Abstract
The CLSI Antifungal Subcommittee followed the M23-A2 “blueprint” to develop interpretive MIC breakpoints for anidulafungin, caspofungin, and micafungin against Candida species. MICs of ≤2 μg/ml for all three echinocandins encompass 98.8 to 100% of all clinical isolates of Candida spp. without bisecting any species group and represent a concentration that is easily maintained throughout the dosing period. Data from phase III clinical trials demonstrate that the standard dosing regimens for each of these agents may be used to treat infections due to Candida spp. for which MICs are as high as 2 μg/ml. An MIC predictive of resistance to these agents cannot be defined based on the data from clinical trials due to the paucity of isolates for which MICs exceed 2 μg/ml. The clinical data set included only three isolates from patients treated with an echinocandin (caspofungin) for which the MICs were >2 μg/ml (two C. parapsilosis isolates at 4 μg/ml and one C. rugosa isolate at 8 μg/ml). Based on these data, the CLSI subcommittee has decided to recommend a “susceptible only” breakpoint MIC of ≤2 μg/ml due to the lack of echinocandin resistance in the population of Candida isolates thus far. Isolates for which MICs exceed 2 μg/ml should be designated “nonsusceptible” (NS). For strains yielding results suggestive of an NS category, the organism identification and antimicrobial-susceptibility test results should be confirmed. Subsequently, the isolates should be submitted to a reference laboratory that will confirm the results by using a CLSI reference dilution method.
Members of the echinocandin class of antifungal agents act by inhibition of the synthesis of 1,3-β-d-glucan in the fungal cell wall (8, 16). All three available echinocandins—anidulafungin, caspofungin, and micafungin—possess fungicidal activity against most species of Candida, including polyene- and azole-resistant species (9, 14, 33, 48, 59, 65). All have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of esophageal candidiasis and invasive candidiasis, including candidemia (15, 30, 35, 43, 45, 56; Mycamine [micafungin] package insert, 2005, Astellas Pharma US, Deerfield, IL; Cancidas [caspofungin] package insert, 2001, Merck & Co., Whitehouse Station, NJ; and Eraxis [anidulafungin] package insert, 2006, Pfizer, Inc., New York, NY). These agents all provide excellent clinical efficacy coupled with low toxicity for the treatment of serious candidal infections.
Collaborative studies conducted by the Clinical and Laboratory Standards Institute (CLSI) Antifungal Subcommittee have resulted in a consensus recommendation for a standardized method for in vitro susceptibility testing of the echinocandins against Candida spp. (11, 41, 49). The broth microdilution (BMD) method employs RPMI 1640 broth medium, incubation at 35°C for 24 h, and an MIC endpoint criterion of prominent reduction in growth (≥50% inhibition relative to growth of control). This standardized method provides reliable and reproducible MIC results with good separation of the “wild-type” MIC distribution from isolates of Candida with mutations in the FKS1 gene for which reduced susceptibility to echinocandins has been documented (7, 41, 46, 47, 49).
There is broad experience with testing the echinocandins by using the CLSI BMD method (24, 42, 50, 51, 55, 58-60). In addition to standardized testing methods, the CLSI Antifungal Subcommittee has approved quality control limits for BMD test methods with all three echinocandins (11, 12).
Previously, the CLSI Antifungal Subcommittee used the accumulated microbiological and clinical data to provide a blueprint for the establishment of interpretive breakpoints for antifungal susceptibility testing of fluconazole (52) and voriconazole (53) against species of Candida. The analytical model followed that outlined for all types of antimicrobial susceptibility testing in CLSI document M23-A2 (37). During their June 2007 meeting, the CLSI Antifungal Subcommittee utilized this approach to propose interpretive breakpoints for MIC testing of anidulafungin, caspofungin, and micafungin against Candida species. These analyses are summarized below.
MATERIALS AND METHODS
Organisms.
All isolates of Candida used to generate the MIC distribution profiles and cross-resistance studies were obtained from the ARTEMIS Global Antifungal Surveillance Program (55). A total of 5,346 isolates of Candida (15 different species from 91 study centers) collected from blood and normally sterile body sites from 2001 through 2006 were sent to the ARTEMIS central reference laboratory (University of Iowa) for identification and susceptibility testing by the CLSI BMD method (11).
In addition to the isolates noted above, all Candida spp. isolated at baseline from subjects with definite infections in phase II and III primary studies of caspofungin (28), anidulafungin (56), and micafungin (30, 45) were identified and tested by using the CLSI BMD MIC method in reference laboratories located at Merck (Rahway, NJ), International Health Management Associates (Schaumberg, IL), and the University of Texas (Houston), respectively. A total of 406 isolates were obtained from caspofungin esophageal and invasive candidiasis clinical trials (28), while 135 isolates were obtained from the anidulafungin-versus-fluconazole candidemia study (56), and 410 isolates were obtained from two micafungin candidemia studies (30, 45). These isolates represented the baseline isolates from subjects eligible for this analysis.
Antifungal susceptibility testing.
All isolates were tested in accordance with the standards in CLSI document M27-A3 (10) using RPMI 1640 medium, an inoculum of from 0.5 × 103 to 2.5 × 103 cells/ml, and incubation at 35°C. MICs were determined visually after 24 h of incubation as the lowest concentration of drug that caused a significant diminution (≥50%) of growth below control levels (47, 55).
Quality control.
Quality control was performed on each day of testing by using CLSI-recommended reference strains C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 (12).
Phase II and III clinical trials.
The clinical trial data (patient outcomes and baseline isolates) used in this analysis included the results from four phase II or III studies of esophageal candidiasis treated with caspofungin (4, 28, 60, 62), two phase III studies of invasive candidiasis treated with caspofungin (28, 35, 63), one phase III study of invasive candidiasis treated with anidulafungin (56), and two phase III studies of invasive candidiasis treated with micafungin (30, 45). These were all multi-institutional studies, the details of which are described elsewhere (4, 28, 30, 35, 45, 56, 60, 61, 63). The responses to echinocandin therapy in each study were determined by the investigator at the end of therapy as cured, improved, or failed. A cured or improved response was classified as success, and all other responses as failure.
In vivo correlation.
Clinical outcomes as determined by the investigators at the end of therapy were compared to the relevant echinocandin MIC for each baseline Candida isolate. Where more than one baseline pathogen was identified per patient, the isolate for which the MIC was highest was used.
