Abstract
The marine bacterium Vibrio fischeri uses two acyl-homoserine lactone (acyl-HSL) quorum-sensing systems. The earlier signal, octanoyl-HSL, produced by AinS, is required for normal colonization of the squid Euprymna scolopes and, in culture, is necessary for a normal growth yield. In examining the latter requirement, we found that during growth in a glycerol/tryptone-based medium, wild-type V. fischeri cells initially excrete acetate but, in a metabolic shift termed the acetate switch, they subsequently utilize the acetate, removing it from the medium. In contrast, an ainS mutant strain grown in this medium does not remove the excreted acetate, which accumulates to lethal levels. The acetate switch is characterized by the induction of acs, the gene encoding acetyl coenzyme A (acetyl-CoA) synthetase, leading to uptake of the excreted acetate. Wild-type cells induce an acs transcriptional reporter 25-fold, coincident with the disappearance of the extracellular acetate; in contrast, the ainS mutant did not display significant induction of the acs reporter. Supplementation of the medium of an ainS mutant with octanoyl-HSL restored normal levels of acs induction and acetate uptake. Additional mutant analyses indicated that acs regulation was accomplished through the regulator LitR but was independent of the LuxIR quorum-signaling pathway. Importantly, the acs mutant of V. fischeri has a competitive defect when colonizing the squid, indicating the importance of proper control of acetate metabolism in the light of organ symbiosis. This is the first report of quorum-sensing control of the acetate switch, and it indicates a metabolic connection between acetate utilization and cell density.
“Quorum sensing” refers to a signaling mechanism used by many bacteria to monitor cell density. The bacteria release small signal molecules into the environment, and they respond once a threshold level of the signal accumulates. Various bacteria use quorum sensing to regulate diverse activities, such as luminescence, motility, protease expression, antibiotic production, and biofilm formation (13). Many of these activities are thought to be most effective when coordinately expressed by many bacteria (2). The first quorum-sensing system characterized was the Vibrio fischeri LuxIR system, in which accumulation of an acyl-homoserine lactone (HSL) signal leads to the induction of luminescence (30). Since that time, dozens of other bacterial quorum-sensing systems have been identified, including those that utilize different signal molecules, such as oligopeptides (8). Some bacteria have multiple systems that can be either independent or integrated. Examples of such integration include parallel systems that converge on the same regulators, or sequential ones in which one system regulates another in addition to its own target genes (27, 29).
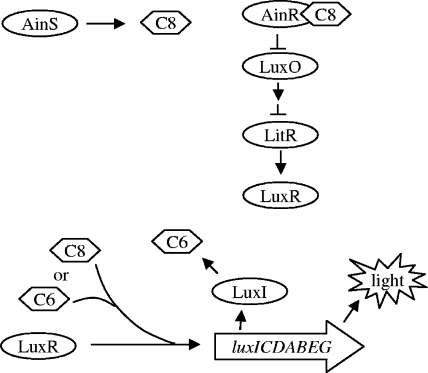
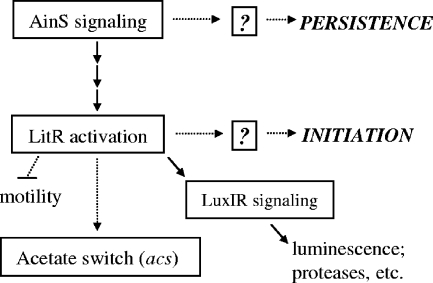
V. fischeri has two acyl-HSL quorum-sensing systems, the AinS system and the LuxIR system, which work together in a sequential manner (Fig. 1) (15, 24, 47). The signal synthase AinS produces octanoyl-l-HSL (C8-HSL) which, at threshold densities achieved in culture, will interact with the receptor AinR and initiate a signaling cascade. AinR binding of C8-HSL represses LuxO, allowing translation of the master transcriptional activator LitR (24). With LuxO repressed, LitR is able to upregulate a number of genes, most notably luxR (14, 40). LuxR can interact with C8-HSL to weakly induce transcription of the luxICDABEG operon, leading to a low level of luminescence and the production of 3-oxohexanoyl-l-HSL (3OC6-HSL) by LuxI (24, 38). Once 3OC6-HSL accumulates to a sufficient concentration, it binds to LuxR, activating it and leading to an even greater induction of the LuxR regulon (11, 24). While AinS regulates a number of activities through LitR, such as rpoS expression and normal motility, examinations of mutant strains have identified activities controlled by AinS independently of LitR, such as normal persistence in the light organ, indicating that LitR controls only one branch of the AinS regulon (14, 23, 40). The sequential nature of this system gives V. fischeri the ability to differentiate between, and respond to, at least three bacterial population conditions: low cell density, when neither autoinducer is sensed; intermediate cell density, when only C8-HSL is sensed; and high cell density, when both C8-HSL and 3OC6-HSL are sensed. In environments that support the growth of V. fischeri, this sequential arrangement also leads to temporal control over the expression of various genes (1, 23).
FIG. 1.
The AinS signaling cascade controlling the lux operon. Proteins depicted in this simplified cascade are transcriptional regulators (AinR, LitR, LuxR), phosphorelay components (LuxO), or signal synthases (AinS, LuxI) (23). The quorum signals serving as coregulators include C8-HSL (C8) or 3OC6-HSL (C6).
V. fischeri exists both free living in seawater and as a bioluminescent symbiont in the light-emitting organs of certain species of squid and fish (21). In V. fischeri ES114, a symbiont of the Hawaiian bobtail squid, Euprymna scolopes, the AinS quorum-sensing system controls a number of symbiotic activities. ainS mutant cells are delayed in initiating the colonization of juvenile squid and, in a separate response, they persist poorly beyond the first 24 h of colonization (23, 24). The mechanisms underlying these defects are unknown. In addition, when cultured in a rich medium, the ainS mutant was reported to have a lower growth yield than wild-type cells, although no specific cause for this difference was identified (23, 24). We hypothesized that the latter result reflects a problem in normal metabolism, i.e., the AinS system directly or indirectly controls an important metabolic pathway associated with efficient metabolism.
