Abstract
Few data exist to describe in vitro patterns of cross-resistance among large collections of clinical Aspergillus isolates, including those of species other than Aspergillus fumigatus. We examined 771 Aspergillus spp. clinical isolates collected from 2000 to 2006 as part of a global antifungal surveillance program (553 A. fumigatus, 76 A. flavus, 59 A. niger, 35 A. terreus, and 24 A. versicolor isolates and 24 isolates of other Aspergillus species). Antifungal susceptibility testing was performed by the Clinical and Laboratory Standards Institute (CLSI) M38-A broth dilution method with itraconazole (ITR), posaconazole (POS), ravuconazole (RAV), and voriconazole (VOR). We examined the potential for cross-resistance by using measures of correlation overall and by species. For most Aspergillus isolates (from 88% of isolates for ITR to 98% of isolates for VOR and POS), MICs of each triazole were ≤1 μg/ml. When all 771 isolates were examined, there were statistically significant correlations for all six triazole-triazole pairs. For A. fumigatus, the strongest correlations seen were those between VOR and RAV MICs (r = 0.7) and ITR and POS MICs (r = 0.4). Similarly, for A. flavus, only VOR and RAV MICs and ITR and POS MICs demonstrated statistically significant positive correlations. We have demonstrated correlations among triazole MICs for Aspergillus, which for the most common species (A. fumigatus and A. flavus) were strongest between VOR and RAV MICs and ITR and POS MICs. However, Aspergillus species for which MICs of VOR or POS were >2 μg/ml remain extremely rare (<1% of isolates).
Azole resistance among Aspergillus spp. is uncommon. Denning and colleagues have shown maximum rates of itraconazole resistance (MIC ≥ 4 μg/ml) of 4.2% for Aspergillus spp. and 2.1% for A. fumigatus (6, 15). These findings are supported by those of Verweij et al. (21) and Diekema et al. (8). Clinical and in vivo resistance to itraconazole (5, 6) and elevated voriconazole MICs (20) for A. fumigatus clinical isolates have been described previously, and clear advances in defining the mechanisms of azole resistance in this species have been made (1, 2, 6, 9, 11-16, 22). However, no study has investigated azole cross-resistance in more than a handful of clinical Aspergillus isolates (15).
As part of our global antifungal resistance surveillance programs, we collected hundreds of clinical isolates of Aspergillus spp. from medical centers worldwide between 2000 and 2006. We used this large collection of clinical Aspergillus isolates to describe the patterns of in vitro activity of four azoles (itraconazole, voriconazole, posaconazole, and ravuconazole) against Aspergillus spp.
MATERIALS AND METHODS
Organisms.
A total of 771 unique patient clinical isolates of Aspergillus spp. were obtained from 62 different medical centers worldwide. The isolates were obtained from a variety of sources, including sputum, bronchoscopy, and tissue biopsy specimens. The collection of isolates included 553 A. fumigatus, 76 A. flavus, 59 A. niger, 35 A. terreus, and 24 A. versicolor isolates and 24 isolates of other Aspergillus species. All isolates were identified using standard microscopic morphology and were stored as spore suspensions in sterile distilled water at room temperature until they were used in the study. Before testing, each isolate was subcultured at least twice on potato dextrose agar (Remel, Lenexa, KS) to ensure viability and purity. As a screen for cryptic species within the A. fumigatus complex (e.g., A. lentulus), all A. fumigatus isolates for which the MIC of any azole was ≥2 μg/ml were tested for growth at 50°C. All isolates screened grew at 50°C, confirmation that they were A. fumigatus rather than another species within the complex.
Susceptibility testing.
Posaconazole (Schering-Plough), voriconazole (Pfizer), ravuconazole (Bristol-Myers Squibb), and itraconazole (Janssen) were all obtained as reagent-grade powders from their respective manufacturers. The broth microdilution method was performed according to the CLSI M38-A standard (3). Trays containing a 0.1-ml aliquot of the appropriate drug solution (2× final drug concentration) in each well were sealed and stored at −70°C until being used in the study. The stock conidial suspension (106 spores/ml) was diluted to a final inoculum concentration of 0.4 × 104 to 5 × 104 CFU/ml and dispensed into the microdilution wells. The final concentrations of drugs in the wells ranged from 0.008 to 8.0 μg/ml. The inoculated microdilution trays were incubated at 35°C and read at 48 h. The MIC end point for the azoles was defined as the lowest concentration that produced the complete inhibition of growth.