Development of MIC interpretive breakpoints.
The MIC interpretive breakpoints for the three echinocandins and Candida spp. were developed by taking into account the available microbiologic data, the known resistance mechanisms and their relation to both MICs and in vivo outcomes, pertinent pharmacokinetic (PK) and pharmacodynamic (PD) parameters, and clinical outcome data as described previously for fluconazole (52) and voriconazole (53). The PD indices associated with treatment efficacy for the echinocandins include the area under the concentration-time curve (AUC)/MIC and time to maximum concentration of drug in serum (Cmax)/MIC ratios. The PD target associated with a stasis endpoint for echinocandins is equivalent to AUC/MIC and Cmax/MIC ratios near 10 and 1, respectively (2, 3, 20, 24, 32, 64). Free (not bound to protein) echinocandin concentrations are considered in these estimates. The results of PD studies with most anti-infective drugs have shown that only unbound drug is generally available for microbiologic activity.
RESULTS AND DISCUSSION
Interpretive breakpoints for caspofungin against Candida species and MIC distribution profile.
The MIC90 and the percentage of isolates for which caspofungin MICs were 2 μg/ml or less are shown in Table 1. These results were all determined in a single reference laboratory using CLSI-recommended BMD methods. This large data set represents recent (2001 to 2006), clinically important (blood and normally sterile site) isolates from 91 different medical centers throughout the world. The overall MIC90 for caspofungin was 0.25 μg/ml, and 99.9% of the 5,346 isolates were inhibited by ≤2 μg/ml of caspofungin.
TABLE 1.
Comparative in vitro activity of three echinocandin antifungal agents against bloodstream isolates of Candida speciesa
| Species | No. of isolates tested | Results for:b
|
|||||
|---|---|---|---|---|---|---|---|
| ANID
|
CASP
|
MICA
|
|||||
| MIC90 | % ≤ 2 | MIC90 | % ≤ 2 | MIC90 | % ≤ 2 | ||
| C. albicans | 2,869 | 0.06 | 100 | 0.06 | 100 | 0.03 | 100 |
| C. glabrata | 747 | 0.12 | 99.9 | 0.06 | 99.9 | 0.015 | 100 |
| C. tropicalis | 625 | 0.06 | 100 | 0.06 | 99.8 | 0.06 | 100 |
| C. krusei | 136 | 0.06 | 100 | 0.25 | 100 | 0.12 | 100 |
| C. parapsilosis | 759 | 2 | 92.5 | 1 | 99.9 | 2 | 100 |
| C. guilliermondii | 61 | 2 | 90.2 | 1 | 95.1 | 1 | 100 |
| All Candida spp. | 5,346 | 2 | 98.8 | 0.25 | 99.9 | 1 | 100 |
MICs were determined in RPMI broth with 24-h incubation and prominent-inhibition endpoint. Data were compiled from reference 55.
% ≤ 2, percentage of isolates for which the MIC was 2 μg/ml or less. Abbreviations: ANID, anidulafungin; CASP, caspofungin; MICA, micafungin.
The caspofungin MIC90 for Candida krusei (0.25 μg/ml), C. parapsilosis (1 μg/ml), and C. guilliermondii (1 μg/ml) was considerably higher than that observed for the three common species, C. albicans (0.06 μg/ml), C. glabrata (0.06 μg/ml), and C. tropicalis (0.06 μg/ml). The mechanism for this intrinsic reduced susceptibility appears to be a direct reflection of amino acid polymorphisms within the FKS1 “hot spot” regions of the respective species (22, 47). Nevertheless, 95.1% of C. guilliermondii, 99.9% of C. parapsilosis, and 100% of C. krusei isolates were inhibited by ≤2 μg/ml of caspofungin, a concentration that is attained throughout the dosing interval at standard doses (70-mg loading dose and 50-mg daily dose) of caspofungin (8). As noted previously (50, 51, 54), 100% of fluconazole-resistant isolates of Candida spp. were inhibited by ≤2 μg/ml (MIC90, 0.25 μg/ml) of caspofungin (Table 2). These data, including the species distribution rank-order (Table 1), are highly representative of those published in numerous in vitro studies (21, 42, 54).
TABLE 2.
In vitro activity of three echinocandin antifungal agents against fluconazole-resistant isolates of Candida speciesa
| Species | No. of isolates tested | Results for:b
|
|||||
|---|---|---|---|---|---|---|---|
| ANID
|
CASP
|
MICA
|
|||||
| MIC90 | % ≤ 2 | MIC90 | % ≤ 2 | MIC90 | % ≤ 2 | ||
| C. albicans | 41 | 0.06 | 100 | 0.06 | 100 | 0.03 | 100 |
| C. glabrata | 110 | 0.12 | 100 | 0.06 | 100 | 0.015 | 100 |
| C. krusei | 146 | 0.12 | 100 | 0.25 | 100 | 0.06 | 100 |
| All Candida spp. | 315 | 1 | 100 | 0.25 | 100 | 0.5 | 100 |
Relationship between resistance mechanisms, MICs, and in vivo response.
The mechanisms of resistance to caspofungin include (i) specific “hot spot” mutations in the FKS1 gene (which encodes essential components of the glucan synthesis enzyme complex) and (ii) overexpression of Sbe2p, a Golgi protein involved in transport of cell wall components (7, 46, 47). Among these mechanisms, only the FKS1 mutations have been implicated in clinical resistance (47). Unlike the azole class drugs, drug efflux transporters do not appear to be a factor in the resistance of Candida spp. to caspofungin or other members of the echinocandin class (5, 39, 47).
Clinical isolates of C. albicans displaying elevated MICs for caspofungin have been shown to contain FKS1 mutations (Table 3) (46, 47). Furthermore, these strains showed a decreased sensitivity for inhibition of glucan synthase by caspofungin and reduced echinocandin efficacy in animal models (Table 3). It is notable that such mutations have only been observed in resistant strains (46, 47).
TABLE 3.
Resistance properties associated with caspofungin and clinical isolates of Candida albicans from single patientsa
| Isolate | Fks1 change | MIC (μg/ml)b | Glucan synthesis IC50 (ng/ml)c | Mouse ED90 (mg/kg/day)d |
|---|---|---|---|---|
| C. albicans 16998 | None | 0.5 | 0.56 | <0.06 |
| C. albicans 18195 | None | 0.25 | 0.91 | 0.01 |
| C. albicans 16996 | S645F | >8 | 162 | 1.09 |
| C. albicans 16997 | S645P | >8 | 1,997 | 9.98 |
MICs were determined according to CLSI M27-A3 (11).
