MLST CC45. The genotype for two was USA300 and MLST CC8 (one of which was methicillin resistant; SCCmec IV), which is characteristic of the predominant U.S. community-associated S. aureus strain. The genotype for the remaining TSST-1-positive isolate was typical of that of previously described Japanese isolates associated with NTED, i.e., USA100, MLST CC5, and SCCmec II. This last isolate represented the only clindamycin-resistant strain identified in the study. There was 100% agreement between in vitro susceptibility results for methicillin obtained by the Kirby-Bauer method and results of amplification of the mecA gene by PCR.
FIG. 3.
PFGE macrorestriction analysis of the 14 TSST-1-producing isolates cultured during the study. Angle brackets indicate isolates with indistinguishable PFGE patterns from the same subject. Methicillin-resistant isolates are indicated by an asterisk.
Medical history, habits, and practices results. (i) Japanese (Tokyo) subjects.
Both the history of pregnancy and the history of having given birth were statistically associated with an increased prevalence of positive antibody titers (P = 0.030 and P = 0.047, respectively). When age was included in the model, however, neither of these factors was an independent predictor of positive antibody titers. The rates of vaginal colonization with S. aureus were not influenced by recent sexual activity, number of partners in the year prior to the study, history of tampon use, or history of pregnancy. However, subjects who had used a barrier contraceptive had a higher prevalence of vaginal S. aureus colonization than subjects who had not (P = 0.022).
(ii) U.S. control groups.
Caucasian subjects had a significantly higher prevalence of positive antibody titers than Japanese subjects living in self-declared traditional Japanese households (P = 0.002) and a higher (approaching statistical significance) prevalence of positive antibody titers than Japanese subjects living in nontraditional Japanese households (P = 0.065). There was a significant association between the prevalence of positive antibody titers to TSST-1 and the number of sexual partners within the year prior to the study (P = 0.0048). Only 59% (13 of 22) of U.S. Japanese subjects with no sexual partners in the year prior to the study had positive antibody titers, compared with 78% (75 of 96) of those reporting one sexual partner and 86% (12 of 14) of those with at least two sexual partners over this period. For all U.S. Japanese subjects, the prevalence of positive antibody titers was not influenced by history of tampon use, sexual intercourse, or pregnancy. Japanese tampon users from nontraditional households were not significantly different from Caucasians with respect to the prevalence of antibody.
DISCUSSION
mTSS has been a subject of study in the United States and a number of other countries. Although rare (e.g., one case per 100,000 menstruating women in the United States), mTSS has been documented to occur worldwide, with country-to-country variation in incidence reports (2, 9, 14, 17, 20, 31, 36).
In contrast, there have been approximately 12 well-documented cases of mTSS in Japan over the past 15 years (2, 19, 20, 23, 31, 35, 45, 50), despite the fact that TSST-1-producing strains of S. aureus are prevalent throughout the country (18, 52, 53). It is unclear whether the small number of reported mTSS cases reflects the failure to recognize the disease, underreporting of the disease, or a rate that is actually lower than that in the United States because of microbiologic or immunologic reasons.
The global incidence of TSST-1-producing strains has been documented worldwide (Seymour, unpublished report). Colonization with S. aureus is generally highest (20 to 30%) in the nose or oropharynx among non-health-care workers (36). Vaginal colonization with S. aureus has been determined to be lower (10% to 20%) in the United States, Europe, and Asia (36; Seymour, unpublished report). Similarly, TSST-1-producing strains of S. aureus have been isolated vaginally from 1 to 4% of healthy, menstruating women in the general population (1, 4, 24, 36, 39). Reports from Japan over the past few years have shown TSST-1 to be responsible for a large number of NTED cases, a disease of neonates that lack maternal antibody to the toxin. In this light, the relative infrequency of mTSS in Japan is especially striking (22, 29, 48, 49).
The purpose of this study was to obtain additional information regarding microbiologic and immunologic risk factors related to mTSS. The study enrolled 209 healthy adult women in Tokyo and included collection of demographic and health practices information, sera for antibody testing, and samples from three mucosal sites for isolation of S. aureus; we characterized the staphylococcal isolates for the production of TSST-1, methicillin resistance, and additional molecular characteristics. Our key finding, with respect to antibody, was that only 47% of women had positive titers of anti-TSST-1 antibody, a rate that was significantly lower than the reported seropositivity rates in the United States and Europe (36). After the data were corrected for age, there were no significant predictors of the presence of antibody to TSST-1.