In the absence of azole breakpoints for the filamentous fungi, we used the following provisional breakpoints for all agents tested: susceptible, MIC ≤ 1 μg/ml; intermediate, MIC = 2 μg/ml; and resistant, MIC ≥ 4 μg/ml.
Quality control.
Quality control was ensured by testing the following strains recommended in the CLSI M38-A document: A. flavus ATCC 204304, Candida parapsilosis ATCC 22019, and Candida krusei ATCC 6258.
Statistical methods.
We examined correlations between the MICs of each azole-azole combination by using the Pearson correlation coefficient. SPSS software version 13 (SPSS, Chicago, IL) was used for statistical analyses.
RESULTS AND DISCUSSION
Antifungal susceptibility test results for all four azoles against the 771 isolates of Aspergillus spp. are displayed in Table 1. Posaconazole and voriconazole were the most active agents in vitro, with 98% of isolates inhibited at an MIC of ≤1 μg/ml, while itraconazole was the least active, with 88% of isolates inhibited at an MIC of ≤1 μg/ml.
TABLE 1.
In vitro susceptibilities of 771 isolates of Aspergillus spp. to four azole antifungal agents
| Species (no. of isolates) | Antifungal agent | MIC (μg/ml)
|
Cumulative % of isolates inhibited at MIC (μg/ml) of:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | 50%/90% | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||
| A. fumigatus (553) | Itraconazole | 0.12-2 | 0.5/1 | 0 | 0 | 3 | 17 | 51 | 93 | 100 | ||
| Posaconazole | 0.03-2 | 0.25/0.5 | 4 | 17 | 37 | 79 | 97 | 99 | 100 | |||
| Ravuconazole | 0.06-4 | 0.5/0.5 | 0 | <1 | 3 | 34 | 92 | 99 | >99 | 100 | ||
| Voriconazole | 0.06-4 | 0.25/0.5 | 0 | <1 | 13 | 81 | 98 | >99 | >99 | 100 | ||
| A. flavus (76) | Itraconazole | 0.12-2 | 0.5/1 | 0 | 0 | 1 | 20 | 80 | 99 | 100 | ||
| Posaconazole | 0.06-2 | 0.25/0.5 | 0 | 1 | 25 | 75 | 93 | 97 | 100 | |||
| Ravuconazole | 0.12-2 | 0.5/1 | 0 | 0 | 1 | 12 | 50 | 99 | 100 | |||
| Voriconazole | 0.06-1 | 0.5/1 | 0 | 1 | 4 | 22 | 75 | 100 | ||||
| A. niger (59) | Itraconazole | 0.5->8 | 2/>8 | 0 | 0 | 0 | 0 | 10 | 41 | 76 | 78 | 78 |
| Posaconazole | 0.12-2 | 0.25/1 | 0 | 0 | 19 | 58 | 85 | 92 | 100 | |||
| Ravuconazole | 0.25-8 | 2/4 | 0 | 0 | 0 | 3 | 14 | 46 | 88 | 98 | 100 | |
| Voriconazole | 0.12-2 | 0.5/1 | 0 | 0 | 3 | 12 | 53 | 97 | 100 | |||
| A. terreus (35) | Itraconazole | 0.12-1 | 0.5/0.5 | 0 | 0 | 6 | 34 | 97 | 100 | |||
