Abstract
Rep and UvrD are two related Escherichia coli helicases, and inactivating both is lethal. Based on the observation that the synthetic lethality of rep and uvrD inactivation is suppressed in the absence of the recombination presynaptic proteins RecF, RecO, or RecR, it was proposed that UvrD is essential in the rep mutant to counteract a deleterious RecFOR-dependent RecA binding. We show here that the synthetic lethality of rep and uvrD mutations is also suppressed by recQ and recJ inactivation but not by rarA inactivation. Furthermore, it is independent of the action of UvrD in nucleotide excision repair and mismatch repair. These observations support the idea that UvrD counteracts a deleterious RecA binding to forks blocked in the rep mutant. An ATPase-deficient mutant of UvrD [uvrD(R284A)] is dominant negative in a rep mutant, but only in the presence of all RecQJFOR proteins, suggesting that the UvrD(R284A) mutant protein is deleterious when it counteracts one of these proteins. In contrast, the uvrD252 mutant (G30D), which exhibits a strongly decreased ATPase activity, is viable in a rep mutant, where it allows replication fork reversal. We conclude that the residual ATPase activity of UvrD252 prevents a negative effect on the viability of the rep mutant and allows UvrD to counteract the action of RecQ, RecJ, and RecFOR at forks blocked in the rep mutant. Models for the action of UvrD at blocked forks are proposed.
Replication forks initiated at the chromosome replication origin can encounter obstacles that block their progression and lead to replication arrest. Replication restart is then essential to complete chromosome replication (32). In Escherichia coli, depending on the cause of arrest, the reassembly of a functional replication complex takes place directly on arrested replication forks or after DNA processing by recombination proteins (21). Defects in the processing of arrested replication forks can generate chromosome rearrangements, leading to genome instability (5). Replication impairment is an important source of instability in several organisms, and understanding the various pathways that process arrested replication forks to allow replication restart is an important issue (4, 34). For this purpose, we studied the fate of replication forks arrested in the absence of the Rep helicase in Escherichia coli.
In E. coli, chromosome replication is catalyzed by a replisome constituted of the replicative helicase (DnaB), the primase (DnaG), and the DNA polymerase III holoenzyme (Pol IIIh), a 10-subunit complex (29). Rep is a nonessential replicative helicase; chromosome replication is twice as slow in rep mutants as in wild-type cells (17). Rep translocates on DNA in the 3′-5′ direction and is proposed to play two roles in vivo. Based on the observation that in vitro Rep dislodges DNA-bound proteins, it was originally proposed that Rep acts by dislodging proteins in front of replication forks (38). More recently, Rep was proposed to act in a Rep-PriC pathway of replication restart (31). In vitro experiments showed that Rep and PriC proteins act at replication forks with a gap on the leading strand: Rep helicase displaces the lagging-strand end, thus forming a DNA structure on which PriC can load the replicative helicase (12, 13). The role of the Rep-PriC pathway of replication restart in vivo has yet to be determined. rep inactivation (but not priC inactivation [our unpublished data]) is synthetic lethal with the inactivation of the recombination gene recB or recC, which inactivates RecBCD-dependent homologous recombination (33, 35). This indicated that replication arrest in the rep mutant causes the formation of RecBCD substrates. RecBCD is a highly processive helicase/exonuclease that acts at DNA double-strand ends; it unwinds and degrades double-stranded DNA (dsDNA) until it encounters a specific DNA sequence named Chi. At Chi sites, RecBCD shifts to a recombinase, forming a 3′ single-stranded DNA (ssDNA) on which it directly loads RecA (14). Studies of the lethality of the rep recBC mutants showed that blocked replication forks undergo a specific reaction called replication fork reversal (RFR) (33). This reaction anneals the leading- and lagging-strand ends at blocked forks, creating a Holliday junction adjacent to a double-strand end, which renders RecB and RecC essential for viability (the RecD subunit is dispensable for homologous recombination). RecBCD processes reversed forks either by degrading the dsDNA end or by promoting homologous recombination. Both pathways lead to a structure on which PriA and other preprimosomal proteins load the replicative helicase DnaB, allowing the reassembly of a new replisome and replication restart. In addition to the rep mutant, the RFR reaction takes place in several other replication mutants, including Pol III(Ts) mutants at a high temperature (23).
The RecFOR recombination pathway is required for ssDNA gap repair. RecFOR proteins allow the formation of a RecA filament on ssDNA coated by the ssDNA binding (SSB) protein, by removing SSB from DNA and loading RecA (27). RecA filaments invade a dsDNA homologous molecule, leading to the formation of a Holliday junction recognized by RuvAB and RecG. The RuvAB complex and the RecG helicase catalyze branch migration of Holliday junctions, while RuvAB in complex with the endonuclease RuvC catalyzes resolution of Holliday junctions (16). RecFOR can act in conjunction with two other presynaptic proteins: the RecQ helicase, which translocates in the 3′-5′ direction, and RecJ, a 5′-3′ ssDNA exonuclease. These two proteins are needed for double-strand break repair by homologous recombination in a recBC sbcB sbcCD background, in which the absence of the SbcB and SbcCD nucleases allows DNA double-strand ends to be recombined by the successive action of RecQ, RecJ, RecFOR, RecA, and RuvABC (reviewed in reference 6). RecQ and RecJ are also required for RecFOR-promoted RecA binding at Pol III(Ts)-blocked forks (8). In dnaE(Ts) (Pol III polymerase) and dnaN(Ts) (Pol III clamp) mutants, RecQ, RecJ, and RecFOR promote RecA binding in a way that forms a toxic structure which prevents RFR and replication restart. This deleterious action of RecQ, RecJ, RecFOR, and RecA (called RecQJFORA hereafter) at Pol III(Ts)-blocked forks is counteracted by a helicase very similar to Rep, the UvrD helicase (8).
Like Rep, UvrD is a superfamily 1 helicase that translocates on DNA in a 3′-5′ direction. UvrD is required for nucleotide excision repair (NER) and mismatch repair (MMR) (16, 19). In addition, uvrD deletion leads to an increase in homologous recombination, showing that UvrD counteracts homologous recombination in vivo. Actually, in vitro, UvrD is able to dismantle a RecA filament and RecA-made recombination intermediates, suggesting a RecA-removal activity of UvrD in vivo (26, 36). UvrD allows RFR at arrested replication forks in dnaE(Ts) and dnaN(Ts) mutants by counteracting RecQJFORA. This function corresponds to two different modes of action of UvrD: the uvrD252 allele, largely impaired for its ATPase activity, still counteracts RecQJFORA at dnaE(Ts)-blocked forks, whereas it is inactive at dnaN(Ts)-blocked forks (18).