IC50 (50% inhibitory concentration) for inhibition of glucan synthase enzyme complex in vitro.
ED90 (90% effective dose) required for reduction of kidney organism burden in a murine candidiasis model.
FKS1 mutations conferring resistance to caspofungin and other echinocandins have been identified in several C. albicans strains from patients (6, 31, 34, 46), as well as in two C. glabrata strains (10, 17) and in two strains of C. krusei (25, 27, 46) isolated from patients refractory to therapy (Table 4). These and other case reports, for which studies to document mutations were not performed (Table 4), provide compelling examples of the relationship between high or increasing caspofungin MICs and a poor clinical outcome. In each of these instances, progressive resistance to caspofungin, as well as to other echinocandins, was observed (Table 4). Notably, caspofungin MICs for strains of Candida with documented FKS1 gene mutations and for other published resistant strains generally show values of from 4 μg/ml to more than 8 μg/ml (Table 4). Furthermore, in four instances, resistance to caspofungin was confirmed in an animal model (26, 27, 46).
TABLE 4.
Published cases of Candida sp. infections associated with increased MICs of echinocandins as determined by the CLSI reference methoda
| Reference | Organism | Infection (comment) | Antifungal treatment | Isolate (source) | MIC (μg/ml)
|
FKS mutation | Comment(s) | ||
|---|---|---|---|---|---|---|---|---|---|
| CAS | MFG | AFG | |||||||
| Hernandez et al. (26) | C. albicans | Esophagitis (HIV) | FLC, AMB, CAS | A | 0.25 | ND | ND | ND | Resistance confirmed in an animal model |
| B | 0.25 | ND | ND | ND | |||||
| C | >64 | ND | ND | ND | |||||
| Laverdiere et al. (31) | C. albicans | Esophagitis (HIV) | FLC, VRC, AMB, CAS, ITZ, MFG | A | 0.06 | 0.03 | 0.03 | None | S645F and R1361H mutations |
| B | 2 | 2 | 1 | Yes | |||||
| C1 | 2 | 2 | 1 | Yes | |||||
| C2 | 1 | 2 | 0.5 | Yes | |||||
| Miller et al. (34) | C. albicans | Esophagitis (HIV) | FLC, VRC, AMB, CAS | A | 8 | ND | ND | Yes | S645P mutation; susceptible parent not available |
| Park et al. (46) | C. albicans | Disseminated (patient A) | CAS | A (oral) | 0.5 | ND | ND | None | S645F and S645P mutations; resistance confirmed in animals |
| B (blood) | >8 | ND | ND | Yes | |||||
| C (lung) | >8 | ND | ND | Yes | |||||
| D (liver) | 0.25 | ND | ND | None | |||||
| Park et al. (46) | C. albicans | Disseminated (patient B) | CAS | A (urine) | 0.5 | ND | ND | None | S645F mutation; resistance confirmed in animals |
| B (urine) | 0.5 | ND | ND | None | |||||
| C (oral) | 4 | ND | ND | Yes | |||||
| D (oral) | 4 | ND | ND | Yes | |||||
| Daneman et al. (13) | C. glabrata | Fungemia (3 episodes) | CAS (3 courses) | A (blood) | 0.5 | ND | ND | ND | |
| B (blood) | 8 | ND | ND | ND | |||||
| C (blood) | 8 | ND | ND | ND | |||||
| D (blood) | >16 | ND | ND | ND | |||||
| Dodgson et al. (17) | C. glabrata | Fungemia | AMB, VRC, CAS | A (blood) | 0.12 | ND | ND | ND | S663P mutation |
| B (blood) | >8 | >8 | >8 | Yes | |||||
| C (bone marrow) | >8 | ND | ND | ND | |||||
| Krogh-Madsen et al. (29) | C. glabrata | Fungemia | CAS, VRC | A (gall bladder) | 0.5 | ND | ND | ND | Resistance confirmed in animal model |
| B (urine) | >8 | ND | ND | ND | |||||
| C (gall bladder) | 8 | ND | ND | ND | |||||
| D (gall bladder) | 1 | ND | ND | ND | |||||
| Villareal et al. (62) | C. glabrata | Fungemia | CAS | A (blood) | 0.125 | ND | 0.03 | ND | |
| B (peritoneal fluid) | 8 | ND | 0.125 | ND | |||||
| Hakki et al. (25) | C. krusei | Fungemia | CAS, AMB, VRC | A (blood) | 2 | 0.5 | 0.25 | None | F655C mutation in one allele (27) |
| B (throat) | 8 | 4 | 4 | Yes | |||||
| Park et al. (46) | C. krusei | Fungemia | CAS | A (stool) | 32 | ND | ND | Yes | R1361G mutation; susceptible parent not available |
| Moudgal et al. (36) | C. parapsilosis | Prosthetic valve endocarditis | AMB, 5FC, FLC, CAS | A (blood) | 2 | 8 | 1 | ND | |
| B (blood) | >16 | >16 | 2 | ND | |||||
| Cleary et al. (10) | C. glabrata | Fungemia | CAS | A (blood) | 0.06 | 0.06 | 0.03 | None | D632E mutation |
| B (blood) | >4 | >4 | >4 | Yes | |||||
| C (blood) | >4 | >4 | >4 | Yes | |||||
Data were compiled from references 6, 10, 13, 17, 25, 26, 29, 31, 34, 36, 46, and 62. Abbreviations: CLSI, Clinical and Laboratory Standards Institute; AMB, amphotericin B; FLC, fluconazole; VRC, voriconazole; 5FC, flucytosine; ITZ, itraconazole; CAS, caspofungin; MFG, micafungin; AFG, anidulafungin; ND, not done.
Cross-resistance among echinocandins and between echinocandins and fluconazole.
It is now well established that cross-resistance between caspofungin and fluconazole does not exist (39, 54). Caspofungin and the other echinocandins are poor substrates for most multidrug efflux transporters, and the results of studies involving fluconazole-resistant strains of C. albicans expressing high levels of CDR1, CDR3, and/or MDR1 demonstrated full susceptibility to caspofungin (5). Furthermore, 100% of 315 clinical isolates of fluconazole-resistant Candida spp. were susceptible to caspofungin at an MIC of 2 μg/ml or less (MIC90, 0.25 μg/ml) (Table 2).