| Posaconazole | 0.06-0.5 | 0.25/0.25 | 0 | 9 | 49 | 94 | 100 | |||||
| Ravuconazole | 0.03-1 | 0.5/0.5 | 3 | 3 | 9 | 40 | 94 | 100 | ||||
| Voriconazole | 0.06-1 | 0.25/0.5 | 0 | 3 | 6 | 54 | 94 | 100 | ||||
| A. versicolor (24) | Itraconazole | 0.12->8 | 1/2 | 0 | 0 | 4 | 17 | 33 | 67 | 96 | 96 | 96 |
| Posaconazole | 0.06-2 | 0.5/1 | 0 | 8 | 21 | 46 | 75 | 92 | 100 | |||
| Ravuconazole | 0.03-2 | 0.5/1 | 8 | 8 | 25 | 38 | 67 | 96 | 100 | |||
| Voriconazole | 0.03-2 | 0.25/1 | 4 | 8 | 21 | 67 | 75 | 96 | 100 | |||
| A. nidulans (5) | Itraconazole | 0.5-0.5 | 0.5/NAc | 0 | 0 | 0 | 0 | 100 | ||||
| Posaconazole | 0.06-0.25 | 0.12/NA | 0 | 20 | 80 | 100 | ||||||
| Ravuconazole | 0.03-2 | 0.12/NA | 40 | 40 | 80 | 80 | 80 | 80 | 100 | |||
| Voriconazole | 0.03-2 | 0.12/NA | 40 | 40 | 80 | 80 | 80 | 80 | 100 | |||
| A. sydowii (4) | Itraconazole | 0.5-4 | 2/NA | 0 | 0 | 0 | 0 | 25 | 25 | 75 | 100 | |
| Posaconazole | 0.25-1 | 0.25/NA | 0 | 0 | 0 | 50 | 75 | 100 | ||||
| Ravuconazole | 0.25-2 | 0.25/NA | 0 | 0 | 0 | 50 | 50 | 75 | 100 | |||
| Voriconazole | 0.25-2 | 0.25/NA | 0 | 0 | 0 | 50 | 75 | 75 | 100 | |||
| A. calidoustus (A. ustus)a (4) | Itraconazole | 0.12-2 | 2/NA | 0 | 0 | 25 | 25 | 25 | 25 | 100 | ||
| Posaconazole | 0.25-8 | 2/NA | 0 | 0 | 0 | 25 | 25 | 25 | 50 | 75 | 100 | |
| Ravuconazole | 0.25-2 | 1/NA | 0 | 0 | 0 | 25 | 25 | 50 | 100 | |||
| Voriconazole | 0.25-4 | 2/NA | 0 | 0 | 0 | 25 | 25 | 25 | 75 | 100 | ||
| All Aspergillus isolatesb (771) | Itraconazole | 0.12->8 | 0.5/2 | 0 | 0 | 3 | 17 | 53 | 88 | 98 | 98 | 98 |
| Posaconazole | 0.03->8 | 0.25/0.5 | 3 | 13 | 34 | 76 | 95 | 98 | >99 | >99 | >99 | |
| Ravuconazole | 0.03->8 | 0.5/1 | 1 | 1 | 4 | 30 | 80 | 94 | 99 | >99 | >99 | |
| Voriconazole | 0.03->8 | 0.25/0.5 | 1 | 1 | 12 | 67 | 90 | 98 | >99 | >99 | >99 | |
Formerly identified as A. ustus (19).
In addition to the species in the table, the total includes two A. glaucus isolates, one A. oryzae isolate, and one A. niveus isolate.
NA, not applicable.
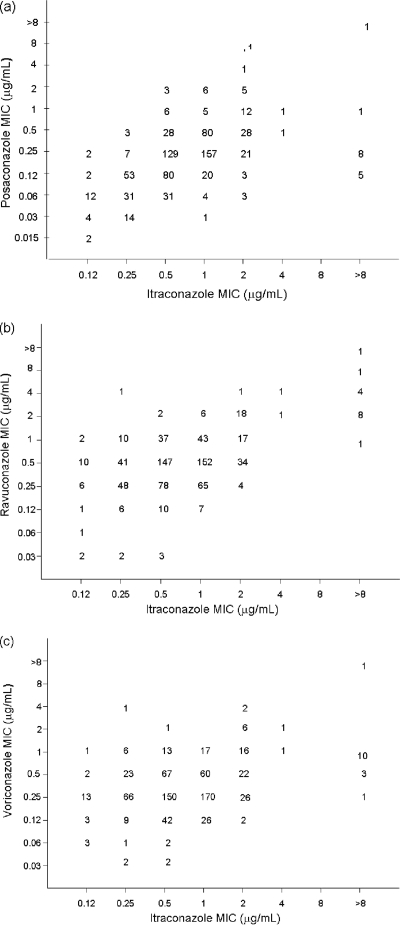
Table 2 details the distributions of MIC50s, MIC90s, and MICs of posaconazole, ravuconazole, and voriconazole with respect to the itraconazole MICs. For each of the newer azoles, the MIC increased as the itraconazole MIC increased. Scattergrams of the relationships between itraconazole and other azole MICs are presented in Fig. 1.
