Abstract
Trimethylamine N-oxide (TMAO) reductases are widespread in bacteria and often function in anaerobic respiration. The regulation and expression of TMAO reductase operons have been well studied in model genera such as Escherichia, Shewanella, and Rhodobacter, although TMAO reductases are present in many other bacteria, including the marine Vibrio species. The genome sequence of Vibrio fischeri revealed three putative TMAO reductase operons, and a previous report identified TMAO reductase activity in symbiotic V. fischeri isolates associated with the light organs of adult Hawaiian bobtail squid, Euprymna scolopes. We examined the roles and regulation of these three operons using mutational analyses and promoter-reporter fusions. We found that the torECA promoter, and to a lesser extent the torYZ and dmsABC promoters, were active during symbiotic colonization of juvenile E. scolopes; however, a V. fischeri strain lacking TMAO reductase activity displays no discernible colonization defect over the first 48 h. Our studies also revealed that torECA has the most active promoter of the putative TMAO reductase operons, and TorECA is the major contributor to TMAO-dependent growth in V. fischeri under the conditions tested. Interestingly, the transcriptional regulation of TMAO reductase operons in V. fischeri appears to differ from that in previously studied organisms, such as Escherichia coli, which may reflect differences in gene arrangement and bacterial habitat. This study lays the foundation for using V. fischeri as a model system for studying TMAO reductases in the Vibrionaceae.
Trimethylamine N-oxide (TMAO) can be used by various bacteria as a terminal electron acceptor during anaerobic respiration. TMAO is commonly found in marine environments, including in the tissues of marine animals, where it is used as an osmolyte (5) and can reach levels of mmol kg−1 of wet weight (21, 41). Much of our understanding of the regulation and function of TMAO reductases comes from studies of bacteria from the genera Escherichia, Shewanella, and Rhodobacter (24), although TMAO reductases can also be found in other genera. A previous report demonstrated that marine Vibrio species are capable of reducing TMAO (27), and genome sequencing projects are revealing that TMAO reductases are common within the Vibrionaceae, which includes many economically and/or medically important species. However, the regulation of these operons and their relative contribution to TMAO reduction in Vibrio species have not been studied, despite the fact that TMAO is most common in marine environments (5). In addition, many of the previous studies have focused on TMAO reduction during growth in laboratory cultures, leaving the relevance of TMAO reduction in a natural (e.g., marine host associated) environment largely unexplored. To address these areas, we have used Vibrio fischeri as a model organism to examine TMAO reduction in the Vibrionaceae. Because the symbiosis between V. fischeri and its symbiotic partner Euprymna scolopes (40) can be reconstituted in the lab (29), we were able to address how TMAO reduction contributes to bacterial growth in a natural habitat where TMAO is presumably present.
The genome sequence of V. fischeri (31) revealed that this organism likely has three TMAO reductase operons, and previous work by others identified TMAO reductase activity in V. fischeri cells grown under laboratory conditions and during symbiotic growth in association with E. scolopes light organ tissue (27). These data suggest that TMAO reduction may be an important respiratory pathway in V. fischeri during growth in environments where TMAO is present. Our goals were to characterize the regulation of each of the three putative TMAO reductase operons in response to growth conditions, to determine whether known regulatory proteins influenced the promoter activity of each operon, and to determine the contribution of each putative reductase to TMAO-dependent growth during the culture of V. fischeri under both batch-culture and symbiotic conditions. This study represents the first in-depth characterization of TMAO-based respiration in a marine organism other than Shewanella spp.
MATERIALS AND METHODS
Reagents.
All restriction enzymes were purchased from New England Biolabs (Ipswich, MA), and chemicals were obtained from Sigma-Aldrich (St. Louis, MO). PCR analysis was performed using KOD HiFi DNA polymerase (Novagen, San Diego, CA), using the manufacturer's suggested reaction conditions. All PCR-amplified DNA fragments were verified by sequencing at the University of Michigan DNA Sequencing Core Facility or at the Oklahoma Medical Research Foundation DNA Sequencing Center. Primer sequences are available upon request.
Strains and plasmids.
