Abstract
Transport media should preserve the viability and stability of microorganisms in clinical specimens. In this study, the Port-A-Cul transport system and the Copan transport system without charcoal, both designed to preserve anaerobes, were evaluated. Dacron swabs were inoculated with two combinations of facultative and anaerobic organisms typically found in vaginal swab samples. Combination I contained Candida albicans, Escherichia coli, Enterococcus spp., group B streptococci, Lactobacillus crispatus, and Staphylococcus aureus. Combination II contained Lactobacillus iners, Peptoniphilus asaccharolyticus, Mycoplasma hominis, Prevotella bivia, Prevotella corporis, Porphyromonas asaccharolytica, Mobiluncus curtisii, Peptostreptococcus anaerobius, and Gardnerella vaginalis. Duplicate swabs were placed into the two transporters and held for 24, 48, 72, and 96 h at 4 and 24°C. Both transporters maintained the viability of organisms better at 4°C than at 24°C. Prevotella bivia and Prevotella corporis had a loss of viability in both transporters at both temperatures. However, at 24°C, there was a significantly greater loss of viability for Mycoplasma hominis, Prevotella bivia, Prevotella corporis, and Peptoniphilus asaccharolyticus when the organisms were stored in Copan transport medium than when they were stored in Port-A-Cul transport medium for 96 h (P < 0.002). Some organisms proliferated in the transport media, but when transporters were held at 24°C for 96 h, a significantly greater increase in the concentrations of group B streptococci and Candida albicans, Escherichia coli, and Enterococcus spp. organisms in Copan medium than in Port-A-Cul medium was observed (P < 0.002). At room temperature, the Port-A-Cul system is superior to the Copan system with respect to the preservation of fastidious microorganisms and the prevention of the proliferation of facultative organisms.
The ecosystem of the vagina is a complex mixture of fastidious anaerobes, nonfastidious aerobes, and genital mycoplasmas (1, 5-7, 9, 14, 16). Therefore, vaginal swab samples are also microbiologically complex. Due to the increased utilization of centralized laboratories, specimens may be in transit for time periods greater than 24 h, and during that time they may be exposed to various temperature conditions. Factors which may influence organism survival include deviations in temperature, interactions among microbes in the transport medium, prolonged transport time, and the type of transport medium that is used. It has been reported previously that there is a loss of viability observed during transport, with the degree of loss dependent on the microorganism, the transport system, and the temperature (2-4, 8, 10, 15, 18, 19, 21-23). An ideal transport system maintains the viability of fastidious organisms during transport without allowing the overgrowth of bacteria such as coliforms. Few studies have evaluated the survival of fastidious organisms in mixtures or clinical samples (2, 3, 4), and few have evaluated transport times greater than 24 h (3, 4, 15, 19, 21).
The objective of this study was to compare two transport systems for their capacities to preserve the viability of microorganisms and the stability of microorganism quantities during transport for up to 96 h when there were several organisms in combination on a single swab. Two marketed products labeled for the preservation of anaerobes in clinical specimens were evaluated. The Port-A-Cul transport system (Becton Dickinson and Co., Sparks, MD) and the Copan transport system without charcoal (Thermo-Fisher, Waltham, MA) were subjected to conditions that mimic those potentially encountered during transport to determine whether they had similar performance characteristics.
(A portion of this work was presented at the 105th General Meeting of the American Society for Microbiology, Atlanta, GA, 1 to 5 June 2005.)
