Abstract
The pandemic spread of Vibrio parahaemolyticus is an international public health issue. Because of the outbreak potential of the organism, it is critical to establish an internationally recognized molecular subtyping protocol for V. parahaemolyticus that is both rapid and robust as a means to monitor its further spread and to guide control measures in combination with epidemiologic data. Here we describe the results of a multicenter, multicountry validation of a new PulseNet International standardized V. parahaemolyticus pulsed-field gel electrophoresis (PFGE) protocol. The results are from a composite analysis of 36 well-characterized V. parahaemolyticus isolates from six participating laboratories, and the isolates represent predominant serotypes and various genotypes isolated from different geographic regions and time periods. The discriminatory power is very high, as 34 out of 36 sporadic V. parahaemolyticus strains tested fell into 34 distinguishable PFGE groups when the data obtained with two restriction enzymes (SfiI and NotI) were combined. PFGE was further able to cluster members of known pandemic serogroups. The study also identified quality measures which may affect the performance of the protocol. Nonadherence to the recommended procedure may lead to high background in the PFGE gel patterns, partial digestion, and poor fragment resolution. When these quality measures were implemented, the PulseNet V. parahaemolyticus protocol was found to be both robust and reproducible among the collaborating laboratories.
Vibrio parahaemolyticus, a halophilic gram-negative bacterium, can cause acute gastroenteritis in humans who consume contaminated raw or undercooked seafood. The clinical symptoms include watery diarrhea often accompanied with abdominal cramping, nausea, vomiting, low-grade fever, and chills. The organism was first identified as a cause of food-borne illness in Japan in 1950 (13). Isolates carrying one or both of the virulence factors, thermostable direct hemolysin (TDH) and the TDH-related hemolysin (TRH), are considered virulent (22, 27, 32).
Previously, V. parahaemolyticus infections have been typically sporadic cases attributed to multiple serotypes; 75 different combinations of somatic (O) and capsular (K) serotypes have been identified (18, 24). However, the epidemiology of this infection has changed since 1996. The incidence of V. parahaemolyticus infections among hospitalized patients in Kolkata, India, suddenly increased in February 1996 (24). It was determined that serotype O3:K6 strains accounted for 50 to 80% of the strains isolated during this period. Further surveillance studies demonstrated that this particular serotype was responsible for outbreaks worldwide in the Americas (7, 8, 14, 15), Asian countries, including Bangladesh, India, Japan, Korea, Laos, Taiwan, Thailand, and Vietnam (2, 9, 21, 25, 34), Europe (20, 29), and Mozambique (1).
Following the global spread of V. parahaemolyticus O3:K6 and the resulting large food-borne outbreaks, several phenotypic and molecular techniques have been employed to identify pandemic markers and characterize these strains. Serotyping is the primary means of subtyping strains of V. parahaemolyticus, but this method is resource intensive, and additional subtyping methods are now widely used. Strains of serotype O3:K6 responsible for pandemic disease since 1996 belong to a unique clone that is different from strains isolated previously (24). This new clone carries the tdh gene encoding TDH but lacks the trh gene encoding TRH, and it displays unique profiles in an arbitrarily primed PCR assay (24). Further studies identified two molecular markers of this pandemic group: one (toxRS/new) targeted for specific DNA sequences within the 1,364-bp toxRS region of the toxRS new clone, and the other (ORF8) targeted for orf8 of the f237 phage genome (17, 21). The toxRS operon encodes transmembrane proteins involved in the regulation of virulence-associated genes and is well conserved in the Vibrio genus (21). More recently, detection of genetic markers has further revealed the emergence of other serogroups that would be classified as pandemic clones, such as O4:K68 and O1:KUT (K untypeable), due to serotype conversion (21). Currently, 21 serotypes similar to the pandemic clone of O3:K6 have been identified (23). However, the utility of orf8 or the toxRS/new sequence as a reliable genetic marker for the identification of pandemic candidate strains is controversial (5, 6, 25, 26). Additional subtyping methods other than serotyping and genetic marker detection would enhance surveillance of the worldwide spread of known pandemic groups and the emergence of new serotypes of pandemic potential.
Pulsed-field gel electrophoresis (PFGE) is a molecular technique for separating large restriction fragments of chromosomal DNA by alternating the direction of the electric field. PulseNet International, a collaboration of international PulseNet networks dedicated to molecular surveillance of food-borne diseases, has successfully developed and implemented highly standardized PFGE protocols for Escherichia coli O157:H7, Salmonella spp., Shigella sonnei, Listeria monocytogenes, Campylobacter jejuni, and Vibrio cholerae which have enabled timely sharing of PFGE profiles within the PulseNet network (12, 30).
Preliminary reports demonstrated that PFGE exhibits good discriminatory power for subtyping V. parahaemolyticus (3, 10, 19, 31, 34, 35); however, it was not possible to compare the results of various studies, as different PFGE parameters were used. To enhance our ability to monitor this pathogen, there is urgent need for an internationally standardized V. parahaemolyticus PFGE protocol which can be readily implemented in laboratories all over the world. Application of a standardized protocol should allow for reliable comparison of DNA fingerprints in a timely manner during cross-border multinational outbreaks. In addition, data gathered by various laboratories can be shared with the intent to effectively monitor global dissemination of both existing and emerging pandemic strains.