Because of the lower than expected seropositivity rate for TSST-1 among Japanese women, we conducted a follow-up study in the United States aimed at elucidating the extent to which genetics and environmental factors influenced the development of antibody. As a group, U.S. women had a high rate of seropositivity for TSST-1 (82%) (P < 0.001 versus women in Japan), which was comparable to the rate found in a large study previously conducted in the United States (36).
Of interest, however, was that ethnic Japanese women living in the United States had a seropositivity rate (75%) that was significantly higher than that of Japanese women living in Tokyo (47%), suggesting a strong environmental influence on the development of antibody, but also had a lower rate than that of a cohort of Caucasian subjects (89%). In the larger previously conducted U.S. study, the black population demonstrated a 78% positive antibody titer level (36). The black population in the United States does not appear to be at an increased risk for mTSS based on the reported incidence rates (3, 16).
The difference between Japanese-American and Caucasian subjects is most likely a reflection of differences in lifestyle and health practices. Japanese-American women who described themselves as living in traditional Japanese households were less likely to have positive antibody titers than those who had assumed a Western lifestyle. A history of recent sexual intercourse was associated with a higher rate of seropositivity among Japanese-American women, as was tampon use among Japanese-American women living in nontraditional households. These results, taken together, suggest that the development of antibody to TSST-1 is largely, if not exclusively, a function of environmental factors rather than a genetic inability to develop antibody. For example, while tampons are not a source of S. aureus (44), digital procedures associated with the insertion of tampons and barrier contraceptives may increase the potential exposure to S. aureus, resulting in vaginal colonization.
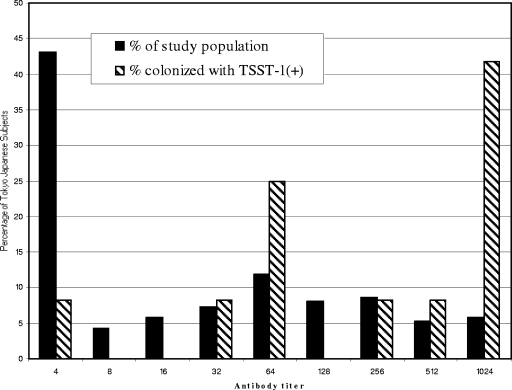
The relatively low rate of seropositivity for TSST-1 in Japan was not a result of lack of colonization by S. aureus and specifically by TSST-1-producing strains: 52% of women were colonized with S. aureus at one or more mucosal sites (Table 2), 8.8% of all isolates produced TSST-1, and 6% of women were colonized with a TSST-1-producing strain. Two of the 209 women (0.9%) had TSST-1-producing S. aureus vaginal colonization, which is similar to the rate found in other geographical locations (1, 4, 24, 36, 39). Colonization with a toxin-producing strain was associated with a high rate of antibody positivity: 92% of women colonized with a toxin-producing strain had a high level of antibody (Fig. 4). Subjects with nose and/or throat colonization with a toxin-producing strain had higher antibody titers than those with vaginal colonization (Table 3). It has been demonstrated that nasal exposure to superantigens (e.g., TSST-1) is an excellent avenue for generating neutralizing antibodies to superantigens and for enhancing the bactericidal activity of neutrophils (30). Furthermore, the nasal mucosae consist of a thin, pseudostratified, columnar epithelium interspersed with glandular ducts and resting on a basement membrane. This nasal basement membrane is semipermeable due to capillary penetration, which makes it fundamentally different from basement membranes found elsewhere in the human body (54). One could speculate that the nasal basement membrane of the throat and nasal mucosal surfaces may be easier for toxin to penetrate than the more robust barrier of the vagina (7, 42). These differences may warrant further investigation of this hypothesis. It was noted that nasal colonization with toxigenic S. aureus was 6% in North America (36) and 3% in Japan (chi-square P value, 0.0695), which suggests a trend, although not statistically significant, toward higher colonization and potential exposure to the toxin via the nose in North American women versus the study population.
FIG. 4.
Titers of anti-TSST-1 antibodies in Japanese women in Tokyo and Japanese women colonized with TSST-1-producing S. aureus.
