Abstract
We report the first case of purulent pericarditis with greenish pericardial effusion caused by Shewanella algae in a patient with gastric and gallbladder cancer. This case expands the reported spectrum of infection caused by S. algae and raises the possibility that S. algae is a causative pathogen for purulent pericarditis.
CASE REPORT
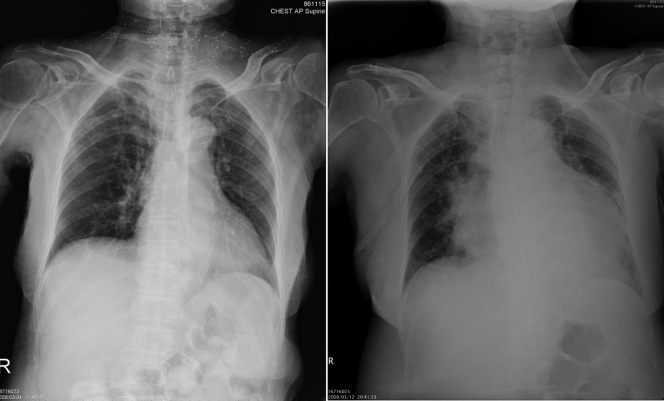
A 76-year-old woman presented with fever and abdominal discomfort for 7 days. She had a history of gastric cancer, for which she had undergone radical gastrectomy, and gallbladder cancer, for which she had undergone cholecystectomy, and had resided in a nursing home for 8 months. On admission, the patient was afebrile and showed stable vital signs: temperature, 36.4°C; pulse rate, 94/min; respiratory rate, 18/min; blood pressure, 122/76 mm Hg. Abdominal examination found no evidence of hepatosplenomegaly or ascites. The jugular vein was not engorged, and there was no peripheral pitting edema. A chronic ulcerative lesion was noted on the lateral aspect of her left ankle. The white blood count was 18,800/mm3 with neutrophil predominance (93%), hemoglobin was 9.9 g/dl, and the platelet count was 273,000/mm3. C-reactive protein was elevated, at 135 mg/dl (reference range, <6 mg/dl). Chest X-ray on admission showed no abnormalities (Fig. 1, left). Empirical antibiotic treatment with flomoxef (1 g every 8 h [q8h]) was prescribed for possible intra-abdominal infection after two sets of blood culture were done. On the eighth hospitalization day, the patient developed dyspnea and hypotension. Tachycardia with cardiac friction rub was noted on auscultation. A follow-up chest X-ray showed marked cardiomegaly with a flask shape (Fig. 1, right). Echocardiography revealed no evidence of infective endocarditis but a massive pericardial effusion with collapsed right ventricle. Emergent pericardiocentesis was performed, and 800 ml greenish pericardial fluid (Fig. 2) was drained out.
FIG. 1.
The initial chest X-ray (left) showed no obvious abnormality on admission. A chest X-ray taken on the eighth hospitalization day (right) showed marked cardiomegaly with a flask shape, indicating the presence of pericardial effusion.
FIG. 2.

Greenish and turbid pericardial fluid aspirated from the patient with Shewanella algae purulent pericarditis.
Two sets of blood culture (Bactec 9240; Becton Dickinson, Sparks, MD) yielded a gram-negative bacillus. The aspirated pericardial fluid was inoculated onto the blood agar plate, eosin methylene blue agar, and chocolate agar (BBL Microbiology Systems, Cockeysville, MD) and subsequently yielded the same organism on these media. The isolate was initially susceptible to tigecycline, cefpirome, ceftazidime, amikacin, trimethoprim-sulfamethoxazole, imipenem, and piperacillin-tazobactam but resistant to ciprofloxacin (as determined by the disk diffusion test). Piperacillin-tazobactam (4.5 g q6h) was given for 10 days, and subsequent pericardial culture showed resistance to piperacillin-tazobactam. The antibiotic was then changed to imipenem (500 mg q6h). The patient was discharged to a general ward on the 16th day of intensive care unit stay and remained well at follow-up 2 weeks later.