The inactivation of the helicases Rep and UvrD, which share 34% identity, is lethal. Because the viability of the Δrep ΔuvrD double mutant is restored by the inactivation of recF, recO, or recR, it was proposed that UvrD is required at rep-blocked forks for its anti-RecFOR action (30). The deleterious action of RecFOR in the rep mutant is likely to promote RecA binding, although RecA inactivation does not suppress the synthetic lethality of rep uvrD cells, presumably because RecA is needed in this double mutant for yet another unknown function. In the present work, we studied the role of UvrD in a rep mutant. First, we showed that the synthetic lethality of rep and uvrD inactivation is independent of the action of UvrD in NER and MMR and of the action of Rep in the Rep-PriC replication restart pathway. Then, we observed that, similarly to the inactivation of recFOR, the inactivation of recQ or recJ also restores the viability of the Δrep ΔuvrD double mutant. Finally, we show that, as previously observed for dnaE(Ts)-blocked forks, the uvrD252 mutation (Gly30Asp), which does not prevent RecFOR- and RecA-dependent SOS induction and hyperrecombination (18), nevertheless prevents RecQJFOR action at rep-blocked forks.
MATERIALS AND METHODS
Strains and plasmids.
The strain background was JJC40, which is an hsdR Thr+ Pro+ derivative of AB1157 (leu-6 thi-1 his-4 argE3 lacY1 galK2 ara-14 xyl-5 mtl-1 tsx-33 rpsL31 supE44). Most of the strains were constructed by P1 transduction. Details of strain constructions and strain genotypes are described in Table 1. recBC(Ts) stands for recB270 recC271 mutations (15) (Table 1). All thermosensitive mutants were constructed and propagated at 25°C. Null mutants were checked by PCR with external oligonucleotides that amplify DNA fragments of different lengths for the wild-type and the interrupted alleles. recBC mutants were checked for the inactivation of exonuclease V (they are permissive for the growth of T4 gpII mutants). uvrA and uvrD mutants were checked for their UV-sensitive phenotypes. mutL and uvrD mutants were checked for their mutator phenotypes (increase in the proportion of Rifr clones in overnight cultures). To ascertain that the rep uvrD252 recBC(Ts) mutant had not acquired a suppressor mutation that would allow rep uvrD viability, we compared the cotransduction efficiency of ΔuvrD and met::Tn10 in JJC2540 [uvrD252 recBC(Ts)] and JJC4571 [rep uvrD252 recBC(Ts)]. As expected, 4 ΔuvrD mutants out of 11 met::Tn10 transductants were obtained in JJC2540, while no ΔuvrD mutant cloned was obtained in JJC4571 out of 28 met::Tn10 transductants; this shows that in the JJC4571 context, rep and uvrD inactivation is synthetic lethal, as expected. The minimum media (MM) was M9 supplied with thiamine (0.05%), CaCl2 (100 μM), MgSO4 (2 mM), glucose (0.4%), and Casamino Acids (0.2%) (25). Plasmid pGBts-rep has been described previously (3); it carries the rep gene under the control of its own promoter. Plasmid pAM-rep was constructed by recloning the rep gene from pGBts-rep in the polylinker of the IPTG (isopropyl-β-d-thiogalactopyranoside)-dependent vector pAM34 (9).
TABLE 1.
Strains
| Strain | Relevant genotype | Construction, source, or reference |
|---|---|---|
| CAG18491 | metE3079::Tn10 | 28 |
| JJC104 | recJ248::Tn10 | Susan Lovett, Brandeis University, MA |
| JJC330 | recB270(Ts) recC271(Ts) | 33 |
| JJC1177 | rep::Ap | Laboratory collection |
| JJC1702 | ΔrarA::Cm | 2 |
| JJC1707 | Δrep::cam uvrD::Tn5 recR::Tn5 metE::Tn10 (pGBts-rep+) | MAC 574 in reference 30 |
| JJC1708 | Δrep::cam uvrD::Tn5 (pGBts-rep+) | MAC 556 in reference 30 |
| JJC1892 | rep::Ap (pAM-rep+) | Laboratory collection |
| JJC1945 | sfiA11 | 10 |
| JJC1960 | uvrD::Tn5 rep::Ap (pAM-rep+) | Laboratory collection |
| JJC2044 | ΔuvrA::Cm | Laboratory collection |
| JJC2062 | recF400::Tn5 zid501::Tn10 | Laboratory collection |
| JJC213 | Δrep::kan | 35 |
| JJC2230 | ΔruvABC::Cm (pGB-ruvABC+) | JJC754 transformed with pGB-ruvABC+ |
| JJC2386 | Δ(uvrD-yigB)::cam | 8 |
| JJC2417 | rep::Ap uvrD::Tn5 recR::Tn5 metE::Tn10 (pGBts-rep+) | JJC1707 *P1 JJC1177a |
| JJC2457 | sfiA11 Δ(uvrD-yigB)::cam | JJC1945 *P1 JJC2386 |
| JJC2530 | sfiA11 uvrD252 | 18 |
| JJC2534 | sfiA11 uvrD252 (pGBts-rep+) | JJC2530 transformed with pGBts-rep+ |
| JJC2540 | sfiA11 uvrD252 recB270(Ts) recC271(Ts) | 18 |
| JJC2542 | sfiA11 uvrD252 (pGBts-rep+) rep::Ap | JJC2534 *P1 JJC1177 |
