Abstract
The loop-mediated isothermal amplification (LAMP) method was developed to distinguish between the varicella-zoster virus (VZV) vaccine (vOka) strain and wild-type strains. Two single nucleotide polymorphisms (SNPs) (nucleotide [nt] 105705 for VR-1 VZV LAMP and nt 106262 for VR-2 VZV LAMP) located in the open reading frame 62 gene were selected as LAMP targets. Amplified vOka DNA demonstrated a typical ladder pattern; however, no LAMP product was detected in reactions performed with DNAs from other human herpesviruses by either VR-1 VZV LAMP or VR-2 VZV LAMP. This result was confirmed by a turbidity assay. The sensitivities of both VR-1 and VR-2 VZV LAMP determined by either the turbidity assay or agarose gel electrophoresis were 100 copies per reaction. To discriminate the vOka strain from wild-type strains, VR-1 and VR-2 VZV LAMP products were digested with the appropriate restriction enzymes (SacII for VR-1 LAMP and SmaΙ for VR-2 LAMP). The digested products were clearly different in the vOka strain and wild-type strains. To evaluate the utility of the LAMP methods for rapid differentiation, viral DNA (without DNA extraction) in swab samples was directly tested. Wild-type VZV DNA was detected in 20 swab samples by either VR-1 VZV LAMP or VR-2 VZV LAMP. Sequence analysis confirmed the expected SNPs in the LAMP products amplified from the vOka strain and the five wild-type strains.
Varicella (chickenpox) is a common and highly contagious febrile exanthematous disease in childhood that is caused by primary infection with varicella-zoster virus (VZV). VZV can latently infect the dorsal root ganglia after primary infection and reactivate in immunocompromised patients and elderly individuals, resulting in shingles (zoster). Although varicella is generally a benign, self-limiting illness, primary VZV infection can cause severe systemic infection with potentially fatal outcomes in immunocompromised patients. A live attenuated varicella vaccine was originally developed by Takahashi et al. to prevent severe or fatal varicella in immunocompromised children (28). Subsequently, as the VZV Oka vaccine was shown to be safe and effective in healthy children (2, 3, 14), universal varicella immunization was started in 1995 in the United States. Moreover, the Oka vaccine (vOka) strain has been shown to prevent the virus reactivation that causes shingles in older individuals (23). Therefore, the vOka strain is also a current zoster vaccine in the United States.
Adverse reactions to the vaccine are rare and typically mild. The main adverse event following immunization is a skin rash, which occurs in 5% of healthy children (6). Molecular analysis of the VZV genome obtained from vesicular regions in these patients demonstrated that 60% of the viruses were wild-type VZV (26). Similar adverse events have also been demonstrated in recipients of the zoster vaccine (20, 30). Moreover, it has been reported that shingles occurs rarely in vaccinees, which might be caused by reactivation of a dormant vOka strain as an adverse reaction (10, 17). Therefore, if both varicella and zoster vaccines are widely used, distinguishing the vOka strain and the wild-type strain is very important for determining the frequency of VZV vaccine-related complications.
The previous method used to discriminate the vOka and wild-type strains was based on restriction fragment length polymorphism analysis of the viral genomes from isolated viruses (15). This method had limited general clinical use because the virus had to be isolated from the vesicular region. To solve this problem, PCR methods, which detect single nucleotide polymorphism (SNP) mutations in open reading frame 38 (ORF38), ORF54, and ORF62, have been developed to distinguish the vOka and wild-type strains (1, 25). However, it has been shown that SNPs in ORF38 and ORF54 genes can discriminate the vOka strain from all wild-type strains except the Japanese genotype. Only the SNP in the ORF62 gene can differentiate between the vOka strain and all wild-type strains, including the Japanese genotype (7, 8, 18, 19). Recently, real-time PCR-based discrimination methods were introduced by three different institutes (4, 24, 29). Compared to conventional PCR, real-time PCR does not require a post-PCR step to detect the PCR products, reducing the contamination risk and creating a faster and easier detection assay.