Only 2 of 159 colonizing strains of S. aureus were MRSA, which is in striking contrast to the very high rate of MRSA among infecting strains of S. aureus in Japan (22, 40, 51), and only 2 strains were both methicillin resistant and producers of TSST-1. This also contrasts sharply with the many reports of NTED in Japan, which is caused by TSST-1-producing MRSA. In our study, only 1 of 159 isolates was of the NTED type, despite the ubiquity of this organism in Japan, which may relate to the fact that a population of non-health-care workers was studied. An additional unexpected finding was that 6 of the 12 unique TSST-1-producing S. aureus isolates were of the USA200 and MSLT CC30 background, the predominant strain type associated with TSST-1 production in the United States. In addition, two of the TSST-1-producing isolates, one methicillin susceptible, were of the USA300 background linked to community-associated infections in the United States.
The low rate of seropositivity seen in the Tokyo Japanese women was unexpected. Since 92% of the Tokyo Japanese subjects who carried a TSST-1 strain had positive antibody titers, either exposure to the toxin is limited, Japanese women are genetically nonresponsive, or there are other environmental factors necessary for an immunogenic response to TSST-1. The percentage of Tokyo subjects who carry S. aureus and the percentage carrying TSST-1 strains are comparable to those in other countries (Seymour, unpublished report). Although a woman's exposure to S. aureus can vary over time (37), it can be reasonably assumed that Japanese women also have exposure over time. Thus, exposure to TSST-1 strains should occur at a frequency similar to that in other countries. The U.S. study assessed the genetic capabilities of Japanese women to respond to TSST-1. Inclusion criteria for the U.S. study required that Japanese women who participated in this study be of Japanese descent in order to control for potential marked genetic differences such as HLA haplotype. The fact that Japanese women living in the United States had a higher rate of seropositivity than those living in Tokyo suggests that the Tokyo Japanese women have the immunological capability to achieve the same anti-TSST-1 antibody titers as their U.S. Japanese cohorts. Therefore, the difference between the seropositivities of the Tokyo Japanese women and the U.S. Japanese women suggests a strong environmental influence on the development of neutralizing antibodies.
This study was not powered to expose specific health and hygiene practices that might be critical determinants of seropositivity, but there is a suggestion, in this and a previous study (39), that sexual intercourse (and most likely other forms of intimate activity) predisposes women to antibody development, presumably by increasing exposure to new strains of the bacteria or by increasing the frequency of exposure to TSST-1-producing S. aureus strains. Other unidentified environmental factors may also be important. Women who lack antibody and become colonized with TSST-1-producing S. aureus are at risk of developing TSS, but this and previous studies (21, 39) have shown that, at any given time, most colonized women are already immune to TSST-1. Although the USA100 NTED type of S. aureus strain is apparently widespread in Japan, an unexpected finding of this study was the similarity between the predominant TSST-1-producing S. aureus strain colonizing Japanese study subjects and the S. aureus strain commonly associated with mTSS in the United States.
Acknowledgments
We thank Paul Modern (Dartmouth), J. Q. Liu (Procter & Gamble), Ron Berg (Procter & Gamble), and Dan Wolter (Creighton University) for technical support; Rita Reynolds (Procter & Gamble) for data management; Sachiko Shiode (Procter & Gamble) for review and helpful comments; and West Coast Clinical Trials (Long Beach and Costa Mesa, CA) and Sogo Clinical Pharma Ltd (Tokyo) for facilitating the clinical studies.
This study was funded by The Procter & Gamble Company, Cincinnati, OH.
J. Parsonnet and R. V. Goering received research support from the study sponsor.
Footnotes
Published ahead of print on 11 June 2008.