| JJC2632 | Δtus::kan pheA::Spc-TerB uvrD(R284A) yigB::Cm | Laboratory collection |
| JJC2634 | ΔuvrD294::Kan | 18 |
| JJC2643 | sfiA11 uvrD(R284A) yigB::Cm | 7 |
| JJC3121 | Δrep::cam uvrD::Tn5 (pGBts-rep+) recJ248::Tn10 | JJC1708 *P1 JJC104 |
| JJC3122 | Δrep::cam uvrD::Tn5 (pGBts-rep+) recQ1803::Tn3 | JJC1708 *P1 recQ1803::Tn3 uvrD::Tn5 |
| JJC3236 | uvrD::Tn5 rep::Ap (pAM-rep+) ΔrarA::Cm | JJC1960 *P1 JJC1702 |
| JJC3870 | Δrep::kan (pAM-rep+) | JJC213 transformed with pAM-rep+ |
| JJC3971 | Δrep::kan (pAM-rep+) ΔuvrD294::Kan | JJC3870 *P1 JJC2634 |
| JJC405 | recQ1803::Tn3 | R. G. Lloyd, University of Nottingham, United Kingdom |
| JJC4090 | Δrep::kan mutL218::Tn10 | JJC213 *P1 mutL218::Tn10 |
| JJC4092 | Δrep::kan ΔuvrA::Cm | JJC213 *P1 JJC2044 |
| JJC4144 | rep::Ap (pAM-rep+) uvrD(R284A) yigB::Cm | JJC1892 *P1 JJC2632 |
| JJC4201 | Δrep::kan mutL218::Tn10 (pAM-rep+) | JJC4090 transformed with pAM-rep+ *P1 JJC2457 |
| JJC4207 | Δrep::kan ΔuvrA::Cm metE3079::Tn10 | JJC4092 *P1 CAG18491 |
| JJC4263 | Δrep::kan ΔuvrA::Cm ΔuvrD294::Kan met+ | JJC4207 *P1 JJC2634 |
| JJC4297 | Δrep::cam uvrD::Tn5 recJ248::Tn10 | JJC3121 cured of pGBts-rep+ |
| JJC4298 | Δrep::cam uvrD::Tn5 recQ1803::Tn3 | JJC3122 cured of pGBts-rep+ |
| JJC4326 | rep::Ap (pAM-rep+) uvrD(R284A) yigB::Cm recF400::Tn5 zid501::Tn10 | JJC4144 *P1 JJC2062 |
| JJC4362 | rep::Ap (pAM-rep+) uvrD(R284A) yigB::Cm recJ248::Tn10 | JJC4144 *P1 JJC104 |
| JJC4373 | sfiA11 uvrD252 rep::Ap | JJC2542 cured of pGBts-rep+ |
| JJC4485 | rep::Ap uvrD(R284A) yigB::Cm recF400::Tn5 zid501::Tn10 | JJC4326 cured of pAM-rep+ |
| JJC4486 | rep::Ap uvrD(R284A) yigB::Cm recJ248::Tn10 | JJC4362 cured of pAM-rep+ |
| JJC4494 | Δrep::kan mutL218::Tn10 ΔuvrA::Cm | JJC4090 *P1 JJC2044 |
| JJC4509 | sfiA11 uvrD252 (pGBts-rep+) rep::Ap mutL218::Tn10 | JJC2542 *P1 mutL218::Tn10 |
| JJC4526 | sfiA11 uvrD252 rep::Ap mutL218::Tn11 | JJC4509 cured of pGBts-rep+ |
| JJC4569 | uvrD::Tn5 rep::Ap (pAM-rep+) ΔuvrA::Cm | JJC1960 *P1 JJC2044 |
| JJC4571 | sfiA11 uvrD252 recB270(Ts) recC271(Ts) Δrep::kan | JJC2540 *P1 JJC213 |
| JJC4572 | uvrD::Tn5 Δrep::Ap (pAM-rep+) ΔuvrA::Cm mutL218::Tn10 | JJC4569 *P1 mutL218::Tn10 |
| JJC4588 | sfiA11 uvrD252 recB270(Ts) recC271(Ts) Δrep::kan recF400::Tn5 zid501::Tn10 | JJC4571 *P1 JJC2062 |
| JJC4616 | sfiA11 uvrD252 recB270(Ts) recC271(Ts) Δrep::kan mutL218::Tn10 | JJC4571 *P1 mutL218::Tn10 |
| JJC4637 | sfiA11 uvrD252 recB270(Ts) recC271(Ts) Δrep::kan recF400::Tn5 zid501::Tn10 | JJC4588 *P1 JJC2230 |
| JJC4702 | sfiA11 uvrD(R284A) yigB::Cm recQ1803::Tn3 | JJC2643 *P1 JJC405 |
| JJC4704 | sfiA11 uvrD(R284A) yigB::Cm recQ1803::Tn3 (pGBts-rep+) | JJC4702 transformed with pGBts-rep+ |
| JJC4763 | sfiA11 uvrD(R284A) yigB::Cm recQ1803::Tn3 (pGBts-rep+) Δrep::kan | JJC4704 *P1 JJC213 |
| JJC4799 | sfiA11 uvrD(R284A) yigB::Cm recQ1803::Tn3 Δrep::kan | JJC4763 cured of pGBts-rep+ |
| JJC505 | Δrep::kan recB270(Ts) recC271(Ts) | 22 |
| JJC754 | ΔruvABC::Cm | 33 |
| JJC760 | Δrep::cam (pGBts-rep+) | 24 |
*P1 indicates transduction by P1.
Segregation of pGBts-rep and pAM-rep.
Each segregation experiment was performed at least three times. Cells carrying the pGBts-rep plasmid were grown overnight in Luria-Bertani (LB) broth with 60 μg ml−1 of spectinomycin at 30°C. They were diluted 1,000-fold in MM and were grown for 6 h at 42°C. For segregation curves, after dilution in MM, cells were grown for 2 h at 30°C prior to the shift at 42°C, and aliquots were then plated on MM with or without spectinomycin every 2 h for 6 h. Plates were incubated at 37°C and counted every 1, 2, and 3 days. For pAM-rep, segregation experiments were performed at 37°C. Cells containing pAM-rep were grown overnight in LB broth in the presence of 500 μM IPTG and 60 μg·ml−1 of spectinomycin. Cells were diluted 1,000-fold in MM and grown for 8 h at 37°C. Segregation cultures were then treated as for pGBts-rep. For most of the strains, cells that kept the rep-carrying plasmid appeared overnight, whereas cells that lost it appeared in 2 days. Cells were picked on MM with or without spectinomycin (and IPTG for pAM-rep) to identify those that lost the plasmid. The plasmidless cells were checked systematically for resistance to antibiotics, UV resistance, and PCR amplification of relevant genes.
Measure of linear DNA by PFGE.
Quantification of linear DNA by pulsed-field gel electrophoresis (PFGE) was performed as described previously (33).
RESULTS
rep uvrD lethality is independent of a role of UvrD in repair pathways.