Both PCR and real-time PCR require a thermal cycler to amplify target sequences. In particular, real-time PCR requires a special thermal cycler with precision optics that monitor the fluorescence emissions from sample wells. In contrast, the loop-mediated isothermal amplification (LAMP) method can amplify template DNA under isothermal conditions with efficiency and specificity as high as those of a nested double PCR (21). This remarkable ability allows the method to be performed with simple and cost-effective equipment, which is a significant advantage for small hospital laboratories (5, 11, 12, 22, 27, 31). The method can amplify template DNA within 1 h, and when swab samples are immersed in distilled water, LAMP can directly amplify target DNA without DNA extraction (5, 12). This is an additional advantage to establish an easy method to detect viral DNA. Therefore, the purpose of this study was to construct a rapid genotyping method to distinguish between the vOka and wild-type strains using the LAMP method.
MATERIALS AND METHODS
Viruses and samples.
A live attenuated varicella vaccine (Biken; lot BVZ0038), manufactured by the Research Foundation for Microbial Diseases of Osaka University, was used as the vOka strain. The Kawaguchi strain, which was a kind gift from the Foundation for Microbial Diseases of Osaka University, was used as a reference strain for the wild-type virus. Twenty swab samples (sample numbers 1 to 20) were collected from patients clinically diagnosed with varicella. Informed consent was obtained from the parents of all children. Specimens were collected from patients at the outpatient clinic of the Fujita Health University hospital, placed in 1 ml of sterilized water, and stored at −20°C until analysis was performed.
DNA extraction.
Viral DNA was extracted from varicella vaccine fluid and Kawaguchi strain fluid using a Qiaamp blood kit (Qiagen, Chatsworth, CA). The same DNA extraction kit was used to extract DNA from 200 μl of the five swab samples, which were used for sequence analysis. After extraction, the DNA was eluted in 100 μl of buffer and stored at −20°C.
Primer design.
To develop a LAMP method that could discriminate the vOka strain from wild-type strains, two SNPs (nucleotides [nt] 105705 and 106262) located in the ORF62 gene were selected as targets with unique restriction endonuclease sites based on sequence data published by Gomi et al. (8). To amplify the target sequences, including two different SNPs, primers were designed from published sequences (GenBank accession number NC_001348) using Primer Explorer v3 software (Fujitsu, Tokyo, Japan). The location and sequence of each primer in the target DNA are shown in Fig. 1.
FIG. 1.
(A) VR-1 VZV LAMP. The locations and names of target sequences used as primers within the ORF62 gene are shown at the top. The name and sequence of each primer used for LAMP are shown below. B2c, sequence complementary to B2; F1c, sequence complementary to F1; LPFc, sequence complementary to LPF; B3c, sequence complementary to B3; Sequence PCR F2, PCR primer for sequence analysis of the VR-1 VZV LAMP product. (B) VR-2 VZV LAMP. The locations and names of target sequences used as primers within the ORF62 gene are shown at the top. The name and sequence of each primer used for LAMP are shown in below. B2c, sequence complementary to B2; F1c, sequence complementary to F1; LPFc, sequence complementary to LPF; B3c, sequence complementary to B3. Sequence PCR F2, PCR primer for sequence analysis of the VR-2 VZV LAMP product.
LAMP.
Two different LAMP systems were designed: (i) VR-1 VZV LAMP, targeting SNP 105705, and (ii) VR-2 VZV LAMP, targeting SNP 106262. The LAMP reaction was performed using the Loopamp DNA amplification kit (Eiken Chemical Co., Ltd., Tokyo, Japan) according to the manufacturer's instructions. Each LAMP reaction mixture (25 μl) contained 0.6 μM of the FIP and BIP primers, 0.1 μM of each outer primer (F3 primer and B3 primer), 0.3 μM of each loop primer (LPF primer and LPB primer), 12.5 μl of 2× reaction mixture, 1 μl of Bst polymerase, and 5 μl of sample. This mixture was incubated at 65°C for 60 min. The LAMP products were detected by turbidity using LA-200 (Teramecs, Kyoto, Japan). The turbidity cutoff value used to distinguish positive and negative samples was defined as 0.1. After the turbidity was measured, the LAMP products were subjected to electrophoresis on 1.5% agarose gels and visualized under UV light after ethidium bromide staining.
Restriction endonuclease analysis.
