Abstract
Cord formation is a characteristic property of the cultured Mycobacterium tuberculosis complex species. We describe a case of Mycobacterium marinum demonstrating robust cord formation. Nontuberculous mycobacteria can form true cords in broth culture but do so rarely, despite the fact that many species contain the cell wall glycolipid that mediates cord formation.
CASE REPORT
A 44-year-old, previously healthy female nurse presented with erythema and swelling at the distal interphalangeal joint of the right index finger, a palpable mass in the second web space of the right hand, and a second mass at the distal right forearm. Approximately 5 weeks prior to presentation, she had sustained a subungual foreign body injury to the right index finger. Around this time, she recalled cleaning her aquarium, which held Siamese fighting fish. Her past medical history included gastroesophageal reflux disease, borderline hypertension, and dermatofibromas of the right leg excised 9 years earlier. Her only medication at the time of presentation was omeprazole at 30 mg twice per day. Physical examination revealed tenderness of the right hand lesions, whose arrangement suggested an incipient lymphangitis. More-proximal lymphadenopathy was not present. The associated joints showed a normal range of motion, and an X-ray of the right hand and wrist did not reveal osteomyelitis or joint destruction. The remainder of her physical examination was unremarkable. The complete blood count was within normal parameters (white blood cells, 7,100/mm3; hemoglobin, 14.2 gm/dl; hematocrit, 39.2%; platelets, 234,000/mm3).
Biopsies of the three lesions were obtained. Direct Gram stains of the ground tissue showed a few mononuclear and polymorphonuclear white blood cells but no organisms. Standard aerobic and anaerobic bacterial cultures were negative. Direct acid-fast staining with an auramine-rhodamine fluorochrome stain was negative. Histological examination revealed acute and chronic inflammation of the superficial and deep dermis, with associated noncaseating granulomas. No organisms were identified histologically by Ziehl-Neelsen, Fite, Grocott methenamine silver, or tissue Gram stains. Mycobacterial broth culture at 30°C in Bactec 12B vials became positive at 9 days, whereas broth culture at 37°C remained negative for the entire 6-week incubation period. Solid medium culture also produced colonies at 30°C (chocolate agar slant) but not at 37°C (Lowenstein-Jensen slant). A Ziehl-Neelsen smear of the 30°C broth culture showed robust cord formation (Fig. 1). DNA probe assays for the Mycobacterium tuberculosis complex, the Mycobacterium avium complex, and Mycobacterium kansasii (AccuProbe; Gen-Probe Inc., San Diego, CA) were negative. Subcultures were prepared on solid medium (Middlebrook 7H11) and were grown shielded and unshielded from light until colonies were mature. Colonies on the shielded plates were nonpigmented (Fig. 2A). Exposure of the shielded cultures to light for 2 h, followed by overnight reincubation, elicited production of carotenoid pigmentation (Fig. 2B). Biochemical studies demonstrated that the isolate produced <45 mm of bubbles by the semiquantitative catalase test, was able to hydrolyze urea, was able to hydrolyze Tween 80 after 5 days of incubation, and was negative for the ability to reduce potassium tellurite in 3 days. On the basis of these findings, the isolate was identified as Mycobacterium marinum. This identification was confirmed by DNA sequencing of a 500-base-pair region of the 16S rRNA gene at a reference laboratory.
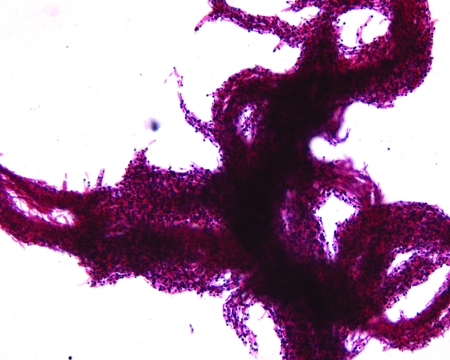
FIG. 1.
Ziehl-Neelsen smear of 30°C broth culture after 9 days of incubation, demonstrating robust cord formation.