The initial experiments described here led us to suspect a defect in the acetate switch, a central regulatory mechanism present in bacteria (50). The acetate switch in Escherichia coli and Salmonella enterica has been well studied, and it describes the shift that occurs as cells change from net excretion of acetate to net uptake and utilization of acetate. This switch is most directly regulated by acetyl coenzyme A (acetyl-CoA) synthetase (Acs). Acs converts acetate to acetyl-CoA, trapping the metabolite in the cytoplasm and making it available for both catabolic and anabolic processes. Although the regulation of acs and the acetate switch is a complex process and is not fully understood, the induction of acs is largely independent of the extracellular acetate concentration and is instead linked to the nutritional state of the cell. Identified regulators include RpoS and cyclic AMP receptor protein (CRP) (3, 20, 39, 50). We report here that the previously described growth defect of an ainS mutant of V. fischeri is due to accumulation of extracellular acetate caused by low acs transcription and the absence of a functioning acetate switch. In this manner, the acidification of the medium points us to a novel connection between cell-cell signaling and metabolic homeostasis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains and plasmids used in this work are listed in Table 1. V. fischeri strains are derivatives of ES114 and were grown at 28°C in a seawater-based tryptone (SWT) medium (per liter, 5 g Bacto-tryptone, 3 g yeast extract, 3 ml glycerol, 700 ml filtered Instant Ocean [Aquarium Systems, Inc, Mentor, OH], and 300 ml distilled water) (4) for all experiments, except as otherwise noted. For mutant construction and plasmid selection and maintenance, Luria-Bertani salt medium (16) was used. Escherichia coli strains used in construction of plasmids were grown at 37°C in either Luria-Bertani medium (36) or brain heart infusion medium (BD, Sparks, MD). Media were solidified with 1.5% agar as needed. When appropriate, antibiotics were added to media at the following concentrations: erythromycin (Erm), 5 μg/ml for V. fischeri and 150 μg/ml for E. coli; chloramphenicol (Cam), 5 μg/ml for V. fischeri and 25 μg/ml for E. coli; and kanamycin (Kan), 100 μg/ml for V. fischeri and 50 μg/ml for E. coli. 3OC6-HSL was obtained from Sigma-Aldrich Corp. (St. Louis, MO); C8-HSL was obtained from Aurora Biosciences (Coralville, IA). Other medium reagents were purchased from Fisher Scientific, Inc. (Fair Lawn, NJ).
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| V. fischeri | ||
| ES114 | Wild-type E. scolopes light organ isolate | 4 |
| CL21 | ainS::cam | 24 |
| CL42 | luxO::kan | 24 |
| CL53 | luxR::erm | 22 |
| PMF8 | litR::kan | 14 |
| VCW2G7 | luxI (frameshift) | 24 |
| MB21124 | acs::Tnerm | This study |
| KV909 | rpoS::erm | K. Visick |
| JB24 | Δcrp | 5 |
| E. coli | ||
| DH5α-λpir | Cloning vector | 17 |
| Plasmids | ||
| pCL112 | ainS in a 2.1-kb fragment in pVO8 with an Erm resistance cassette | 24 |
| pAKD701 | pVSV209 with a promoterless lacZ cassette and a Kan resistance cassette | A. Dunn; 12 |
| pSVS101 | pAKD701 with a V. fischeri acs promoter region inserted in front of promoterless lacZ | This study |
| pVO8 | pACYC with Cam and Erm resistance cassettes | 46 |
| pMF2 | litR in a 1-kb fragment in pVO8 with an Erm resistance cassette | 14 |
Isolation of an acs mutant.
In the first published V. fischeri ES114 genome (GenBank accession no. CP000020.1), acs was reported to be encoded by two open reading frames (ORFs), VF2383 and VF2384 (35), and we therefore suspected that errors existed in the description of the acs locus of ES114. PCR amplification and subsequent sequencing identified frameshift errors in the original sequence, confirming that acs is encoded by a single ORF in ES114, with the new locus tag of VF_2383. The updated sequence (GenBank accession no. CP000020.2) has been published separately as part of a resequencing/reannotation study (26).
During the construction of a transposon mutant library of V. fischeri strain ES114, strains containing insertion mutations in acs were isolated by random transposition into the ES114 genome, followed by identification of the mutants of interest as follows. Transposition was achieved by conjugation with plasmid pMJM10, which encodes (i) a hyperactive Tn5 transposase, an origin of transfer with RP4 transfer functions (oriTRP4), and Kan resistance on its backbone; and (ii) a Tn5 transposon that encodes oriVR6Kγ and Erm resistance. In brief, multiple independent conjugations were conducted by standard methods (45), and Ermr V. fischeri colonies were individually arrayed in 96-well microplates. Colonies that were Kanr were eliminated, and the remaining mutant collection was frozen. Twenty of the Ermr Kans mutants were studied by Southern blotting and verified to contain single insertions (data not shown).
To identify acs mutants in the collection (MB Mutant Collection), we conducted PCRs with 96 template pools of mutants, each of which containing 96 mutants (a total of 9,216 mutants screened). In a variation on the genetic footprinting strategy (42), one primer, MJM-127 (5′-ACAAGCATAAAGCTTGCTCAATCAATCACC), was targeted to both ends of the transposon, facing outward, whereas the other primer, MJM-219 (5′-GTAAGTTTGTTCAAAGCGGTCAT), was specific to the 3′ end of the acs gene, facing upstream. Amplification was conducted using Platinum Taq DNA polymerase High Fidelity (Invitrogen, Carlsbad, CA). Reactions of 10 μl contained 1 μl of cell lysate (pooled, diluted 1:100 in H2O), 1× reaction buffer (supplied with Invitrogen DNA polymerase), 0.2 mM of each deoxynucleoside triphosphate, 2 mM MgSO4, 0.25 μM primer MJM-127, 0.25 μM primer MJM-219, and 0.2 U DNA polymerase. Amplification was conducted by using a PTC-200 thermal cycler (MJ Research, Watertown, MA) using a program of 95°C for 2 min; then 30 cycles of 95°C for 30 s, 55°C for 30 s, and 68°C for 90 s; and a final step of 68°C for 5 min.
Four pools were identified initially as having candidate acs mutants, and the well locations of the candidates were identified in the mutant collection. One of these strains, MB21124, was further streak purified. Arbitrarily primed PCR-based insertion site mapping (10) identified the transposon insertion site to be located after nucleotide 891 in the (revised) acs ORF. All experiments with MB21124 were done in comparison with its parent strain, ES114 isolate MJM1100.
Construction of an acs transcriptional reporter.
To construct an acs′-lacZ+ reporter fusion, the region of DNA from 471 bp upstream and 110 bp downstream of the V. fischeri acs gene start site, including the predicted promoter region, was PCR amplified using primers 5′-ACATGCATGCAGATCGAGCTTGCTTGCGTCAT and 5′-GACTAGTCCTTCAGGGTTGATAACGGATTGC, which contain SphI and SpeI restriction sites (underlined), respectively. The PCR product was isolated, digested, and ligated into SpeI/SphI-digested pAKD701, which contains a promoterless lacZ gene, by using standard genetic techniques. The insertion was confirmed by PCR analysis.