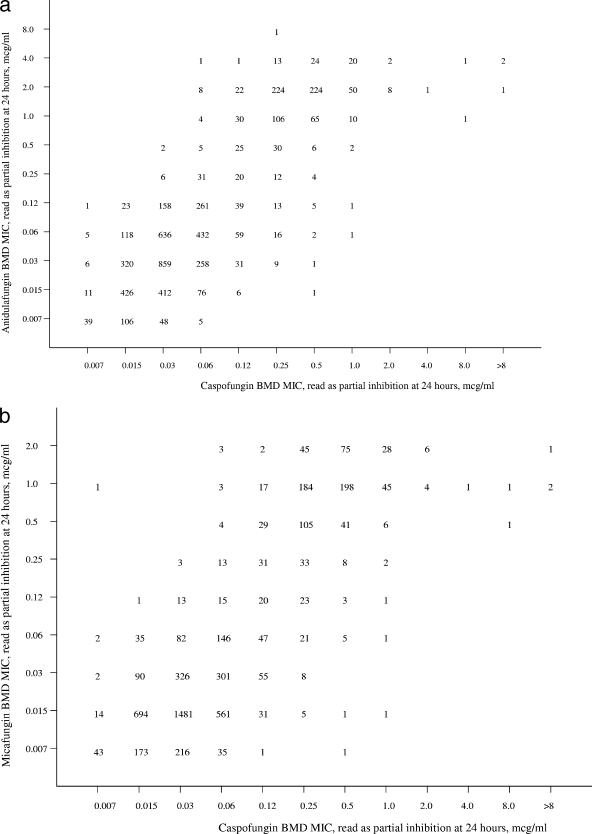
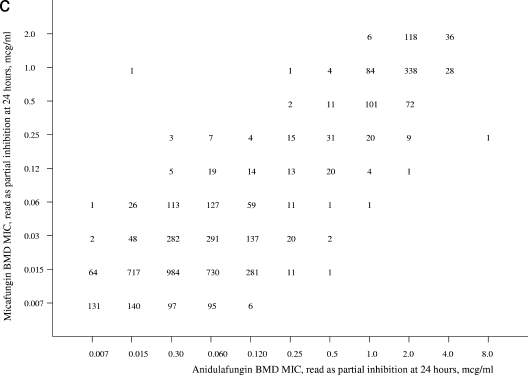
Given the mechanism of action that is shared among the echinocandins, it is not surprising that they demonstrate a similar spectrum and potency (47). Scatterplots of anidulafungin (Fig. 1a) and micafungin MICs (Fig. 1b) versus caspofungin MICs show a high degree of correlation (R = 0.85 and 0.84, respectively). The essential agreement (MIC ± 2 dilutions) for the comparisons is striking at 93% for anidulafungin versus caspofungin and 97% for micafungin versus caspofungin. These findings support the observations of Balashov et al., Park et al., and Perlin (7, 46, 47) indicating that among FKS1 mutants expressing resistance to caspofungin, the strains are cross resistant to micafungin and anidulafungin. The strength of these relationships is modified somewhat by the distinctly rare occurrence of clinical isolates for which the MICs of caspofungin, micafungin, and anidulafungin exceed 4 μg/ml (55).
FIG. 1.
Scatterplots of anidulafungin (a) and micafungin (b) versus caspofungin MICs and of anidulafungin versus micafungin (c) MICs for 5,346 isolates of Candida spp. Excellent correlations were observed for all three comparisons (r = 0.85, 0.84, and 0.89, respectively). MICs were determined for each drug using RPMI 1640 medium, a 24-h incubation, and a partial-inhibition (≥50%) endpoint.
In vitro correlation of in vivo data.
A total of 406 patients enrolled in phase II/III clinical trials for treatment of esophagitis (292 patients) and invasive candidiasis (114 patients) were infected with Candida spp., received caspofungin therapy, and were characterized as treatment successes or failures at the end of therapy by the site investigators (Table 5). The overall species and MIC distribution was comparable to that shown in Table 1. No significant differences in clinical response to caspofungin therapy were noted among the various species of Candida (28), and so for purposes of this analysis, the results for all species were merged.
TABLE 5.
Relationship between MIC and outcome for treatment of candidiasis with caspofungina
| MIC (μg/ml)b | Results for:c
|
|||||
|---|---|---|---|---|---|---|
| Esophageal candidiasis
|
Invasive candidiasis
|
Total
|
||||
| S/T | % | S/T | % | S/T | % | |
| 0.008 | 1/1 | 100 | 1/1 | 100 | ||
| 0.016 | ||||||
| 0.03 | 1/1 | 100 | 1/1 | 100 | ||
| 0.06 | 3/4 | 75 | 3/4 | 75 | ||
| 0.125 | 5/7 | 71 | 2/3 | 67 | 7/10 | 70 |
| 0.25 | 29/38 | 76 | 15/23 | 65 | 44/61 | 72 |
| 0.5 | 89/116 | 77 | 28/35 | 80 | 117/151 | 77 |
| 1 | 81/96 | 84 | 19/28 | 68 | 100/124 | 81 |
| 2 | 26/28 | 93 | 20/23 | 87 | 46/51 | 90 |
| 4 | 1/1 | 100 | 1/1 | 100 | 2/2 | 100 |
| 8 | 1/1 | 100 | 1/1 | 100 | ||
| Total | 236/292 | 81 | 86/114 | 75 | 322/406 | 79 |
| Total ≤2 | 235/291 | 81 | 84/112 | 75 | 319/403 | 79 |
Previously, Kartsonis et al. (28) concluded that there was no relationship between caspofungin MIC results and clinical outcome for either infection type. Indeed, there is no apparent difference in outcome at each of the MICs in what could be considered a “wild-type” MIC distribution (Tables 1 and 5). Unfortunately, the data set includes only three isolates from patients treated with caspofungin for which the caspofungin MICs were >2 μg/ml (two C. parapsilosis isolates at 4 μg/ml and one C. rugosa isolate at 8 μg/ml). Thus, the clinical data contain too few results for patients infected with isolates with reduced susceptibility to caspofungin to arrive at any firm conclusion regarding the relationship between elevated caspofungin MICs and clinical outcome. The data simply show that clinical failures are distributed evenly across the susceptible wild-type population of infecting isolates. These failures are likely due to factors other than the “drug-bug” interaction. Such limitations of clinical trial data have been noted previously (57). Overall, these data define the susceptible population of Candida species as those for which caspofungin MICs are 2 μg/ml or less.