Microbiology.
The organism was initially identified as Shewanella putrefaciens by the Phoenix 100 ID/AST (Becton Dickinson) automated identification system. The subsequent positive growth in 6% NaCl was compatible with identification as Shewanella algae but not S. putrefaciens. S. algae exhibited green to orange colonies on the blood agar plate.
This organism was further confirmed as S. algae by the partial 16S rRNA gene sequence analysis (540 bp). In brief, PCR was performed with primers DG74 (5′-AACTGGAGGAAGGTGGGGAT-3′) and RW01 (5′-AGGAGGTGATCCAACCGCA-3′). The amplification products obtained by PCR were sequenced, and the sequences were compared to known 16S rRNA gene sequences in the GenBank database of the National Center for Biotechnology Information by using the BLAST algorithm. The species with the best match was S. algae (accession number EF542799.1).
Discussion.
Gram-negative bacilli and gram-positive cocci such as Streptococcus pneumoniae, Staphylococcus aureus, and Streptococcus pyogenes are the most important causative agents of bacterial pericarditis (7). The most common S. algae infections involve ears and soft tissue, but serious infections such as bacteremia, meningitis, and infective endocarditis have also been described (4). This is the first reported case of a purulent pericarditis due to S. algae.
Shewanella species are gram-negative, facultative anaerobic, nonfermentative bacilli and are present ubiquitously in many foods, sewage, freshwater or stagnant water, seas, lakes, rivers, oil emulsions, natural gas, and petroleum brines (8). Although these organisms have been isolated from a variety of clinical specimens, they are rarely reported as pathogens of human infections (4, 8, 10). S. putrefaciens and S. algae (formerly classified as S. putrefaciens biotype II) are the only two species of Shewanella that have been reported to cause human infections (4). These reported infections included biliary tract infection, empyema thoracis, bacteremia, endocarditis, skin and soft tissue infection, dacryocystitis, intracranial abscess, arthritis, peritonitis, ventilator-associated pneumonia, and ear infection (2, 4, 10). To our best knowledge, S. algae has never been reported as a cause of purulent pericarditis in humans.
Most cases of S. algae in humans were acquired from contaminated water through the disintegrated skin. The bacteria resided in devitalized tissues or denuded skin and served as a nidus for opportunistic infection (4). The portal of entry of the organism in our patient was not easily clarified because of the absence of any recent contact with environmental conditions suitable for propagation of the organism. The organism might have gained access into the pericardium by hematogenous spread via a contaminated cutaneous lesion such as her left ankle wound. In addition, our patient had gastric cancer and gallbladder cancer, and her immunocompromised condition may also have contributed to the development of severe S. algae infection with bacteremia and pericardial involvement.
Most of the Shewanella sp. infections reported in the literature have been attributed to S. putrefaciens because S. algae is not included in the databases of automated identification systems. Although available automated identification systems are not able to differentiate between S. algae and S. putrefaciens, S. algae could be easily differentiated from S. putrefaciens by phenotypic characterization (4). In contrast to S. putrefaciens, S. algae is able to produce mucoid colonies with beta-hemolysis on sheep blood agar, to grow at 42°C and in 6% NaCl, to reduce nitrite, and to produce acid from maltose (5). Phenotypic characterization has revealed that more than 80% of human infections were in fact caused by S. algae (9).
S. algae is characteristically susceptible to aminoglycosides, carbapenems, erythromycin, and quinolones but resistant to penicillin (4). In our patient, S. algae was initially susceptible to piperacillin-tazobactam and became resistant to piperacillin-tazobactam 10 days later. Rapid development of drug resistance to imipenem, but not to piperacillin-tazobactam, has been reported (6). The mechanism for the rapid development of drug resistance to piperacillin-tazobactam required further study. Previous reports (3, 5) showed that most Shewanella infections could be effectively treated by a combination of surgical therapy/drainage and appropriate antibiotics. As in this case, prompt drainage of the pericardial fluid might have contributed to a favorable outcome.