High-performance liquid chromatography (HPLC) analysis.
V. fischeri strains were cultured in the indicated liquid media, and 1-ml samples were taken at the times indicated. The samples were processed as previously described by Weimer et al. (49) and were analyzed by the Weimer laboratory (University of Wisconsin—Madison).
β-Galactosidase assay.
Plasmids pAKD701 and pSVS101 were introduced into the strains indicated by triparental mating as previously described (44). Strains were cultured in SWT medium, and β-galactosidase activity was measured from three independent cultures at the time points indicated (see Fig. 2), using a microtiter dish method modified from Slauch and Silhavy (41). Cell-pellets were frozen at −20°C before resuspension, and the sodium dodecyl sulfate/chloroform step was omitted. The A420 values of the wells were read every 30 s for 1 h using a GeniosPro 96-well plate reader (Tecan, Research Triangle Park, NC). The relative units of β-galactosidase activity were calculated using the following formula: rate (Vmax)/(optical density at 600 nm [OD600] × volume [ml]).
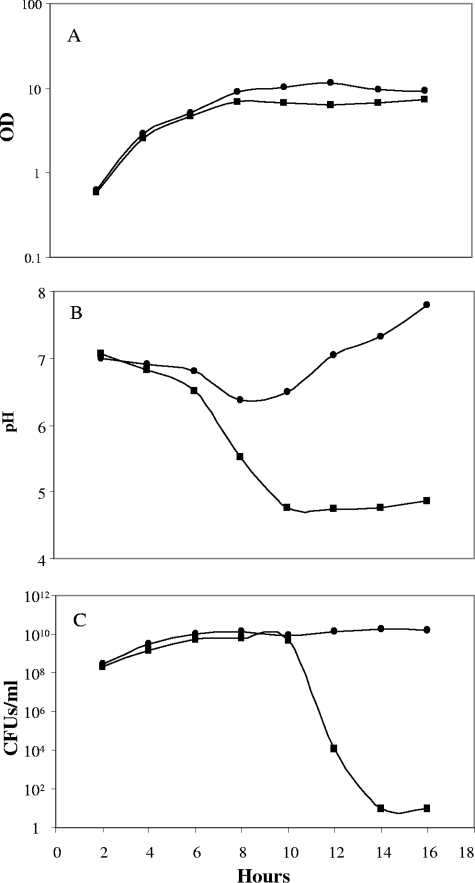
FIG. 2.
Growth characteristics of an ainS mutant of V. fischeri. Growth in SWT medium of wild-type (circles) and ainS (squares) cells was monitored. At the indicated time points, samples were removed and the OD600 (A) and pH (B) were measured, and an aliquot was diluted and plated to determine CFU/ml (C). The pH of an uninoculated flask remained between 7.3 and 7.5. The average results of two separate experiments was plotted.
Colonization assay.
Newly hatched squid were placed in 100 ml of 0.2-μm-filter-sterilized seawater (FSW) containing the appropriate strain or combination of strains at approximately 2,000 CFU/ml. A control group of animals was placed into uninoculated FSW. The single-strain treatments were incubated with the inoculum overnight, while the competition animals were transferred to fresh, uninoculated FSW after 3 h. All animals were transferred to fresh FSW at 16 h postinoculation and sacrificed at 48 h postinoculation and stored at −80°C. Individual animals were homogenized, and the homogenate was diluted and spread onto SWT agar, which was incubated overnight. The colonies were counted, and the mean number of CFU per light organ was calculated. For the competition experiments, about 100 colonies were patched onto selective media to determine the ratio of mutant to wild-type CFU present. The relative competitive index (RCI) was determined by the following formula: (final ratio, mutant CFU/wild-type CFU)/(initial ratio, mutant CFU/wild-type CFU).
Motility assay.
The swimming behavior of V. fischeri was determined by growing strains in SWT medium to approximately 0.3 OD600. The cells were collected by centrifugation of 1 ml of the culture, and the cell pellet was washed with a defined seawater minimal medium (MM) base (23) and suspended in MM to 0.3 OD600. Three microliters of each culture was spotted onto the surface of a 0.25% agar plate containing MM supplemented with 0.3% Casamino Acids. The diameters of the rings of migrating cells were examined after 8 h.
RESULTS
The ainS mutant acidifies the medium to lethal levels.
To examine the basis of the growth yield defect of an ainS mutant, cells of a wild-type isolate of V. fischeri (strain ES114, isolated from a squid light organ) and its ainS mutant derivative were grown in SWT medium overnight, after which the OD and viable counts (CFU/ml) were determined. The ainS culture not only had a relatively low OD yield, as previously noted (24), but also had no viable cells, as determined by CFU (Table 2). A measurement of the culture pH revealed that the ainS cells had acidified the medium to a lethal pH of about 4.6, whereas the wild-type culture showed no loss of viability and did not reduce the medium pH (Table 2). The acidification and subsequent loss of viability were not observed upon the addition of a buffer (50 mM Tris-HCl) to the growth medium (data not shown), further suggesting that acidification is the cause, and not a result, of the cell death. Consistent with previous results (23, 24), ainS mutants carrying a complementing ainS+ allele in trans and wild-type cells grew similarly (data not shown), suggesting that the acidification is not caused by a secondary mutation. It remained possible that the wild-type phenotype was caused not by the cells sensing the signal but instead by the synthesis of the C8-HSL molecule, by consumption of the acid released by the ainS cells, for example, or by excretion of an alkaline product. However, the addition of 40 nM of the C8-HSL signal to the culture medium of an ainS culture also restored the growth yield, viability, and pH to wild-type levels (Table 2), indicating that acidification by the ainS strain is a result of the absence of accumulation of extracellular C8-HSL signal and not a result of blocking the synthesis of the signal. Signal transduction through the AinS pathway is therefore required for proper pH homeostasis in cultures of V. fischeri.
TABLE 2.
Growth yield characteristics of quorum-signaling mutants of V. fischeria
| Strain | OD600b | CFU/ml (1010) | pHb |
|---|---|---|---|
| Wild type | 7.6 | 1.4 ± 0.2 | 7.6 |
| Wild type + 40 nM C8-HSL | 8.0 | 1.9 ± 0.1 | 7.8 |
| ainS | 6.7 | NDc | 4.6 |
| ainS + 40 nM C8-HSL | 8.0 | 2.0 ± 0.1 | 7.7 |
| luxO | 8.3 | 0.7 ± 0.1 | 7.3 |
| ainS luxO | 8.0 | 0.8 ± 0.1 | 7.2 |
| litR | 6.9 | ND | 4.5 |
| luxR | 8.6 | 1.8 ± 0.1 | 7.6 |
| luxI | 6.6 | 0.7 ± 0.6 | 7.6 |
Data are terminal values for triplicate cultures of each strain grown in SWT medium for 18 h (stationary phase).