UvrD is required for NER and MMR. We tested a possible role of the absence of NER and MMR in the Δrep ΔuvrD synthetic lethality. To inactivate NER, we introduced a uvrA deletion in a Δrep mutant by P1 transduction. The efficiency of ΔuvrA P1 transduction was similar in the Δrep mutant and in wild-type cells (10−6 transductant/viable cell), and inactivation of uvrA did not affect the viability of Δrep cells measured by the plating efficiency of overnight cultures (Table 2). Similarly mutL, which inactivates MMR, was transduced in Δrep cells as efficiently as in wild-type cells (10−6 transductant/viable cell), and mutL did not affect the viability of Δrep cells (Table 2). We inactivated both NER and MMR in Δrep cells by constructing a Δrep ΔuvrA mutL strain. The efficiency of transduction of ΔuvrA in the Δrep mutL cells was the same as in wild-type cells, and Δrep ΔuvrA mutL cells showed the same viability as the Δrep cells or the Δrep mutL cells (Table 2). These results indicate that NER and MMR are not essential in a Δrep mutant.
TABLE 2.
Inactivation of NER or MMR does not affect the viability of the Δrep mutant
| Strain | Relevant genotype | CFU/ml at 37°Ca | Plating efficiencyb |
|---|---|---|---|
| JJC213 | Δrep | 4.00E+09 ± 7.39E+08 | 1.00 |
| JJC4092 | Δrep ΔuvrA | 2.47E+09 ± 1.07E+09 | 0.62 |
| JJC4090 | Δrep mutL::Tn10 | 4.15E+09 ± 5.55E+08 | 1.04 |
| JJC4494 | Δrep ΔuvrA mutL::Tn10 | 3.26E+09 ± 8.44E+08 | 0.81 |
Overnight cultures grown at 37°C were plated at 37°C to determine the CFU/ml.
Ratio of the CFU/ml of the strain to the CFU/ml of the Δrep strain.
Since UvrD acts at the last step of NER and MMR, the lethality of the Δrep ΔuvrD mutant could result from the formation of toxic intermediates by NER or MMR early proteins. To test this hypothesis, we analyzed the viability of the Δrep ΔuvrD mutant in cells where the genes acting upstream of uvrD in NER or MMR are inactivated. We took advantage of a conditional plasmid carrying the wild-type rep gene, pAM-rep, which replicates only in the presence of IPTG. Δrep ΔuvrD ΔuvrA (pAM-rep), Δrep ΔuvrD mutL (pAM-rep), and Δrep ΔuvrD ΔuvrA mutL::Tn10 (pAM-rep) mutants were constructed. No plasmidless segregant was recovered after propagation in the absence of IPTG (Table 3). These results indicate that the synthetic lethality of rep and uvrD genes is independent of UvrD action in DNA repair.
TABLE 3.
rep uvrD lethality is suppressed by the inactivation of recJ and recQ
| Strain | Relevant genotype | Plasmidless colonies (CFU/ml)a |
|---|---|---|
| JJC3870 | Δrep (pAM-rep) | 2.01E+08 ± 1.88E+08 |
| JJC760 | Δrep (pGBts-rep) | 1.65E+09 ± 1.31E+09 |
| JJC3971 | Δrep ΔuvrD (pAM-rep) | <1E+04 |
| JJC1708 | Δrep uvrD::Tn5 (pGBts-rep) | <1E+04 |
| JJC4263 | Δrep ΔuvrD ΔuvrA (pAM-rep) | <1E+04 |
| JJC4201 | Δrep ΔuvrD mutL::Tn10 (pAM-rep) | <1E+04 |
| JJC4572 | Δrep uvrD::Tn5 ΔuvrA mutL (pAM-rep) | <1E+04 |
| JJC3122 | Δrep uvrD::Tn5 recQ::Tn3 (pGBts-rep) | 1.44E+09 ± 7.65E+08 |
| JJC3121 | Δrep uvrD::Tn5 recJ::Tn10 (pGBts-rep) | 1.36E+09 ± 4.12E+08 |
| JJC2417 | Δrep uvrD::Tn5 recR::Tn5 (pGBts-rep) | 1.78E+09 ± 1.06E+09 |
| JJC3236 | Δrep uvrD::Tn5 ΔrarA (pAM-rep) | <1E+04 |
| JJC4144 | Δrep uvrD(R284A) (pAM-rep) | <1E+04 |
| JJJ4763 | Δrep uvrD(R284A) recQ::Tn3 (pGBts-rep) | 6.58E+08 ± 3.09E+08 |
| JJC4362 | Δrep uvrD(R284A) recJ::Tn10 (pAM-rep) | 6.83E+08 ± 2.97E+08 |
| JJC4326 | Δrep uvrD(R284A) recF::Tn5 (pAM-rep) | 1.16E+09 ± 5.92E+08 |
| JJC2542 | Δrep uvrD252 (pGBts-rep) | 8.27E+08 ± 7.53E+08 |
| JJC4509 | Δrep uvrD252 mutL::Tn10 (pGBts-rep) | 1.21E+09 ± 5.09E+08 |
Cells containing pGBts-rep or pAM-rep were cured by propagation at 42°C or in medium lacking IPTG, respectively, and colonies isolated on MM were checked for plasmid loss by picking on spectinomycin plates (see Materials and Methods).
The restart pathway Rep-PriC is not essential in a ΔuvrD mutant.
Since Rep and PriC act together in a replication restart pathway, the synthetic lethality of rep and uvrD could result from a requirement for this replication restart pathway in a ΔuvrD mutant. To test this hypothesis, the efficiency of cotransduction of ΔuvrD with the Met+ gene was compared in priC and PriC+ met::Tn10 contexts (met is 15 kb downstream of uvrD and 30% linked in cotransduction). Transductants were selected for the loss of methionine auxotrophy (transduction of the wild-type Met+ gene), and the proportion of uvrD::kan cotransductants was measured. The percentage of cotransduction of ΔuvrD with met in PriC+ cells was 30% of that in priC mutant cells. The priC uvrD double mutant did not show any growth defect, indicating that the inactivation of PriC in ΔuvrD cells has no effect on viability. Thus, Δrep ΔuvrD lethality is independent of the action of Rep in the Rep-PriC pathway of replication restart.
Inactivation of RecQ and RecJ restores the viability of rep uvrD cells.
