Abstract
The diversity in the expression of Lewis antigens (Le) of 226 single colonies of Helicobacter pylori isolated from four regions of the stomach of eight adults is shown. Ley was expressed more in strains colonizing antrum than in strains colonizing fundus, whereas Lex was more common in fundus strains. cagA+ strains were more associated with Le-negative strains.
The Helicobacter pylori Lewis antigens (Le) mimic Lewis blood group antigens found on the surfaces of human gastric cells, providing the bacteria with a possible mechanism of immune evasion (5). This intimate interaction with the epithelia suggests that adaptation to specific regions of the stomach might occur. Most reports have studied H. pylori Le expression in isolates from the antrum and corpus exclusively (6-8, 10); the present study sought to characterize Le antigen expression in multiple colonies isolated from four regions of the stomach, namely, the antrum, corpus, fundus, and incisura of individual patients. The possible correlation of Le antigen expression with the presence of cagA is still contradictory (8, 10). We also analyzed the possible association of Le antigens expression with cagA.
Eight Mexican patients with an average age of 58 years (range, 28 to 71 years; five male, three female) presenting at the Gastroenterology Unit of Hospital de Especialidades, CMNSXXI, Instituto Mexicano del Seguro Social, in Mexico City because of gastroduodenal symptoms were studied. Participants were informed about the nature of the study and asked to sign a consent form; the present study was approved by the Instituto Mexicano del Seguro Social Ethics Committee. Two biopsy samples from each site—the antrum, incisura, corpus, and fundus—were obtained; one biopsy of each site was homogenized and cultured for H. pylori on selective blood agar medium in a 10% CO2 atmosphere, as previously described (6). A total of 226 individual colonies were studied (Table 1), a mean of 28 colonies per patient, and seven patients per region. From each colony, a 48-h culture in blood-agar was harvested in saline solution for DNA isolation and for Le antigen determination. The presence of Le antigens was determined by an enzyme-linked immunosorbent assay using commercial monoclonal antibodies to Lex and Ley (Signet Laboratories, Dedham, MA), as previously described (5). Results were expressed in optical density units (ODU), and values of >100 ODU were considered positive. Each clone was tested in quadruplicate on 2 different days, and the mean of the ODU was used for analysis. Differences in frequencies among regions were analyzed by using the chi-square test. DNA was isolated by using guanidine-isothiocyanate-sarkosyl and then used to determine cagA by PCR (6) and to genotype isolates by RAPD [random(ly) amplified polymorphic DNA] as previously described (1).
TABLE 1.
Frequency of Le antigen expressed by 226 isolates from four different regions of the stomachs of eight Mexican patients
| Region | No. of colonies | No. (%) of isolates with Lewis antigen type:
|
|||
|---|---|---|---|---|---|
| Lex+y− | Lex−y+ | Lex+y+ | Lex−y− | ||
| Antrum | 57 | 1 (2) | 18 (32) | 20 (35) | 18 (31) |
| Incisura | 56 | 5 (9) | 14 (25) | 13 (33) | 24 (43) |
| Corpus | 57 | 4 (7) | 17 (30) | 15 (26) | 21 (37) |
| Fundus | 56 | 6 (11) | 14 (25) | 13 (23) | 23 (41) |
| Total | 226 | 16 (7) | 63 (28) | 61 (27) | 86 (38) |
In contrast to previous studies (6-8, 10), we mapped the expression of Le antigens by strains colonizing four regions of the stomach. The frequencies of expression of Le antigens among all of the 226 isolates were 38% for Le−, 28% for Ley, 27% for Lexy, and 7% for Lex (Table 1). It is important to note that the frequency of colonies expressing Lex+y− was significantly lower (P = 0.02) in the antrum than in the fundus. In addition, there was a trend for colonies Lex−y− to be less common in the antrum than in the incisura and fundus, although this trend was not significant. Taken together, the frequency of colonies expressing Ley was significantly higher in antrum (38 of 57) than in both incisura and fundus (27 of 56 in both) (P = 0.048) (Table 1). In addition, there was a trend for colonies expressing Lex+y+ to be more frequent in the antrum than in the fundus, although this trend was not significant (P = 0.15).
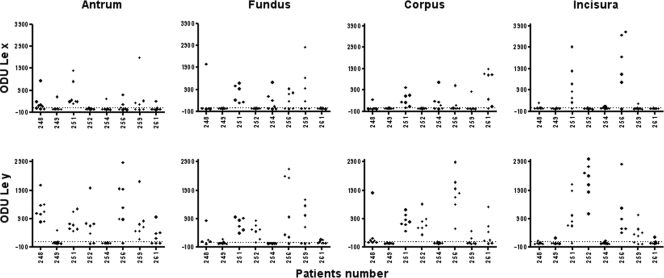
As in a previous study in Mexican patients (6), a considerable variability in the levels of expression of Le antigens (mean ± the standard deviation) was found in all colonies from the four regions of the stomach (Fig. 1 and Table 2), even among isolates from the same patient. For example, the isolates from patient 256 were highly variable for Ley, and the isolates from patient 251 were highly variable for Lex in the four stomach regions studied. Even so, in the antrum the level of expression of Ley (972 ± 621) was significantly higher than the expression of Lex (599 ± 578), whereas in the other three regions no significant difference was found between the expressions of Ley and Lex. The variability observed in the expression of Le antigen was not due to colonization with different strains, since in seven of the eight patients all of the isolated colonies showed the same RAPD pattern, and only in one case (patient 259) was a mixed infection documented, showing two different RAPD patterns. In accordance with these results, a previous study also reported a high diversity in the expression of Le antigens among multiple isolates from single patients even if they had the same RAPD pattern; the authors of that study suggested that variability in expression of Le antigens is not due to genetic diversity but to mechanisms regulating the expression and activity of fucosyltransferases (11). This variability would justify considering each colony as a phenotypically independent event for the statistical analyses.