All errors were within 4%.
ND, none detected (less than 102 CFU/ml).
Culture acidification by the ainS mutant becomes apparent during mid-exponential phase.
To determine when medium acidification and cell death occur in an ainS culture, the OD, pH, and CFU/ml of both the wild type and the ainS derivative were monitored during 16 h of growth in SWT medium (Fig. 2). The pH of both cultures dropped during the period of early exponential growth, with a difference between the two becoming apparent at 6 h; by 8 h, a difference between the ODs of the two cultures had begun to become evident. At 10 h after inoculation, the pH of the ainS culture reached its lowest point and was soon followed by a dramatic drop in CFU/ml. The initial rate of acidification by the wild-type cells was more moderate than that of the ainS culture, and the acidification was reversed starting between 8 and 10 h of growth. Eventually, the pH of this culture reached a level that was higher than that of the uninoculated medium. This increase in pH of the wild-type culture is likely due to net production of ammonia resulting from the catabolism of amino acids in the medium, and it suggests that these cells may have produced acids while growing on the glycerol in the medium and then switched to utilization of the peptides and production of ammonia later in growth (50).
The ainS mutant accumulates acetate in the culture medium.
Because earlier studies of V. fischeri acidification of a different culture medium identified accumulated pyruvate as the cause of low culture pH (34), we examined the organic acids present in cell cultures to determine which, if any, were responsible for the ainS culture acidification. Cell-free overnight culture supernatants of wild-type and ainS strains grown in SWT medium were collected and analyzed by HPLC. Results of this analysis are listed in Table 3. Of the levels of organic acids detected, only those of acetic acid differed between the wild-type and ainS cultures, reaching 14 mM in the mutant culture. The addition of acetic acid to uninoculated SWT medium to this concentration produced a pH of 3.5, indicating that the presence of the acetic acid is sufficient to account for the pH drop caused by growth of the ainS strain. Because the wild-type culture, like that of the ainS mutant, acidified the medium during early exponential phase (Fig. 2), the culture supernatants of these two strains were analyzed at 4-h intervals to determine whether the early acidification was also caused by acetate excretion (Table 2). Four hours after inoculation, the wild-type and ainS cultures had accumulated similar amounts of acetate (between 5 and 7 mM); however, the acetate level in the wild-type culture decreased dramatically by 8 h, while that in the ainS mutant culture continued to rise. This result indicated that both wild-type and ainS cells initially excrete acetate into the extracellular medium, but only wild-type cells are able to subsequently remove the acid.
TABLE 3.
Appearance of organic acids during the growth of V. fischeri strains in SWT mediuma
| Time (h) | Acetate (mM)
|
Lactate (mM)
|
||
|---|---|---|---|---|
| Wild type | ainS | Wild type | ainS | |
| 0 | 0.1 | 0.2 | NDb | ND |
| 4 | 5.5 | 6.9 | ND | ND |
| 8 | 1.1 | 9.7 | 1.7 | 0.3 |
| 12 | 0.3 | 13.4 | 4.9 | 5.1 |
| 16 | 0.2 | 14.1 | 5.5 | 5.2 |
None of the following appeared at >1.5 mM above SWT medium controls: formate, isobutyrate, isovalerate, propionate, succinate, valerate, and 2-methylbutyric acid. Uninoculated media had between 0.1 and 0.2 mM acetate. Values are the average results from three cultures.
ND, none detected.
Wild-type cells remove acetate from the medium following acs induction.
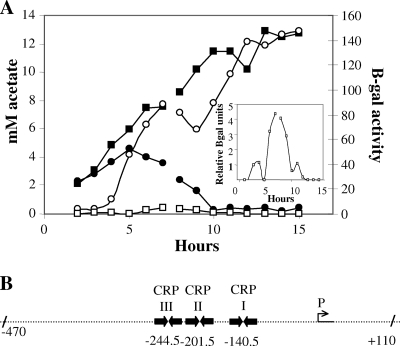
The metabolic behavior of wild-type V. fischeri (Fig. 2) is similar to that of E. coli growing on a mixture of acetogenic and nonacetogenic compounds (50). To determine whether acetate removal by V. fischeri is associated with the acetate switch, i.e., with the induction of acs, a transcriptional fusion of the V. fischeri acs promoter region (Fig. 3B) to a promoterless lacZ gene was constructed on a plasmid that was conjugated into wild-type and ainS strains. The resulting reporter strains were used to determine the relationship between the kinetics of acetate accumulation and acs expression (Fig. 3A). Growth rates of all the strains were the same regardless of the presence of the fusion plasmid or the empty vector (data not shown). Both wild-type and ainS cultures began to accumulate extracellular acetate by 2 h postinoculation, and the cultures accumulated similar amounts until 4 h. The wild-type culture showed a net decrease of acetate in the medium after 5 h and had removed almost all by 10 h. In contrast, the ainS mutant continued excreting acetate until approximately 13 h, by which point the concentration was 13 mM. Induction of acs expression could be detected for wild-type cells as early as 4 h, preceding the appearance of net acetate removal, which was seen 2 h later. In the ainS cells, acs was barely detectable across most of the growth curve. There was, however, a small but reproducible increase in acs′-lacZ+ expression in the ainS cells between 5 and 7 h (Fig. 3, inset), indicating that transcription may still be responding to other, unknown, and relatively minor, activators of the acetate switch.
FIG. 3.
Transcriptional control of V. fischeri acs. (A) Relationship between the expression of acs and acetate uptake during growth in SWT medium. Acetate accumulation in the culture supernatant (closed symbols) and acs expression in the bacteria (open symbols) were determined for wild-type (circles) and ainS (squares) strains containing an acs fusion plasmid. Each point is the average of results for two cultures; two growth curves were done, overlapping at 7 and 8 h postinoculation. The overlapping points were similar for the two experiments; the break in the graph indicates where the data set for one experiment ends and the other begins. Inset, the small, but detectable, transient induction of acs expression that can be seen with the ainS culture. (B) Features of the V. fischeri acs promoter region. Sequence analysis identified three potential CRP binding sites: CRP I (TCTGAn6TCTAA), CRP II (ACTGAn6TCTCA), and CRP III (GGTGAn6TGACA). Based on spacing, a potential −10 promoter site (AATAAT) has been identified at −44 (P). Hatch marks indicate the region used in the promoter plasmid. Because we could not unambiguously identify the transcriptional start site by homology, we have numbered the base pairs with respect to the translational start site.
The acidification phenotype is regulated through LitR and is independent of LuxR signaling.