MATERIALS AND METHODS
A total of 15 organisms were used to evaluate the integrity of the Port-A-Cul and Copan transport systems in maintaining the quantities and viability of organisms. The transporters were held for 0, 24, 48, 72, and 96 h at 4 and 24°C. The organisms selected represent common vaginal isolates. Isolates from the American Type Culture Collection (ATCC; Manassas, VA) and the Culture Collection of the University of Göteborg (CCUG; Göteborg, Sweden) were used, along with four clinical isolates of each organism (except Mycoplasma hominis, Prevotella bivia, and Prevotella corporis, of which only three clinical isolates each were used). Strains included were Candida albicans ATCC 10231, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, group B Streptococcus sp. strain ATCC 13813, Lactobacillus crispatus ATCC 33197, Staphylococcus aureus ATCC 25923, Lactobacillus iners CCUG 28746, Gardnerella vaginalis ATCC 14018, Mycoplasma hominis ATCC 23114, Prevotella bivia ATCC 29303, Prevotella corporis ATCC 33574, Porphyromonas asaccharolytica ATCC 25260, Mobiluncus curtisii ATCC 35241, Peptostreptococcus anaerobius ATCC 27337, and Peptoniphilus asaccharolyticus ATCC 14963. The identification of clinical isolates of Candida albicans, Enterococcus spp., Staphylococcus aureus, Escherichia coli, group B streptococci, and Gardnerella vaginalis (13) and the identification methods used for the anaerobic gram-positive and gram-negative rods and Mycoplasma and Lactobacillus species (1, 11, 12, 17, 20) were described previously. The strains of Enterococcus were identified only to the genus level and are referred to as Enterococcus spp. Each reference strain and clinical isolate was held at −80°C in litmus milk (Becton Dickinson and Co.). Mycoplasma hominis was stored in a Mycoplasma broth without antibiotics, prepared in-house, at −80°C. Before transport survival studies, all isolates were thawed and subcultured twice to ensure purity.
A fresh subculture of each organism was suspended in sterile phosphate-buffered saline to a concentration of 108 CFU/ml by using a 0.5 McFarland standard (PML Microbiologicals, Wilsonville, OR) as a guide. The concentrations were confirmed by counting colonies on plates inoculated with serial dilutions of the suspensions. From these suspensions, the organisms were combined into two groups in the following quantities. Group I contained 103 CFU/ml each of Candida albicans and Lactobacillus crispatus and 105 CFU/ml each of Enterococcus spp., group B Streptococcus, Escherichia coli, and Staphylococcus aureus. The low number of Candida albicans cells represents what is typically found in asymptomatic women of reproductive age, and Lactobacillus crispatus can be detected at levels of <105 CFU/ml among women with bacterial vaginosis (unpublished data). Group II contained 105 CFU/ml each of Lactobacillus iners, Gardnerella vaginalis, Mycoplasma hominis, Prevotella bivia, Prevotella corporis, Porphyromonas asaccharolytica, Mobiluncus curtisii, Peptostreptococcus anaerobius, and Peptoniphilus asaccharolyticus. From each mixture, 100-μl aliquots of the suspension were transferred onto 10 Dacron swabs (Thermo-Fisher) and onto 10 nylon swabs prepackaged with the Copan transporters. The Dacron swabs were inserted into the Port-A-Cul transporters, and the nylon swabs were inserted into the Copan transporters. After all of the swabs had been inoculated, serial dilutions of each mixture were prepared and these dilutions were cultured to ensure that all of the organisms were viable and present in the appropriate concentrations.
Five replicates of each type of transporter were held at 4 and 24°C in the dark. Samples from one Port-A-Cul transporter and one Copan transporter from each temperature condition were serially diluted 1:10 in phosphate-buffered saline and inoculated onto Columbia agar with 5% sheep blood (PML Microbiologicals), Brucella agar with 5% sheep blood (PML Microbiologicals), laked blood agar with kanamycin and vancomycin (PML Microbiologicals), human bilayer Tween (Becton-Dickenson), and an A-8 agar (8) and Mycoplasma broth without antibiotics, both prepared in house, at 0, 24, 48, 72, and 96 h. The media were then incubated at 37°C in 5 to 7% CO2 for 48 h or at 37°C under anaerobic conditions for 120 h. All recovered organisms were compared to those on reference plates for colony morphology and reidentified by the methods described previously.
Statistical analyses were performed using SPSS statistical software release 14.0.1 (SPSS Inc., Chicago, IL), and statistical tests were evaluated at the 0.05 two-sided significance level. Student's t tests were used to evaluate differences in the mean log change in concentrations of microorganisms from the baseline to 24 h between transport media and between transport temperatures. Analysis of variance (ANOVA) was used to evaluate the effects of transport media and time on the mean log concentrations of microorganisms at each transport temperature.