TABLE 2.
In vitro susceptibilities of 771 isolates of Aspergillus spp. to posaconazole, ravuconazole, and voriconazole with respect to itraconazole susceptibility
| Itraconazole category (no. of isolates) | Antifungal agent | MIC (μg/ml)
|
Cumulative % of isolates inhibited at MIC (μg/ml) of:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | 50%/90% | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||
| Susceptible (MIC ≤ 1 μg/ml) (680) | Posaconazole | 0.03-2 | 0.25/0.5 | 3 | 15 | 37 | 81 | 97 | 99 | 100 | ||
| Ravuconazole | 0.03-4 | 0.5/1 | 1 | 1 | 5 | 34 | 85 | 99 | >99 | 100 | ||
| Voriconazole | 0.03-4 | 0.25/0.5 | 1 | 2 | 13 | 72 | 94 | >99 | >99 | 100 | ||
| Intermediate (MIC = 2 μg/ml) (74) | Posaconazole | 0.06-8 | 0.5/1 | 0 | 4 | 8 | 37 | 74 | 91 | 97 | 99 | 100 |
| Ravuconazole | 0.25-4 | 0.5/2 | 0 | 0 | 0 | 5 | 51 | 74 | 99 | 100 | ||
| Voriconazole | 0.12-4 | 0.5/2 | 0 | 0 | 3 | 38 | 68 | 89 | 97 | 100 | ||
| Resistant (MIC ≥ 4 μg/ml) (17) | Posaconazole | 0.12->8 | 0.25/1 | 0 | 0 | 29 | 77 | 82 | 94 | 94 | 94 | 94 |
| Ravuconazole | 1->8 | 2/8 | 0 | 0 | 0 | 0 | 0 | 6 | 59 | 88 | 94 | |
| Voriconazole | 0.25->8 | 1/2 | 0 | 0 | 0 | 6 | 24 | 88 | 94 | 94 | 94 | |
FIG. 1.
Scatterplots of posaconazole (a), ravuconazole (b), and voriconazole (c) MICs versus itraconazole MICs for 771 clinical isolates of Aspergillus spp.
When all 771 isolates were examined, there were statistically significant correlations for all six triazole-triazole pairs (data not shown). Table 3 lists the azole-azole pairs with the most strongly correlated MICs for each of the most common species. For the three most common species, A. fumigatus, A. flavus, and A. niger, the strongest MIC correlations seen were those for ravuconazole and voriconazole and for itraconazole and posaconazole.
TABLE 3.
Strongest species-specific correlations between MICs of four azoles, by species
| Species | No. of isolates tested | Agents for which MICs were correlateda | Pearson correlation coefficient | P value |
|---|---|---|---|---|
| A. fumigatus | 553 | RAV and VOR | 0.72 | <0.001 |
| ITR and POS | 0.41 | <0.001 | ||
| A. flavus | 76 | RAV and VOR | 0.43 | <0.001 |
| ITR and POS | 0.31 | 0.006 | ||
| A. niger | 59 | ITR and RAV | 0.58 | <0.001 |
| RAV and VOR | 0.47 | <0.001 | ||
| A. terreus | 35 | RAV and VOR | 0.55 | 0.001 |
| A. versicolor | 24 | RAV and VOR | 0.90 | <0.001 |
| RAV and POS | 0.77 | <0.001 |
Abbreviations: RAV, ravuconazole; VOR, voriconazole; ITR, itraconazole; POS, posaconazole.