FIG. 1.
Expression of Lex and Ley antigens by 226 H. pylori colonies isolated from the antrum, fundus, corpus, and incisura of the stomachs of eight Mexican patients.
TABLE 2.
Comparison of levels of expression of Lewis antigens in four different regions of the stomach by single-colony isolates from eight Mexican patients
| Region | Expression of Le antigen (mean ODU ± SD)
|
P | |
|---|---|---|---|
| Lex | Ley | ||
| Antrum | 599 ± 578 | 972 ± 621 | 0.025 |
| Incisura | 1,301 ± 1,13 | 1,156 ± 926 | 0.65 |
| Corpus | 752 ± 575 | 901 ± 684 | 0.42 |
| Fundus | 885 ± 665 | 820 ± 694 | 0.75 |
The results presented above suggest that there is selective expression of Le antigens by H. pylori in the different regions of the stomach. The expression of Ley seems to be predominant, or selected for in the antrum, whereas that of Lex−y− and Lex would be favored in the environment of the fundus. A limitation of the present study is the number of patients analyzed, and a study with larger number of cases is needed to confirm these results. It has been suggested that the expression of surface antigens involved in interaction and binding to epithelial cells, such as Le antigens by H. pylori, is influenced by the environment present in the different regions of the gastroduodenal regions (9). Thus, a previous study found significant differences between strains colonizing the antrum and those colonizing the duodenum (9).
The expression of Le antigens has been associated with the presence of the cagA+ gene. Lex expression has been reported as associated to cagA+ (10); however, and similar to other studies (4, 6, 8), we did not find such an association. In fact, even considering that Lex was the less expressed of the Le antigens (16 of 226 isolates, Fig. 1), only 4 (25%) of the 16 Lex colonies were cagA+. These four colonies were isolated from three different patients. We found a significant decreasing tendency of association with cagA and Le antigens as follows: Lex < Ley (P = 0.02) < Lexy (P = 0.005) < Le− (P < 0.001). Thus, in contrast to previous studies, cagA was significantly more associated with strains not expressing Le antigens.
In contrast to what we found for Le expression, the presence of cagA+ strains was not significantly different among the four regions of the stomach studied, which is consistent with previous results of an in situ study of cagA+ strains (2).
Acknowledgments
This project was supported by CONACYT and Coordinacíon de Investigacíon, IMSS, Mexico. J.T. is a recipient of an exclusivity scholarship from Fundacíon IMSS, Mexico.
Footnotes
Published ahead of print on 11 June 2008.
REFERENCES
- 1.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 205137-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camorlinga-Ponce, M., C. Romo, G. González-Valencia, O. Muñoz, and J. Torres. 2004. Topographical localisation of cagA positive and cagA negative Helicobacter pylori strains in the gastric mucosa; an in situ hybridisation study. J. Clin. Pathol. 57822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Valencia, G., J. C. Atherton, O. Muñoz, M. Dehesa, A. Madrazo de la Garza, and J. Torres. 2000. Helicobacter pylori vacA and cagA genotypes in Mexican adults and children. J. Infect. Dis. 182:1450-1454. [DOI] [PubMed] [Google Scholar]
- 4.Marshall, D. G., S. O. Hynes, D. C. Coleman, C. A. O'Morain, C. J. Smyth, and A. P. Moran. 1999. Lack of a relationship between Lewis antigen expression and cagA, CagA, vacA, and VacA status of Irish Helicobacter pylori isolates. FEMS Immunol. Med. Microbiol. 2479-90. [DOI] [PubMed] [Google Scholar]
- 5.Moran, A. P., M. M. Prendergast, and B. J. Appelmelk. 1996. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol. Microbiol. 16105-115. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz-Perez, L., G. González-Valencia, C. S. Giono, O. Muñoz, and J. Torres. 2001. A comparison of Lewis X and Lewis Y expression in Helicobacter pylori obtained from children and adults. J. Infect. Dis. 1831147-1151. [DOI] [PubMed] [Google Scholar]
- 7.Ryan, K. A., A. P. Moran, S. O. Hynes, T. Smith, D. Hyde, C. A. O'Morain, and M. Maher. 2000. Genotyping of cagA and vacA, Lewis antigen status, and analysis of the poly-(C) tract in the alpha(1,3)-fucosyltransferase gene of Irish Helicobacter pylori isolates. FEMS Immunol. Med. Microbiol. 28113-120. [DOI] [PubMed] [Google Scholar]
- 8.Taylor, D. E., D. A. Rasko, R. Sherburne, C. Ho, and L. D. Jewell. 1998. Lack of correlation between Lewis antigen expression by Helicobacter pylori and gastric epithelial cells in infected patients. Gastroenterology 27311513-11522. [DOI] [PubMed] [Google Scholar]
- 9.Thoreson, A. C., N. Hosseini, A. M. Svennerholm, and I. Bolin. 2000. Different Helicobacter pylori strains colonize the antral and duodenal mucosa of duodenal ulcer patients. Helicobacter 569-78. [DOI] [PubMed] [Google Scholar]
- 10.Wirth, H. P., M. Yang, M. Karita, and M. J. Blaser. 1996. Expression of the human cell surface glycoconjugates Lewis X and Lewis Y by Helicobacter pylori isolates is related to cagA status. Infect. Immun. 644598-4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth, H. P., M. Yang, R. M. Peek, Jr., J. Hook-Nikanne, M. Fried, and M. J. Blaser. 1999. Phenotypic diversity in Lewis expression of Helicobacter pylori isolates from the same host. J. Lab. Clin. Med. 133488-500. [DOI] [PubMed] [Google Scholar]