In VR-1 VZV LAMP, LAMP products amplified from the vOka strain contained two SacII sites at gene position 105705 and in the loop primer, and LAMP products amplified from the wild-type strain contained a unique restriction site only in the loop primer. In VR-2 VZV LAMP, only the LAMP product amplified from the vOka strain contained a SmaΙ site at gene position 106262. To digest the LAMP product, 2 μl of LAMP product was incubated with the appropriate restriction enzyme (SacII and SmaΙ; New England Biolabs, Ipswich, MA). The VR-1 VZV LAMP product or the VR-2 VZV LAMP product was digested with the appropriate enzyme at 37°C for 60 min or at 25°C for 60 min, respectively. After the LAMP product was digested, each sample was electrophoresed on a 1.5% agarose gel, and the gel was stained with ethidium bromide.
Cloning VZV DNA.
To determine the sensitivity of the VZV LAMP method, a plasmid containing the target DNA sequence was constructed. First, upstream (VZVVR-1CF, 5′-CAAAGCGTGTTCTCTGTCGT-3′, and VZVVR-2CF, 5′-ACAAACACAGGGGTTGTTCG-3′) and downstream (VZVVR-1CB, 5′-CTTCGACCCGTCTTCCTTC-3′, and VZVVR-2CB, 5′-GGACGTGTCCGCTTTGAAC-3′) primers spanning the sequences between the F3 and B3 primers were synthesized. VZV DNA (vOka strain) was amplified with these two primers by conventional PCR. The PCR products were cloned into a pGEM-T vector using the pGEM-T Vector System II (Promega, Madison, WI) following the manufacturer's instructions. The plasmids (pGEMVZVVR-1 and pGEMVZVVR-2) constructed with this system were serially diluted to evaluate the lower detection limit of the LAMP protocol.
Sequencing.
Direct sequencing of purified LAMP products amplified from the vOka strain and five wild-type isolates was performed using the BigDye terminator cycle-sequencing kit and Prism 3100-Avant (Applied Biosystems, Foster City, CA). The LAMP products were purified using a PCR purification kit (Qiagen) and then sequenced. The purified LAMP products were sequenced using F2 primers (VR-1VZVLAMP products, F2 [5′-GTGTTCCGTCGCTCCCCT-3′]; VR-2 VZV LAMP products, F2 [5′-GCGAGTCCTTCTGTGACCGCC-3′]) according to the manufacturer's instructions. The viral sequences were compared using the Clustal W computer programs (DNA Data Bank of Japan).
RESULTS
Specificities and sensitivities of LAMP methods.
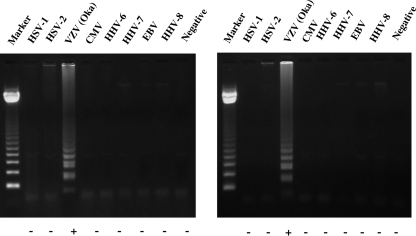
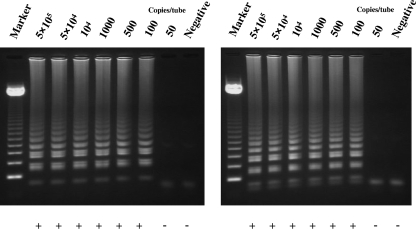
To evaluate the specificities of VR-1 and VR-2 VZV LAMP, each LAMP reaction was performed on DNA extracted from the vOka strain and seven human herpesviruses (herpes simplex virus type 1 [HSV-1] and HSV-2, cytomegalovirus, Epstein-Barr virus, human herpesvirus 6, human herpesvirus 7, and human herpesvirus 8). As shown in Fig. 2, amplified vOka DNA showed a typical ladder pattern. However, no LAMP product was detected in reactions performed with DNAs from other human herpesviruses by either VR-1 VZV LAMP or VR-2 VZV LAMP. This result was confirmed by a turbidity assay (Fig. 2). To determine the sensitivities of the VR-1 and VR-2 VZV LAMP methods, serially diluted plasmids (pGEMVZVVR-1 and pGEMVZVVR-2) containing the target DNAs were used to define the detection limit. The sensitivities of both VR-1 VZV LAMP and VR-2 VZV LAMP determined by either the turbidity assay or agarose gel electrophoresis were 100 copies per reaction (Fig. 3).
FIG. 2.
To determine the specificities of the VR-1 (left) and VR-2 (right) VZV LAMP methods, DNAs from eight human herpesviruses (HSV-1, HSV-2, VZV, Epstein-Barr virus, human cytomegalovirus, human herpesvirus 6, human herpesvirus 7, and human herpesvirus 8) were amplified by the LAMP methods. Detection of the LAMP products was confirmed by 1.5% agarose gel electrophoresis and a turbidity assay (LA-200). Plus (positive) and minus (negative) indicate the results of the turbidity assay. Marker, 123-bp DNA ladder.