Strains and plasmids used in this study are given in Table 1. General cloning procedures were used, and plasmids were transformed and maintained in E. coli strains DH5α (17) or DH5α λpir (13). Constructs containing the RP4 origin of transfer were introduced into V. fischeri isolates using triparental mating as previously described (37).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| Vibrio fischeri | ||
| ES114 | Wild-type isolate from E. scolopes light organ tissue | 7 |
| AKD800 | ES114 ΔtorECA (allele exchanged from pAKD800) | This study |
| AKD801 | ES114 ΔtorYZ (allele exchanged from pAKD801) | This study |
| AKD802 | ES114 ΔdmsABC (allele exchanged from pAKD802) | This study |
| AKD803 | ES114 ΔtorECA ΔtorYZ | This study |
| AKD804 | ES114 ΔtorECA ΔdmsABC | This study |
| AKD805 | ES114 ΔtorYZ ΔdmsABC | This study |
| AKD806 | ES114 ΔtorECA ΔtorYZ ΔdmsABC | This study |
| AKD807 | ES114 Tn7 PtorYZ-lacZ insertion | This study |
| AKD808 | ES114 Tn7 PtorECA-lacZ insertion | This study |
| AKD809 | ES114 Tn7 PdmsABC-lacZ insertion | This study |
| JB1 | ES114 Δfnr | J. L. Bose |
| KV1585 | ES114 torR::pKV174 | 18 |
| Escherichia coli | ||
| DH5α | F′/endA1 hsdR17 glnV44 thi-1 recA1 gyrA relA1 Δ(lacIZYA-argF)U169 deoR [φ80dlacIΔ(lacZ)M15] | 17 |
| DH5α λpir | λpir derivative of DH5α | 13 |
| CC118 λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1, lysogenized with λpir | 33 |
| Plasmids | ||
| pVSV105 | E. coli-V. fischeri shuttle vector, RP4 oriT, pES213 and R6K replication origins, Cmr | 14 |
| pVSV209 | E. coli-V. fischeri shuttle vector, RP4 oriT, pES213 and R6K replication origins, promoterless gfp and Cmr, constitutive rfp, Knr | 14 |
| pVSV209torECA | pVSV209 with the torECA promoter region | This study |
| pVSV209torYZ | pVSV209 with the torYZ promoter region | This study |
| pVSV209dmsABC | pVSV209 with the dmsABC promoter region | This study |
| pAKD701 | gfp and Cmr-containing StuI-SanDI fragment in pVSV209 was replaced with a StuI-SanDI lacZ fragment PCR-amplified from E. coli MG1655 using lacZF and lacZR, rfp removed by NotI digestion followed by self-ligation | This study |
| pAKD701torECA | pAKD701 with the torECA promoter region | This study |
| pAKD701torYZ | pAKD701 with the torYZ promoter region | This study |
| pAKD701dmsABC | pAKD701 with the dmsABC promoter region | This study |
| pAKD800 | ΔtorECA allele, R6K and ColE1 replication origins, RP4 oriT, Emr, Knr | This study |
| pAKD801 | ΔtorYZ allele, R6K and ColE1 replication origins, RP4 oriT, Emr, Knr | This study |
| pAKD802 | ΔdmsABC allele, R6K and ColE1 replication origins, RP4 oriT, Emr, Knr | This study |
| pAKD803 | pVSV105 containing the torECA complementing fragment | This study |
| pEVS104 | Conjugative helper plasmid, R6K replication origin, Knr | 37 |
| pEVS107 | pEVS94S derivative, mini-Tn7, mob, Emr, Knr | 23 |
| pEVS107torYZ | pEVS107 containing PtorYZ-lacZ PCR amplified from pAKD701torYZ | This study |
| pEVS107torECA | pEVS107 containing PtorECA-lacZ PCR amplified from pAKD701torECA | This study |
| pEVS107dmsABC | pEVS107 containing PdmsABC-lacZ PCR amplified from pAKD701dmsABC | This study |
Cmr, chloramphenicol resistance; Knr, kanamycin resistance; Emr, erythromycin resistance; rfp, red fluorescent protein gene.
V. fischeri strains lacking TMAO reductase operons.
Strains lacking one, two, or all three of the putative operons were constructed by allelic exchange (10), resulting in in-frame deletion mutants. Briefly, approximately 1.6 kb of DNA upstream of the start codon for each operon was PCR amplified and fused to an approximately 1.6-kb DNA fragment downstream of the stop codon for each operon using an engineered restriction site, resulting in replacement of the operon with a 6-bp restriction enzyme recognition site between the start and stop codons.
Green fluorescent protein reporter plasmids.
Approximately 500 bp (torECA) or 300 bp (torYZ and dmsABC) of DNA upstream of the ATG start codon for the first gene in each operon was PCR amplified using primers engineered to contain recognition sites for SalI and AvrII restriction endonucleases. These fragment sizes were chosen to reflect the intergenic region between the operon of interest and the nearest upstream coding sequence. These fragments were cloned into the SalI and AvrII restriction sites of pVSV209 (14), generating transcriptional promoter fusions to a promoterless gfp. These plasmids contain the V. fischeri pES213 origin of replication and are present in approximately 10 copies per chromosome in V. fischeri isolates (13).
lacZ reporter plasmids.
The promoter-containing fragments described in the previous section were similarly PCR amplified and cloned into pAKD701 to generate transcriptional promoter fusions to lacZ. pAKD701 was constructed by replacing the gfp and chloramphenicol resistance gene-containing StuI-SanDI fragment in pVSV209 (14) with a StuI-SanDI lacZ fragment PCR amplified from E. coli MG1655 and removal of an rfp-containing NotI fragment. These plasmids contain the V. fischeri pES213 origin of replication and are present in approximately 10 copies per chromosome in V. fischeri isolates (13).
Chromosomal promoter-lacZ fusion reporters.
The TMAO reductase promoter regions fused to the lacZ reporter on pAKD701torECA, pAKD701torYZ, or pAKD701dmsABC were PCR amplified and cloned into pEVS107 (23), generating mini-Tn7 constructs containing the respective TMAO reductase promoters fused to lacZ. The chromosomal insertion was performed as described previously (23).
Growth media.
V. fischeri strains were grown at 24 or 28°C in LBS (35) or mineral salts medium (MS), which contained (per liter) 1.0 g Casamino Acids, 6 ml 50% glycerol, 378 μl 1 M NaPO4 (pH 7.5), 50 ml 1 M Tris (pH 8.0), 6 mg FeSO4-7H2O, 13.6 g MgSO4-7H2O, 0.83 g KCl, 19.5 g NaCl, 1.62 g CaCl2-2H2O. When noted, 20 mM glucose was added to MS. For overnight cultures harboring plasmids, 100 μg ml−1 of kanamycin was added to the growth medium. For anaerobic growth, 20 ml of medium was placed in sealed 165-ml bottles, and the bottles were sparged with a 5% H2, 10% CO2, and an 85% N2 gas mixture prior to autoclaving, except where noted.