RESULTS
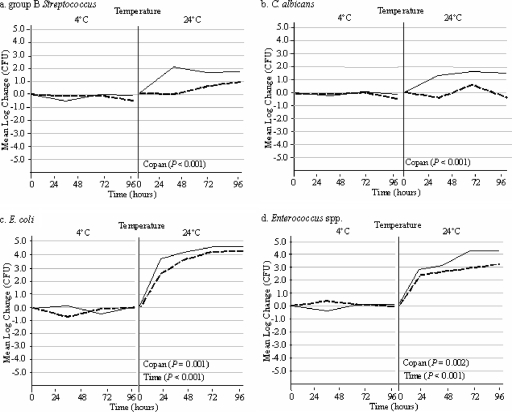
The stability profiles of group B streptococci, Candida albicans, Escherichia coli, and Enterococcus spp. held at 4 or 24°C in Port-A-Cul and Copan transport media are shown in Fig. 1. At 4°C, all four organisms were stable in both media for up to 96 h, suggesting that the prolonged transport of samples may be acceptable when these organisms are being targeted. However, when the transport media were held at room temperature, a 1/2-log increase in group B streptococci in Port-A-Cul medium after 48 h was observed, while there was a 2-log increase in group B streptococci in the Copan transport system after 48 h (Fig. 1a). Candida albicans proliferated in the Copan transport medium at 24°C but not in the Port-A-Cul medium (Fig. 1b). Escherichia coli and Enterococcus spp. concentrations had both increased by 2 logs in both Port-A-Cul and Copan transport media at 24 h and continued to increase over the 96-h holding period, suggesting that these bacteria may proliferate during transport when specimens containing them are transported at room temperature (Fig. 1c and d). As shown in Fig. 1, all four organisms were detected at higher concentrations in the Copan medium than in the Port-A-Cul transport medium (P < 0.002).
FIG. 1.
Side-by-side comparison of organisms held in Port-A-Cul and Copan transporters at 4 and 24°C over a 96-h time period. Copan P values indicate a significant increase in organism concentrations in Copan transporters (solid lines) compared to those in Port-A-Cul transporters (dashed lines) over 96 h. Time P values indicate a significant increase in organism concentrations compared to the starting concentrations in both transporters over 96 h. P values were calculated using ANOVA.
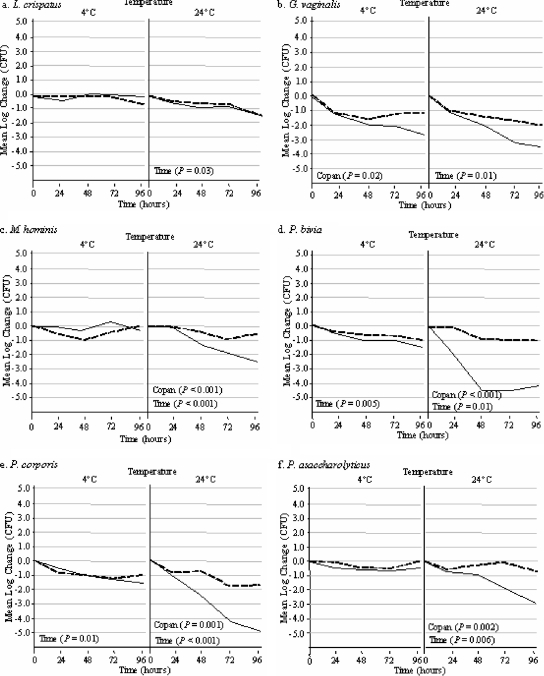
The rates of survival of six nutritionally fastidious and oxygen-sensitive organisms in the Port-A-Cul system versus the Copan system over 96 h are illustrated in Fig. 2. At 4°C, there was no significant difference in the numbers of viable organisms detected over 96 h in the Port-A-Cul and Copan transporters, with the exception of Gardnerella vaginalis (Fig. 2b), which had a lower survival rate in the Copan system than in the Port-A-Cul system (P = 0.02). As shown in Fig. 2, all six microorganisms had a significant decrease in viability over 96 h at room temperature (P < 0.05 for each). However, there was a significantly greater loss of bacterial viability for organisms stored in Copan medium than for those stored in Port-A-Cul medium for Mycoplasma hominis (Fig. 2c), Prevotella bivia (Fig. 2d), Prevotella corporis (Fig. 2e), and Peptoniphilus asaccharolyticus (Fig. 2f) (P < 0.002 for each comparison).