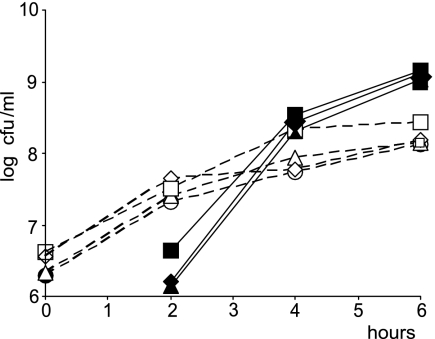
To determine whether RecQ and RecJ are involved in the RecFOR-dependent formation of deleterious DNA structures in Δrep ΔuvrD cells, we tested whether recQ or recJ inactivation restores the viability of a Δrep ΔuvrD double mutant. pGBts-rep, a conditional plasmid that replicates only at 30°C, was used. We constructed Δrep ΔuvrD recQ::Tn3 (pGBts-rep) and Δrep ΔuvrD recJ::Tn10 (pGBts-rep) strains. In both cases, propagation of the strain at 42°C allowed the recovery of plasmidless colonies, as observed with Δrep ΔuvrD recF::Tn5 (pGBts-rep) cells (30) (Fig. 1 and Table 3). The observation that the inactivation of RecQ, RecJ, or RecF restores the viability of Δrep ΔuvrD cells suggests that the lethality of Δrep ΔuvrD cells is caused by the concerted action of all these presynaptic proteins.
FIG. 1.
rep uvrD lethality is suppressed by recQ or recJ inactivation. Segregation experiments were carried out as described in Materials and Methods. Briefly, plasmid-containing cells grown at 30°C were shifted to 42°C at time zero. After 2 to 4 h, they plateau due to the lack of plasmid replication at high temperature (open symbols), while plasmidless cells appear (closed symbols). No plasmidless cells appeared with the rep uvrD double mutant, while they did appear with similar kinetics with rep uvrD recR, recJ, or recQ triple mutants (and with the rep single mutant, JJC760; data not shown). Open symbols are CFU on spectinomycin plates, i.e., pGBts-rep-containing strains. Closed symbols are pGBts-rep-cured strains, measured as CFU on MM, identified as Spts. Circles, JJC1708 [rep uvrD (pGBts-rep)]; triangles, JJC2417 [rep uvrD recR (pGBts-rep)]; squares, JJC3121 [rep uvrD recJ (pGBts-rep)]; diamonds, JJC3122 [rep uvrD recQ (pGBts-rep)].
We have previously shown that in dnaE(Ts) cells (Pol III polymerase mutant), RarA is required with RecQ, RecJ, and RecFOR for the formation of deleterious RecA filaments at inactivated replication forks, whereas it is not required for the formation of RecA filaments in dnaN(Ts) cells (Pol III clamp mutant) (18). We tested whether RarA is required for the formation of deleterious RecA filaments in Δrep ΔuvrD cells by investigating whether the inactivation of rarA restores the viability of Δrep ΔuvrD cells. A Δrep ΔuvrD ΔrarA (pGBts-rep) strain was constructed; no plasmidless colonies could be recovered after curing the plasmid by propagation at 42°C. Therefore, RarA is not required for the formation of deleterious RecA filaments by RecQJFOR in the Δrep mutant (Table 3).
Role of the uvrD(R284A) allele in Δrep cells.
We tested the effect of the uvrD(R284A) allele, which totally inactivates the helicase function of UvrD by a mutation in the helicase domain IV (11, 39). The combination of the uvrD(R284A) allele with rep deletion in the presence of the pAM-rep plasmid does not allow the recovery of plasmidless colonies after cell propagation in the absence of IPTG (Table 3), indicating that the rep uvrD(R284A) double mutant is lethal. In order to test whether the lethality of Δrep uvrD(R284A) cells is due to a deleterious action of RecQ, RecJ, and RecFOR proteins, Δrep uvrD(R284A) recJ(pAM-rep), Δrep uvrD(R284A) recF(pAM-rep), and Δrep uvrD(R284A) recQ(pGBts-rep) strains were constructed. Plasmidless colonies were recovered with all three mutants (Table 3). In conclusion, the uvrD(R284A) mutation confers to Δrep the same phenotypes as the null uvrD mutation.
Role of the uvrD252 allele in Δrep cells.
UvrD252 is a partial mutant. As in uvrD null mutants, it is defective for NER and antirecombination action and is constitutively induced for the SOS response (18). However, it has retained the UvrD MMR function (37). We previously showed that UvrD252 retains the capacity to antagonize RecQJFORA-dependent prevention of RFR in a dnaE(Ts) mutant but not in a dnaN(Ts) mutant (18). We asked whether UvrD252 antagonizes RecQJFOR action at arrested replication forks in Δrep cells.
A Δrep uvrD252 (pGBts-rep) mutant was constructed. Plasmidless colonies were obtained after cell propagation at 42°C to cure the plasmid (Table 3), showing that this combination of mutations is viable. This finding indicates that UvrD252 retains the capacity to antagonize RecQJFOR deleterious action in a Δrep mutant. The weak residual ATPase activity of the UvrD252 enzyme detected in vitro is only significant in vivo when the helicase activity of UvrD is activated by MutL during MMR. In order to test if the anti-RecQJFOR action of UvrD252 in Δrep cells is dependent on a stimulation of UvrD252 by MutL, we constructed a Δrep uvrD252 mutL (pGBts-rep) mutant. As with the rep uvrD252 (pGBts-rep) mutant, plasmidless colonies were recovered after segregation (Table 3). Thus, UvrD252 does not require MutL to antagonize the deleterious action of RecQJFOR in Δrep cells.