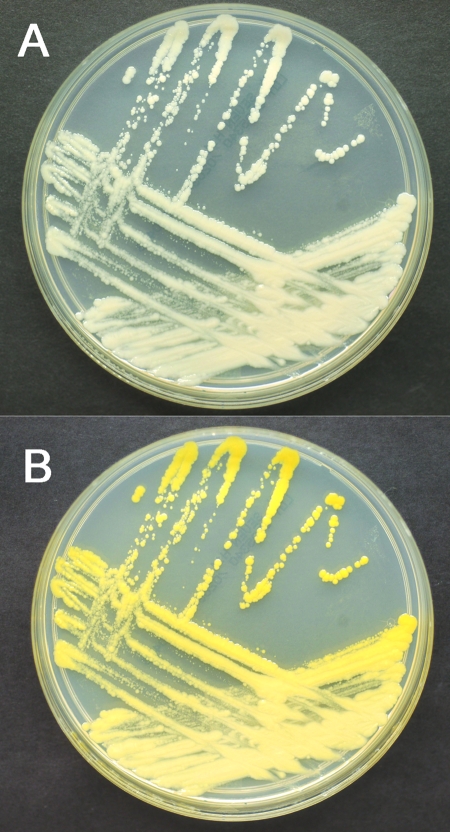
FIG. 2.
Subculture of the isolate grown on Middlebrook 7H11 plates. The top panel (A) shows the isolate prior to light exposure. The bottom panel (B) shows the same plate after light exposure and reincubation, demonstrating that the isolate is a photochromogen.
In this case, the biochemical properties of the mycobacterial isolate, its photochromogenicity, and its preference for low temperature incubation leave no doubt that it is properly identified as M. marinum, and the clinical presentation, as well as the histopathology, is classic for cutaneous infection caused by this organism. However, the finding of tight rope-like “cords” during examination of the broth culture by Ziehl-Neelsen smear was surprising and unexpected.
Cord formation—the aggregation of acid-fast bacilli (AFB) end-to-end and side-to-side to form serpentine structures—is associated with the Mycobacterium tuberculosis complex, and its detection has been found to be a sensitive and specific screen for the M. tuberculosis complex in liquid culture of clinical specimens (5, 7, 9, 13). In most laboratories, the detection of cord formation guides the selection of further testing for culture confirmation and full identification. Some laboratories even report a presumptive identification of the M. tuberculosis complex to physicians on the basis of cord formation in broth culture (7). Cord formation is strictly an in vitro phenomenon, and the proportion of M. tuberculosis isolates that demonstrate this phenomenon has been shown to vary greatly between clinical laboratories (7, 8). This suggests that strain differences and/or differences in the handling of cultures prior to AFB staining may influence cord formation (7, 8). The interpretation of cording morphology, particularly in nontuberculous species, such as M. kansasii, the M. avium complex, M. marinum, and certain rapid growers that can frequently form looser aggregates or “pseudocords,” is also subject to interobserver differences (7, 13).
The factor responsible for cord formation has been identified as trehalose 6, 6′-dimycolate (TDM), a glycolipid with two long-chain beta-hydroxyl alpha-branched fatty acids of variable length (6, 10). TDM is a virulence factor and has myriad immunologic properties, including induction of chronic granulomas in animal models of tuberculosis, making it an attractive therapeutic target (10). TDM has been detected in nontuberculous mycobacteria, including M. avium complex species (3), which rarely form true cords, suggesting that the glycolipid is not sufficient for this property. Alternatively, the specific lengths of the fatty acid chains in TDM or species-specific interactions with other cell wall components may determine its tendency to promote cord formation.
Three homologous secreted proteins with enzyme activity—FbpA, FbpB, and FbpC, or the antigen 85 complex—are required for cord factor biosynthesis in M. tuberculosis (1). Antigen 85 orthologs have been reported to occur in nontuberculous mycobacterial species, such as M. kansasii and M. bovis, as well as in other bacterial genera (12), suggesting that they and their products (such as TDM) must play an important physiological role aside from cord formation. A synthetic, competitive antagonist of these three enzymes has been shown to inhibit cell wall biosynthesis in M. tuberculosis H37Ra, indicating that the cord factor is important to the integrity of the cell wall (1).
