Abstract
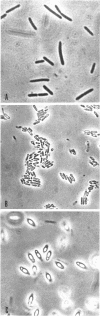
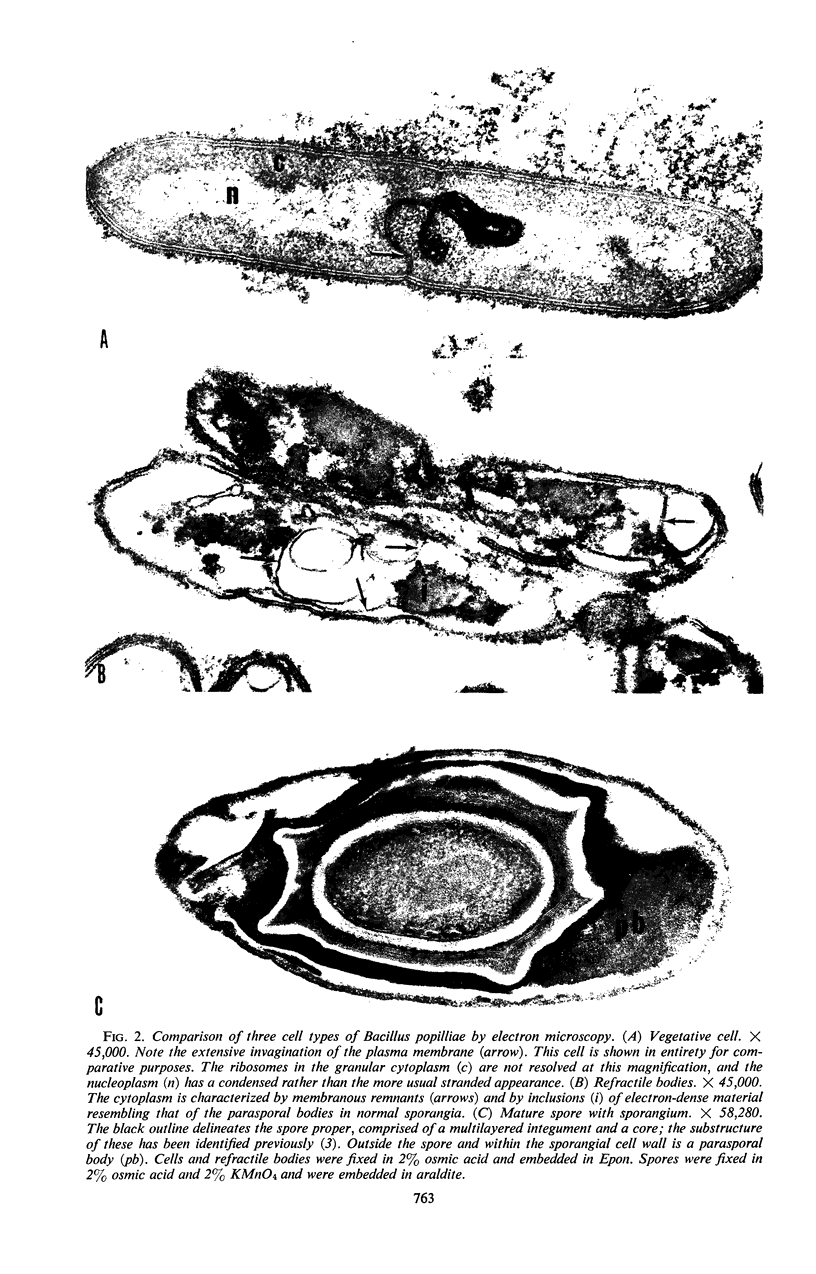
Spores of Bacillus popilliae from infected larvae and refractile bodies produced in a Trypticase-barbiturate medium were similar but distinct from vegetative cells of this organism in protein, nucleic acid, and enzyme composition. The spores and refractile bodies were found to have catalase activity, some of which was heat-resistant. This enzyme was not found in the vegetative cells. The spores contained dipicolinic acid, but the refractile bodies did not. The latter were similar to cells in having considerably higher levels of phosphate extractable with cold trichloroacetic acid and of poly-β-hydroxybutyrate than had the spores. Electron microscopy demonstrated conclusively that the refractile bodies are distinctly different from either cells or spores of B. popilliae. The possibility that these bodies are formed as a result of an aborted sporulation process is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH J. A., SADOFF H. L. Aerobic sporulating bacteria. I. Glucose dehydrogenase of Bacillus cereus. J Bacteriol. 1962 Apr;83:699–707. doi: 10.1128/jb.83.4.699-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., HASHIMOTO T., GERHARDT P. Calcium reversal of the heat susceptibility and dipicolinate deficiency of spores formed "endotrophically" in water. Can J Microbiol. 1960 Apr;6:213–224. doi: 10.1139/m60-023. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S. H., Arredondo M. I. Evidence for an intracytoplasmic membrane in the core of spores of Bacillus popilliae. Experientia. 1966 Feb 15;22(2):77–78. doi: 10.1007/BF01900157. [DOI] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H. Dependence of the heat resistance of bacterial endospores on their dipicolinic acid content. Nature. 1959 Jan 10;183(4654):124–125. doi: 10.1038/183124a0. [DOI] [PubMed] [Google Scholar]
- Costilow R. N., Sylvester C. J., Pepper R. E. Production and stabilization of cells of Bacillus popilliae and Bacillus lentimorbus. Appl Microbiol. 1966 Mar;14(2):161–169. doi: 10.1128/am.14.2.161-169.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R. H., HALVORSON H. Comparison of electron transport systems in vegetative cells and spores of Bacillus cereus. J Bacteriol. 1961 Jan;81:51–58. doi: 10.1128/jb.81.1.51-58.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWLER W. M., SHAW P. D., GOTTLIEB D. TERMINAL OXIDATION IN CELL-FREE EXTRACTS OF FUNGII. J Bacteriol. 1963 Jul;86:9–17. doi: 10.1128/jb.86.1.9-17.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafield F. P., Cooksey K. E., Doudoroff M. beta-Hydroxybutyric dehydrogenase and dimer hydrolase of Pseudomonas lemoignei. J Biol Chem. 1965 Oct;240(10):4023–4028. [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE N. L., HALVORSON H. O. Studies on the spores of aerobic bacteria. IV. A heat resistant catalase from spores of Bacillus terminalis. J Bacteriol. 