Because of the lethality of the rep uvrD double mutant, the effects of uvrD inactivation on RFR in a rep mutant cannot be tested. However, the viability of the Δrep uvrD252 cells offered the possibility to study the effect of the uvrD252 allele on RFR in Δrep cells. As presented in the introduction, the first step of RFR is the formation of a Holliday junction with a dsDNA end. In the absence of RecBC, the dsDNA end is not processed and the reversed fork is recognized only by RuvABC, which resolves the Holliday junction, causing chromosome linearization. Linear chromosome fragments can be detected by PFGE, as intact chromosomes or σ molecules are entrapped in the wells while linear fragments enter gels. Thus, the amount of linear DNA detected in a recBC(Ts) context at the restrictive temperature of 42°C is a measure of RFR. A recBC(Ts) Δrep uvrD252 strain was constructed at 25°C by transducing a Δrep::Kan deletion in a recBC(Ts) uvrD252 strain. The rep gene is 37 kb distant from the uvrD gene, and these two genes are 25% to 30% linked in P1 transduction. Accordingly, more than half of Δrep::Kan transductants remained uvrD252 based on their UV sensitivity, showing that a recBC(Ts) Δrep uvrD252 triple mutant is fully viable at 25°C. The thermosensitivity of recBC(Ts) Δrep uvrD252 cells was determined by measuring the plating efficiency at 37°C and 42°C of overnight cultures grown at 25°C (Table 4). The results showed that recBC(Ts) Δrep uvrD252 cells are as thermosensitive at 37°C and 42°C as recBC(Ts) Δrep cells. Thus, the uvrD252 allele can be combined with rep deletion but does not suppress the requirement for RecBC for viability.
TABLE 4.
uvrD252 does not suppress the rep recBC(Ts) lethality at high temperature
| Strain | Mutant genotype | Plating efficiency ata:
|
|
|---|---|---|---|
| 37°C | 42°C | ||
| JJC505 | recBC(Ts) Δrep | <10−6 | <10−6 |
| JJC2540 | recBC(Ts) uvrD252 | 1.24 ± 0.09 | 1.12 ± 0.46 |
| JJC4571 | recBC(Ts) Δrep uvrD252 | <10−6 | <10−6 |
Overnight cultures grown at 25°C were plated at 25°C and 37°C or 42°C. For each culture, the ratio of clones obtained at 37°C or 42°C to those obtained at 30°C was calculated, and the results are expressed as the averages of the ratios.
We measured the proportion of broken chromosomes at 42°C in recBC(Ts) Δrep uvrD252 cells. The high level of fork breakage in recBC(Ts) Δrep uvrD252 was not significantly different from that in recBC(Ts) Δrep cells (about 70%; Table 5). To ascertain that chromosome breakage results from RFR, we tested that it requires the Holliday junction resolving complex RuvABC. However, owing to its hyperrecombination phenotype, the uvrD252 ruvABC double mutant is viable only if gap repair is prevented by recFOR inactivation (18). recFOR inactivation has no effect on fork breakage in rep recBC(Ts) uvrD252 cells, as previously reported for rep recBC(Ts) cells (3) (see JJC4588 in Table 5). The level of linear DNA decreases significantly upon inactivation of RuvABC in a recBC(Ts) Δrep uvrD252 recF mutant (26%; Table 5). Thus, fork breakage in the rep uvrD252 recBC(Ts) context is RuvABC dependent, indicating that it results from RFR. The inactivation of mutL did not modify the level of fork breakage in the recBC(Ts) Δrep uvrD252 mutant (Table 5), indicating that MutL is not required for the action of UvrD252 at rep-blocked forks. In conclusion, the UvrD252 protein allows RFR in the presence of all RecQJFORA proteins. As for the UvrD wild-type protein, it can prevent or counteract the deleterious action of these proteins.
TABLE 5.
uvrD252 does not decrease the percentage of linear chromosome in the rep recBC(Ts) mutant
| Strain | Relevant genotype | Percentage of linear DNAa at:
|
nb | |
|---|---|---|---|---|
| 30°C | 42°C | |||
| JJC330 | recBC(Ts) | 9.1 ± 3.4 | 19.1 ± 5 | 3/3 |
| JJC505/790 | recBC(Ts) rep | 16.8 ± 4.9 | 71.1 ± 8.6 | 3/7 |
| JJC2540 | recBC(Ts) uvrD252 | 20 | 39 ± 1 | 1/3 |
| JJC4571 | recBC(Ts) rep uvrD252 | 14.5 ± 3.5 | 71.5 ± 3.5 | 2/6 |
| JJC4616 | recBC(Ts) rep uvrD252 mutL | 11 ± 1 | 73 ± 3 | 2/3 |
| JJC4588 | recBC(Ts) rep uvrD252 recF | 12 ± 4.2 | 58.8 ± 6 | 2/4 |
| JJC4637 | recBC(Ts) rep uvrD252 recF ruvABC | 5.5 ± 0.7 | 26.1 ± 1 | 2/7 |
The percentage of linear DNA was quantified by PFGE performed with 3H-Thy-labeled chromosomes, by measuring the proportion of linear chromosome that enters the pulsed-field gels (33). RecBC+ cells always contain less than 10% linear DNA at all temperatures by this technique, as shown here by the low level of linear DNA in the recBC(Ts) mutant at 30°C (33; also data not shown). Results for JJC330 and JJC505/790 (33) and for JJC2540 (18) were previously published and reproduced here as controls.
Number of independent experiments (30°C/42°C).
DISCUSSION
In this work, we characterized in vivo the action of UvrD in a rep mutant. Our results support the proposal that UvrD acts at rep-blocked forks to counteract a deleterious RecA filament and show that the strongly impaired uvrD252 mutant has retained this function.
In Pol III(Ts) mutants, replication perturbation at high temperature (37°C or 42°C) leads to the formation of (i) gaps left behind replication forks and (ii) inactivated forks. We previously showed that, while the formation of RecA filaments at gaps is independent of RecQ, the formation of RecA filaments at arrested replication forks requires RecQ and renders UvrD anti-RecA action essential for RFR and for viability (18). We show here that the RecQ helicase and the RecJ exonuclease are also needed for RecFOR lethal action in rep uvrD cells, which suggests that RecQ and RecJ participate in the formation of RecA filaments counteracted by the UvrD helicase at rep-blocked forks. As previously observed in the dnaN(Ts) mutant, RarA is not required for the fixation of RecA at rep-blocked forks. Therefore, the action of RarA does not correlate with that of UvrD252, arguing against a role for RarA in UvrD252 activity.
To address the role of the ATPase activity of UvrD for its anti-RecA action, we took advantage of two uvrD mutants, uvrD252 and uvrD(R284A). In vitro, both mutant proteins bind ssDNA; the G30D mutation in uvrD252 is adjacent to the first helicase motif and the ATPase activity of UvrD252 is largely decreased, while the R284A mutation is within the helicase motif IV and the ATPase activity of the UvrD(R284A) mutant is fully abolished (11, 39). In agreement with its lack of ATPase activity in vitro, the uvrD(R284A) mutant is defective in NER and MMR in vivo. In contrast, the uvrD252 mutant is defective for NER but supports MMR, probably through a stimulation of its residual ATPase activity by MutL. In addition, UvrD252 is fully deficient for the prevention of SOS induction and for the prevention of homologous recombination, indicating that it cannot remove RecA from DNA gaps, in the wild type as well as in Pol III(Ts) mutants (18).