Development of caspofungin MIC interpretive breakpoints.
Given the MIC distribution shown in Tables 1, 2, and 5 and the clinical relationship between MIC and efficacy, what are the possible breakpoints for BMD MIC testing of caspofungin against Candida? Regarding the category of susceptible, breakpoints at ≤1 μg/ml and ≤2 μg/ml were considered. The MIC distribution profile obtained with the optimal BMD method for over 5,000 clinical isolates indicates that 99.9% of all clinical isolates of Candida spp. are inhibited by ≤2 μg/ml of caspofungin (Table 1). In light of this MIC distribution, it is notable that caspofungin MICs for strains of Candida with documented FKS1 gene mutations (7, 46, 47) and for the published resistant strains (6, 13, 17, 25, 27, 29, 34, 36, 62) were all >2 μg/ml and were usually ≥8 μg/ml (Tables 3 and 4). It is known that such strains respond poorly to echinocandin treatment in animals and humans and contain a glucan synthesis enzyme complex that is less sensitive to inhibition by caspofungin than that of wild-type strains, further confirming their status as caspofungin-resistant strains (47). Such strains are rarely encountered clinically (0.1% of 5,346 clinical isolates); however, when observed they appear to exhibit a class-specific resistance profile (47, 55).
Pertinent PK data for caspofungin include a peak serum concentration of approximately 10 μg/ml and a sustained concentration of >1 μg/ml (total drug concentrations) throughout the dosing interval following a loading dose of 70 mg and a daily dosing regimen of 50 mg (8, 16). The AUC is approximately 120 mg·h/liter (total drug concentration).
PD investigations of caspofungin against Candida have been performed, and both in vitro and in vivo models have demonstrated a correlation between drug dose, organism MIC, and outcome (1, 18, 19, 32). Caspofungin exhibits concentration-dependent killing that is optimized at a peak-to-MIC ratio of ∼4:1 and produces a prolonged (>12 h) postantifungal effect (17, 18, 31). Louie et al. (32) have noted the importance of the total drug exposure (AUC) for determining caspofungin efficacy in a murine infection model of invasive candidiasis. A formal examination of the target AUC/MIC has not been undertaken with caspofungin. The study of Louie et al. (32) employed a single strain of C. albicans and found that the AUC/MIC ratio associated with a stasis endpoint was near 20. Although the echinocandins are significantly bound to human serum proteins, the impact of protein binding on echinocandin activity remains under study, and data generated in animals with yet-again different patterns of protein binding must be interpreted cautiously. In vitro, the agents bind to different human serum proteins and the in vitro impact of this binding varies by agent (40, 44), with caspofungin least affected. As a consequence, the in vivo PD estimates were weighted less heavily in the committee's analysis of the data.
Taken together, the MIC distribution and the PK/PD data would support a caspofungin MIC of either ≤1 μg/ml or ≤2 μg/ml as predictive of efficacy. A caspofungin MIC of ≤2 μg/ml encompasses 99.9% of all clinical isolates of Candida spp. without bisecting any species group. While extensive PD target studies have not been undertaken with caspofungin, the PK of the drug (70-mg loading dose and 50-mg maintenance dose) would produce concentrations above 1 μg/ml (total drug concentration) throughout the treatment period (8, 16). The ability of caspofungin to successfully treat infections due to isolates for which the MIC is as high as 2 μg/ml is strongly supported by the data from clinical trials, as shown in Table 5.
Due to the paucity of isolates for which the caspofungin MIC was elevated (>2 μg/ml), an MIC predictive of resistance cannot be defined based on data from clinical trials. The fact that FKS1 mutants and isolates of Candida spp. in case reports of caspofungin failures (Table 4) generally show MICs of 4 μg/ml to more than 8 μg/ml suggests that the rare isolates for which the MICs exceed 2 μg/ml may not respond optimally to treatment with caspofungin (54). Nevertheless, the consensus of the CLSI Antifungal Subcommittee was that although the data were sufficient to support a susceptible breakpoint of ≤2 μg/ml, additional data were needed before a resistant breakpoint could be established. Given this reasoning, the subcommittee has recommended that isolates for which the caspofungin MIC is ≤2 μg/ml should be considered susceptible and that isolates for which the MIC is greater than 2 μg/ml should be considered “nonsusceptible.” The latter isolates should be subjected to repeat testing and referred to an appropriate reference laboratory for confirmation. It is anticipated that as experience with these uncommon isolates grows, the CLSI Antifungal Subcommittee will ultimately be able to establish a resistant MIC breakpoint.
Interpretive breakpoints for anidulafungin and micafungin against species of Candida.
Although the accumulated in vitro and clinical data to support MIC breakpoints for anidulafungin and micafungin are somewhat less than those used for caspofungin, a parallel logic to that used for caspofungin was employed. This was based in large part on shared mechanisms of action and resistance, a similar MIC distribution profile, cross-resistance data, and the results of clinical trials with each agent.
As shown for caspofungin, the MIC distribution profiles for anidulafungin and micafungin were bimodal, with 98.8% (anidulafungin) to 100% (micafungin) of 5,346 isolates of Candida inhibited by ≤2 μg/ml of each agent (Table 1). Low MICs for anidulafungin and micafungin were observed with C. albicans, C. glabrata, and C. tropicalis (modal MICs of 0.015 to 0.03 μg/ml), whereas the MICs for both agents were higher for C. parapsilosis and C. guilliermondii (modal MICs of 1 to 2 μg/ml).
As noted previously, cross-resistance was not observed between fluconazole and either anidulafungin or micafungin (Table 2). Cross-resistance was observed between both of these agents and caspofungin (Fig. 1a and 1b) and also between each other (Fig. 1c). The essential agreement between anidulafungin and micafungin was 92% (Fig. 1c). The categorical agreement between anidulafungin and caspofungin (Fig. 1a), calculated using the susceptible breakpoint of ≤2 μg/ml and caspofungin as the reference result, was 98.1% with 1.1% very major errors (false susceptible) and 0.1% major errors (false resistant). Likewise, the categorical agreement between micafungin and caspofungin (Fig. 1b) was 99.9%, with only 0.1% major errors.