We describe a multicenter, multicountry study of an internationally standardized, rapid V. parahaemolyticus PFGE protocol for use in the PulseNet network as an epidemiological tool for subtyping V. parahaemolyticus strains. This study is a collaborative effort of the PulseNet V. parahaemolyticus working group. The working group is composed of six participating laboratories: International Centre for Diarrheal Diseases Research of Bangladesh (ICDDR,B), Public Health Laboratory Centre (PHLC) of Hong Kong (co-coordinating laboratory), National Institute of Cholera and Enteric Diseases (NICED) of India, National Institute of Infectious Diseases (NIID) of Japan, National Institute of Health of Thailand (NIH), and the PulseNet Methods Development and Validation Laboratory of the Centers for Disease Control and Prevention (CDC) of the United States of America (USA) (co-coordinating laboratory).
MATERIALS AND METHODS
Bacterial strains.
A panel of 36 epidemiologically unrelated isolates of V. parahaemolyticus representing common serotypes isolated from different geographic regions and time periods were collected from six participating laboratories (Table 1). All isolates were sent to a coordinating laboratory (PHLC in Hong Kong) to keep the stock of bacterial strains in freeze-dried ampoules. Strains were coded and then distributed in nutrient agar stabs to participating laboratories.
TABLE 1.
Characteristics of 36 Vibrio parahaemolyticus isolates used in the multicenter study of a rapid standardized pulsed-field gel electrophoresis protocola
| Isolate | Yr of isolation | Country of isolation | Serotypeb
|
Presence of the following genec:
|
PFGE profiled
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | K | tl | tdh | trh | toxRS/new sequence | orf8 | SfiI | NotI | |||
| VPS01 | 1998 | Bangladesh | 3 | 6 | + | + | − | + | + | 1 | 1 |
| VPS02 | 2000 | Bangladesh | 3 | 6 | + | + | − | + | + | 2 | 2 |
| VPS03 | 1998 | Bangladesh | 4 | 68 | + | + | − | + | + | 3 | 3 |
| VPS04 | 1998 | Bangladesh | 4 | 68 | + | + | − | + | + | 3 | 3 |
| VPS05 | 1998 | Bangladesh | 1 | UT | + | + | − | + | + | 4 | 4 |
| VPS06 | 2000 | Bangladesh | 1 | UT | + | + | − | + | + | 4 | 5 |
| VPS07 | 2004 | Hong Kong | 3 | 6 | + | + | − | + | + | 5 | 6 |
| VPS08 | 2005 | Hong Kong | 3 | 6 | + | + | − | + | − | 6 | 7 |
| VPS09 | 2004 | Hong Kong | 4 | 8 | + | + | − | − | − | 7 | 8 |
| VPS10 | 2005 | Hong Kong | 4 | 8 | + | + | − | − | − | 8 | 9 |
| VPS11 | 2004 | Hong Kong | 3 | 29 | + | + | − | − | − | 9 | 10 |
| VPS12 | 2005 | Hong Kong | 3 | 29 | + | + | − | − | − | 10 | 11 |
| VPS13 | 2002 | India | 4 | 68 | + | + | − | + | + | 11 | 12 |
| VPS14 | 2001 | India | 3 | 6 | + | + | − | + | + | 12 | 13 |
| VPS15 | 2002 | India | 1 | UT | + | + | − | + | + | 13 | 4 |
| VPS16 | 2004 | India | 1 | UT | + | + | − | + | + | 14 | 14 |
| VPS17 | 2002 | India | 1 | 25 | + | + | − | + | + | 12 | 15 |
| VPS18 | 2004 | India | 1 | 25 | + | − | − | + | + | 15 | 16 |
| VPS19 | 2004 | Japan | 1 | UT | + | + | − | + | + | 3 | 4 |
| VPS20 | 2004 | Japan | 1 | UT | + | + | + | − | − | 16 | 17 |
| VPS21 | 1981 | Japan | 3 | 6 | + | + | − | − | − | 17 | 18 |
| VPS22 | 1981 | Japan | 3 | 6 | + | − | + | − | − | 18 | 19 |
| VPS23 | 1994 | Japan | 4 | 12 | + | + | + | − | − | 19 | 20 |
| VPS24 | 1996 | Japan | 4 | 12 | + | + | − | − | − | 20 | 21 |
| VPS25 | 2004 | Thailand | 1 | 38 | + | − | − | − | − | 21 | 22 |
| VPS26 | 2005 | Thailand | 1 | 38 | + | + | − | − | − | 22 | 23 |
| VPS27 | 2005 | Thailand | 2 | 28 | + | − | − | − | − | 23 | 24 |
| VPS28 | 2005 | Thailand | 2 | 28 | + | − | − | − | − | 24 | 25 |
| VPS29 | 2005 | Thailand | 3 | 54 | + | − | − | − | − | 25 | 26 |
| VPS30 | 2005 | Thailand | 3 | 54 | + | − | − | − | − | 26 | 27 |
| VPS31 | 1997 | USA | 4 | 12 | + | + | + | − | − | 27 | 28 |
| VPS32 | 1998 | USA | 3 | 6 | + | + | − | + | + | 28 | 29 |
| VPS33 | 2001 | USA | 3 | 6 | + | + | − | + | + | 2 | 2 |
| VPS34 | 2001 | USA | 4 | 12 | + | + | + | − | − | 29 | 30 |
| VPS35 | 2003 | USA | 6 | 18 | + | − | − | − | − | 30 | 31 |
| VPS36 | 2004 | USA | 6 | 18 | + | + | + | − | − | 31 | 32 |
The seven isolates chosen for the phase II study are shown in boldface type.