FIG. 2.
Side-by-side comparison of organisms held in Port-A-Cul and Copan transporters at 4 and 24°C over a 96-h time period. Copan P values indicate a significant decrease in organism concentrations in Copan transporters (solid lines) compared to those in Port-A-Cul transporters (dashed lines) over 96 h. Time P values indicate a significant decrease in organism concentrations compared to the starting concentrations in both transporters over 96 h. P values were calculated using ANOVA.
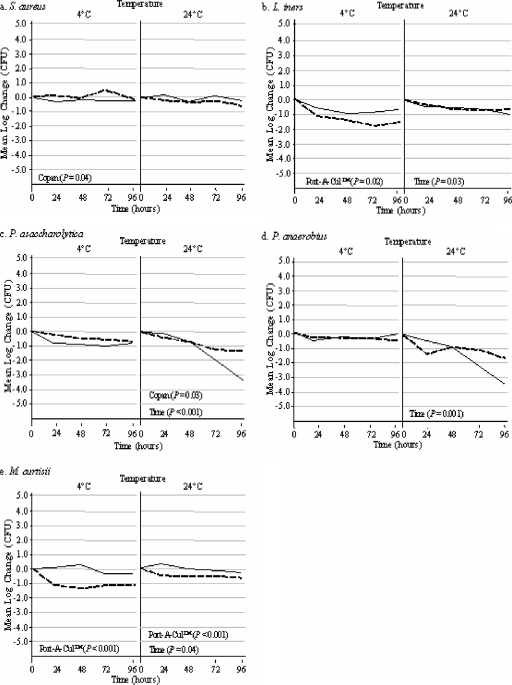
The results of comparisons for the remaining five organisms in the Port-A-Cul system versus the Copan system over 96 h are displayed in Fig. 3. At 4°C, Staphylococcus aureus (Fig. 3a) had a slightly lower survival rate in the Copan medium than in the Port-A-Cul medium (P = 0.04); however, in both transport media, the fluctuation in the concentration was less than 1 log. The concentrations of Staphylococcus aureus cells in both transporters at room temperature remained stable for up to 96 h. The Lactobacillus iners and Mobiluncus curtisii concentrations were significantly less stable in Port-A-Cul medium than in Copan medium at 4°C (P < 0.02), with both organisms having a loss of viability corresponding to greater than 1 log in the Port-A-Cul system versus the Copan system. Porphyromonas asaccharolytica and Peptostreptococcus anaerobius concentrations in both transporters remained stable at 4°C; however, at 24°C, the concentrations of both organisms decreased significantly over time (P < 0.001), with a significantly greater loss of Porphyromonas asaccharolytica viability in the Copan system than in the Port-A-Cul system (P = 0.03). The data shown in Fig. 2 and 3 indicate that 4°C was optimal for the preservation of the viability of the fastidious organisms, with the exception of Mobiluncus curtisii and Lactobacillus iners, which had better stability at 24°C in both transporters.
FIG. 3.
Side-by-side comparison of organisms held in Port-A-Cul and Copan transporters at 4 and 24°C over a 96-h time period. Copan P values indicate a significant decrease in organism concentrations in Copan transporters (solid lines) compared to those in Port-A-Cul transporters (dashed lines) over 96 h. Port-A-Cul P values indicate a significant decrease in organism concentrations in Port-A-Cul transporters compared to those in Copan transporters over 96 h. Time P values indicate a significant decrease in organism concentrations compared to the starting concentrations in both transporters over 96 h. P values were calculated using ANOVA.