Additional evidence for cross-resistance among all three echinocandins comes from studies of FKS1 mutants, both laboratory-derived and clinical isolates (Table 4) (47). Balashov et al., Park et al., and Perlin (7, 46, 47) have shown that the FKS1 modification mechanism broadly encompasses the class of echinocandin drugs. Strains of Candida found to contain FKS1 mutations displayed highly elevated MICs for caspofungin, anidulafungin, and micafungin (Table 4) (47).
The results of PK/PD studies for both anidulafungin and micafungin reveal a Cmax of approximately 10 μg/ml and trough concentrations of 1 to 2 μg/ml (8, 16). Both agents exhibit concentration-dependent killing and a prolonged (12 to 24 h) postantifungal effect (19, 20). The AUC (total drug concentration) for anidulafungin (200-mg loading dose and 100-mg maintenance dose) is 112 mg·h/liter, and that for micafungin (100-mg daily dose) is 126 mg·h/liter (8, 16). More-extensive animal model PD target investigation has been undertaken with these echinocandins (2, 3). Similar to caspofungin, the PD indices associated with efficacy for these agents were the AUC/MIC and Cmax/MIC ratios (2, 3, 23, 24, 32). A stasis endpoint in an in vivo model of invasive C. albicans and C. glabrata infection for both anidulafungin and micafungin was achieved at an AUC/MIC ratio of 10 to 20 when free-drug concentrations were considered. The PK of these compounds in patients would be expected to meet and exceed this target for these species (2, 3). This target would not be achieved for the MIC distribution commonly observed with C. parapsilosis (Table 1). However, the impact of the higher MICs observed with C. parapsilosis on this PD target has not yet been examined in these models. As discussed above in the section on caspofungin, pending questions regarding echinocandins and binding to human serum proteins led the committee to weight these data less heavily.
The relationship between MIC and clinical outcome for invasive candidiasis, anidulafungin, and micafungin is shown in Table 6. Importantly, no isolates for which MICs were greater than 2 μg/ml for either agent were observed in the respective clinical trials. The MIC distributions for both anidulafungin and micafungin and isolates from the clinical trials were consistent with those of survey data (Table 1) and define the “susceptible” population. The clinical response to each agent was similar irrespective of the MIC, and there were too few isolates (none) with elevated MICs to make any conclusion regarding resistance. Notably, of the seven isolates of C. parapsilosis for which micafungin MICs were 2 μg/ml, five (71%) were treated successfully (overall response of C. parapsilosis to micafungin was 74%) (Table 6).
TABLE 6.
Relationship between MIC and outcome for treatment of invasive candidiasis with andidulafungin and micafungina
| MIC (μg/ml)b | Results for:c
|
|||
|---|---|---|---|---|
| Anidulafungin
|
Micafungin
|
|||
| S/T | % | S/T | % | |
| 0.008 | 67/70 | 96 | ||
| 0.016 | 11/14 | 79 | 120/149 | 81 |
| 0.03 | 11/13 | 85 | 116/152 | 76 |
| 0.06 | 8/9 | 89 | 12/13 | 92 |
| 0.125 | 1/3 | 33 | 12/14 | 86 |
| 0.25 | 1/1 | 100 | 15/17 | 88 |
| 0.5 | 4/5 | 80 | 19/25 | 76 |
| 1 | 3/5 | 60 | 28/31 | 90.3 |
| 2 | 2/2 | 100 | 5/9 | 56 (71)d |
| Total | 119/135 | 88 | 327/410 | 80 |
| Total ≤2 | 119/135 | 88 | 327/410 | 80 |
MICs were determined in accordance with the standards of CLSI document M27-A3 (11). Total ≤2, total number of patients for whose isolates the drug MIC was 2 μg/ml or less.
S/T, number of patients successfully treated/total number treated.
Five of 7 patients infected with C. parapsilosis for which the MIC was 2 μg/ml were treated successfully.
As seen with caspofungin, the MIC distribution, cross-resistance and resistance mechanism study results, and PK/PD data support anidulafungin and micafungin MICs of ≤1 μg/ml or ≤2 μg/ml as predictive of efficacy. Anidulafungin and micafungin MICs of ≤2 μg/ml encompass 98.8 to 100% of all clinical isolates of Candida spp. without bisecting any species group and represent a concentration that is easily maintained throughout the dosing period. As shown in the data from the clinical trials (Table 6), standard dosing regimens for anidulafungin (200-mg loading dose and 100-mg maintenance dose) and micafungin (100 mg daily) may be used to treat infections due to Candida species for which MICs are as high as 2 μg/ml. An MIC predictive of resistance cannot be defined based on the data from clinical trials.
Recommendations for echinocandin MIC breakpoints.
As done previously (52, 53) the CLSI Antifungal Subcommittee followed a “blueprint” to develop interpretive breakpoints for caspofungin, anidulafungin, and micafungin. The process took into account mechanisms of resistance, analysis of the MIC population distribution, consideration of cross-resistance patterns, analysis of parameters associated with success in PD models of infection, and the results of clinical efficacy studies.
Given the overall in vitro and clinical comparability of these agents, it was decided to utilize the same susceptible breakpoint for all three agents. The CLSI Antifungal Subcommittee decided to recommend a “susceptible only” breakpoint of ≤2 μg/ml, due to the lack of echinocandin resistance in the population of Candida isolates thus far. Although a lower breakpoint would encompass virtually all strains of C. albicans, C. glabrata, and C. tropicalis, a susceptible breakpoint of ≤2 μg/ml was deemed necessary to avoid bisecting the population of C. parapsilosis, a common species that responds clinically to echinocandin therapy despite elevated MICs. Isolates of C. albicans and C. glabrata for which echinocandin MICs are 2 μg/ml, although considered susceptible, are clearly outside of the normal wild-type distribution of echinocandin MICs for these species. Indeed, Garcia-Effron et al. (22) have shown that isolates of C. albicans and C. glabrata with this “reduced susceptibility” phenotype contain substitutions in the conserved distal proline in Fks1p hot spot 1 that are analogous to those occurring naturally in C. parapsilosis. Impaired glucan synthase enzyme kinetics in these strains suggest that such mutations may result in a fitness cost to the cell. This decrease in fitness, coupled with the excellent PK of the echinocandins, likely contributes to the ability of these agents to effectively treat infections due to Candida species for which the MICs are as high as 2 μg/ml (Tables 5 and 6). Regardless, isolates with this unusual phenotype warrant further study, and although they may respond clinically to echinocandin treatment, they could pose problems under conditions of decreased drug penetration.