UT, untypeable.
+, present; −, absent.
PFGE profiles were assigned numbers arbitrarily.
Gene detection.
The 36 V. parahaemolyticus isolates were further characterized by PCR for different genes. Detection of total hemolysins, thermolabile hemolysin (tl), tdh, and trh was carried out by multiplex PCR amplification as previously described by Bej et al. (4). Identification of genetic markers toxRS/new and orf8 for the pandemic group was performed for each isolate as previously described (17, 21).
Phase I—method selection and international protocol evaluation.
V. parahaemolyticus PFGE protocols previously employed by participating laboratories were collected and reviewed. On the basis of different factors, such as the complexity of laboratory procedures, turnaround time, and ability to analyze PFGE profiles, the rapid PFGE protocol adopted by PulseNet USA as described by Parsons et al. (28) was selected for international evaluation. Each laboratory evaluated this protocol by subtyping the panel of 36 strains by using two restriction enzymes, SfiI and NotI.
(i) PFGE protocol.
The CDC protocol was performed as described previously (28) with slight modifications and further recommendations to optimize its use on an international level. The modifications incorporated are described in detail below (see “Phase II—protocol validation” below).
(ii) Data analysis.
PFGE patterns were analyzed using the BioNumerics version 4.0 software (Applied Maths, Sint Martens Latem, Belgium). The TIFF images were normalized by using the PulseNet universal Salmonella enterica serotype Braenderup (H9812) size standard (16) on each gel against the reference in the database. PFGE profiles were compared using the Dice coefficient and UPGMA (unweighted pair group method using arithmetic averages) clustering with a 1.5% band position tolerance window and 1.5% optimization. The clustering of the PFGE patterns and band assignments were confirmed visually. All gel images generated from the six participating laboratories were independently analyzed at the PHLC in Hong Kong and at the CDC in the USA, and the results were compared.
(iii) Review after phase I.
After the completion of phase I, a detailed survey of laboratory procedures (including equipment and reagents used) was performed. In addition, participants were invited to provide feedback on the proposed protocol as utilized in their own laboratories. Based on the survey findings, further experiments were carried out by the PHLC in Hong Kong and the CDC in the USA to assess any possible impacts identified that procedural modifications would have on protocol performance. The results of both the survey and additional testing established final recommendations (see below) for protocol optimization and the second phase of the study, protocol validation.
Phase II—protocol validation.
The protocol validation was undertaken using the PulseNet USA protocol with the following modifications. Cultures of V. parahaemolyticus for use in PFGE plug preparation were grown on plates containing Trypticase soy agar with 5% defibrinated sheep blood (TSA-SB) or a comparable nonselective medium with the intent of avoiding the use of a selective medium, such as thiosulfate-citrate-bile-sucrose (TCBS). Cultures were incubated at 37°C for ≤20 h prior to plug preparation as opposed to 18 to 24 h. The participating laboratories were recommended to adhere to the proposed protocol (28) when preparing cell suspensions for PFGE plugs (i.e., an optical density ranging from 0.35 to 0.45 as measured by the Dade microscan turbidity meter [Dade Behring, Deerfield, IL]). In addition, the PulseNet USA protocol was adjusted to prepare a restriction digestion with 50 U of the concentrated SfiI (40 units/μl) instead of the unconcentrated form (10 units/μl) of the enzyme. The participating laboratories retyped a subset of 7 isolates selected from the original panel of 36 V. parahaemolyticus strains with special attention to adhere to the proposed modifications. Seven strains were selected for this phase in order to conserve resources and to assess the usefulness of the protocol for discriminating different serotypes within the evaluation set. In summary, participating laboratories were asked to run two 10-well gels of seven strains digested with SfiI and NotI under the same running conditions (the initial and final switch times were 10 seconds and 35 seconds, respectively).
RESULTS
Strain characterization.