Visualization of promoter-gfp reporter activity in juvenile squid light organs.
Aposymbiotic E. scolopes hatchlings were inoculated with V. fischeri ES114 containing pVSV209, pVSV209torECA, pVSV209torYZ, or pVSV209dmsABC, as described previously (14). At the indicated time points, individual animals were dissected and visualized using a Nikon (Melville, NY) Eclipse E600 epifluorescence microscope outfitted with a Nikon Chroma 51004 v2 fluorescein isothiocyanate/tetramethyl rhodamine isothiocyanate filter cube, a Nikon Chroma 41017 Endow green fluorescent protein filter cube, and a Nikon Coolpix 5000 camera. All images were treated equally, with cropping and brightness reduction performed in Microsoft PowerPoint 2003.
Assays for symbiotic competence.
Aposymbiotic E. scolopes hatchlings were inoculated with an approximately 1:1 mix of AKD806 (ΔtorECA ΔtorYZ ΔdmsABC) and its parent, ES114, as previously described (14). After 48 h, individual animals were homogenized and dilution plated. Plates were incubated aerobically at 28°C overnight, and individual colonies were patched to plates containing TMAO and incubated anaerobically in GasPak jars (BD, Franklin Lakes, NJ) at 28°C for 16 to 20 h to determine the ratio of AKD806 (V. fischeri patches that did not grow) to ES114. The ability of AKD806 to grow after patching was confirmed in a subset of the samples by incubating a replicate plate aerobically. The ability of AKD806 to compete with ES114 is presented as a relative competitiveness index (RCI), which is the ratio of AKD806 to ES114 in the host divided by the ratio of these strains in the inoculum. An RCI not significantly different from 1 indicates the strains compete equally well during host colonization. Three independent experiments were performed, and the data were combined. Statistical significance was determined using Student's t test on log-transformed data.
Anaerobic growth curves.
V. fischeri strains ES114, AKD803 (ΔtorECA ΔtorYZ), AKD804 (ΔtorECA ΔdmsABC), AKD805 (ΔtorYZ ΔdmsABC), and AKD806 (ΔtorECA ΔtorYZ ΔdmsABC) were grown at 24°C with either 4 or 40 mM TMAO in MS, in MS supplemented with glucose, or in LBS. Anaerobic bottles were inoculated with a 1,000-fold dilution of an aerobically grown overnight culture of the respective strain, and the optical density was measured at 595 nm. Each experiment consisted of three independent cultures for each strain and was repeated three times. In each case, one representative experiment is shown.
β-Galactosidase assays testing the influence of environmental conditions on promoter-lacZ transcriptional fusions.
Assays were performed as described previously (10), using a modified Miller assay. Cells were grown aerobically or anaerobically in LBS, MS, or MS supplemented with glucose, either with or without 40 mM TMAO. For aerobic growth, an overnight culture (16 h) was diluted 1,000-fold in 15 ml of medium in a 125-ml Erlenmeyer flask. For anaerobic growth, an aerobic overnight culture (16 h) was diluted 1,000-fold into 20 ml of medium in sealed anaerobic 165-ml bottles. Samples were collected at the mid-log and stationary phases, with the specific optical density at 595 nm varying per medium type and growth conditions. Each experiment consisted of three independent cultures for each strain and was repeated three times. One representative experiment of the three is shown. Average Miller unit values for the negative control (V. fischeri harboring pAKD701) for each growth condition were subtracted prior to calculating the average and the standard error. Values for the negative control varied per growth condition and ranged from 0.2 to 1.4 Miller units.
β-Galactosidase assays testing the influence of known regulatory proteins on promoter-lacZ transcriptional fusions.
Assays were performed as described in the previous section. Culture tubes (18-mm in diameter) containing 3 ml of LBS were inoculated with 3 μl of an overnight culture and placed in sealed canisters with a GasPak EZ anaerobe pouch system (BD, Franklin Lakes, NJ) to achieve anaerobic growth conditions. The canisters were placed at 24°C until the cultures reached the stationary phase. For experiments with KV1585, 40 mM of TMAO was added to the LBS when indicated. Cultures grown in the GasPak system displayed levels of promoter activity similar to those grown in anaerobic bottles (e.g., Table 2 and data not shown); therefore, the GasPak system was used for these assays. Each experiment consisted of three independent cultures for each strain, and one representative experiment of three is shown. The average Miller unit values are presented as described in the previous section.
TABLE 2.