Despite correlations between all azole-azole MICs, when itraconazole-resistant isolates (n = 17) were examined, the MICs of posaconazole and voriconazole for most of these isolates (94 and 88%, respectively) were ≤1 μg/ml. By contrast, the MIC of ravuconazole was ≤1 μg/ml for only 1 (6%) of 17 itraconazole-resistant isolates.
Resistance to the triazole antifungal agents is uncommon among clinical isolates of Aspergillus spp. (6, 15). Most studies concerning mechanisms of azole resistance in Aspergillus have been performed with A. fumigatus and have demonstrated that resistance was associated with the modification of the 14-α sterol demethylase target enzyme (CYP51), specifically, modification corresponding to mutations in the gene cyp51A (1, 2, 6, 9, 11-16). Importantly, mutations resulting in resistance to posaconazole and itraconazole appear to differ from those conferring resistance to voriconazole and ravuconazole (12, 14, 17, 22). Cross-resistance to itraconazole and posaconazole has been associated with amino acid substitutions at glycine 54 (G54) (7, 12, 16, 17), whereas cross-resistance to voriconazole and ravuconazole has been associated with amino acid substitutions at G448 (13, 22). It has been postulated previously, based on results from molecular modeling studies, that a substitution at G54 in the α helix of A. fumigatus CYP51A (AfCYP51A) confers resistance to posaconazole and itraconazole by perturbing the binding of the long side chain in the hydrophobic channel (channel 2) of the enzyme (22). Given that voriconazole and ravuconazole lack a long side chain, substitutions at G54 would be predicted to have no effect on the binding of these compact triazoles to the target. Conversely, substitution near the heme cofactor (e.g., at G448) would disturb the binding of voriconazole and ravuconazole to a greater extent than that of posaconazole and itraconazole due to the stabilizing influence of the side chain present in the latter two agents (22).
Recently, a third pattern of azole resistance in A. fumigatus was reported (7, 12, 16). This new pattern is characterized by high MICs of itraconazole, voriconazole, ravuconazole, and posaconazole. The majority of strains with this phenotype harbor amino acid substitutions at methionine 220 (M220) (13, 17). The role of the M220 substitutions in multiazole resistance was confirmed by Mellado et al., who introduced mutated cyp51A genes into an A. fumigatus wild-type strain, with resulting resistance to all triazole agents (13). They also detected M220 substitutions in five clinical isolates of A. fumigatus that exhibited the multiazole-resistant phenotype. Thus, it appears that the ways in which the various mutations in AfCYP51A impact the susceptibility to specific azoles reflect differences in the ways the azoles interact with the target protein.
An additional mechanism of resistance to azoles in A. fumigatus is reduced intracellular accumulation of the drugs. This mechanism was first demonstrated in laboratory-derived itraconazole-resistant mutants of A. fumigatus in which the reduced accumulation of itraconazole was postulated to be due to either diminished permeability or defects in an energy-dependent uptake process (11). More recently, Nascimento et al. found that in addition to a mutation at G54 in the AfCYP51A target, itraconazole-resistant isolates of A. fumigatus exhibited high-level expression of two genes, AfMDR3 and AfMDR4, which encode drug efflux pumps (16). As in other fungi, it appears that multiple resistance mechanisms in A. fumigatus are involved in resistance to the triazole antifungal agents.