Greenish pleural effusion has been reported in a patient with cholecystopleural fistula (1). However, there was no fistula noted between the pericardium and the biliary system in our case. The reason for the greenish color of pericardial fluid is not clear. The ability of S. algae to reduce Fe(III) might have contributed to the green color, but further study is needed (11).
In summary, we report a case of purulent pericarditis with greenish pericardial effusion caused by S. algae in a patient with gastrointestinal malignancy. This case expands the reported spectrum of infection caused by S. algae and raises the possibility that S. algae is a causative pathogen for purulent pericarditis.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Delcò, F., G. Domenighetti, D. Kauzlaric, D. Donati, and G. Mombelli. 1994. Spontaneous biliothorax (thoracobilia) following cholecystopleural fistula presenting as an acute respiratory insufficiency: successful removal of gallstones from the pleural space. Chest 106961-963. [DOI] [PubMed] [Google Scholar]
- 2.Dhawan, B., R. Chaudhry, B. M. Mishra, and R. Agarwal. 1998. Isolation of Shewanella putrefaciens from a rheumatic heart disease patient with infective endocarditis. J. Clin. Microbiol. 362394. (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez, H., B. F. Vogel, L. Gram, S. Hoffmann, and S. Schaebel. 1996. Shewanella alga bacteremia in two patients with lower leg ulcers. Clin. Infect. Dis. 221036-1039. [DOI] [PubMed] [Google Scholar]
- 4.Holt, H. M., B. Gahrn-Hansen, and B. Bruun. 2005. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin. Microbiol. Infect. 11347-352. [DOI] [PubMed] [Google Scholar]
- 5.Holt, H. M., P. Sogarrd, and B. Gahrn-Hansen. 1997. Ear infections with Shewanella algae. A bacteriologic, clinical and epidemiology study of 67 cases. Clin. Microbiol. Infect. 3329-334. [DOI] [PubMed] [Google Scholar]
- 6.Kim, D. M., C. I. Kang, C. S. Lee, H. B. Kim, E. C. Kim, N. J. Kim, M. D. Oh, and K. W. Choe. 2006. Treatment failure due to emergence of resistance to carbapenem during therapy for Shewanella algae bacteremia. J. Clin. Microbiol. 441172-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klacsmann, P. G., B. H. Bulkley, and G. M. Hutchins. 1977. The changed spectrum of purulent pericarditis: an 86 year autopsy experience in 200 patients. Am. J. Med. 63666-673. [DOI] [PubMed] [Google Scholar]
- 8.Kueh, C. S. W., P. Kutarski, and M. Brunton. 1992. Contaminated marine wounds: the risk of acquiring acute bacterial infection from marine recreational beaches. J. Appl. Bacteriol. 73412-420. [DOI] [PubMed] [Google Scholar]
- 9.Nozue, H., T. Hayashi, Y. Hashimoto, T. Ezaki, K. Hamasaki, K. Ohwada, and Y. Terawaki. 1992. Isolation and characterization of Shewanella algae from human clinical specimens and emendation of the description of S. alga Simidu et al., 1990, 335. Int. J. Syst. Bacteriol. 42628-634. [DOI] [PubMed] [Google Scholar]
- 10.Tsai, T. H., and H. Y. You. 2006. Necrotizing fasciitis caused by Shewanella putrefaciens in a uremic patient. J. Microbiol. Immunol. Infect. 39516-518. [PubMed] [Google Scholar]
- 11.Turick, C. E., F. Caccavo, Jr., and L. S. Tisa. 2003. Electron transfer from Shewanella algae BrY to hydrous ferric oxide is mediated by cell-associated melanin. FEMS. Microbiol. Lett. 22099-104. [DOI] [PubMed] [Google Scholar]