Activity of TMAO reductase promoter-lacZ fusions
| Conditionsa | Promoter activity in Miller unitsb
|
|||||
|---|---|---|---|---|---|---|
| PtorECA-lacZ
|
PtorYZ-lacZ
|
PdmsABC-lacZ
|
||||
| Without TMAO | With TMAO | Without TMAO | With TMAO | Without TMAO | With TMAO | |
| Aerobic | ||||||
| LBS | ||||||
| Mid-log phase | 120 (±6)d | 360 (±35) | 8 (±1) | 7 (±1) | 2 (±0.1)c | 5 (±1) |
| Stationary phase | 170 (±6.3)c | 390 (±58) | 100 (±14)c | 60 (±4) | 3 (±0.3)c | 5 (±0.4) |
| MS with glucose | ||||||
| Mid-log phase | 170 (±37) | 220 (±39) | 6 (±1) | 5 (±1) | 2 (±0.1) | 2 (±0.4) |
| Stationary phase | 340 (±16) | 481 (±145) | 20 (±5) | 10 (±1) | 4 (±1)c | 2 (±0.2) |
| MS without glucose | ||||||
| Mid-log phase | 300 (±33) | 440 (±73) | 17 (±1.1)c | 12 (±1.2) | 3 (±0.1) | 2 (±0.2) |
| Stationary phase | 810 (±63) | 890 (±65) | 30 (±1)d | 17 (±0.9) | 5 (±0.3)d | 3 (±0.2) |
| Anaerobic | ||||||
| LBS | ||||||
| Mid-log phase | 340 (±1) | 500 (±86) | 90 (±1)d | 60 (±6) | 70 (±6)d | 10 (±1) |
| Stationary phase | 320 (±15)d | 1,220 (±81.9) | 100 (±3.5)d | 200 (±13) | 70 (±2)d | 40 (±2) |
| MS with glucose | ||||||
| Mid-log phase | 30 (±5)d | 160 (±23) | 70 (±9)d | 10 (±2) | 76 (±21)c | 6 (±1) |
| Stationary phase | 50 (±3) | 604 (±234) | 110 (±7.2)d | 70 (±3) | 170 (±11)d | 30 (±2) |
| MS without glucose | ||||||
| Mid-log phase | ND | 780 (±29) | ND | 120 (±22) | ND | 80 (±5) |
| Stationary phase | ND | 3,170 (±164) | ND | 280 (±12) | ND | 100 (±9) |
Culture conditions included growth in LBS (rich medium) or MS, either aerobically or anaerobically, and with or without 40 mM TMAO. OD595 measurements were used to determine the mid-log and stationary phases (see Table 3).
Promoter activity is reported for the average of three independent cultures (± standard error of the mean). Significant differences between Miller unit values for a particular promoter with and without TMAO under specific growth conditions were determined using Student's t test. ND, not determined; V. fischeri will not grow under these conditions.
P < 0.05.
P < 0.01.
RESULTS
The annotation of the V. fischeri ES114 genome (31) identified three putative TMAO reductase operons composed of open reading frames (ORFs) VFA0297-VFA0299, VFA0189-VFA0188, and VFA0082-VFA0080. We further used BLAST (1) to identify sequence similarity to known TMAO reductase operons in E. coli and sequence similarity to TorE in “Shewanella massilia” and found homologs in V. fischeri ranging from 37 to 76% amino acid identity over >93% of the respective protein. Based on this information, we assigned these ORFs in V. fischeri as TorECA, TorYZ, and DmsABC, respectively.
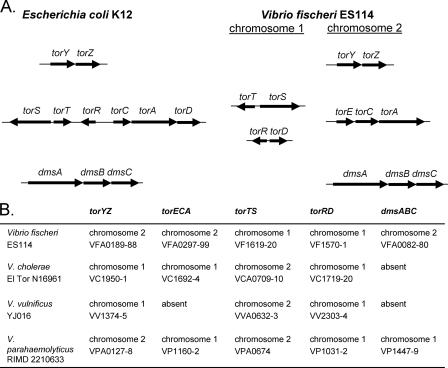
Based on these analyses, ES114 appears to contain a suite of TMAO reductase-like operons similar to those found in E. coli (Fig. 1A). However, for the operon encoding TorA, in E. coli the regulatory elements (TorS, TorT, and TorR) and the structural components (TorC, TorA, and TorD) are encoded contiguously on the genome, whereas in V. fischeri, the genes are arranged in three separate operons, one of which is on a separate chromosome (Fig. 1A). In addition, unlike in E. coli, in V. fischeri the TorA operon includes torE, a gene also found in Shewanella species (12). The function of TorE is currently unknown, but it is predicted to be a small membrane protein (12). Interestingly, the presence of the TMAO reductases varies between Vibrio species, with Vibrio vulnificus lacking torECA and both V. vulnificus and Vibrio cholerae lacking dmsABC (Fig. 1B). When the operons are present, their chromosomal location is not consistent among all species (Fig. 1B). In addition, one alternative operon arrangement has been identified in the unfinished genomes of Photobacterium profundum 3TCK and “Vibrio angustum” S14, where TorR and TorD are encoded contiguously with TorYZ on the chromosome. Although the location and presence of TMAO reductase operons vary between the Vibrio species shown in Fig. 1, V. fischeri does appear to encode the full set of known TMAO reductases and regulatory elements, making it a good starting point for understanding the regulation and use of the TMAO reductases in Vibrio species and how these differ from the currently characterized systems in E. coli and other organisms.
FIG. 1.
TMAO reductase operon structure in E. coli and Vibrio species. (A) Comparison of gene organization in E. coli and V. fischeri. (B) Presence and location of TMAO reductase operons in representative Vibrio species. Chromosomal location and ORF numbers are indicated for individual genes.
Testing the expression and role of TMAO reductase operons during symbiotic colonization.
To gain insight into the function of each TMAO reductase in V. fischeri and the importance of TMAO reduction during growth in a natural environment (during symbiotic colonization), we constructed plasmid-based promoter-gfp reporters and in-frame deletion mutants.