To determine the pathway through which acs expression and acetate utilization are regulated, we examined the final culture pH of strains of V. fischeri with mutations at various points in the quorum-sensing pathway. As expected, the luxO mutant, which would signal downstream transcriptional activators even in the absence of C8-HSL, does not produce excess acid. Consistent with the proposed model, an ainS luxO double mutant also resembles wild-type cultures, indicating that the control of acs is signaled downstream of LuxO. The litR mutant, which still produces and senses the AinS signal, C8-HSL, but cannot produce the transcriptional activator LitR, acidified the culture in a manner similar to that of ainS (Table 2), suggesting that the LitR branch of the AinS signaling pathway regulates acs activity (Fig. 1). Complementing the litR mutant with the litR+ gene on a plasmid restored wild-type growth in culture (data not shown). In contrast, neither the luxR nor the luxI mutant acidified the medium, but instead, each grew like the wild type. In addition, an rpoS mutant was tested and found to have a wild-type phenotype (data not shown), indicating that the LitR and AinS control of acidification is not through rpoS. Taken together, these data indicate that the control of acs expression by AinS is regulated at a point downstream of LitR but is independent of LuxIR quorum signaling. Therefore, the LitR-dependent pathways that regulate luxR and acs are distinct.
C8-HSL, but not 3OC6-HSL, induces acs′-lacZ+ expression in the ainS mutant.
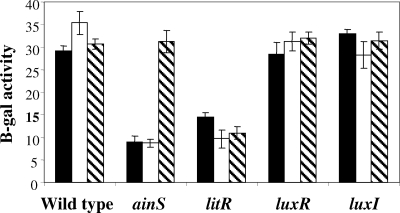
To examine the effect of acyl-HSL quorum-sensing signals on acs expression in V. fischeri, bacteria were grown in media supplemented with synthetic C8-HSL or 3OC6-HSL. The addition of 40 nM of either signal to wild-type cultures had no effect on acs′-lacZ+ expression (Fig. 4). In contrast, the addition of the C8-HSL signal to ainS mutant cells restored acs′-lacZ+ expression to wild-type levels (Fig. 4), showing that, like acidification, the absence of normal acs induction in the ainS mutant results from the lack of signal accumulation and not from a secondary mutation or from a disruption caused by blocking signal synthesis. As expected, the addition of 3OC6-HSL to the ainS mutant culture had no effect on acs expression. Consistent with the position of LitR downstream of the C8-HSL receptor in the signal cascade (Fig. 4), C8-HSL addition had no effect in the litR mutant background.
FIG. 4.
Effect of quorum-sensing signals on the expression of acs by V. fischeri. The relative level of transcription of an acs′-lacZ+ promoter reporter was determined with strains of wild-type and quorum-sensing mutants containing the acs fusion plasmid. The SWT culture medium was supplemented with either 40 nM 3OC6-HSL (white bars), 40 nM C8-HSL (striped bars) or a buffer blank (black bars). The standard errors for three replicate experiments are shown. B-gal, β-galactosidase.
Consistent with the culture phenotype, the acs expression level of the ainS luxO mutant was partially restored, to twice the level observed for the ainS mutant (data not shown). Though expression was not fully restored, this level of expression is sufficient to lead to the removal of excess acetate before it reaches lethal levels (Table 2).
The V. fischeri acetate switch is repressed by glucose.
Because the acs promoter region in V. fischeri contained putative CRP binding sequences (Fig. 3B), we examined acs expression in wild-type cells grown with and without glucose to determine whether similar repression levels occur under these conditions. Because V. fischeri produces excess pyruvate when grown on glucose (34), comparisons based on culture OD and pH were not performed, and the media used were buffered with 50 mM Tris-HCl. Wild-type cultures were grown in buffered SWT medium with or without added 0.3% glucose, and acs expression was determined at 8 h. The culture grown with glucose reached only 14% of the level of acs′-lacZ+ expression that was found with cells grown without glucose. This difference in yield suggests that, as in E. coli, glucose may repress the V. fischeri acetate switch by inhibiting CRP binding to the acs promoter. The addition of a saturating concentration (120 μM) of synthetic C8-HSL raised acs expression by only 1.5%, indicating that AinS signaling alone cannot overcome the effects of catabolite repression. A V. fischeri CRP mutant was examined to confirm the role of this activator in acs expression; however, the extremely poor growth of this strain prevented any meaningful conclusions.
The acs mutant is unable to utilize acetate.
When the growth of the acs mutant of V. fischeri in SWT medium was examined, it was similar to that of the wild type, with a final OD600 of 8.3, and a pH of 7.8. When the culture supernatant was analyzed by HPLC, we found that, like the wild type (Table 3), the culture accumulated only 3.6 mM acetate by 4 h; however, this level was not diminished during further growth (data not shown). These results are consistent with reports that less acetate is produced by an E. coli acs mutant than by the wild type (9).
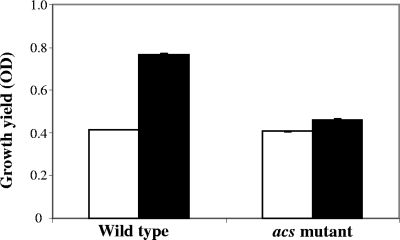
V. fischeri is unable to grow on acetate as a sole carbon source, but it can utilize it in the presence of other nutrients. To determine whether the acs mutant retains the ability to use acetate, we determined the effect of acetate addition on the yield of wild-type and acs cultures grown in a MM with a limiting concentration of Casamino Acids (0.1% [wt/vol]). The final yield of the wild type was almost two times higher with added acetate (Fig. 5), indicating its ability to consume and utilize this nutrient. In contrast, while an acs culture grows as well as the wild type on Casamino Acids alone, the addition of acetate produces only a minor yield increase. Taken together, these data suggest that the acs mutant has a significant defect in acetate utilization.
FIG. 5.
Effect of acetate on the growth yield of V. fischeri wild type and acs mutant. Strains were grown in MM containing Casamino Acids (0.1% wt/vol) as the sole carbon and nitrogen source (white bar) or in the same medium supplemented with 50 mM acetate (black bar). The standard errors for three replicate experiments are indicated. OD, OD600.
acs mutants are not hypermotile.
To determine whether alterations in the acetate switch are responsible for the previously observed hypermotility of ainS and litR mutants (23), the swimming behavior of the acs mutant was examined by soft agar motility assay. As expected, the extent of migration of the ainS strain was 2.7 times that of its parent; in contrast, the extent of migration of the acs mutant was 0.92 times that of its parent. These observations indicate that the hypermotility phenotype of the ainS and litR mutants is not a result of misregulation of the acetate switch.
acs mutants show a competitive defect in animal colonization.