REFERENCES
- 1.Adesiyun, A. A., D. Singh, and R. I. Gunness. 1994. Toxic shock syndrome toxin-1 (TSST-1) production and phage susceptibility of Staphylococcus aureus strains from human vaginas and anterior nares in Trinidad. Zentralbl. Bakteriol. 280371-381. [DOI] [PubMed] [Google Scholar]
- 2.Azuma, M. 1999. A case of menstrual toxic shock syndrome possibly caused by methicillin-resistant Staphylococcus aureus (MRSA). Kansenshogaku Zasshi 73267. [Google Scholar]
- 3.Centers for Disease Control and Prevention. 30 April 2004. Summary of notifiable diseases—United States. Morbid. Mortal. Wkly. Rep. 511-84. [PubMed] [Google Scholar]
- 4.Chow, A. W., K. H. Bartlett, R. Percival-Smith, and B. J. Morrison. 1984. Vaginal colonization with Staphylococcus aureus, positive for toxic-shock marker protein, and Escherichia coli in healthy women. J. Infect. Dis. 15080-84. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Cui, L., H. Kasegawa, Y. Murakami, H. Hanaki, and K. Hiramatsu. 1999. Postoperative toxic shock syndrome caused by a highly virulent methicillin-resistant Staphylococcus aureus strain. Scand. J. Infect. Dis. 31208-209. [DOI] [PubMed] [Google Scholar]
- 7.Davis, C. C., M. J. Kremer, P. M. Schlievert, and C. A. Squier. 2003. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution, and tissue damage. Am. J. Obstet. Gynecol. 1891785-1791. [DOI] [PubMed] [Google Scholar]
- 8.de Saxe, M. J., P. Hawtin, and A. A. Wieneke. 1985. Toxic shock syndrome in Britain—epidemiology and microbiology. Postgrad. Med. J. 61(Suppl. 1)5-21. [PubMed] [Google Scholar]
- 9.DeVries, A., L. Lesher, T. Rogers, R. Danila, and R. Lynfield. 2007. Incidence of staphylococcal toxic shock syndrome 2000-2003, abstr. 269. 45th Annu. Meet. Infect. Dis. Soc. Am.
- 10.Dickgiesser, N., and B. Kustermann. 1987. IgG antibodies to toxic shock syndrome toxin-1 (TSST-1) in human sera. Wien. Klin. Wochenschr. 65256-258. (In German.) [DOI] [PubMed] [Google Scholar]
- 11.Enright, M. C., N. P. J. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 381008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fey, P. D., B. Saïd-Salim, M. E. Rupp, S. H. Hinrichs, D. J. Boxrud, C. C. Davis, B. N. Kreiswirth, and P. M. Schlievert. 2003. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara, Y., and S. Endo. 2001. A case of toxic shock syndrome secondary to mastitis caused by methicillin-resistant Staphylococcus aureus. Kansenshogaku Zasshi 75898-903. [DOI] [PubMed] [Google Scholar]
- 14.Gaventa, S., A. L. Reingold, A. W. Hightower, C. V. Broome, B. Schwartz, C. Hoppe, J. Harwell, L. K. Lefkowitz, S. Makintubee, and D. R. Cundiff. 1989. Active surveillance for toxic shock syndrome in the United States, 1986. Rev. Infect. Dis. 11(Suppl. 1)S28-S34. [DOI] [PubMed] [Google Scholar]
- 15.Goering, R. V. 2004. Pulsed field gel electrophoresis. In D. H. Persing, F. C. Tenover, J. Versalovic, Y. Tank, B. Unger, D. Relman, and T. J. White (ed.), Molecular microbiology: diagnostic principles and practice. ASM Press, Washington, DC.
- 16.Gustafson, T. L., G. L. Swinger, A. L. Booth, R. H. Hutcheson, Jr., and W. Schaffner. 1982. Survey of tampon use and toxic shock syndrome, Tennessee, 1979 to 1981. Am. J. Obstet. Gynecol. 143369-374. [DOI] [PubMed] [Google Scholar]
- 17.Hajjeh, R. A., A. Reingold, A. Weil, K. Shutt, A. Schuchat, and B. A. Perkins. 1999. Toxic shock syndrome in the United States: surveillance update, 1979-1996. Emerg. Infect. Dis. 5807-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisata, K., K. Kuwahara-Arai, M. Yamanoto, T. Ito, Y. Nakatomi, L. Cui, T. Baba, M. Terasawa, C. Stozono, S. Kinoshita, Y. Yamashiro, and K. Hiramatsu. 2005. Dissemination of methicillin-resistant staphylococci among healthy Japanese children. J. Clin. Microbiol. 433364-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishi, K. 2001. A case report of menstrual toxic shock syndrome caused by toxin producing Staphylococcus aureus isolated from vaginal fluid. Acta Obstet. Gynaecol. Jpn. 53659-663. [Google Scholar]