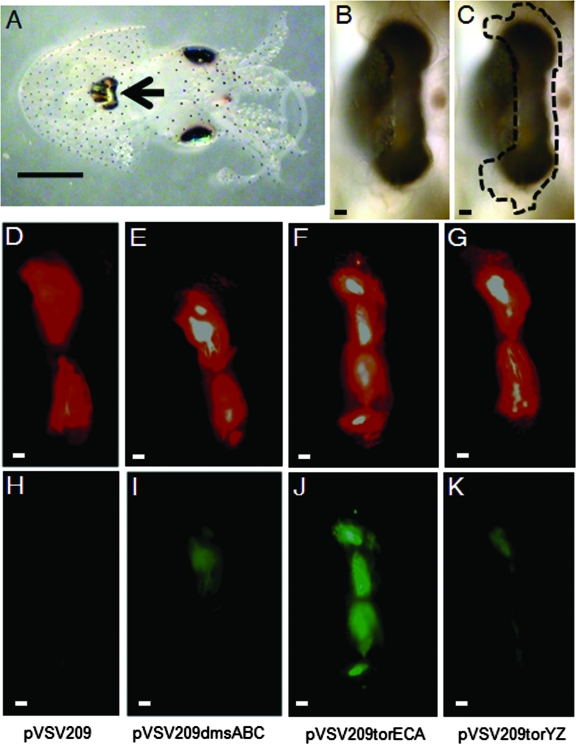
To determine whether TMAO reductase operon promoters were active during host colonization, we inoculated aposymbiotic juvenile squid with ES114 carrying the pVSV209-based (14) gfp transcriptional reporter plasmids and qualitatively monitored colonization and promoter activity using epifluorescence microscopy. Seventy-two hours after inoculation, the juvenile light organs were fully colonized by V. fischeri (Fig. 2D to G), but promoter activity varied among the operons (Fig. 2H to K). The torECA promoter appeared to be most active, with relatively slight activity for PtorYZ and PdmsABC. Similar levels of promoter activity were observed at 24 and 48 h postinoculation (data not shown), suggesting that relative promoter activity does not change during the early stages of colonization.
FIG. 2.
Expression of TMAO reductase operon promoter-gfp reporters during growth in the host light organ. (A) Light microscopy image of the ventral side of a juvenile squid. The arrow points to the location of the light organ tissue. The black bar represents 1 mm. (B) Higher magnification of the light organ tissue area depicted in panel A. The black bar represents approximately 10 μm. (C) Same image as panel B but with the location of the transparent light organ tissue highlighted. Epifluorescence microscopy was used to image juvenile squid light organs infected with V. fischeri containing pVSV209 (D, H), pVSV209dmsABC (E, I), pVSV209torECA (F, J), and pVSV209torYZ (G, K). Panels D to G are images that show red (from the constitutive rfp) and green (from the gfp reporter) fluorescence and indicate the location of the bacteria in the organ. Panels H to K are images that show only green fluorescence and indicate the level of activity for each reductase operon promoter-gfp reporter. Images were taken 72 h postinoculation at 200× magnification. Similar results were observed 24 and 48 h postinoculation. White bars represent approximately 10 μm.
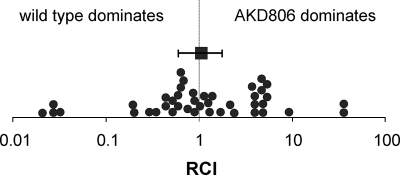
The reporter assays described above suggested that certain TMAO reductases are produced during symbiotic colonization. To determine whether the presence of TMAO reductases was important for host colonization, we assayed the ability of AKD806, a V. fischeri mutant lacking the three putative TMAO reductases, to colonize aposymbiotic juvenile squid. In experiments where E. scolopes squid were exposed to clonal inocula containing either AKD806 or the wild type, the onset of bioluminescence and colonization levels were similar in the two treatments, suggesting that AKD806 does not have a symbiotic defect compared to the wild type (data not shown). However, in some mutants, symbiotic defects are only apparent when they are competed with the wild type in mixed inocula (36, 39). Interestingly, AKD806 displayed no significant colonization defect 48 h after inoculation when competed with the wild type, as evidenced by an average RCI value of 1.05 for three combined experiments (Fig. 3). Similar results were obtained at 72 and 96 h after inoculation (data not shown).
FIG. 3.
Competitiveness of AKD806 (ΔtorECA ΔtorYZ ΔdmsABC) during symbiotic colonization. RCI values for squid inoculated with a 1:1 ratio of AKD806 to the wild type, measured 48 h after the exposure of squid hatchlings to the bacterial strains. Each filled circle represents the RCI value calculated from the ratio of strains in an individual animal. The RCI value equals the ratio of AKD806 to ES114 in the host divided by the ratio of these strains in the inoculum, with a value equal to 1 indicating that the strains compete equally well during host colonization. Three independent experiments were performed, and the data were combined for a total of 43 animals. The square represents the average RCI value for the combined experiments, with error bars representing the 95% confidence interval.
Culture conditions influence TMAO reductase promoter activity.
Examination of promoter-gfp fusions (above) demonstrated that the promoter strength of the three putative TMAO reductase operons had different expression levels during symbiotic growth and suggested that the expression of each operon might be controlled in response to different signals. To determine whether promoter activity was influenced by growth phase, oxygen tension, medium type, and/or the presence of TMAO, each promoter region was cloned into pAKD701 to generate transcriptional fusions with lacZ, and these reporter plasmids were introduced into V. fischeri ES114. The resulting strains were then grown under varied conditions (Table 2), with samples harvested for β-galactosidase assays at the mid-log and stationary phases (Table 3). The various conditions were chosen in an attempt to determine how the promoter activity of the putative V. fischeri TMAO reductases compared to previously studied organisms under aerobic and anaerobic conditions in the presence and absence of TMAO. In addition, previous work with Rhodopseudomonas capsulata determined that TMAO can be used as an electron sink during fermentation rather than as an electron acceptor for anaerobic respiration (22); therefore, media were chosen that were either not fermentable (MS) or contained various fermentable carbon sources (MS with glucose; LBS).
TABLE 3.