FIG. 3.
To determine the sensitivities of the VR-1 (left) and VR-2 (right) VZV LAMP methods, serially diluted plasmids (pGEMVZVVR-1 and pGEMVZVVR-2) containing the target DNAs were amplified by the LAMP methods. Detection of the LAMP products was confirmed by 1.5% agarose gel electrophoresis and a turbidity assay (LA-200). Plus (positive) and minus (negative) indicate the results of the turbidity assay. Marker, 123-bp DNA ladder.
Distinguishing between the vOka strain and the wild-type strain.
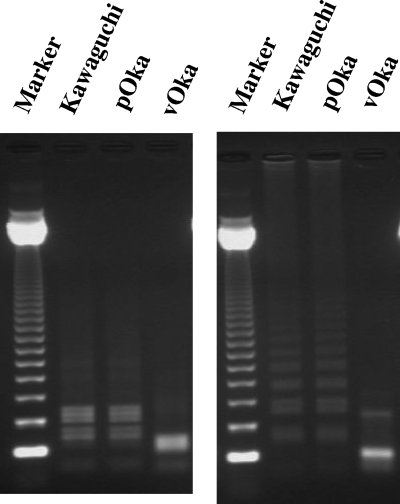
In VR-1 VZV LAMP, products amplified from the vOka strain and the wild-type strain were predicted to have two or one SacII site in the loop primer, respectively. As expected, the typical ladder pattern disappeared in the LAMP products after SacII digestion, and the digested band sizes were clearly different in the vOka strain and the Kawaguchi strain or wild-type isolate (Fig. 4). Meanwhile, in VR-2 LAMP, it was predicted that the LAMP product amplified from the vOka strain, but not the wild-type strain, would have a SmaΙ site. As expected, after SmaI digestion, the typical ladder pattern disappeared in the vOka strain LAMP product but remained in the LAMP products of both the Kawaguchi strain and the wild-type isolate (Fig. 4).
FIG. 4.
To discriminate between the vOka strain and wild-type strains, VR-1 VZV LAMP products (left) and VR-2 VZV LAMP products (right) were digested with SacII and SmaΙ, respectively. The Kawaguchi strain was used as the reference strain for wild-type virus. pOka, parental Oka strain; M, marker (123-bp DNA ladder).
Evaluation of clinical applications.
Because of its high amplification efficiency, the LAMP method has the capacity to amplify viral DNA directly without DNA extraction. In order to evaluate the utility of the LAMP methods for rapid differentiation, viral DNA was directly tested from swab samples. Five microliters each of 20 swab samples was (without DNA extraction) used to amplify VZV DNA by both the VR-1 and VR-2 VZV LAMP methods. LAMP products were directly detected in all 20 swab samples by turbidity assay. Then, the 20 amplified products were used in restriction endonuclease analysis. All clinical samples showed the wild-type digested patterns of both VR-1 and VR-2 VZV LAMP. To confirm the SNPs detected in the LAMP products, LAMP products amplified from the vOka strain and the five wild-type strains were sequenced. As expected, the appropriate SNPs were found in the LAMP products amplified from the vOka strain and the five wild-type strains. The SacII site upstream of the F1 sequence was missing in the wild-type VR-1 VZV LAMP products because the site was replaced by the loop primer sequence (LPF), as shown in Fig. 1A.
DISCUSSION
Genetically discriminating the vOka strain and wild-type VZV has become important because of universal varicella vaccine immunization and general use of the zoster vaccine in the United States. A real-time PCR method was recently developed to distinguish between the strains (11, 12, 23), eliminating the need for a postamplification step. Thus, real-time PCR is currently considered an appropriate method for high-throughput VZV genotyping in central laboratories. However, wild-type VZV or vOka vaccine strains can spread in pediatric wards. Moreover, VZV infection in immunocompromised patients causes atypical clinical features. In these settings, rapid detection and genotyping of VZV is required. Thus, if a small number of samples have to be analyzed immediately to control a hospital infection (9, 16), detecting and genotyping VZV DNA by the LAMP method is an additional option that can easily be performed in each hospital laboratory.