- 20.Ishihara, T., Y. Yanase, and H. Igarashi. 1987. A case of toxic shock syndrome from which TSST producing Staphylococcus aureus was isolated from a tampon and vaginal fluid. Kansenshogaku Zasshi 61619-623. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson, J. A., E. M. Kasworm, B. A. Crass, and M. S. Bergdoll. 1986. Nasal carriage of toxigenic Staphylococcus aureus and prevalence of serum antibody to toxic-shock-syndrome toxin 1 in Utah. J. Infect. Dis. 153356-359. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi, K., N. Takahashi, C. Piao, K. Totsuka, H. Nishida, and T. Uchiyama. 2003. Molecular epidemiology of methicillin-resistant Staphylococcus aureus strains causing neonatal toxic shock syndrome-like exanthematous disease in neonatal and perinatal wards. J. Clin. Microbiol. 413001-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura, T. 1988. A possible case of toxic shock syndrome. Rinsho To Kenkyu 653191-3194. [Google Scholar]
- 24.Linnemann, C. C., Jr., J. L. Staneck, S. Hornstein, T. P. Barden, J. L. Rauh, P. F. Bonventre, C. R. Buncher, and A. Beiting. 1982. The epidemiology of genital colonization with Staphylococcus aureus. Ann. Intern. Med. 96940-944. [DOI] [PubMed] [Google Scholar]
- 25.Loch, E. G., B. A. Crass, and M. S. Bergdoll. 1986. Staphylococcus aureus—toxic shock syndrome toxin-1 antibody titers in serum of German women. Arch. Gynecol. 237229-233. [DOI] [PubMed] [Google Scholar]
- 26.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 415113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer, R. D., S. R. Monday, G. A. Bohach, and S. M. Schlievert. 2001. Prolonged course of toxic shock syndrome associated with methicillin-resistant Staphylococcus aureus enterotoxins G and I. Int. J. Infect. Dis. 5163-166. [DOI] [PubMed] [Google Scholar]
- 28.Monday, S. R., and G. A. Bohach. 1999. Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J. Clin. Microbiol. 373411-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano, M., H. Miyazawa, Y. Kawano, M. Kawagishi, K. Torii, T. Hasegawa, Y. Iinuma, and M. Ohta. 2002. An outbreak of neonatal toxic shock syndrome-like exanthematous disease (NTED) caused by methicillin-resistant Staphylococcus aureus (MRSA) in a neonatal intensive care unit. Microbiol. Immunol. 46277-284. [DOI] [PubMed] [Google Scholar]
- 30.Narita, K., D.-L. Hu, T. Tsuji, and A. Nakane. 2008. Intranasal immunization of mutant toxic shock syndrome toxin 1 elicits systemic and mucosal immune response against Staphylococcus aureus infection. FEMS Immunol. Med. Microbiol. 52389-396. [DOI] [PubMed] [Google Scholar]
- 31.Ochiai, K., S. Isonishi, M. Murae, K. Kusuhara, and S. Hachiya. 1983. Toxic shock syndrome (TSS)—report of 2 cases and a study on vaginal flora and sanitary products used during menstruation. Nippon Sanka Fujinka Gakkai Zasshi 351689-1692. [PubMed] [Google Scholar]
- 32.Okada, T., S. Furukawa, K. Miwa, R. Sakai, and J. Sugiyama. 1999. A new exanthematous disease in newborn infants caused by exotoxins producing Staphylococcus aureus; exotoxins production of the isolates and serum levels of antitoxin antibody in the patients and umbilical cord blood. Kansenshogaku Zasshi 73893-900. [DOI] [PubMed] [Google Scholar]
- 33.Okada, T., A. Makimoto, A. Kitamura, S. Furukawa, K. Miwa, and R. Sakai. 2000. A new exanthematous disease in newborn infants caused by exotoxins producing methicillin-resistant Staphylococcus aureus; pathology from viewpoints of local and systemic levels of exotoxin and cytokine. Kansenshogaku Zasshi 74573-579. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 462155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ota, K. 2000. A case of toxic shock syndrome (TSS) possibly caused by enterotoxin-B, p. 24. Abstr. 486th Kanto Reg. Meet. Jpn. Soc. Int. Med.
- 36.Parsonnet, J., M. A. Hansmann, M. L. Delaney, P. A. Modern, A. M. DuBois, W. Wieland-Alter, K. W. Wissemann, J. E. Wild, M. B. Jones, J. L. Seymour, and A. B. Onderdonk. 2005. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J. Clin. Microbiol. 434628-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsonnet, J., M. Delaney, A. Dubois, P. Modern, W. Weiland-Alter, M. Hansmann, J. Seymour, J. Wild, and A. Onderdonk. 2002. Persistence of toxic shock syndrome toxin-1 (TSST-1)-producing Staphylococcus aureus & antibody (Ab) to TSST-1, abstr. B-1437, p. 58. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., San Diego, CA.