Approximate OD595 values for cultures harvested for the β-galactosidase assays reported in Table 2
| Conditions | OD595 valuesa
|
|||
|---|---|---|---|---|
| Without TMAO
|
With TMAO
|
|||
| Mid-log phase | Stationary phase | Mid-log phase | Stationary phase | |
| Aerobic | ||||
| LBS | 1.3 | 3.7 | 1.3 | 3.7 |
| MS with glucose | 0.4 | 1.7 | 0.4 | 1.7 |
| MS | 0.6 | 0.9 | 0.6 | 0.9 |
| Anaerobic | ||||
| LBS | 0.3 | 0.4 | 0.3 | 1.3 |
| MS with glucose | 0.3 | 0.9 | 0.3 | 2.0 |
| MS | ND | ND | 0.4 | 1.0 |
ND, not determined; Vibrio fischeri will not grow under these conditions.
Overall promoter activity was highest for PtorECA, with much lower levels for PtorYZ and PdmsABC. Upon examining the effect of substrate on promoter expression, we found that TMAO induced PtorECA activity during anaerobic growth in both MS and LBS, but under aerobic conditions, TMAO induced PtorECA activity only in LBS and to a lesser degree than under anaerobic conditions. Interestingly, the highest levels of PtorECA activity were observed during the stationary phase in medium containing TMAO but lacking a fermentable carbon source (MS without glucose) (Table 2). In addition, high levels of PtorECA activity were observed during aerobic growth, both with and without TMAO present. In contrast, PtorYZ and PdmsABC activities were either unaffected or decreased in the presence of TMAO, with the exception of PtorYZ activity during anaerobic growth in LBS.
To address the possibility that the 300-bp promoter fragments for the TorYZ and DmsABC operons did not encompass the essential regulatory region, we constructed reporters containing between 500 and 600 bp upstream of the start codon for each operon. The promoter activity of the larger constructs was compared to that of the 300-bp constructs under a subset of conditions, and no significant differences in promoter activity were observed (data not shown), suggesting that the 300-bp fragments contained the necessary regulatory regions for controlling promoter activity under the conditions tested.
To address the possibility that the measurements of promoter activity could be influenced by the reporter plasmids being multicopy in V. fischeri (∼10 copies per chromosome) (13), we constructed single-copy chromosomal reporters by cloning the pAKD701-based promoter-lacZ fusions into a mini-Tn7 on pEVS107 (23) and introducing these constructs into V. fischeri isolates. This mini-Tn7 generates site-specific inserts at an intergenic att locus downstream of glmS, making it a useful tool for creating isogenic chromosomal insertions that do not disrupt other genes (23). The promoter activity of the mini-Tn7-based reporters was compared to the plasmid-based reporters under a subset of conditions. For the torECA and dmsABC reporters, the pattern of activity of the plasmid and chromosomal reporters was similar, although the activity was lower overall for the Tn7-based chromosomal reporters (data not shown). In contrast, whereas the plasmid-based torYZ reporter assays indicated an increase in activity in response to TMAO when grown in LBS, the chromosomal-based reporter (strain AKD807) showed similar levels of promoter activity in the presence and absence of TMAO (data not shown), suggesting that the increased copy number either affects the activity of an unknown regulatory protein or enhances overall expression such that subtle differences in LacZ activity are easier to detect.
Influence of TorR and FNR on TMAO reductase promoter activity.
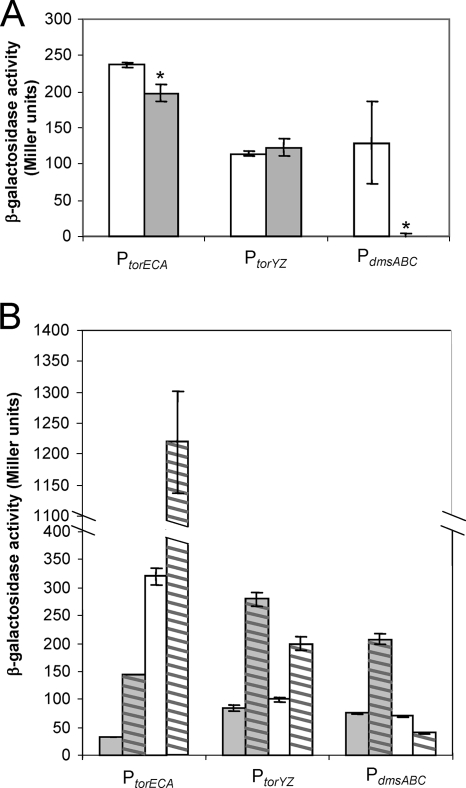
Previous work by others with E. coli and Shewanella species has shown that the regulators FNR (11) and TorR (8, 32) influence the expression of TMAO/dimethyl sulfoxide (DMSO) reductase operons. In addition, a recent bioinformatics-based study of V. fischeri identified putative FNR binding sites upstream of the torECA and dmsABC operons, as well as a putative ArcA binding site upstream of dmsABC in this organism (28). To determine whether these regulators were involved in the control of promoter activity of the putative TMAO reductase operons in V. fischeri, the lacZ reporter plasmids were introduced into V. fischeri strains JB1, AMJ2 (10), and KV1585, which lack FNR, ArcA, and TorR, respectively. The absence of ArcA did not appear to influence promoter activity in any of the three operons (data not shown). However, FNR and TorR did influence promoter activity of the torECA and dmsABC operons (Fig. 4). During anaerobic growth, PtorECA and PdmsABC activity levels were significantly (P < 0.05) lower in JB1 relative to those of wild-type V. fischeri (Fig. 4A), suggesting that FNR positively regulates the torECA and dmsABC promoters, although to low degrees. FNR also positively regulates dmsABC in E. coli (6); however, FNR does not influence E. coli torCAD promoter activity (34) or Shewanella oneidensis TMAO reductase gene expression (15).