A Basic Local Alignment Search Tool (BLAST) search of publicly available genomic databases (National Center for Biotechnology Information) revealed that M. marinum encodes an apparent FbpA ortholog (GenBank accession number AAO39762) with 83% amino acid identity to FbpA from M. tuberculosis H37Rv (GenBank accession number NP_218321) and M. avium (GenBank accession number NP_959150) (Fig. 3), suggesting conservation of at least one cord factor biosynthetic enzyme in M. marinum. The presence of FbpB and FbpC orthologs in M. marinum is unclear and may reflect the incompleteness of available genomic data. Of note, M. marinum 1218 R, a fish outbreak isolate, has been shown to form cords on biofilms generated on synthetic surfaces (4). A series of M. marinum strains have recently been grouped into at least two subtypes, defined by their virulence in zebrafish, pathogenicity in humans, and amplified fragment length polymorphism differences (11). It will be of interest to determine whether properties of cord formation are in any way correlated with these presumptive subtypes.
FIG. 3.
Amino acid sequence alignment of FbpA from M. avium subsp. paratuberculosis K-10, M. marinum, and M. tuberculosis H37Rv, performed using the ClustalW program (2). Amino acid identity is indicated by “*”; “.” indicates similar amino acids; “:” indicates unrelated amino acids.
Detection of cording AFB in broth culture often guides the selection of further testing (8) and in some laboratories leads to a presumptive identification of M. tuberculosis that is reported to physicians (7). This case clearly demonstrates that cord formation is not specific to M. tuberculosis complex species and by itself cannot identify an isolate as belonging to that group of organisms.
Acknowledgments
We thank Colleen Hoffman for her assistance and technical expertise.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 2761420-1422. [DOI] [PubMed] [Google Scholar]
- 2.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 313497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita, Y., T. Naka, M. R. McNeil, and I. Yano. 2005. Intact molecular characterization of cord factor (trehalose 6,6′-dimycolate) from nine species of mycobacteria by MALDI-TOF mass spectrometry. Microbiology 1513403-3416. [DOI] [PubMed] [Google Scholar]
- 4.Hall-Stoodley, L., O. S. Brun, G. Polshyna, and L. P. Barker. 2006. Mycobacterium marinum biofilm formation reveals cording morphology. FEMS Microbiol. Lett. 25743-49. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski, D. A., and D. J. Hardy. 1995. Selective utilization of DNA probes for identification of Mycobacterium species on the basis of cord formation in primary BACTEC 12B cultures. J. Clin. Microbiol. 331548-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu, J., C. E. Barry III, G. S. Besra, and H. Nikaido. 1996. Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J. Biol. Chem. 27129545-29551. [DOI] [PubMed] [Google Scholar]
- 7.McCarter, Y. S., I. N. Ratkiewicz, and A. Robinson. 1998. Cord formation in BACTEC medium is a reliable, rapid method for presumptive identification of Mycobacterium tuberculosis complex. J. Clin. Microbiol. 362769-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris, A. J., and L. B. Reller. 1993. Reliability of cord formation in BACTEC media for presumptive identification of mycobacteria. J. Clin. Microbiol. 312533-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson, S. M., and C. P. Cartwright. 1998. Comparison of algorithms for selective use of nucleic-acid probes for identification of Mycobacterium tuberculosis from BACTEC 12B bottles. Diagn. Microbiol. Infect. Dis. 31537-541. [DOI] [PubMed] [Google Scholar]
- 10.Ryll, R., Y. Kumazawa, and I. Yano. 2001. Immunological properties of trehalose dimycolate (cord factor) and other mycolic acid-containing glycolipids—a review. Microbiol. Immunol. 45801-811. [DOI] [PubMed] [Google Scholar]
- 11.van der Sar, A. M., A. M. Abdallah, M. Sparrius, E. Reinders, C. M. Vandenbroucke-Grauls, and W. Bitter. 2004. Mycobacterium marinum strains can be divided into two distinct types based on genetic diversity and virulence. Infect. Immun. 726306-6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yagupsky, P. V., D. A. Kaminski, K. M. Palmer, and F. S. Nolte. 1990. Cord formation in BACTEC 7H12 medium for rapid, presumptive identification of Mycobacterium tuberculosis complex. J. Clin. Microbiol. 281451-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]