A summary of the log increase or decrease in viable cells for all 15 organisms at 24 h is presented in Table 1. If the Port-A-Cul system is used for specimen transport, a temperature of 4°C is recommended since it prevents the overgrowth of Escherichia coli and Enterococcus spp. and preserves the quantities of the fastidious organisms. The Copan system at room temperature was permissive of the proliferation of Candida albicans, Escherichia coli, Enterococcus spp., and group B streptococci, while the Port-A-Cul system at 24°C was permissive of the proliferation of Escherichia coli and Enterococcus spp. (Table 1). Overall, these data suggest that genital swab specimens should be transported at 4°C in either Copan or Port-A-Cul transporters. When transporters were maintained at 4°C, there were no statistically significant differences in the numbers of viable organisms at 24 h between the two media, with the exception of the Mobiluncus curtisii concentrations, which decreased by 1 log in the Port-A-Cul system and remained stable in the Copan system (P = 0.03). However, if the transporters were held at room temperature for 24 h, the Port-A-Cul transporter was superior to the Copan transporter with respect to preventing the proliferation of Candida albicans, Escherichia coli, and group B streptococci (P = 0.04).
TABLE 1.
Comparisons of results for Port-A-Cul transporters at 4 and 24°C, Copan transporters at 4 and 24°C, and Port-A-Cul and Copan transporters at 4°C at 24 h
| Microorganism(s) | Mean changea in concn (log CFU/ml) (range) in Port-A-Cul transporters at:
|
P valueb | Mean changea in concn (log CFU/ml) (range) in Copan transporters at:
|
P valueb |
P valueb for comparison of:
|
|||
|---|---|---|---|---|---|---|---|---|
| 4°C | 24°C | 4°C | 24°C | Port-A-Cul and Copan transporters at 4°C | Port-A-Cul and Copan transporters at 24°C | |||
| Candida albicans | −0.04 (−0.60-0.90) | 0.08 (−0.80-0.90) | 0.8 | 0.10 (−0.70-1.00) | 1.10 (0.20-1.60) | 0.03 | 0.7 | 0.04 |
| Escherichia coli | −0.14 (−0.30-0.00) | 2.38 (2.00-2.80) | <0.001 | −0.20 (−0.70-0.10) | 3.62 (2.80-4.50) | <0.001 | 0.7 | 0.04 |
| Enterococcus spp. | 0.10 (−0.30-0.90) | 2.35 (1.70-3.30) | <0.001 | −0.12 (−0.30-0.10) | 2.78 (2.30-3.10) | <0.001 | 0.4 | 0.3 |
| Group B streptococci | −0.14 (−0.30-0.00) | 0.55 (−0.20-1.30) | 0.07 | −0.10 (−0.70-0.10) | 1.37 (1.00-2.00) | <0.001 | 0.8 | 0.04 |
| Lactobacillus crispatus | −0.43 (−0.90-0.00) | −0.63 (−1.30-0.10) | 0.5 | −0.41 (−1.00-0.30) | −0.48 (−1.00-0.10) | 0.8 | 0.9 | 0.6 |
| Staphylococcus aureus | 0.10 (−0.80-1.00) | −0.21 (−1.00-0.50) | 0.4 | −0.24 (−1.00-0.80) | 0.17 (−0.50-0.90) | 0.3 | 0.5 | 0.3 |
| Lactobacillus iners | −1.08 (−2.10-0.20) | −0.28 (−1.10-0.10) | 0.1 | −0.44 (−0.80-0.00) | −0.31 (−0.70-0.10) | 0.6 | 0.2 | 0.9 |
| Gardnerella vaginalis | −1.19 (−2.00-−0.10) | −1.03 (−2.00-−0.30) | 0.7 | −1.40 (−2.60-−0.20) | −1.04 (−2.00-0.20) | 0.5 | 0.7 | 0.98 |
| Mycoplasma hominis | −0.37 (−1.00-0.00) | 0.02 (−0.30-0.30) | 0.2 | 0.00 (−0.20-0.20) | −0.10 (−0.30-0.20) | 0.5 | 0.2 | 0.5 |
| Prevotella bivia | −0.21 (−0.70-0.20) | −0.07 (−0.20-0.00) | 0.5 | −0.25 (−1.80-0.00) | −1.5 (−3.00-0.50) | 0.2 | 0.9 | 0.2 |
| Prevotella corporis | −0.73 (−1.00-0.00) | −0.73 (−1.00-0.10) | 0.99 | −0.35 (−0.70-0.10) | −0.70 (−1.30-0.10) | 0.4 | 0.1 | 0.95 |
| Porphyromonas asaccharolytica | −0.18 (−0.80-0.30) | −0.35 (−0.80-0.00) | 0.5 | −0.83 (−2.60-0.30) | −0.08 (−0.80-1.00) | 0.3 | 0.3 | 0.5 |
| Peptostreptococcus anaerobius | −0.20 (−0.90-0.10) | −1.3 (−2.30-−0.30) | 0.02 | −0.36 (−1.00-0.00) | −0.36 (−1.00-0.80) | 1.00 | 0.5 | 0.07 |
| Peptoniphilus asaccharolyticus | −0.14 (−0.30-0.00) | −0.28 (−0.70-0.00) | 0.4 | −0.46 (−0.80-0.00) | −0.43 (−0.80-0.00) | 0.9 | 0.1 | 0.5 |
| Mobiluncus curtisii | −1.04 (−1.80-0.40) | −0.43 (−1.00-0.00) | 0.2 | 0.08 (−0.40-0.50) | 0.28 (0.00-0.60) | 0.3 | 0.03 | 0.01 |
All values represent the changes in the log concentration from the baseline.