In single-strain inoculation assays, the acs mutant reached the same levels of symbiotic light organ colonization as its wild-type parent at 48 h, suggesting that this mutant was able to proliferate in the environment of the light organ. However, in a more challenging colonization assay, newly hatched squid were coinoculated with equal numbers of the acs mutant and wild-type cells. Forty-eight hours after inoculation, the symbiont population was dominated 3.6-fold by wild-type cells (log RCI, −0.55 ± 0.24), and this competitive disadvantage was still evident at 72 and 96 h postinoculation. Thus, the capacity to employ the acetate switch to adjust its metabolism is an important part of a symbiont's adaptation to the conditions in the light organ.
DISCUSSION
The work presented here yields a number of interesting conclusions. It is the first report of quorum-sensing control of the acetate switch and greatly informs our understanding of the relationship between these two regulatory systems. These results not only explain the previously observed growth defect of the ainS mutant but also extend our understanding of the architecture of the ainS signaling pathway and suggest that quorum sensing, and LitR in particular, has a more critical role in cell homeostasis than previously thought. Thus, it appears that regulation of the acetate has an additional layer that had not been anticipated, namely, the integration of extracellular signaling that is independent of the metabolic state of the cell. Finally, we have confirmed a role for acs in the squid-vibrio symbiosis, underscoring the importance of the acetate switch in the adaptation of V. fischeri to this niche and providing a potential model system for investigating the role of the acetate switch in host-microbe associations.
The acetate switch is used by bacteria to balance the excretion and utilization of acetate; more generally, this regulation allows the cell to effectively manage nutrient resources. Bacteria excrete acetate (in the form of acetic acid) when the flow of carbon through glycolysis exceeds the capacity of the tricarboxylic acid cycle to utilize and recycle the resulting products. This condition arises either because of a high rate of glycolysis or because one or more steps in the tricarboxylic acid cycle is slowed or blocked (50). As nutrient conditions change, these bacteria can switch their metabolism and begin assimilating extracellular acetate through the action of Acs. This switch allows the cell to efficiently utilize available nutrients, even turning a waste product into a carbon and energy source, as well as managing the levels of acetate and acetyl-CoA. These compounds are important both as central metabolites and, along with acetyl-phosphate, in signaling within the cell through changes in their ratios (50). This signaling can affect processes such as phosphate acquisition and motility (32, 50).
acs regulation by quorum sensing.
To our knowledge, this is the first report of quorum signals regulating the acetate switch, adding an important class of environmental sensing and response elements (i.e., autoinducers) to the list of control mechanisms for this critical metabolic shift point. For E. coli, a number of regulators of acs have been identified. Most are either direct or indirect indicators of the physiological state of the cell, such as the catabolite repressor protein CRP, and nucleoid proteins FIS and IHF (3, 6). Except as it affects the nutrients available to the cell, the extracellular environment of the cell has not previously been shown to have a direct impact on the acetate switch itself (50). Here, we have identified an extracellular signal that, in V. fischeri, is required (though not sufficient) for typical induction of acs and is produced and sensed regardless of the physiological state of the cell. This discovery suggests that for V. fischeri, at least, the extracellular environment has an impact on this central metabolic switch point. It is unknown whether such regulation is restricted to V. fischeri or will be found for other organisms as the switch is studied in more species (50).
The two quorum-sensing systems in V. fischeri have previously been shown to be sequential, with control over different activities (23, 24). The work reported here identifies another gene, and with it an entire metabolic system, that is regulated by LitR, further discriminating the two quorum-sensing systems (Fig. 6). The timing of the induction of acs is consistent with the induction of luminescence (24), which is also regulated though LitR; however, it is difficult to draw any conclusions as to the basis of this cooccurrence. Acs is not regulated through LuxR, further supporting the notion that these two quorum-sensing systems do not work in a parallel manner or as coincidence detectors converging on the same regulators, as is the case for quorum sensing in Vibrio harveyi and Vibrio cholerae (18, 28, 29). In contrast, and similar to the V. fischeri system, both the Vibrio anguillarum and the Pseudomonas aeruginosa quorum-sensing systems operate sequentially (29, 43). In the latter species, for instance, the Las system controls expression of the downstream Rhl system. Such a sequential arrangement suggests that these species must respond in different manners to different cell densities.
FIG. 6.
Model of AinS regulation of the acetate switch in V. fischeri. AinS signaling, which regulates an unknown activity(ies) required for persistent colonization in the symbiosis (23), sits atop a phosphorelay cascade that ultimately leads to translation of the master regulator LitR. LitR, which controls luminescence and protease activity through LuxIR induction (1), also blocks motility, and it activates transcription of acs. By an independent route, LitR regulates an unknown gene(s) required for normal initiation of symbiosis (14). Solid lines indicate direct steps that have been demonstrated. Dotted lines indicate an unknown number of steps.
It remains unclear how LitR controls acs expression. Though this regulator's binding site at the luxR promoter has been identified, no “LitR box” sequence has been identified that can account for all LitR-regulated genes (14, 40). Thus, a bioinformatic examination of the acs promoter region has failed to indicate whether LitR might directly activate acs, and it is possible that LitR controls an intermediate gene that is responsible for acs induction. The glucose repression of the acetate switch and the small induction of acs in ainS cultures (Fig. 3) indicate that regulators besides LitR also play a role in modulating the acetate switch. We conclude that quorum sensing has been integrated into a major role in the control of the V. fischeri acetate switch.
Role of acs regulation in symbiosis.
Previous studies have shown that quorum sensing in V. fischeri controls functions important for host interactions, such as luminescence and motility (23, 48) (Fig. 6); we report here that quorum sensing is also involved in controlling the bacterium's cellular homeostasis. The latter role may reflect the fact that in nature, bacteria experience high population densities only under certain conditions, such as during an association with a host. We hypothesize that V. fischeri has coopted quorum sensing as a mechanism to control different nutrient acquisition strategies in different environments. Under moderate to high cell density, V. fischeri is poised to utilize extracellular acetate through the activation of acs by the AinS/LitR quorum-sensing pathway. Our data suggest that nonsymbiotic V. fischeri free-living in the seawater would fail to activate acs to levels sufficient for the utilization of extracellular acetate, forcing cells to use different sources of carbon or to enter periods of quiescence. Such a model is testable and allows us to pose future questions to examine the role of acetate utilization during different stages of growth by V. fischeri populations. Additionally, the “diffusion-sensing” model (19, 33) postulates that conditions other than bacterial population levels may, at least theoretically, be able to alter the sensing of quorum signals. The acetate switch will be most metabolically efficient if the excreted acetate remains in the cells' immediate environment. As such, parameters including both cell density and extracellular diffusion rate are likely to be relevant to its control. Acetate utilization not only may be a direct nutritional benefit to the individual bacterium but also may benefit the population by removing this acidifying waste product before it reaches toxic levels. It is of note that acs is controlled by the lower-density quorum-sensing system (AinS), which would be most sensitive to differences in diffusion. In contrast, LuxIR, the hallmark quorum-sensing system in V. fischeri, is likely to be induced only if the local diffusion rate is low enough that the AinS signal threshold can be maintained.