Although both the VR-1 and VR-2 VZV LAMP methods amplified VZV DNA, no amplification was observed with seven other human herpesvirus DNAs (Fig. 2). The detection limits of these two different LAMP methods were 100 copies per reaction, which is higher than that of the previously reported VZV LAMP method (22). Although the turbidity assay is generally less sensitive than agarose gel electrophoresis, the detection limits of the turbidity assay were the same as those of agarose gel electrophoresis in this study. These findings demonstrate that both the VR-1 and VR-2 VZV LAMP methods amplify VZV DNA with specificity and efficiency. To detect viral DNA from the clinical sample, only the turbidity assay should be carried out to save time in the examination. Moreover, as suggested with the HSV LAMP method (5) and the human herpesvirus 6 LAMP method (12), the present study demonstrated that VZV DNA could be directly amplified from swab samples. This is a remarkable advantage for developing an easy and cost-effective genotyping procedure because the DNA extraction step, which is time-consuming and requires a DNA extraction kit, can be avoided. Although we found that the direct LAMP method was slightly less sensitive than the original LAMP method using extracted DNA (5, 12), the direct LAMP method is sufficient to detect VZV DNA in swab samples from vesicular skin regions containing high copy numbers of viral DNA (13).
According to recent sequence analysis of the vOka strain and other, wild-type strains, the SNPs in the ORF62 gene are useful to discriminate between the vOka strain and wild-type strains, including the Japanese genotype (8, 18). Loparev et al. recently demonstrated that four vOka SNPs (nt 105705, 106262, 107252, and 108111) that are located in the ORF62 gene were consistently found in all 21 clinical isolates collected from postvaccination skin rashes caused by the vOka strain (19). Therefore, two (nt 105705 and 106262) of the four SNPs, which are recognized by unique restriction endonucleases (SacII and SmaΙ), were selected for developing the LAMP-based genotyping procedure in this study. In VR-1 VZV LAMP, the SNP is located in the loop primer, making the band size after SacII digestion of the LAMP product difficult to predict. Nonetheless, the patterns of the digested bands were clearly different in the vOka strain and the wild-type strains (Fig. 4). In VR-2 LAMP, although the LAMP products amplified from the wild-type strains were not digested with SmaΙ, the LAMP product amplified from the vOka strain was digested with SmaΙ, producing the predicted band sizes (112, 121, 137, and 139 bp) (Fig. 4). Furthermore, the characteristic SNP was discovered in each LAMP product by sequence analyses. These findings suggest that both the VR-1 VZV LAMP and the VR-2 VZV LAMP methods are reliable for distinguishing the vOka strain and the wild-type strains. Therefore, this LAMP method is a new option for rapid genotyping to discriminate the vOka strain and wild-type strains.
In summary, this study demonstrated that both VR-1 and VR-2 VZV LAMP are reliable methods to distinguish the vOka strain and wild-type strains. Either of the two methods could be used to test the clinical samples. This method is a valuable alternative to VZV genotyping. As the LAMP method can be performed with cost-effective equipment and can amplify VZV DNA directly (without DNA extraction), the method will be useful in small hospital laboratories.
Acknowledgments
We gratefully acknowledge Eiken Chemical for their contributions to this work. We also thank Akiko Yoshikawa and Akemi Miki for their technical support.
This work was supported in part by a grant-in-aid for the 21st Century COE Program of Medicine and the Open Research Center, both at Fujita Health University, from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was also conducted with the support of a grant for Research Promotion of Emerging and Reemerging Infectious Diseases (H18-Shinko-004) from the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Published ahead of print on 11 June 2008.