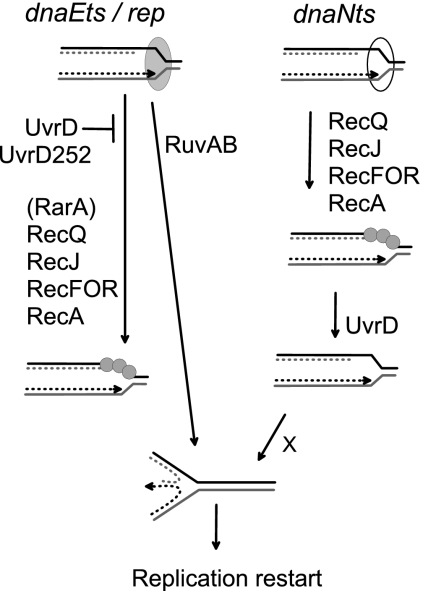
We previously reported that the uvrD252 allele can be combined with dnaE(Ts) and dnaN(Ts) mutations at low temperature, while uvrD(R284A) is lethal in these two Pol III(Ts) backgrounds even at permissive temperatures (30°C or 25°C). We propose that the dominant-negative phenotype conferred by the uvrD(R284A) mutation in Pol III(Ts) mutants results from a poisoning effect of stable UvrD(R284A)-DNA complexes, owing to a total absence of ATPase activity (18). Here, we found that the uvrD(R284A) mutation is also synthetic lethal with rep inactivation, whereas uvrD252 is not, suggesting that the residual ATPase activity of UvrD252 is responsible for the lack of the dominant-negative effect of this mutation. Our study of Pol III(Ts) uvrD252 mutants indicated that UvrD counteracts RecA filaments at blocked replication forks by two different modes of action. In the dnaN(Ts) mutant, UvrD252 is unable to antagonize RecA binding to forks, suggesting that UvrD acts by dismantling RecA filaments from dnaN(Ts)-blocked forks (Fig. 2). In contrast, in the dnaE(Ts) mutant, UvrD252 is able to prevent RFR inhibition by RecA, and we proposed that it acts prior to RecA binding to DNA (Fig. 2). The occurrence of RFR in the rep uvrD252 double mutant suggests that UvrD also acts prior to RecA in the rep mutant and that this action is unaffected by the uvrD252 mutation (Fig. 2). We show here that the UvrD(R284A) mutant protein does not poison rep-blocked forks when RecQJFOR are absent, suggesting that UvrD(R284A) does not access rep-blocked forks in the absence of these proteins and that the actions of RecQJFOR and UvrD are concerted. uvrD252 was originally isolated as a recombination-deficient mutant in a recBC sbcB sbcCD background. This property does not simply reflect a loss of function of the uvrD252 mutant allele, since uvrD null mutations do not inactivate homologous recombination in this background (20). It is interesting to note that recBC sbcB sbcCD is the only background where homologous recombination is RecQ dependent and the only background where uvrD252 confers a recombination-deficient phenotype, and hence counteracts recombination proteins. We can propose two models that account for these observations. In the first model, although UvrD252 has lost most of the UvrD in vivo activities, it has acquired a particular property of counteracting RecQ, observed at dsDNA ends in the recBC sbcB sbcC mutant and at blocked forks in dnaE(Ts) and rep mutants but not at dnaN(Ts)-blocked forks. In the second model, by blocking several RecQ activities, UvrD252 reveals that UvrD and RecQ proteins share common targets, suggesting that certain RecQ functions, performed with RecJ and RecFOR, are controlled by UvrD. The “raison d'être” of this control could be a need to prevent inappropriate RecQ (and RecJFOR) action at certain inactivated replication forks.
FIG. 2.
Model of action of UvrD/uvrD252 in replication mutants (adapted from reference 18). At dnaN(Ts)-blocked forks (right portion), UvrD252 is unable to counteract RecQJFORA, and we propose that UvrD acts by removing RecA from DNA. At dnaE(Ts)-blocked (13) and rep-blocked (this work) forks, UvrD252 can counteract RecQJFORA, and we propose that it acts prior to RecA binding, by preventing it. RarA is shown in parentheses because it is only essential for RecA binding in the dnaE(Ts) mutant and not in the rep mutant. RuvAB is the only way of RFR in the dnaE(Ts) mutant and one of two pathways in the rep mutant. In the dnaN(Ts) mutant, RFR is RuvAB independent and the enzymes that reverse forks are unknown (1).
Acknowledgments
We thank Steve Sandler and Richard Centore for very helpful reading of the manuscript.
R.L. is supported by the Fondation de la Recherche Médicale (FRM grant number FDT 20070910238). The work was supported by ANR-05-BLAN-0204-01 and FRM INE 2005110527.
Footnotes
Published ahead of print on 20 June 2008.