Because the cell densities required for activation of the quorum-sensing systems in strains like V. fischeri ES114 may occur in nature only during association with a host (4), it has been thought that many, if not most, quorum-controlled activities in this species are important for establishing or maintaining these associations (1). However, it is unclear what role the acetate switch has in the squid-vibrio symbiosis. When inoculated as a clonal population, acs mutants are capable of initiating colonization and establishing a normal population size in the light organ, indicating that the acetate switch is not crucial for symbiosis. However, the competition defect of the acs mutant clearly indicates that there is a disadvantage to cells with improperly regulated acetate metabolism. We expect that the threefold defect we detected after only 2 days would be more dramatic over longer periods, as this association is a long-term mutualism that lasts the lifetime of the host (>6 months). Because the ability to efficiently utilize the available nutrients is an important part of any bacterial-host association, such a disadvantage is not surprising.
Newly hatched squid do not encounter clonal populations of environmental V. fischeri, so a defect in the acetate switch would be detrimental to a bacterium in nature. It also remains possible that the acetate switch takes on an even greater importance later in symbiotic development (i.e., in the adult light organ), when population levels and increased competition for nutrients may occur. Further research into the nutritional conditions of, and gene regulation within, symbionts in the adult light organ may inform our understanding of the coordination between the AinS system and the acetate switch, as well as the role of acetate regulation in maintaining symbioses in general.
The impact of acetate metabolism and host association has been examined with mammalian gut systems, where acetate is a major product of and substrate for microbial metabolism and can be taken up by the host (25, 50); however, in vivo experiments are limited by the difficulty of access to the gut as well as the large number species inhabiting it. Comparisons of the role of acetate metabolism in the light organ, colonized by a single species of bacteria, and mammalian gut systems may provide insights into a general impact of acetate on host-microbe interactions.
One possible role of the acetate switch in symbiosis is the production and removal of acetate as a signal molecule in bacterium-host communication. Chemical interactions between microbes and animals are critical in establishing and maintaining symbioses. For example, in the mammalian enteric tract, bacterium-derived short-chain fatty acids can be absorbed by the host and, in addition to being used as a nutrient, can trigger tissue development and the maturation of innate immunity (7, 31, 37). It is possible that colonizing cells of V. fischeri must present acetate at certain times or locations and that the ainS mutants may either secrete too much acetate or produce it at an inappropriate stage of symbiosis development.
This present study both expands our understanding of the impact of the AinS signaling on the physiology of V. fischeri and reveals a new strategy for regulation of the acetate switch in bacteria. Previous work on the acetate switch has identified a number of regulators that respond to changes in the metabolic state of the cell. In V. fischeri, this metabolic program is regulated by both the nutritional state of the cell and an extracellular, cell density-dependent signal.
Acknowledgments
We thank C. Brennan for technical assistance with the transposon mutant library, A. Dunn (University of Oklahoma) for the gift of pAKD701, J. Escalante (University of Wisconsin—Madison) for providing helpful comments on the manuscript, E. Stabb (University of Georgia—Athens) for the transposon vector precursor, K. Visick (Loyola University Chicago) for the gift of KV909, and P. Weimer (University of Wisconsin—Madison) for HPLC analysis. We would also like to thank an anonymous reviewer for extensive and helpful comments.
The research was supported by an NIH grant (RR 12294) to E.G.R. and M. McFall-Ngai. Additional support was provided by NSF grant IOB 0517007 (to M. McFall-Ngai and E.G.R.), NIH grant T32 GM07215 (to the University of Wisconsin—Madison Microbiology Doctoral Training Program in support of S.V.S.), and a Ruth L. Kirschstein National Research Service Award from the National Institute of General Medical Sciences to M.J.M.
Footnotes
Published ahead of print on 16 May 2008.
REFERENCES
- 1.Antunes, L. C., A. L. Schaefer, R. B. Ferreira, N. Qin, A. M. Stevens, E. G. Ruby, and E. P. Greenberg. 2007. Transcriptome analysis of the Vibrio fischeri LuxR-LuxI regulon. J. Bacteriol. 1898387-8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassler, B. L. 2002. Small talk. Cell-to-cell communication in bacteria. Cell 109421-424. [DOI] [PubMed] [Google Scholar]
- 3.Beatty, C. M., D. F. Browning, S. J. Busby, and A. J. Wolfe. 2003. Cyclic AMP receptor protein-dependent activation of the Escherichia coli acsP2 promoter by a synergistic class III mechanism. J. Bacteriol. 1855148-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boettcher, K. J., and E. G. Ruby. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 1723701-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bose, J. L., U. Kim, W. Bartkowski, R. P. Gunsalus, A. M. Overley, N. L. Lyell, K. L. Visick, and E. V. Stabb. 2007. Bioluminescence in Vibrio fischeri is controlled by the redox-responsive regulator ArcA. Mol. Microbiol. 65538-553. [DOI] [PubMed] [Google Scholar]
- 6.Browning, D. F., C. M. Beatty, E. A. Sanstad, K. E. Gunn, S. J. Busby, and A. J. Wolfe. 2004. Modulation of CRP-dependent transcription at the Escherichia coli acsP2 promoter by nucleoprotein complexes: anti-activation by the nucleoid proteins FIS and IHF. Mol. Microbiol. 51241-254. [DOI] [PubMed] [Google Scholar]
- 7.Bugaut, M. 1987. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp. Biochem. Physiol. B 86439-472. [DOI] [PubMed] [Google Scholar]
- 8.Camilli, A., and B. L. Bassler. 2006. Bacterial small-molecule signaling pathways. Science 3111113-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contiero, J., C. M. Beatty, S. Kumari, C. L. DeSanti, W. R. Strohl, and A. J. Wolfe. 2000. Effects of mutations in acetate metabolism on high-cell-density growth of Escherichia coli. J. Ind. Microbiol. Biotechnol. 24421-430. [Google Scholar]
- 10.Danese, P. N., L. A. Pratt, and R. Kolter. 2001. Biofilm formation as a developmental process. Methods Enzymol. 33619-26. [DOI] [PubMed] [Google Scholar]
- 11.Dunlap, P. V. 1999. Quorum regulation of luminescence in Vibrio fischeri. J. Mol. Microbiol. Biotechnol. 15-12. [PubMed] [Google Scholar]
- 12.Dunn, A. K., D. S. Millikan, D. M. Adin, J. L. Bose, and E. V. Stabb. 2006. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 72802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunny, G. M., and S. C. Winans (ed.). 1999. Cell-cell signaling in bacteria. ASM Press, Washington, DC.