P values are from Student's t tests.
DISCUSSION
Both Port-A-Cul and Copan transporters were effective in maintaining organism viability and preventing organism overgrowth at 4°C. This study was unique in that mixtures of organisms were created to replicate genital samples and the impact on survival was evaluated at two temperatures and over a period of up to 96 h. There are, however, a few caveats. Both transporters maintained colony counts better when held at 4°C than when held at 24°C. This finding was true when the transporters were held for as short a period as 24 h or as long a period as 96 h. We chose not to evaluate organism survival when transporters were held at 37°C because the loss of viability at this temperature has been reported previously (18, 21, 23). Vaginal samples are optimally processed within 24 h of collection, but delays can occur during transport. Our data suggest that if the transporters are shipped at room temperature, Port-A-Cul transporters are a better choice due to their capacity to maintain more stable colony counts at a temperature of 24°C than those maintained by Copan transporters. The formula for the medium used in the Port-A-Cul system is proprietary, and therefore, we are unable to determine if there is a difference due to specific components of the media. There was a fairly constant decline of all the anaerobes in both transporters beginning in as little as 24 h. This result is due most likely to the sensitivity of anaerobes to oxygen and would likely occur in any of the commercially available transport systems. In order to achieve the best survival and the most accurate estimates of colonization density, transporters should always be shipped at temperatures that are as close to 4°C as possible. These data suggest that the results of culture-based studies of the vaginal microflora may be influenced by the type of transport medium selected and the conditions under which the specimens are transported.
Acknowledgments
This work was supported by a grant from the NIH, 5U01AI068633.
None of the authors have a commercial or other association that may pose a conflict of interest.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Antonio, M. A. D., L. K. Rabe, and S. L. Hillier. 2005. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J. Infect. Dis. 192394-398. [DOI] [PubMed] [Google Scholar]
- 2.Baron, E. J., M. L. Väisänen, M. McTeague, C. A. Strong, D. Norman, and S. M. Finegold. 1993. Comparison of the Accu-CulShure system and a swab placed in a B-D Port-a-Cul tube for specimen collection and transport. Clin. Infect. Dis. 16(Suppl. 4)S325-S327. [DOI] [PubMed] [Google Scholar]
- 3.Brook, I. 1987. Comparison of two transport systems for recovery of aerobic and anaerobic bacteria from abscesses. J. Clin. Microbiol. 252020-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citron, D. M., Y. A. Warren, M. K. Hudspeth, and E. J. C. Goldstein. 2000. Survival of aerobic and anaerobic bacteria in purulent clinical specimens maintained in the Copan Venturi Transystem and Becton Dickinson Port-a-Cul transport systems. J. Clin. Microbiol. 38892-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredricks, D. N., T. L. Fiedler, and J. M. Marrazzo. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 3531899-1911. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg, R. L., M. A. Klebanoff, R. Nugent, M. A. Krohn, S. L. Hillier, and W. W. Andrews. 1996. Bacterial colonization of the vagina during pregnancy in four ethnic groups. Am. J. Obstet. Gynecol. 1741618-1621. [DOI] [PubMed] [Google Scholar]
- 7.Hillier, S. L., M. A. Krohn, L. K. Rabe, S. J. Klebanoff, and D. A. Eschenbach. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin. Infect. Dis. 16(Suppl. 4)S273-S281. [DOI] [PubMed] [Google Scholar]