The 36 V. parahaemolyticus strains represent pandemic, nonpandemic but virulent, and nonpandemic nonvirulent strains of V. parahaemolyticus of diverse serotypes isolated from 1981 through 2005 (Table 1). Eleven different serotypes were included. The top four serotypes, O3:K6 (n = 9), O1:KUT (n = 6), O4:K12 (n = 4), and O4:K68 (n = 3), together constitute 61% of the isolates. All isolates tested had the tl gene. Four hemolysin genotypes were identified: positive for the tl, tdh, and trh genes (n = 5); positive for the tl and tdh genes but negative for trh (n = 23); positive for tl, negative for tdh, and positive for trh (n = 1); and positive for tl and negative for tdh and trh (n = 7). Based on a previously described genotypic definition (17, 21, 24), 16 of the 36 strains were identified as members of the pandemic group (positive for the tdh gene, negative for trh, and positive for toxRS/new and/or orf8). Among the 16 pandemic strains, four serotypes (7 strains of serotype O3:K6, 3 strains of serotype O4:K68, 5 strains of serotype O1:KUT, and 1 strain of serotype O1:K25) were noted. Although O4:K68, O1:KUT, and O1:K25 were regarded as serovariants of O3:K6 in an earlier report (23), not all of the strains in our study were typed as pandemic strains on the basis of PCR results. These strains were VPS18, VPS20, VPS21, and VPS22. Of the four, three (VPS20, VPS21, and VPS22) were positive for tdh and/or trh while negative for toxRS/new and orf8. Conversely, VPS18 was negative for the thermostable hemolysin genes but positive for toxRS/new and orf8.
PFGE results.
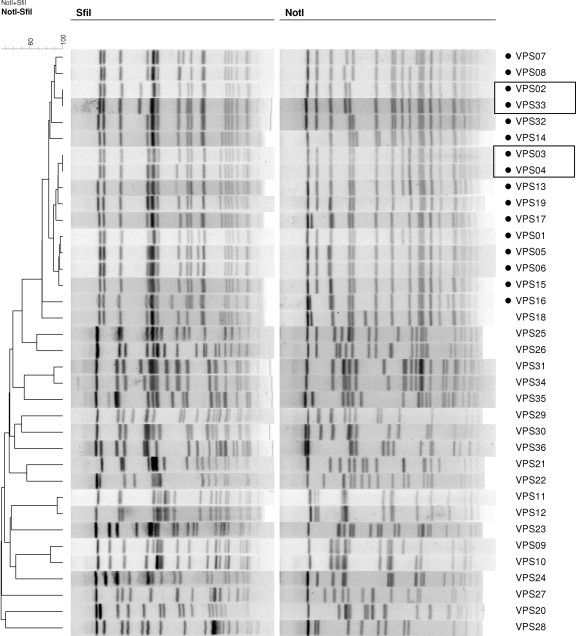
The molecular sizes of PFGE restriction fragments used for analysis ranged from 25 to 700 kb. The number of restriction fragments generated by SfiI ranged from 14 to 20, and the number of restriction fragments generated by NotI ranged from 12 to 20. Thirty-one distinct patterns were noted using SfiI-digested DNA fragments and 32 patterns using NotI (Table 1). By combining data from both restriction enzymes (SfiI and NotI), the 36 V. parahaemolyticus strains could be categorized into 34 distinguishable PFGE groups (Fig. 1). This would indicate that two pairs of strains, strains VPS02 and VPS33 (of serotype O3:K6) and strains VPS03 and VPS04 (of serotype O4:K68) were indistinguishable from one another by two enzymes. PFGE patterns of the 16 pandemic members from four serotypes (O3:K6, O4:K68, O1:KUT, and O1:K25) clustered together (Fig. 1). PFGE identified more subtypes than gene detection did. When subjected to UPGMA clustering using the Dice coefficient, the PFGE profiles of the isolates belonging to the pandemic clone clustered together using either enzyme or both enzymes combined (Fig. 1). The trh- and tdh-negative but toxRS/new- and orf8-positive isolate, VPS18, clustered closely with the isolates from the pandemic clone.
FIG. 1.
Dendrogram combining PFGE patterns of SfiI- and NotI-digested DNA from 36 representative Vibrio parahaemolyticus strains from six participating laboratories. PFGE profiles were generated at the Public Health Laboratory Centre in Hong Kong. Pandemic strains are indicated by small solid black circles.
Phase I results.
All participating laboratories submitted SfiI- and NotI-digested PFGE patterns for the 36 V. parahaemolyticus strains in the panel to the coordinating laboratories (the PHLC in Hong Kong and the CDC in the USA). In this phase, 33% of the gel images displayed an unusually high amount of background and smearing at the bottom of the gel that greatly hindered analysis of the PFGE patterns. A detailed survey of laboratory procedures and equipment was subsequently undertaken to identify factors contributing to suboptimal PFGE results.
Survey findings and assessment of procedural variations.