REFERENCES
- 1.Baharoglu, Z., M. Petranovic, M. J. Flores, and B. Michel. 2006. RuvAB is essential for replication forks reversal in certain replication mutants. EMBO J. 25596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barre, F. X., B. Soballe, B. Michel, M. Aroyo, M. Robertson, and D. Sherratt. 2001. Circles: the replication-recombination-chromosome segregation connection. Proc. Natl. Acad. Sci. USA 988189-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidnenko, V., M. Seigneur, M. PenelColin, M. F. Bouton, S. D. Ehrlich, and B. Michel. 1999. sbcS sbcC null mutations allow RecF-mediated repair of arrested replication forks in rep recBC mutants. Mol. Microbiol. 33846-857. [DOI] [PubMed] [Google Scholar]
- 4.Branzei, D., and M. Foiani. 2007. Interplay of replication checkpoints and repair proteins at stalled replication forks. DNA Repair (Amsterdam) 6994-1003. [DOI] [PubMed] [Google Scholar]
- 5.Branzei, D., and M. Foiani. 2007. Template switching: from replication fork repair to genome rearrangements. Cell 1311228-1230. [DOI] [PubMed] [Google Scholar]
- 6.Clark, A. J., and S. J. Sandler. 1994. Homologous genetic recombination: the pieces begin to fall into place. Crit. Rev. Microbiol. 20125-142. [DOI] [PubMed] [Google Scholar]
- 7.Flores, M. J., V. Bidnenko, and B. Michel. 2004. The DNA repair helicase UvrD is essential for replication fork reversal in replication mutants. EMBO Rep. 5983-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores, M. J., N. Sanchez, and B. Michel. 2005. A fork-clearing role for UvrD. Mol. Microbiol. 571664-1675. [DOI] [PubMed] [Google Scholar]
- 9.Gil, D., and J. P. Bouche. 1991. ColE1-type vectors with fully repressible replication. Gene 10517-22. [DOI] [PubMed] [Google Scholar]
- 10.Grompone, G., V. Bidnenko, S. D. Ehrlich, and B. Michel. 2004. PriA is essential for viability of the Escherichia coli topoisomerase IV parE10(Ts) mutant. J. Bacteriol. 1861197-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall, M. C., and S. W. Matson. 1997. Mutation of a highly conserved arginine in motif IV of Escherichia coli DNA helicase II results in an ATP-binding defect. J. Biol. Chem. 27218614-18620. [DOI] [PubMed] [Google Scholar]
- 12.Heller, R. C., and K. J. Marians. 2007. Non-replicative helicases at the replication fork. DNA Repair (Amsterdam) 6945-952. [DOI] [PubMed] [Google Scholar]
- 13.Heller, R. C., and K. J. Marians. 2005. Unwinding of the nascent lagging strand by Rep and PriA enables the direct restart of stalled replication forks. J. Biol. Chem. 28034143-34151. [DOI] [PubMed] [Google Scholar]
- 14.Kowalczykowski, S. C. 2000. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 25156-165. [DOI] [PubMed] [Google Scholar]
- 15.Kushner, S. R. 1974. In vivo studies of temperature-sensitive recB and recC mutants. J. Bacteriol. 1201213-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzminov, A. 1999. Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev. 63751-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane, H. E., and D. T. Denhardt. 1975. The rep mutation. IV. Slower movement of replication forks in Escherichia coli rep strains. J. Mol. Biol. 9799-112. [DOI] [PubMed] [Google Scholar]
- 18.Lestini, R., and B. Michel. 2007. UvrD controls the access of recombination proteins to blocked replication forks. EMBO J. 263804-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matson, S. W., and A. B. Robertson. 2006. The UvrD helicase and its modulation by the mismatch repair protein MutL. Nucleic Acids Res. 344089-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendonca, V. M., K. Kaiserrogers, and S. W. Matson. 1993. Double helicase II (uvrD)-helicase IV (helD) deletion mutants are defective in the recombination pathways of Escherichia coli. J. Bacteriol. 1754641-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel, B., H. Boubakri, Z. Baharoglu, M. Lemasson, and R. Lestini. 2007. Recombination proteins and rescue of arrested replication forks. DNA Repair (Amsterdam) 6967-980. [DOI] [PubMed] [Google Scholar]
- 22.Michel, B., S. D. Ehrlich, and M. Uzest. 1997. DNA double-strand breaks caused by replication arrest. EMBO J. 16430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel, B., G. Grompone, M. J. Flores, and V. Bidnenko. 2004. Multiple pathways process stalled replication forks. Proc. Natl. Acad. Sci. USA 10112783-12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michel, B., G. D. Recchia, M. PenelColin, S. D. Ehrlich, and D. J. Sherratt. 2000. Resolution of Holliday junctions by RuvABC prevents dimer formation in rep mutants and UV-irradiated cells. Mol. Microbiol. 37180-191. [DOI] [PubMed] [Google Scholar]
- 25.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 26.Morel, P., J. A. Hejna, S. D. Ehrlich, and E. Cassuto. 1993. Antipairing and strand transferase activities of E. coli helicase-II (UvrD). Nucleic Acids Res. 213205-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morimatsu, K., and S. C. Kowalczykowski. 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell 111337-1347. [DOI] [PubMed] [Google Scholar]
- 28.Nichols, B. P., O. Shafiq, and V. Meiners. 1998. Sequence analysis of Tn10 insertion sites in a collection of Escherichia coli strains used for genetic mapping and strain construction. J. Bacteriol. 1806408-6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Donnell, M. 2006. Replisome architecture and dynamics in Escherichia coli. J. Biol. Chem. 28110653-10656. [DOI] [PubMed] [Google Scholar]
- 30.Petit, M. A., and D. Ehrlich. 2002. Essential bacterial helicases that counteract the toxicity of recombination proteins. EMBO J. 213137-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandler, S. J. 2000. Multiple genetic pathways for restarting DNA replication forks in Escherichia coli K-12. Genetics 155487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandler, S. J., and K. J. Marians. 2000. Role of PriA in replication fork reactivation in Escherichia coli. J. Bacteriol. 1829-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seigneur, M., V. Bidnenko, S. D. Ehrlich, and B. Michel. 1998. RuvAB acts at arrested replication forks. Cell 95419-430. [DOI] [PubMed] [Google Scholar]
- 34.Tourriere, H., and P. Pasero. 2007. Maintenance of fork integrity at damaged DNA and natural pause sites. DNA Repair (Amsterdam) 6900-913. [DOI] [PubMed] [Google Scholar]
- 35.Uzest, M., S. D. Ehrlich, and B. Michel. 1995. Lethality of reprecB and reprecC double mutants of Escherichia coli. Mol. Microbiol. 171177-1188. [DOI] [PubMed] [Google Scholar]
- 36.Veaute, X., S. Delmas, M. Selva, J. Jeusset, E. Le Cam, I. Matic, F. Fabre, and M. A. Petit. 2005. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 24180-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Washburn, B. K., and S. R. Kushner. 1993. Characterization of DNA helicase II from a uvrD252 mutant of Escherichia coli. J. Bacteriol. 175341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yancey-Wrona, J. E., and S. W. Matson. 1992. Bound Lac repressor protein differentially inhibits the unwinding reactions catalyzed by DNA helicases. Nucleic Acids Res. 206713-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, G., E. X. Deng, L. R. Baugh, C. M. Hamilton, V. F. Maples, and S. R. Kushner. 1997. Conserved motifs II to VI of DNA helicase II from Escherichia coli are all required for biological activity. J. Bacteriol. 1797544-7550. [DOI] [PMC free article] [PubMed] [Google Scholar]