FIG. 4.
Influence of FNR and TorR on TMAO reductase promoter activity. (A) β-Galactosidase activity for each putative TMAO reductase promoter-lacZ reporter in either wild-type cells (white bars) or JB1 cells (the fnr mutant) (gray bars) grown anaerobically in LBS medium. Asterisks indicate statistically significant decreases in activity in JB1 relative to those in the wild type as measured using Student's t test (PtorECA, P < 0.02; PdmsABC, P < 0.05). (B) β-Galactosidase activity for each putative TMAO reductase promoter-lacZ reporter in KV1585 (the torR mutant) grown anaerobically in LBS medium either without (gray bars) or with (gray bars with hatching) 40 mM TMAO and in the wild type grown in LBS medium either without (white bars) or with (white bars with hatching) 40 mM TMAO. The data for the wild type were taken from Table 2. For both panels, error bars represent the standard error of the mean. Experiments were conducted three times, with one representative experiment shown.
To determine whether TorR plays a role in the regulation of the putative TMAO reductase promoters in response to TMAO, the promoter activity levels were compared in the KV1585 (the torR mutant) background either in the presence or absence of TMAO (Fig. 4B). Interestingly, torECA promoter activity increased in the presence of TMAO even without TorR present, although overall promoter activity levels were decreased in the torR mutant relative to levels observed in the wild type, suggesting that TorR may be involved in activating torECA promoter activity in the absence of TMAO. Additionally, TorR appears to play a role in the negative regulation of dmsABC promoter activity, because PdmsABC activity levels were much higher in KV1585 than in the wild type when TMAO was present. Finally, torYZ promoter activity does not appear to be significantly affected by FNR or TorR.
Role of TMAO reductases in anaerobic growth.
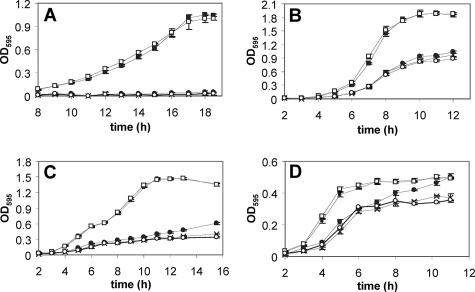
The results of the promoter-reporter assays above suggested that torECA encodes the major TMAO reductase in V. fischeri under laboratory culture conditions and that either TorYZ and DmsABC do not significantly contribute to TMAO reduction and anaerobic respiration or promoter activity levels are not a good predictor of protein levels and physiological importance. To differentiate between these possibilities, the anaerobic growth of V. fischeri strains ES114 (wild type), AKD803 (ΔtorECA ΔtorYZ), AKD804 (ΔtorECA ΔdmsABC), AKD805 (ΔtorYZ ΔdmsABC), and AKD806 (ΔtorECA ΔtorYZ ΔdmsABC) was measured in medium that contained TMAO but varied in the presence of a fermentable carbon source (Fig. 5). Under all conditions tested, the presence of TMAO enhanced growth of wild-type V. fischeri. In contrast, AKD806 (ΔtorECA ΔtorYZ ΔdmsABC) grown in medium containing TMAO reached densities similar to those of the wild type grown in medium lacking TMAO, suggesting that AKD806 does not produce enzymes capable of reducing TMAO and contributing to growth (data not shown). The growth of the strain containing only TorECA (AKD805) was similar to that of the wild type under all conditions tested, supporting the assumption that TorECA is the major TMAO reductase used during respiratory growth of V. fischeri isolates under anaerobic conditions in medium containing TMAO (Fig. 5A to D). In addition, the introduction of the torECA locus in trans on plasmid pAKD803 to AKD806 (ΔtorECA ΔtorYZ ΔdmsABC) restored wild-type growth levels during anaerobic growth in LBS and MS containing 40 mM TMAO (data not shown), further supporting the role of TorECA as the major TMAO reductase used by V. fischeri under these growth conditions. The growth of the strain containing only DmsABC (AKD803) was similar to that of the strain lacking all three operons (AKD806) under all conditions tested, indicating that this reductase is not expressed under these conditions and/or that the main substrate of this enzyme may be a different N- or S-oxide. The strain containing only TorYZ (AKD804) displays an intermediate growth phenotype, where it appears to contribute to an increase in cell density during entry to the stationary phase in LBS (Fig. 5C) but not in MS (Fig. 5A and B). This is consistent with the reporter data presented in Table 2, where torYZ promoter activity is higher during the stationary phase in LBS containing TMAO than in LBS alone, but this effect is not observed in MS medium. These results could reflect a role for TorYZ in the use of TMAO as an electron sink during fermentative growth on compounds found in yeast extract and/or tryptone (LBS) (Fig. 5C and D) but not glucose (MS medium with glucose) (Fig. 5B), as TMAO can play a role in redox balance in R. capsulata (22). In addition, TorYZ may be involved in a response to lower concentrations of TMAO because when AKD804 was grown in medium containing 4 mM TMAO, the cell density reached the same level as that of the wild type and AKD805 (Fig. 5D), suggesting that this enzyme's affinity for TMAO may be relatively higher; however, an increase in PtorYZ activity was not observed when cells were grown with less than 40 mM TMAO (data not shown).
FIG. 5.