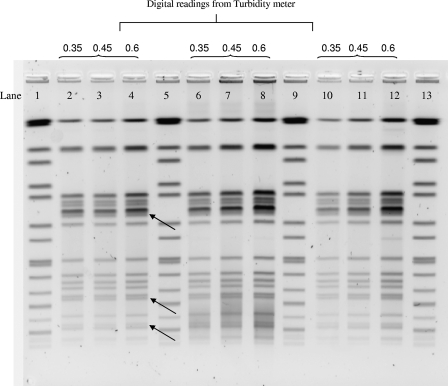
Positive feedback was obtained from the participants about the feasibility of implementing this protocol in their laboratories. The survey responses revealed that minor modifications had been made to the proposed protocol by some laboratories during plug preparation, including the choice of agar plate for subculture, age of culture growth, and concentration of bacterial cell suspensions for plug preparation. Experiments were conducted by the PHLC in Hong Kong and the CDC in the USA to assess the impact of such minor procedural modifications on the results. In these experiments, increased background was observed in PFGE patterns generated from plugs prepared directly from a selective medium, such as a TCBS agar plate (Fig. 2). For isolates on a selective medium, subculture on a nonselective medium (TSA-SB) reduced the degree of background (as shown in lanes 10 to 12 of Fig. 2). Increasing the cell suspension concentrations during plug preparation resulted in higher background (Fig. 2) and in thicker bands that were more difficult to resolve, especially when closely migrating bands were observed (Fig. 2, black arrows).
FIG. 2.
Effect of using different agar plates and cell suspension concentrations on the quality of SfiI PFGE patterns of Vibrio parahaemolyticus isolate VPS01. Lane 1, 5, 9, and 13 contain the size standards for Salmonella enterica serotype Braenderup. Cell concentrations were measured with a Dade Microscan turbidity meter in Falcon 2054 tubes. Lanes 2, 3, and 4 contain plugs prepared directly from isolates grown on TSA-SB. Lane 6, 7, and 8 contain plugs prepared from isolates grown directly on TCBS agar plates. Lane 10, 11, and 12 contain plugs prepared from isolate subculturing from TCBS to TSA-SB.
The age of the culture was also important. Indistinct (fuzzy) banding patterns, especially at the bottom region of some PFGE profiles, were observed with plugs made from cultures that were incubated for more than 20 h. Finally, incomplete digestion was observed when a less-concentrated form (10 units/μl) of SfiI enzyme was used in the restriction digests instead of the concentrated form (40 units/μl), even though the same number of units (50 U per plug slice) of enzyme was present in both situations. These results were used to generate specific recommendations for participating laboratories during phase II.
Phase II results.
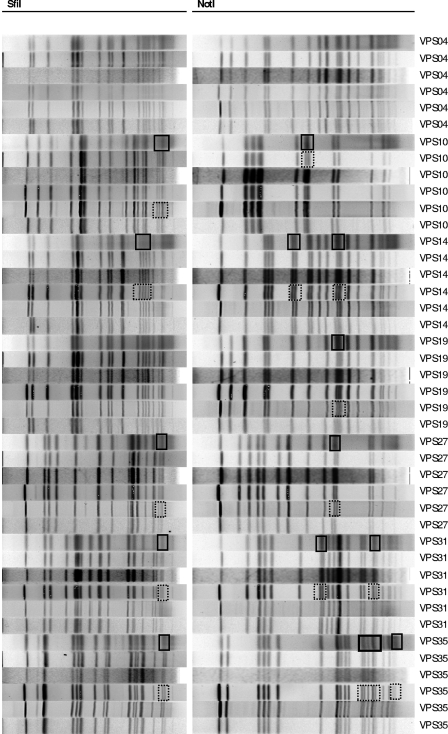
Phase II analysis of SfiI and NotI PFGE results showed significant improvement in the ability to compare PFGE patterns from different laboratories. Analysis of all PFGE fingerprints (digested by both enzymes) from the PHLC in Hong Kong and the CDC in the USA (two coordinating laboratories with comparable equipment and reagents) demonstrated 100% agreement for the subset of seven V. parahaemolyticus isolates. A comparison of all the PFGE patterns from the six participating laboratories generated similarities of 94% or greater for each of the SfiI and NotI PFGE profiles. The differences observed in the patterns found by the different laboratories related to the resolution of two closely migrating bands in some gels versus a single broad band in other gels (Fig. 3). Such small variations are unavoidable but underscore the need for visual confirmation of the automated computerized analysis, since such pseudodifferences will not be recognized by the software. Given the results, the proposed international PulseNet rapid standardized PFGE protocol for V. parahaemolyticus was found to be both robust and reproducible in multiple laboratories.
FIG. 3.
SfiI and NotI PFGE patterns of seven selected Vibrio parahaemolyticus isolates (phase II) from the six participating laboratories. Resolved close-migrating bands and unresolved close-migrating bands are shown outlined (boxed) by dotted lines and solid lines, respectively.
DISCUSSION
This study is a joint effort among six participating laboratories in the PulseNet International network. This multicenter, multicountry collaboration was established to evaluate and validate a rapid standardized PulseNet PFGE protocol for V. parahaemolyticus.
The two restriction enzymes used, SfiI and NotI, generate appropriate numbers of DNA fragments for analysis. Early reports of standardized PulseNet PFGE protocols demonstrated that the combination of two restriction enzymes increases the discriminatory power of the method (12, 33). Our results showed that indistinguishable SfiI PFGE profiles of V. parahaemolyticus strains can be further differentiated by the use of NotI (Fig. 1). We recommend the use of SfiI as the primary enzyme, while NotI can be used when further differentiation is needed. Two pairs of isolates in our panel could not be differentiated by PFGE. Although no known epidemiological connection exists, such a relationship may be possible, especially for strains VPS03 and VPS04, which were both isolated in Bangladesh in 1998.