REFERENCES
- 1.Argaw, T., J. I. Cohen, M. Klutch, K. Lekstrom, T. Yoshikawa, Y. Asano, and P. R. Krause. 2000. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J. Infect. Dis. 1811153-1157. [DOI] [PubMed] [Google Scholar]
- 2.Asano, Y., N. Itakura, Y. Hiroishi, S. Hirose, T. Ozaki, K. Kuno, T. Nagai, T. Yazaki, K. Yamanishi, and M. Takahashi. 1985. Viral replication and immunologic responses in children naturally infected with varicella-zoster virus and in varicella vaccine recipients. J. Infect. Dis. 152863-868. [DOI] [PubMed] [Google Scholar]
- 3.Asano, Y. 1996. Varicella vaccine: the Japanese experience. J. Infect. Dis. 174S310-S313. [DOI] [PubMed] [Google Scholar]
- 4.Campsall, P. A., N. H. Au, J. S. Prendiville, D. P. Speert, R. Tan, and E. E. Thomas. 2004. Detection and genotyping of varicella-zoster virus by TaqMan allelic discrimination real-time PCR. J. Clin. Microbiol. 421409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enomoto, Y., T. Yoshikawa, M. Ihira, S. Akimoto, F. Miyake, C. Usui, S. Suga, K. Suzuki, T. Kawana, Y. Nishiyama, and Y. Asano. 2005. Rapid diagnosis of herpes simplex virus infection by a loop-mediated isothermal amplification method. J. Clin. Microbiol. 43951-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershon, A. A. 1995. Varicella-zoster virus: Prospects for control. Adv. Pediatr. Infect. Dis. 1093-124. [PubMed] [Google Scholar]
- 7.Gomi, Y., T. Imagawa, M. Takahashi, and K. Yamanishi. 2001. Comparison of DNA sequence and transactivation activity of open reading frame 62 of Oka varicella vaccine and its parental viruses. Arch. Virol. Suppl. 1749-56. [DOI] [PubMed] [Google Scholar]
- 8.Gomi, Y., H. Sunamachi, Y. Mori, K. Nagaike, M. Takahashi, and K. Yamanishi. 2002. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J. Virol. 7611447-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossberg, R., R. Harpaz, E. Rubtcova, V. Loparev, J. F. Seward, and D. S. Schmid. 2006. Secondary transmission of varicella vaccine virus in a chronic care facility for children. J. Pediatr. 148842-844. [DOI] [PubMed] [Google Scholar]
- 10.Hardy, I., A. A. Gershon, S. P. Steinberg, P. LaRussa, et al. 1991. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. N. Engl. J. Med. 3251545-1550. [DOI] [PubMed] [Google Scholar]
- 11.Ihira, M., T. Yoshikawa, Y. Enomoto, S. Akimoto, M. Ohashi, S. Suga, N. Nishimura, T. Ozaki, Y. Nishiyama, T. Notomi, Y. Ohta, and Y. Asano. 2004. Rapid diagnosis of human herpesvirus 6 infection by a novel DNA amplification method, loop-mediated isothermal amplification. J. Clin. Microbiol. 42140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihira, M., S. Akimoto, F. Miyake, A. Fujita, K. Sugata, S. Suga, M. Ohashi, N. Nishimura, T. Ozaki, Y. Asano, and T. Yoshikawa. 2007. Direct detection of human herpesvirus 6 DNA in serum by the loop-mediated isothermal amplification method. J. Clin. Virol. 3922-26. [DOI] [PubMed] [Google Scholar]
- 13.Kimura, H., S. Kido, T. Ozaki, N. Tanaka, Y. Ito, R. K. Williams, and T. Morishima. 2000. Comparison of quantitations of viral load in varicella and zoster. J. Clin. Microbiol. 382447-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause, P. R., and D. M. Klinman. 1995. Efficacy, immunogenicity, safety, and use of live attenuated chickenpox vaccine. J. Pediatr. 127518-525. [DOI] [PubMed] [Google Scholar]
- 15.LaRussa, P., O. Lungu, I. Hardy, A. Gershon, S. P. Steinberg, and S. Silverstein. 1992. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J. Virol. 661016-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lässker, U., T. C. Harder, M. Hufnagel, and M. Suttorp. 2002. Rapid molecular discrimination between infection with wild-type varicella-zoster virus and varicella vaccine virus. Infection 30320-322. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence, R., A. A. Gershon, R. Holzman, and S. P. Steinberg. 1988. The risk of zoster after varicella vaccination in children with leukemia. N. Engl. J. Med. 318543-548. [DOI] [PubMed] [Google Scholar]