1954 Sep;68(3):334–337. doi: 10.1128/jb.68.3.334-337.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., SLOAN J. D., Jr, HYATT M. T. Pyrophosphatase activity of Bacillus megaterium spore and vegetative cell extracts. J Bacteriol. 1958 Mar;75(3):291–299. doi: 10.1128/jb.75.3.291-299.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PEPPER R. E., COSTILOW R. N. ELECTRON TRANSPORT IN BACILLUS POPILLIAE. J Bacteriol. 1965 Feb;89:271–276. doi: 10.1128/jb.89.2.271-276.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPPER R. E., COSTILOW R. N. GLUCOSE CATABOLISM BY BACILLUS POPILLIAE AND BACILLUS LENTIMORBUS. J Bacteriol. 1964 Feb;87:303–310. doi: 10.1128/jb.87.2.303-310.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY J. J., FOSTER J. W. Studies on the biosynthesis of dipicolinic acid in spores of Bacillus cereus var. mycoides. J Bacteriol. 1955 Mar;69(3):337–346. doi: 10.1128/jb.69.3.337-346.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., HUNTER J. R. Adenosine deaminase and ribosidase in spores of Bacillus cereus. Biochem J. 1956 Mar;62(3):381–387. doi: 10.1042/bj0620381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes R. A., Roth M. S., Hrubant G. R. Sporulation of bacillus popilliae on solid media. Can J Microbiol. 1965 Oct;11(5):779–783. doi: 10.1139/m65-105. [DOI] [PubMed] [Google Scholar]
- Rhodes R. A. Symposium on microbial insecticides. II. Milky disease of the Japanese Beetle. Bacteriol Rev. 1965 Sep;29(3):373–381. doi: 10.1128/br.29.3.373-381.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPLITTSTOESSER D. F., STEINKRAUS K. H. Factors influencing germination and outgrowth of Bacillus popilliae spores. I. Effect of potassium ions. J Bacteriol. 1962 Aug;84:278–282. doi: 10.1128/jb.84.2.278-282.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUY J. H. The nucleic acids of Bacillus cereus. J Bacteriol. 1958 Aug;76(2):179–184. doi: 10.1128/jb.76.2.179-184.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYLVESTER C. J., COSTILOW R. N. NUTRITIONAL REQUIREMENTS OF BACILLUS POPILLIAE. J Bacteriol. 1964 Jan;87:114–119. doi: 10.1128/jb.87.1.114-119.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L. The effect of gluconate in promoting sporulation in Bacillus cereus. Biochem Biophys Res Commun. 1966 Sep 8;24(5):691–695. doi: 10.1016/0006-291x(66)90379-2. [DOI] [PubMed] [Google Scholar]
- TINELLI R. Etude de la biochimie de la sporulation chez Bacillus megaterium. I. Composition des spores obtenues par carence des différents substrats carbonés. Ann Inst Pasteur (Paris) 1955 Feb;88(2):212–226. [PubMed] [Google Scholar]