The results are affected by minor variations in procedure. The increased background observed using plugs prepared directly from TCBS may be due to selective agents in this medium. For optimal PFGE results, strict use of TSA-SB or a comparable nonselective agar is recommended. In addition, increased cell concentrations during plug preparation resulted in higher background and limited resolution of closely migrating bands. While the values in the standardized protocol (0.35 to 0.45) have been found to work well within the CDC PulseNet laboratories, when different equipment is used for measuring cell concentrations, values should be adjusted accordingly. Previous experiments have shown that indistinct bands appear in the patterns if plugs are made from cultures incubated ≥20 h. The observation could be due to DNA degradation because of the long incubation time. Therefore, fresh cultures incubated for 16 to 18 h are recommended. Incomplete digestion resulting in “ghost or phantom” bands was observed in some SfiI PFGE patterns. The concentrated form (40 units/μl) of SfiI is recommended because it reduces the volume of glycerol (a stabilizing component present with the restriction enzyme) which in high concentrations can reduce the efficiency of the restriction enzyme. Failure to follow these recommendations may lead to high background of the PFGE patterns, partial digestion, and poor resolution. The recommendations made were appropriate for improving the performance of PFGE with V. parahaemolyticus. When these key factors were controlled, the V. parahaemolyticus PulseNet protocol was found to be both robust and reproducible among the collaborating laboratories.
Despite the suggested modifications, minor degrees of variability in PFGE patterns may occur when comparing gels of the same strains generated in different laboratories. These variations are a weakness inherent to PFGE as a method and not unique to the V. parahaemolyticus protocol (12, 22, 33). Such disparities are unlikely to be eliminated and are likely to be due to variations in equipment, different lots of laboratory supplies and reagents, and user variation, which can affect the outcome of the resulting PFGE patterns. Therefore, the present study further emphasizes the importance of following standardized procedures to minimize opportunities for gel-to-gel variation. If specific equipment or supplies are not accessible in certain geographical areas, fine adjustments in an individual laboratory may be useful to enhance interlaboratory reproducibility. The study panel represents a broad spectrum of V. parahaemolyticus strains, including pandemic, nonpandemic but pathogenic, and nonpandemic nonpathogenic strains of V. parahaemolyticus of diverse serotypes, isolated before and after 1996. Serotyping information alone may not accurately identify whether a strain belongs to the pandemic clone. Genetic marker detection offers better discrimination of various strains than phenotypic methods, but PFGE identified more subtypes than PCR detection of multiple genes alone did. The PFGE profiles of the isolates belonging to the pandemic clone clustered together using either enzyme alone or both enzymes combined (Fig. 1), while nonpandemic isolates grouped into multiple secondary clusters. This indicates that PFGE, in conjunction with serotyping, may be useful to preliminarily identify strains belonging to the epidemic clone.
Conclusions.
This study demonstrates the application of a rapid PFGE protocol to subtype a diverse collection of V. parahaemolyticus strains isolated from different geographical areas and time periods. PFGE patterns generated using this protocol can further differentiate strains within the pandemic clone. With the improvements to the protocol, the use of cultures from a nonselective medium incubated for 16 to 18 h with careful adherence to the cell concentration and the use of high-concentration SfiI enzyme, the rapid standardized V. parahaemolyticus PFGE protocol was robust and produced reproducible results. Our study findings highlight the discriminatory power of PFGE over other methods for subtyping strains in the evaluation panel. Using this validated rapid protocol, laboratories can facilitate timely subtyping of new or untypeable serotypes that have or may possess pandemic potential, such as serotype O3:K6 and its serovariants which have emerged since 1996. This collaborative work lays the foundation for setting up a global surveillance database that will enable us to effectively monitor the dissemination of V. parahaemolyticus and to make recommendations in collaboration with epidemiologic findings to facilitate prevention measures that will reduce the spread of infection.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Ansaruzzaman, M., M. Lucas, J. L. Deen, N. A. Bhuiyan, X. Y. Wang, A. Safa, M. Sultana, A. Chowdhury, G. B. Nair, D. A. Sack, L. von Seidlein, M. K. Puri, M. Ali, C. L. Chaignat, J. D. Clemens, and A. Barreto. 2005. Pandemic serovars (O3:K6 and O4:K68) of Vibrio parahaemolyticus associated with diarrhea in Mozambique: spread of the pandemic into the African continent. J. Clin. Microbiol. 432559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa, E., T. Murase, T. Shimada, T. Okitsu, S. Yamai, and H. Watanabe. 1999. Emergence and prevalence of a novel Vibrio parahaemolyticus O3:K6 clone in Japan. Jpn. J. Infect. Dis. 52246-247. [PubMed] [Google Scholar]