For strains yielding results suggestive of a “nonsusceptible” category (>2 μg/ml), organism identification and antimicrobial susceptibility test results should be confirmed. Subsequently, the isolates should be saved and submitted to a reference laboratory that will confirm the results by using a CLSI reference dilution method (37, 38). These isolates should be designated “nonsusceptible.” This approach is consistent with that used for antibacterial testing of agents for which resistance is rare or unknown (38).
Balashov et al., Park et al., and Perlin (7, 46, 47) have clearly shown that the Fks1p modification system broadly encompasses the entire class of echinocandin drugs. A 16- to 128-fold change in MIC relative to the MIC of a fully susceptible wild-type strain is consistently observed for all three echinocandins when tested against a strain with FKS1 mutations (47). The MICs for caspofungin and micafungin tend to be somewhat higher than those determined for anidulafungin in such strains (47). This may result in a strain with an FKS1 mutation being classified as nonsusceptible to caspofungin and micafungin but as susceptible to anidulafungin. The clinical significance of such differences remains to be determined; however, the more-conservative approach would be to consider those isolates tested as nonsusceptible to one of the echinocandins to be nonsusceptible to the other agents in the class. Presently, caspofungin results predict those of either anidulafungin or micafungin with an absolute categorical agreement of >98%. For the time being, the susceptibility results for one echinocandin may be considered to be predictive of those for the other two agents in the class.
The so-called “paradoxical effect” refers to the growth of echinocandin-susceptible organisms at highly elevated drug concentrations far in excess of the MIC. Paradoxical growth is not related to FKS1 mutations or modification of the echinocandin sensitivity of the glucan synthase enzyme complex nor to its upregulation in the presence of drug (47). It most likely represents an adaptive stress response and is more of a laboratory-related phenomenon. The relevance of this effect to patient care has not been demonstrated. As such, paradoxical growth should be ignored in determining echinocandin MICs.
It is anticipated that the susceptible and nonsusceptible categories will be further defined through additional study of isolates that are identified during postmarket surveillance efforts for the three echinocandins. This will include detailed characterization of “high-MIC” or nonsusceptible isolates, with a goal of identifying those strains expressing true echinocandin resistance.
Acknowledgments
Beverly Ringenberg provided excellent support in the preparation of the manuscript.
This work was supported in part by grants from Astellas, Merck, and Pfizer.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Andes, D. 2003. In vivo pharmacodynamics of antifungal drugs in the treatment of candidiasis. Antimicrob. Agents Chemother. 471179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., D. J. Diekema, M. A. Pfaller, et al. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., D. J. Diekema, M. A. Pfaller, et al. In vivo pharmacodynamic target investigation for micafungin against C. albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 4.Arathoon, E. G., E. Gotuzzo, L. M. Noriega, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2002. Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiasis. Antimicrob. Agents Chemother. 46451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann, S. P., T. F. Patterson, and J. L. Lopez-Ribot. 2002. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 402228-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baixench, M. T., N. Aoun, M. Desnos-Ollivier, D. Garcia-Hermoso, S. Bretagne, S. Ramires, C. Piketty, and E. Dannaoui. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 591076-1083. [DOI] [PubMed] [Google Scholar]
- 7.Balashov, S. V., S. Park, and D. S. Perlin. 2006. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS 1. Antimicrob. Agents Chemother. 502058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappelletty, D., and K. Eiselstein-McKitrick. 2007. The echinocandins. Pharmacotherapy 27369-388. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekar, P. H., and J. D. Sobel. 2006. Micafungin: a new echinocandin. Clin. Infect. Dis. 421171-1178. [DOI] [PubMed] [Google Scholar]
- 10.Cleary, J. D., G. Garcia-Effron, S. W. Chapman, and D. S. Perlin. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 522263-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2007. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed. Approved standard M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 12.Clinical and Laboratory Standards Institute. 2007. Reference method for broth dilution antifungal susceptibility testing of yeasts. Informational supplement M27-S3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 13.Daneman, N., A. K. Chan, R. Rennie, et al. 2006. The emergence of caspofungin resistance during treatment of recurrent Candida glabrata candidemia. Clin. Microbiol. Infect. 12(Suppl. 4)386. [Google Scholar]
- 14.Deresinski, C. C., and D. S. Stevens. 2003. Caspofungin. Clin. Infect. Dis. 361445-1457. [DOI] [PubMed] [Google Scholar]
- 15.De Wet, N. T. E., A. J. Bester, J. J. Viljoen, et al. 2005. A randomized, double blind, comparative trial of micafungin (FK463) vs. fluconazole for the treatment of oesophageal candidiasis. Aliment. Pharmacol. Ther. 21899-907. [DOI] [PubMed] [Google Scholar]
- 16.Dodds Ashley, E. S., R. Lewis, J. S. Lewis, C. Martin, and D. Andes. 2006. Pharmacology of systemic antifungal agents. Clin. Infect. Dis. 43(Suppl. 1)S28-S39. [Google Scholar]
- 17.Dodgson, K. J., A. R. Dodgson, C. Pujol, et al. 2005. Caspofungin resistant C. glabrata. Clin. Microbiol. Infect. 11(Suppl. 2)364. [Google Scholar]
- 18.Ernst, E. J., M. E. Klepser, M. E. Ernst, S. A. Messer, and M. A. Pfaller. 1999. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 3375-80. [DOI] [PubMed] [Google Scholar]
- 19.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 441108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernst, E. J., E. E. Roling, C. R. Petzold, D. J. Keele, and M. E. Klepser. 2002. In vitro activity of micafungin (FK-463) against Candida sp: microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 463846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espinel-Ingroff, A. 2003. In vitro antifungal activities of anidulafungin, micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 20121-136. [PubMed] [Google Scholar]