- 38.Parsonnet, J., J. T. Mills, Z. A. Gillis, and G. B. Pier. 1985. Competitive, enzyme-linked immunosorbent assay for toxic shock syndrome toxin-1. J. Clin. Microbiol. 2226-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsonnet, J., C. Sullivan, P. Modern, and K. Wissemann. 1997. Risk factors for TSS among European women: vaginal colonization with S. aureus and antibody to TSST-1, p. 72. In J. Arbuthnott and B. Furman (ed.), European conference on toxic shock syndrome: proceedings of a symposium sponsored by Procter & Gamble. Royal Society of Medicine Press, London, United Kingdom.
- 40.Piao, C., T. Karasawa, K. Totsuka, T. Uchiyama, and K. Kikuchi. 2005. Prospective surveillance of community-onset and healthcare-associated methicillin-resistant Staphylococcus aureus isolated from a university-affiliated hospital in Japan. Microbiol. Immunol. 49959-970. [DOI] [PubMed] [Google Scholar]
- 41.Reingold, A. L., N. T. Hargrett, K. N. Shands, B. B. Dan, G. P. Schmid, B. Y. Strickland, and C. V. Broome. 1982. Toxic shock syndrome surveillance in the United States, 1980 to 1981. Ann. Intern. Med. 96875-880. [DOI] [PubMed] [Google Scholar]
- 42.Roithmann, R. 2007. Specific tests for nasal permeability. Rev. Bras. Otorrinolaringol. 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroder, E., G. Kunstmann, H. Hasbach, and G. Pulverer. 1988. Prevalence of serum antibodies to toxic-shock-syndrome-toxin-1 and to staphylococcal enterotoxins A, B and C in West-Germany. Zentralbl. Bakteriol. Mikrobiol. Hyg. Abt. 1 270110-114. [DOI] [PubMed] [Google Scholar]
- 44.Shands, K. N., G. P. Schmid, B. B. Dan, D. Blum, R. J. Guidotti, N. T. Hargrett, R. L. Anderson, D. L. Hill, C. V. Broome, J. D. Band, and D. W. Fraser. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical feature in 52 cases. N. Engl. J. Med. 3031436-1442. [DOI] [PubMed] [Google Scholar]
- 45.Shibuya, N. 2003. A case of TSS-like clinical condition—microbiological study on menstrual tampon and speculation about actual usage status. J. Dermatol. 113748. [Google Scholar]
- 46.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98503-517. [DOI] [PubMed] [Google Scholar]
- 47.Stokes, M. E., C. S. Davis, and G. S. Koch. 1995. Categorical data analysis using the SAS system. SAS Institute Inc., Gary, NC.
- 48.Takahashi, N. 2003. Neonatal toxic shock syndrome-like exanthematous disease (NTED). Pediatr. Int. 45233-237. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi, N., H. Nishida, M. Ino, Y. Sakata, I. Sakuma, and Y. Takeda. 1995. A new exanthematous disease in newborn infants. Acta Neonat. Jpn. 31371-377. [Google Scholar]
- 50.Tanaka, S., Y. Takemoto, H. Ono, and C. Fuji. 1983. Probable case of toxic shock syndrome. Kansenshogaku Zasshi 57448-453. [DOI] [PubMed] [Google Scholar]
- 51.Totsuka, K., M. Shiseki, K. Kikuchi, and Y. Matsui. 1999. Combined effects of vancomycin and imipenem against methicillin-resistant Staphylcoccus aureus (MRSA) in vitro and in vivo. J. Antimicrob. Chemother. 44455-460. [DOI] [PubMed] [Google Scholar]
- 52.Ueda, S., and Y. Kuwabara. 1992. Enterotoxicogenicity of Staphylococcus aureus strains from healthy women's fingers. J. Antibact. Antifung. Agents 20313-316. [Google Scholar]
- 53.Uemura, E., S. Kakinohana, N. Higa, C. Toma, and N. Nakasone. 2004. Comparative characterization of Staphylococcus aureus isolates from throats and noses of healthy volunteers. Jpn. J. Infect. Dis. 5721-24. [PubMed] [Google Scholar]
- 54.Watelet, J. B., and P. Van Cauwenberge. 1999. Applied anatomy and physiology of the nose and paranasal sinuses. Allergy 54(Suppl. 57)14-25. [DOI] [PubMed] [Google Scholar]