- 3.Bag, P. K., S. Nandi, R. K. Bhadra, T. Ramamurthy, S. K. Bhattacharya, M. Nishibuchi, T. Hamabata, S. Yamasaki, Y. Takeda, and G. B. Nair. 1999. Clonal diversity among recently emerged strains of Vibrio parahaemolyticus O3:K6 associated with pandemic spread. J. Clin. Microbiol. 372354-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bej, A. K., D. P. Patterson, C. W. Brasher, M. C. L. Vickery, D. D. Jones, and C. A. Kaysner. 1999. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh, and trh. J. Microbiol. Methods 36215-225. [DOI] [PubMed] [Google Scholar]
- 5.Bhoopong, P., P. Palittapongarnpim, R. Pomwised, A. Kiatkittipong, M. Kamruzzaman, Y. Nakaguchi, M. Nishibuchi, M. Ishibashi, and V. Vuddhakul. 2007. Variability of properties of Vibrio parahaemolyticus strains isolated from individual patients. J. Clin. Microbiol. 451544-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhuiyan, N. A., M. Ansaruzzaman, M. Kamruzzaman, K. Alam, N. R. Chowdhury, M. Nishibuchi, S. M. Faruque, D. A. Sack, Y. Takeda, and G. B. Nair. 2002. Prevalence of the pandemic genotype of Vibrio parahaemolyticus in Dhaka, Bangladesh, and significance of its distribution across different serotypes. J. Clin. Microbiol. 40284-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabanillas-Beltrán, H., E. LLausás-Magaña, R. Romero, A. Espinoza, A. García-Gasca, M. Nishibuchi, M. Ishibashi, and B. Gomez-Gil. 2006. Outbreak of gastroenteritis caused by the pandemic Vibrio parahaemolyticus O3:K6 in Mexico. FEMS Microbiol. Lett. 26576-80. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1999. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York, 1998. MMWR Morb. Mortal. Wkly. Rep. 4848-51. [PubMed] [Google Scholar]
- 9.Chowdhury, A., M. Ishibashi, V. D. Thiem, D. T. Tuyet, T. V. Tung, B. T. Chien, L. von Seidlein, D. G. Canh, J. Clemens, D. D. Trach, and M. Nishibuchi. 2004. Emergence and serovar transition of Vibrio parahaemolyticus pandemic strains isolated during a diarrhea outbreak in Vietnam between 1997 and 1999. Microbiol. Immunol. 48319-327. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury, N. R., S. Chakraborty, T. Ramamurthy, M. Nishibuchi, S. Yamasaki, Y. Takeda, and G. B. Nair. 2000. Molecular evidence of clonal Vibrio parahaemolyticus pandemic strains. Emerg. Infect. Dis. 6631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2006. Preparation and testing of reagent water in the clinical laboratory; approved guideline, 4th ed. CLSI document C3-A4. Clinical and Laboratory Standards Institute, Wayne, PA.
- 12.Cooper, K. L. F., C. K. Y. Luey, M. Bird, J. Terajima, G. B. Nair, K. M. Kam, E. Arakawa, A. Safa, D. T. L. Cheung, C. P. Law, H. Watanabe, K. Kubota, B. Swaminathan, and E. M. Ribot. 2006. Development and validation of a PulseNet standardized pulsed-field gel electrophoresis protocol for subtyping Vibrio cholerae. Foodborne Pathog. Dis. 351-58. [DOI] [PubMed] [Google Scholar]
- 13.Fujino, T., Y. Okuno, D. Nakada, A. Aoyamna, K. Fukai, T. Mukai, and T. Uebo. 1953. On the bacteriological examination of Shirasu food poisoning. Med. J. Osaka Univ. 4299-304. [Google Scholar]
- 14.Gil, A. I., H. Miranda, C. F. Lanata, A. Prada, E. R. Hall, C. M. Barreno, S. Nusrin, N. A. Bhuiyan, D. A. Sack, and G. B. Nair. 2007. O3:K6 serotype of Vibrio parahaemolyticus identical to the global pandemic clone associated with diarrhea in Peru. Int. J. Infect. Dis. 11324-328. [DOI] [PubMed] [Google Scholar]
- 15.González-Escalona, N., V. Cachicas, C. Acevedo, M. L. Rioseco, J. A. Vergara, F. Cabello, J. Romero, and R. T. Espejo. 2005. Vibrio parahaemolyticus diarrhea, Chile, 1998 and 2004. Emerg. Infect. Dis. 11129-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter, S. B., P. Vauterin, M. A. Lambert-Fair, M. S. Van Duyne, K. Kubota, L. Graves, D. Wrigley, T. Barrett, and E. Ribot. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 431045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iida, T., A. Hattori, K. Tagomori, H. Nasu, R. Naim, and T. Honda. 2001. Filamentous phage associated with recent pandemic strains of Vibrio parahaemolyticus. Emerg. Infect. Dis. 7477-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishibashi, M., K. Ohta, T. Shimada, T. Honda, J. Sugiyama, and Y. Miwatani. 2000. Current status of OK serotype combinations of Vibrio parahaemolyticus. Nippon Saikingaku Zasshi 55539-541. [Google Scholar]