- 22.Garcia-Effron, G., S. K. Katiyar, S. Park, T. D. Edlind, and D. S. Perlin. 2008. A naturally occurring Fks1p proline to alanine amino acid change in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 522305-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumbo, T., G. L. Drusano, W. Liu, L. Ma, M. R. Deziel, M. F. Drusano, and A. Louie. 2006. Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis. Antimicrob. Agents Chemother. 503695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumbo, T., G. L. Drusano, W. Liu, R. W. Kulaway, C. Fregeau, V. Hsu, and A. Louie. 2007. Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob. Agents Chemother. 51968-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakki, M., J. F. Staab, and K. A. Marr. 2006. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 502522-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez, S., J. L. Lopez-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 481382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn, J. N., G. Garcia-Effron, M. J. Hsu, S. Park, K. A. Marr, and D. S. Perlin. 2007. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob. Agents Chemother. 511876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kartsonis, N., J. Killar, L. Mixson, C. M. Hoe, C. Sable, K. Bartizal, and M. Motyl. 2005. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob. Agents Chemother. 493616-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krogh-Madsen, M., M. C. Arendrup, L. Heslet, and J. D. Knudsen. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42938-944. [DOI] [PubMed] [Google Scholar]
- 30.Kuse, E. R., P. Chutchotisakd, C. A. da Cunha, et al. 2007. Micafungin versus liposomal amphotericin B for candidemia and invasive candidiasis: a phase III randomized double-blind trial. Lancet 3691519-1527. [DOI] [PubMed] [Google Scholar]
- 31.Laverdiere, M., R. G. Lalonde, J. G. Baril, D. C. Sheppard, S. Park, and D. S. Perlin. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57705-708. [DOI] [PubMed] [Google Scholar]
- 32.Louie, A., M. Deziel, W. Liu, M. F. Drusano, T. Gumbo, and G. L. Drusano. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 495058-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messer, S. A., D. J. Diekema, L. Boyken, S. Tendolkar, R. J. Hollis, and M. A. Pfaller. 2006. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J. Clin. Microbiol. 44324-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, C. D., B. W. Lomaestro, S. Park, and D. S. Perlin. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26877-880. [DOI] [PubMed] [Google Scholar]
- 35.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 3472020-2029. [DOI] [PubMed] [Google Scholar]
- 36.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Committee for Clinical Laboratory Standards. 2001. Development of in vitro susceptibility testing criteria and quality control parameters, 2nd ed. Approved guideline M23-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 38.National Committee for Clinical Laboratory Standards. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. NCCLS M100-S15. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 39.Niimi, K., K. Maki, F. Ikeda, et al. 2006. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob. Agents Chemother. 501148-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odabasi, Z., V. Paetznick, J. H. Rex, and L. Ostrosky-Zeichner. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 514214-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odds, F. C., M. Motyl, R. Andrade, et al. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 423475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, et al. 2003. Antifungal susceptibility survey of 2000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 473149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostrosky-Zeichner, L., D. Kontoyiannis, J. Raffalli, K. M. Mullane, J. Vazquez, E. J. Anaissie, J. Lipton, P. Jacobs, J. H. J. von Rensburg, J. H. Rex, W. Lau, D. Facklam, and D. N. Buell. 2005. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24654-661. [DOI] [PubMed] [Google Scholar]
- 44.Paderu, P., G. Garcia-Effron, S. Balashov, G. Delmas, S. Park, and D. S. Perlin. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 512253-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pappas, P. G., C. M. F. Rotstein, R. F. Betts, et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45883-893. [DOI] [PubMed] [Google Scholar]
- 46.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 493264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updates 10121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfaller, M. A. 2004. Anidulafungin: an echinocandin antifungal. Expert Opin. Investig. Drugs 131183-1197. [DOI] [PubMed] [Google Scholar]
- 49.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Further standardization of broth microdilution methodology for in vitro susceptibility testing of caspofungin against Candida species by use of an international collection of more than 3,000 clinical isolates. J. Clin. Microbiol. 423117-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2005. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J. Clinical Microbiol. 435425-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. Global surveillance of in vitro activity of micafungin against Candida: a comparison with caspofungin by CLSI-recommended methods. J. Clin. Microbiol. 443533-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfaller, M. A., D. J. Diekema, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfaller, M. A., D. J. Diekema, J. H. Rex, A. Espinel-Ingroff, E. M. Johnson, D. Andes, V. Chaturvedi, M. A. Ghannoum, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, P. Troke, T. J. Walsh, and D. W. Warnock. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistant public health problem. Clin. Microbiol. Rev. 20133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfaller, M. A., L. Boyken, R. J. Hollis, J. Kroeger, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2008. In vitro susceptibility of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reboli, A. C., C. Rotstein, P. G. Pappas, S. W. Chapman, D. H. Kett, D. Kumar, R. Betts, M. Wible, B. P. Goldstein, J. Schranz, D. S. Krause, and T. J. Walsh. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 3562472-2482. [DOI] [PubMed] [Google Scholar]
- 57.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35982-989. [DOI] [PubMed] [Google Scholar]
- 58.Rogers, T. R., E. M. Johnson, and C. Munro. 2007. Echinocandidin antifungal drug resistance. J. Invasive Fungal Infect. 199-105. [Google Scholar]
- 59.Vazquez, J. A. 2005. Anidulafungin: a new echinocandin with a novel profile. Clin. Ther. 27657-673. [DOI] [PubMed] [Google Scholar]
- 60.Villanueva, A., E. G. Arathoon, E. Gotuzzo, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2001. A randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin. Infect. Dis. 331529-1535. [DOI] [PubMed] [Google Scholar]
- 61.Villanueva, A., E. Gotuzzo, E. G. Arathoon, et al. 2002. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am. J. Med. 113294-299. [DOI] [PubMed] [Google Scholar]
- 62.Villareal, N. C., A. W. Fothergill, C. Kelly, et al. 2004. Candida glabrata resistance to caspofungin during therapy, Abstr. M-1034, p. 417. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother.
- 63.Walsh, T. J., H. Teppler, G. R. Donowitz, et al. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 3521391-1402. [DOI] [PubMed] [Google Scholar]
- 64.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 1901464-1471. [DOI] [PubMed] [Google Scholar]
- 65.Zaas, A. K., and B. D. Alexander. 2005. Echinocandins: role in antifungal therapy, 2005. Exp. Opin. Pharmacother. 61657-1668. [DOI] [PubMed] [Google Scholar]