- 8.Hindiyeh, M., V. Acevedo, and K. C. Carroll. 2001. Comparison of three transport systems (Starplex StarSwabII, the new Copan Vi-Pak Amies Agar Gel collection and transport swabs, and BBL Port-A-Cul) for maintenance of anaerobic and fastidious aerobic organisms. J. Clin. Microbiol. 39377-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst, E., B. Wathne, B. Hovelius, and P.-A. Mårdh. 1987. Bacterial vaginosis: microbiological and clinical findings. Eur. J. Clin. Microbiol. 6536-541. [DOI] [PubMed] [Google Scholar]
- 10.Human, R. P., and G. A. Jones. 2004. Evaluation of swab transport systems against a published standard. J. Clin. Pathol. 57762-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moncla, B. J., P. Braham, L. K. Rabe, and S. L. Hillier. 1991. Rapid presumptive identification of black-pigmented gram-negative anaerobic bacteria by using 4-methylumbelliferyl derivatives. J. Clin. Microbiol. 291955-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moncla, B. J., and S. L. Hillier. 2003. Peptostreptococcus, Propionibacterium, Lactobacillus, Actinomyces, and other non-spore-forming anaerobic gram positive bacteria, p. 857-877. In P. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 13.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.). 2007. Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 14.Ness, R. B., S. L. Hillier, H. E. Richter, D. E. Soper, C. Stamm, J. McGregor, D. C. Bass, R. L. Sweet, and P. Rice. 2002. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet. Gynecol. 100765-772. [DOI] [PubMed] [Google Scholar]
- 15.Perry, J. L. 1997. Assessment of swab transport systems for aerobic and anaerobic organism recovery. J. Clin. Microbiol. 351269-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puapermpoonsiri, S., N. Kato, K. Watanabe, K. Ueno, C. Chongsomchai, and P. Lumbiganon. 1996. Vaginal microflora associated with bacterial vaginosis in Japanese and Thai pregnant women. Clin. Infect. Dis. 23748-752. [DOI] [PubMed] [Google Scholar]
- 17.Rabe, L. K., D. Sheiness, and S. L. Hillier. 1995. Comparison of the use of oligonucleotide probes, 4-methylumbelliferyl derivatives, and conventional methods for identifying Prevotella bivia. Clin. Infect. Dis. 20(Suppl. 2)S195-S197. [DOI] [PubMed] [Google Scholar]
- 18.Roelofsen, E., M. Van Leeuwen, G. J. Meijer-Severs, M. H. F. Wilkinson, and J. E. Degener. 1999. Evaluation of the effects of storage in two different swab fabrics and under three different transport conditions on recovery of aerobic and anaerobic bacteria. J. Clin. Microbiol. 373041-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosa-Fraile, M., E. Camacho-Muñoz, J. Rodriguez-Granger, and C. Liébana-Martos. 2005. Specimen storage in transport medium and detection of group B streptococci by culture. J. Clin. Microbiol. 43928-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel, C. A., and M. Roberts. 1984. Mobiluncus gen. nov., Mobiluncus curtisii subsp. curtisii sp. nov., Mobiluncus curtisii subsp. holmesii subsp. nov., and Mobiluncus mulieris sp. nov., curved rods from the human vagina. Int. J. Syst. Bacteriol. 34177-184. [Google Scholar]
- 21.Stoner, K. A., L. K. Rabe, and S. L. Hillier. 2004. Effect of transport time, temperature, and concentration on the survival of group B streptococci in Amies transport medium. J. Clin. Microbiol. 425385-5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teese, N., D. Henessey, C. Pearce, N. Kelly, and S. Garland. 2003. Screening protocols for group B streptococcus: are transport media appropriate? Infect. Dis. Obstet. Gynecol. 11199-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Rensburg, E., J. C. Du Preez, and S. G. Kilian. 2004. Influence of the growth phase and culture medium on the survival of Mannheimia haemolytica during storage at different temperatures. J. Appl. Microbiol. 96154-161. [DOI] [PubMed] [Google Scholar]