- 19.Martinez-Urtaza, J., A. Lozano-Leon, A. DePaola, M. Ishibashi, K. Shimada, M. Nishibuchi, and E. Liebana. 2004. Characterization of pathogenic Vibrio parahaemolyticus isolates from clinical sources in Spain and comparison with Asian and North American pandemic isolates. J. Clin. Microbiol. 424672-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Urtaza, J., L. Simental, D. Velasco, A. DePaola, M. Ishibashi, Y. Nakaguchi, M. Nishibuchi, D. Carrera-Flores, C. Rey-Alvarez, and A. Pousa. 2005. Pandemic Vibrio parahaemolyticus O3:K6, Europe. Emerg. Infect. Dis. 111319-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto, C., J. Okuda, M. Ishibashi, M. Iwanaga, P. Garg, T. Rammamurthy, H. C. Wong, A. Depaola, Y. B. Kim, M. J. Albert, and M. Nishibuchi. 2000. Pandemic spread of an O3:K6 clone of Vibrio parahaemolyticus and emergence of related strains evidenced by arbitrarily primed PCR and toxRS sequence analyses. J. Clin. Microbiol. 38578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meador, C. E., M. M. Parsons, C. A. Bopp, P. Gerner-Smidt, J. A. Painter, and G. J. Vora. 2007. Virulence gene- and pandemic group-specific marker profiling of clinical Vibrio parahaemolyticus isolates. J. Clin. Microbiol. 451133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair, G. B., T. Ramamurthy, S. K. Bhattacharya, B. Dutta, Y. Takeda, and D. A. Sack. 2007. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin. Microbiol. Rev. 2039-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda, J., M. Ishibashi, E. Hayakawa, T. Nishino, Y. Takeda, A. K. Mukhopadhyay, S. Garg, S. K. Bhattacharya, G. B. Nair, and M. Nishibuchi. 1997. Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. J. Clin. Microbiol. 353150-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okura, M., R. Osawa, A. Iguchi, E. Arakawa, J. Terajima, and H. Watanabe. 2003. Genotypic analyses of Vibrio parahaemolyticus and development of a pandemic group-specific multiplex PCR assay. J. Clin. Microbiol. 414676-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osawa, R., A. Iguchi, E. Arakawa, and H. Watanabe. 2002. Genotyping of pandemic Vibrio parahaemolyticus O3:K6 still open to question. J. Clin. Microbiol. 402708-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, K. S., T. Ono, M. Rokuda, M. H. Jang, K. Okada, T. Iida, and T. Honda. 2004. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 726659-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons, M. B., K. L. Cooper, K. A. Kubota, N. Puhr, S. Simington, P. S. Calimlim, D. Schoonmaker-Bopp, C. Bopp, B. Swaminathan, P. Gerner-Smidt, and E. M. Ribot. 2007. PulseNet USA standardized pulsed-field gel electrophoresis protocol for subtyping of Vibrio parahaemolyticus. Foodborne Pathog. Dis. 4285-292. [DOI] [PubMed] [Google Scholar]
- 29.Quilici, M. L., A. Robert-Pillot, J. Picart, and J. M. Fournier. 2005. Pandemic Vibrio parahaemolyticus O3:K6 spread, France. Emerg. Infect. Dis. 111148-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribot, E. M., M. A. Fair, R. Gautom, D. N. Cameron, S. B. Hunter, B. Swaminathan, and T. J. Barrett. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 359-67. [DOI] [PubMed] [Google Scholar]
- 31.Serichantalergs, O., N. A. Bhuiyan, G. B. Nair, O. Chivaratanond, A. Srijan, L. Bodhidatta, S. Anuras, and C. J. Mason. 2007. The dominance of pandemic serovars of Vibrio parahaemolyticus in expatriates and sporadic cases of diarrhoea in Thailand, and a new emergent serovar (O3:K46) with pandemic traits. J. Med. Microbiol. 56608-613. [DOI] [PubMed] [Google Scholar]
- 32.Shirai, H., H. Ito, T. Hirramaya, T. Nakamoto, N. Nakabayashi, K. Kumagai, Y. Takeda, and M. Nishibuchi. 1990. Molecular epidemiologic evidence for association of thermostable direct hemolysin (TDH) and TDH-related hemolysin of Vibrio parahaemolyticus with gastroenteritis. Infect. Immun. 583568-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaminathan, B., T. J. Barrett, S. B. Hunter, R. V. Tauxe, and the CDC PulseNet Task Force. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong, H. C., S. H. Liu, T. K. Wang, C. L. Lee, C. S. Chiou, D. P. Liu, M. Nishibuchi, and B. K. Lee. 2000. Characteristics of Vibrio parahaemolyticus O3:K6 from Asia. Appl. Environ. Microbiol. 663981-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung, P. S., M. C. Hayes, A. DePaola, C. A. Kaysner, L. Kornstein, and K. J. Boor. 2002. Comparative phenotypic, molecular, and virulence characterization of Vibrio parahaemolyticus O3:K6 isolates. Appl. Environ. Microbiol. 682901-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]