Few studies have investigated azole cross-resistance in more than a handful of clinical Aspergillus isolates (15). A large in vitro survey that included 6,423 clinical isolates and 10 species of Aspergillus demonstrated superior potencies of both posaconazole and voriconazole over that of itraconazole; however, no analysis of cross-resistance was provided (18). In a study of 338 Spanish clinical isolates of Aspergillus spp., Gomez-Lopez et al. (10) demonstrated comparable in vitro activities of itraconazole and voriconazole. Among the 12 isolates for which the itraconazole MIC was ≥4 μg/ml, the voriconazole MIC was ≥2 μg/ml for 8 isolates (MIC range, 2 to 8 μg/ml), suggesting some degree of cross-resistance. Likewise, Cuenca-Estrella et al. (4) compared the activities of itraconazole, posaconazole, and voriconazole against 697 isolates of Aspergillus spp. (seven species) and found all three azoles to be quite active, with posaconazole showing slightly greater potency (geometric mean [GM] MIC = 0.1 μg/ml) than either itraconazole (GM MIC = 0.33 μg/ml) or voriconazole (GM MIC = 0.48 μg/ml). Among nine itraconazole-resistant isolates, posaconazole was active (MIC < 1 μg/ml) against five isolates (55%). Cross-resistance involving all three azoles was observed for isolates of A. fumigatus, A. niger, A. nidulans, and other Aspergillus species. Mosquera and Denning (15) studied 11 isolates of A. fumigatus defined as resistant to itraconazole (MIC > 4 μg/ml) in vitro. They found that elevated itraconazole MICs were uniformly associated with elevations in posaconazole MICs of 4- to 256-fold but that elevated itraconazole MICs were not usually associated with elevated MICs of ravuconazole or voriconazole. The patterns of susceptibilities of the isolates to voriconazole and ravuconazole were similar, suggesting similar modes of action and mechanisms of resistance. These findings suggest considerable heterogeneity in azole susceptibility among isolates of A. fumigatus (15). Most recently, Rodriguez-Tudela et al. examined cross-resistance among 393 A. fumigatus isolates, including 32 that were itraconazole resistant (MIC, >4 μg/ml by the method of the European Committee on Antimicrobial Susceptibility Testing) (17). Their findings confirmed that the cross-resistance pattern depends upon the specific mutation present, with G54 mutants displaying cross-resistance to itraconazole and posaconazole while M220 mutants display cross-resistance to all four azoles tested (17).
Our findings confirm the in vitro potencies of the new and investigational azoles (voriconazole, posaconazole, and ravuconazole) against Aspergillus spp. The excellent in vitro activities of these agents limited our ability to examine the true patterns of cross-resistance. The patterns of in vitro susceptibilities we describe correspond to isolates for which azole MICs span a wide spectrum, with the MICs for most organisms (>98%) falling into a range (≤1 μg/ml) that most would consider to indicate susceptibility to the tested agent. Despite this limitation, we have demonstrated that there is significant correlation among the MICs of all four azoles examined. In addition, we found that the azoles whose MICs for the two most common species groups correlated most strongly were ravuconazole and voriconazole and itraconazole and posaconazole. However, when we examined only those isolates for which the MICs of itraconazole were highest (≥4 μg/ml), we did not find that they were predictably resistant to posaconazole or voriconazole; in fact, the MICs of posaconazole and voriconazole for most of these isolates were ≤1 μg/ml.
Unfortunately, there are no data available to assess the clinical impact of different in vitro resistance profiles among Aspergillus spp. However, the available data on resistance mechanisms and MIC correlations suggest not only that one may see correlations between voriconazole and ravuconazole MICs and itraconazole and posaconazole MICs but also that some resistance mutations may result in resistance to all four agents. At this time, given the fact that individual variations in MICs are not predictable, in vitro testing of the susceptibilities of clinical isolates to each of the triazoles is likely to be useful and may allow one to select the most active agent (15).
Acknowledgments
This study was supported in part by research and educational grants from Pfizer Pharmaceuticals and Schering-Plough Research Institute.
We thank all the participating centers who contributed isolates to this study, a list of which can be found at the following URL: http://www.healthcare.uiowa.edu/pathology/site/faculty/pfaller/artemis_participants.pdf.
Footnotes
Published ahead of print on 18 June 2008.
REFERENCES
- 1.Balashov, S. V., R. Gardiner, S. Park, and D. S. Perlin. 2005. Rapid, high-throughput, multiplex, real-time PCR for identification of mutations in the cyp51A gene of Aspergillus fumigatus that confer resistance to itraconazole. J. Clin. Microbiol. 43214-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, J., H. Li, R. Li, D. Bu, and Z. Wan. 2005. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J. Antimicrob. Chemother. 5531-37. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. CLSI, Wayne, PA.