- 14.Fidopiastis, P. M., C. M. Miyamoto, M. G. Jobling, E. A. Meighen, and E. G. Ruby. 2002. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45131-143. [DOI] [PubMed] [Google Scholar]
- 15.Gilson, L., A. Kuo, and P. V. Dunlap. 1995. AinS and a new family of autoinducer synthesis proteins. J. Bacteriol. 1776946-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graf, J., P. V. Dunlap, and E. G. Ruby. 1994. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 1766986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of E. coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 18.Henke, J. M., and B. L. Bassler. 2004. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 1866902-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hense, B. A., C. Kuttler, J. Muller, M. Rothballer, A. Hartmann, and J. U. Kreft. 2007. Does efficiency sensing unify diffusion and quorum sensing? Nat. Rev. Microbiol. 5230-239. [DOI] [PubMed] [Google Scholar]
- 20.Kumari, S., E. J. Simel, and A. J. Wolfe. 2000. σ70 is the principal sigma factor responsible for transcription of acs, which encodes acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 182551-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, K. H., and E. G. Ruby. 1994. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl. Environ. Microbiol. 601565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupp, C., and E. G. Ruby. 2004. Vibrio fischeri LuxS and AinS: comparative study of two signal synthases. J. Bacteriol. 1863873-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lupp, C., and E. G. Ruby. 2005. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol. 1873620-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lupp, C., M. Urbanowski, E. P. Greenberg, and E. G. Ruby. 2003. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol. Microbiol. 50319-331. [DOI] [PubMed] [Google Scholar]
- 25.Macfarlane, S., E. J. Woodmansey, and G. T. Macfarlane. 2005. Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl. Environ. Microbiol. 717483-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandel, M. J., E. V. Stabb, and E. G. Ruby. 2008. Comparative genomics-based investigation of resequencing targets in Vibrio fischeri: focus on point miscalls and artefactual expansions. BMC Genomics 9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55165-199. [DOI] [PubMed] [Google Scholar]
- 28.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110303-314. [DOI] [PubMed] [Google Scholar]
- 29.Milton, D. L. 2006. Quorum sensing in vibrios: complexity for diversification. Int. J. Med. Microbiol. 29661-71. [DOI] [PubMed] [Google Scholar]
- 30.Nealson, K., T. Platt, and J. Hastings. 1970. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 104313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel, P., B. B. Nankova, and E. F. LaGamma. 2005. Butyrate, a gut-derived environmental signal, regulates tyrosine hydroxylase gene expression via a novel promoter element. Brain Res. Dev. Brain Res. 16053-62. [DOI] [PubMed] [Google Scholar]
- 32.Pruss, B. M., and A. J. Wolfe. 1994. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol. Microbiol. 12973-984. [DOI] [PubMed] [Google Scholar]
- 33.Redfield, R. J. 2002. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 10365-370. [DOI] [PubMed] [Google Scholar]
- 34.Ruby, E. G., and K. H. Nealson. 1977. Pyruvate production and excretion by the luminous marine bacteria. Appl. Environ. Microbiol. 34164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruby, E. G., M. Urbanowski, J. Campbell, A. Dunn, M. Faini, R. Gunsalus, P. Lostroh, C. Lupp, J. McCann, D. Millikan, A. Schaefer, E. Stabb, A. Stevens, K. Visick, C. Whistler, and E. P. Greenberg. 2005. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. USA 1023004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J. F., E. F. Fritsch, and T. Maniantis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 37.Sanderson, I. R. 2004. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J. Nutr. 134S2450-S2454. [DOI] [PubMed] [Google Scholar]
- 38.Schaefer, A. L., B. L. Hanzelka, A. Eberhard, and E. P. Greenberg. 1996. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J. Bacteriol. 1782897-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin, S., S. G. Song, D. S. Lee, J. G. Pan, and C. Park. 1997. Involvement of iclR and rpoS in the induction of acs, the gene for acetyl coenzyme A synthetase of Escherichia coli K-12. FEMS Microbiol. Lett. 146103-108. [DOI] [PubMed] [Google Scholar]
- 40.Siegl, A. 2004. The regulatory network of LitR in Vibrio fischeri: a microarray-based characterization. Diplomarbeit. University of Würzburg, Würzburg, Germany.
- 41.Slauch, J. M., and T. J. Silhavy. 1991. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J. Bacteriol. 1734039-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, V., D. Botstein, and P. O. Brown. 1995. Genetic footprinting: a genomic strategy for determining a gene's function given its sequence. Proc. Natl. Acad. Sci. USA 926479-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soberon-Chavez, G., M. Aguirre-Ramirez, and R. Sanchez. 2005. The Pseudomonas aeruginosa RhlA enzyme is involved in rhamnolipid and polyhydroxyalkanoate production. J. Ind. Microbiol. Biotechnol. 32675-677. [DOI] [PubMed] [Google Scholar]
- 44.Stabb, E. V., K. A. Reich, and E. G. Ruby. 2001. Vibrio fischeri genes hvnA and hvnB encode secreted NAD+-glycohydrolases. J. Bacteriol. 183309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stabb, E. V., and E. G. Ruby. 2002. RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol. 358413-426. [DOI] [PubMed] [Google Scholar]
- 46.Visick, K., and E. G. Ruby. 1997. New genetic tools for use in the marine bioluminescent bacterium Vibrio fischeri, p. 119-122. In J. W. Hastings, L. J. Kricka, and P. E. Stanley (ed.), Bioluminescence and chemiluminescence. John Wiley and Sons, New York, NY.
- 47.Visick, K. L. 2005. Layers of signaling in a bacterium-host association. J. Bacteriol. 1873603-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visick, K. L., J. Foster, J. Doino, M. McFall-Ngai, and E. G. Ruby. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 1824578-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weimer, P., Y. Shi, and C. Odt. 1991. A segmented gas/liquid delivery system for continuous culture of microorganisms on insoluble substrates and its use for growth of Ruminococcus flavefaciens on cellulose. Appl. Microbiol. Biotechnol. 36178-183. [Google Scholar]
- 50.Wolfe, A. J. 2005. The acetate switch. Microbiol. Mol. Biol Rev. 6912-50. [DOI] [PMC free article] [PubMed] [Google Scholar]