- 18.Loparev, V. N., A. Gonzalez, M. Deleon-Carnes, G. Tipples, H. Fickenscher, E. G. Torfason, and D. S. Schmid. 2004. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J. Virol. 788349-8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loparev, V. N., E. Rubtcova, J. F. Seward, M. J. Levin, and D. S. Schmid. 2007. DNA sequence variability in isolates recovered from patients with postvaccination rash or herpes zoster caused by Oka varicella vaccine. J. Infect. Dis. 195502-510. [DOI] [PubMed] [Google Scholar]
- 20.Macaladad, N., T. Marcano, M. Guzman, J. Moya, F. Jurado, M. Thompson, C. Meechan, D. Li, K. Schlienger, I. Chan, J. Sadoff, F. Schödel, and J. L. Silber. 2007. Safety and immunogenicity of a zoster vaccine in varicella-zoster virus seronegative and low-seropositive healthy adults. Vaccine 252139-2144. [DOI] [PubMed] [Google Scholar]
- 21.Notomi, T., H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe, N. Amino, and T. Hase. 2000. Loop mediated isothermal amplification of DNA. Nucleic Acids Res. 28e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto, S., T. Yoshikawa, M. Ihira, K. Suzuki, K. Shimokata, Y. Nishiyama, and Y. Asano. 2004. Rapid detection of varicella-zoster virus infection by a loop-mediated isothermal amplification method. J. Med. Virol. 74677-682. [DOI] [PubMed] [Google Scholar]
- 23.Oxman, M. N., M. J. Levin, G. R. Johnson, K. E. Schmader, S. E. Straus, L. D. Gelb, R. D. Arbeit, M. S. Simberkoff, A. A. Gershon, L. E. Davis, A. Weinberg, K. D. Boardman, H. M. Williams, J. H. Zhang, P. N. Peduzzi, C. E. Beisel, V. A. Morrison, J. C. Guatelli, P. A. Brooks, C. A. Kauffman, C. T. Pachucki, K. M. Neuzil, R. F. Betts, P. F. Wright, M. R. Griffin, P. Brunell, N. E. Soto, A. R. Marques, S. K. Keay, R. P. Goodman, D. J. Cotton, J. W. Gnann, Jr., J. Loutit, M. Holodniy, W. A. Keitel, G. E. Crawford, S. S. Yeh, Z. Lobo, J. F. Toney, R. N. Greenberg, P. M. Keller, R. Harbecke, A. R. Hayward, M. R. Irwin, T. C. Kyriakides, C. Y. Chan, I. S. Chan, W. W. Wang, P. W. Annunziato, and J. L. Silber. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 3522271-2284. [DOI] [PubMed] [Google Scholar]
- 24.Parker, S. P., M. Quinlivan, Y. Taha, and J. Breuer. 2006. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J. Clin. Microbiol. 443911-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinlivan, M., A. A. Gershon, S. P. Steinberg, and J. Breuer. 2005. An evaluation of single nucleotide polymorphisms used to differentiate vaccine and wild type strains of varicella-zoster virus. J. Med. Virol. 75174-180. [DOI] [PubMed] [Google Scholar]
- 26.Sharrar, R. G., P. LaRussa, S. A. Galea, S. P. Steinberg, A. R. Sweet, R. M. Keatley, M. E. Wells, W. P. Stephenson, and A. A. Gershon. 2000. The postmarketing safety profile of varicella vaccine. Vaccine 19916-923. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki, R., T. Yoshikawa, M. Ihira, Y. Enomoto, S. Inagaki, K. Matsumoto, K. Kato, K. Kudo, S. Kojima, and Y. Asano. 2006. Development of the loop-mediated isothermal amplification method for rapid detection of cytomegalovirus DNA. J. Virol. Methods 132216-221. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi, M., Y. Okuno, T. Otsuka, J. Osame, and A. Takamizawa. 1975. Development of a live attenuated varicella vaccine. Biken J. 1825-33. [PubMed] [Google Scholar]
- 29.Tang, Y. W., H. T. Allawi, M. DeLeon-Carnes, H. Li, S. P. Day, and D. S. Schmid. 2007. Detection and differentiation of wild-type and vaccine mutant varicella-zoster viruses using an Invader Plus method. J. Clin. Virol. 40129-134. [DOI] [PubMed] [Google Scholar]
- 30.Tyring, S. K., F. Diaz-Mitoma, L. G. Padget, M. Nunez, G. Poland, W. M. Cassidy, N. D. Bundick, J. Li, I. S. Chan, J. E. Stek, and P. W. Annunziato. 2007. Safety and tolerability of a high-potency zoster vaccine in adults ≥50 years of age. Vaccine 251877-1883. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa, T., M. Ihira, S. Akimoto, C. Usui, F. Miyake, S. Suga, Y. Enomoto, R. Suzuki, Y. Nishiyama, and Y. Asano. 2004. Detection of human herpesvirus 7 DNA by loop-mediated isothermal amplification. J. Clin. Microbiol. 421348-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]