- 4.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, M. J. Buitrago, A. Monzon, and J. L. Rodriguez-Tudela. 2006. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 50917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannaoui, E., E. Borel, F. Persat, M. F. Monier, M. A. Piens, et al. 1999. In-vivo itraconazole resistance of Aspergillus fumigatus in systemic murine aspergillosis. J. Med. Microbiol. 481087-1093. [DOI] [PubMed] [Google Scholar]
- 6.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 411364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Guerra, T. M., E. Mellado, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2003. A point mutation in the 14α-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 471120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 413623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Effron, G., E. Mellado, A. Gomez-Lopez, L. Alcazar-Fuoli, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2005. Differences in interactions between azole drugs related to modifications in the 14-α sterol demethylase gene (cyp51A) of Aspergillus fumigatus. Antimicrob. Agents Chemother. 492119-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Lopez, A., G. Garcia-Effron, E. Mellado, A. Monzon, J. L. Rodriguez-Tudela, and M. Cuenca-Estrella. 2003. In vitro activities of three licensed antifungal agents against Spanish clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 473085-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manavathu, E. K., J. A. Vazquez, and P. H. Chandrasekar. 1999. Reduced susceptibility in laboratory-selected mutants of Aspergillus fumigatus to itraconazole due to decreased intracellular accumulation of the antifungal agent. Int. J. Antimicrob. Agents 12213-219. [DOI] [PubMed] [Google Scholar]
- 12.Mann, P. A., R. M. Parmegiani, S. Q. Wei, C. A. Mendrick, X. Li, D. Loebenberg, B. DiDomenico, R. S. Hare, S. S. Walker, and P. M. McNicholas. 2003. Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome P450 14α-demethylase. Antimicrob. Agents Chemother. 47577-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellado, E., G. Garcia-Effron, L. Alcazar-Fuoli, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2004. Substitutions at methionine 220 in the 14α-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob. Agents Chemother. 482747-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mellado, E., G. Garcia-Effron, M. J. Buitrago, L. Alcazar-Fuoli, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2005. Targeted gene disruption of the 14-α sterol demethylase (cyp51A) in Aspergillus fumigatus and its role in azole drug susceptibility. Antimicrob. Agents Chemother. 492536-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosquera, J., and D. W. Denning. 2002. Azole cross-resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 46556-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nascimento, A. M., G. H. Goldman, S. Park, S. A. Marras, G. Delmas, U. Oza, K. Lolans, M. N. Dudley, P. A. Mann, and D. S. Perlin. 2003. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob. Agents Chemother. 471719-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Tudela, J. L., L. Alcazar-Fuoli, E. Mellado, A. Alastruey-Izquierdo, A. Monzon, and M. Cuenca-Estrella. 2008. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 522468-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. McNicholas. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 502009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varga, J., J. Houbraken, H. A. Van Der Lee, P. E. Verweij, and R. A. Samson. 2008. Aspergillus calidoustus sp. nov., causative agent of human infections previously assigned to Aspergillus ustus. Eukaryot. Cell 7630-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verweij, P. E., M. Mensink, A. J. Rijs, J. P. Donnelly, J. F. Meis, and D. W. Denning. 1998. In-vitro activities of amphotericin B, itraconazole and voriconazole against 150 clinical and environmental Aspergillus fumigatus isolates. J. Antimicrob. Chemother. 42389-392. [DOI] [PubMed] [Google Scholar]
- 21.Verweij, P. E., D. T. Te Dorsthorst, A. J. Rijs, H. G. De Vries-Hospers, and J. F. Meis. 2002. Nationwide survey of in vitro activities of itraconazole and voriconazole against clinical Aspergillus fumigatus isolates cultured between 1945 and 1998. J. Clin. Microbiol. 402648-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao, L., V. Madison, A. S. Chau, D. Loebenberg, R. E. Palermo, and P. M. McNicholas. 2004. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14α-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob. Agents Chemother. 48568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]