Anaerobic growth curves of V. fischeri TMAO reductase mutants. Wild-type V. fischeri and the derived TMAO mutant strains were grown in MS (A), MS with glucose (B), and LBS (rich medium) (C), all containing 40 mM TMAO, or in LBS containing 4 mM TMAO (D). Each point corresponds to the average of three independent replicates. Error bars represent the standard error of the mean. One representative experiment of three is shown. □, V. fischeri ES114 (wild type); ×, V. fischeri AKD803 (ΔtorECA ΔtorYZ); •, V. fischeri AKD804 (ΔtorECA ΔdmsABC); ▪, V. fischeri AKD805 (ΔtorYZ ΔdmsABC); ○, V. fischeri AKD806 (ΔtorECA ΔtorYZ ΔdmsABC). OD595, optical density at 595 nm.
DISCUSSION
V. fischeri ES114 has a flexible respiratory system and is capable of utilizing numerous compounds as terminal electron acceptors, including oxygen and compounds such as TMAO, nitrate, and fumarate (27). This respiratory flexibility allows V. fischeri to thrive in a variety of environments, including free-living lifestyles in the water column or in sediments and in associations with hosts either in mixed gut consortia or in very specific associations with symbiotic partners, such as ES114's colonization of the squid E. scolopes (25, 29). In this study, we have laid the groundwork for understanding the role of TMAO reduction in the ability of V. fischeri to live both inside and outside of the host.
Because TMAO is commonly found in marine environments, including in the tissue of marine animals, such as squid (5, 21, 41), it would seem likely that utilization of this compound would be important in V. fischeri ecology. This assumption is supported by a previous report that identified TMAO reductase activity in the light organ tissues of adult E. scolopes, indicating that V. fischeri reduces TMAO in the host (27). Although V. fischeri can reduce nitrate, and the reduction potential for utilizing the nitrate/nitrite redox pair is greater than that for the TMAO/TMA redox pair (38), published measurements indicate that squid tissue can contain approximately 2,000 times more TMAO than nitrate (20, 21, 41). In addition, the growth rate of V. fischeri ES114 in MS containing nitrate is lower compared to growth in medium containing TMAO (27), supporting the idea that although the ΔE′0 values would suggest that using TMAO as a terminal electron acceptor would be less energetically favorable than using nitrate, TMAO may be an important terminal electron acceptor in V. fischeri physiology.
Analysis of the V. fischeri ES114 genome sequence revealed the presence of three putative TMAO reductase operons that could contribute to TMAO reduction. These operons show similarity to those found in E. coli (Fig. 1); however, although an operon similar to the E. coli DMSO reductase-encoding dmsABC was identified, V. fischeri ES114 cannot respire anaerobically using DMSO as a terminal electron acceptor (27). Moreover, it is difficult to distinguish the N- or S-oxide substrate for such reductases purely from bioinformatic approaches. Therefore, because the focus of this study was on TMAO reduction, we considered the possibility that dmsABC might function as a TMAO reductase in V. fischeri. It is worth noting that V. fischeri is not able to use TMAO as a sole source of carbon or nitrogen (27), indicating that the use of TMAO by this organism for growth enhancement likely involves respiration.
Based on the fact that fish and squid tissues can contain high levels of TMAO (5, 21, 41), the previous report of TMAO reduction in E. scolopes adult light organ tissues (27), the predicted low oxygen tensions in the juvenile and adult E. scolopes light organ tissues (30) (our unpublished data), and the observed TMAO reductase promoter activity during colonization (Fig. 2), we predicted that TMAO reductase activity would be an important colonization factor during establishment of the symbiosis; however, based on the results of the colonization assays (Fig. 3), these putative TMAO reductases are apparently not essential for V. fischeri to establish colonization of the E. scolopes light organ. It is possible that during this time period, oxygen levels in the light organ are sufficient for aerobic respiration and TMAO-based respiration becomes increasingly important as the symbiosis matures and the density of bacteria present in the light organ increases. Alternatively, if oxygen tensions in the light organ are low, as has been predicted (30) (our unpublished data), and aerobic respiration is not being utilized, TMAO may not be present in sufficient quantities in the juvenile light organ to support TMAO-based respiration; therefore, this mode of growth is not required for successful colonization. Under this scenario, it is possible that other modes of anaerobic-based energy generation, such as respiration with alternative electron acceptors or fermentation, may be important processes during early colonization. Although we were not able to detect a role for TMAO reduction in our squid colonization assays, this does not rule out the possibility that TMAO reduction plays a role in later stages of the symbiosis and/or in lifestyles outside of the host.
Despite TMAO being common in marine environments (5), the regulation of TMAO reductase activity has not been studied in marine bacteria outside of the genus Shewanella. To address this knowledge gap, we studied the regulation of TMAO reductase operons and their relative contribution to TMAO reduction in V. fischeri, a member of the Vibrionaceae family of marine bacteria. Although V. fischeri has a suite of TMAO reductases similar to that of E. coli, gene arrangement suggests that regulation of TMAO reductase genes in V. fischeri differs from that in other organisms in which it has been studied. Consistent with this hypothesis, the results of this study demonstrate certain differences between regulation of TMAO reductase promoter activity in E. coli and that in V. fischeri. One striking difference involves the TorCAD and TorECA operon promoter activities. Although in E. coli the TMAO reductase system (TorCAD) is expressed under aerobic conditions, the induction of the torCAD operon requires the presence of TMAO under either aerobic or anaerobic growth conditions (3). In contrast, the corresponding operon in V. fischeri (torECA) does not require TMAO for induction, although the addition of TMAO does increase PtorECA activity in certain media during anaerobic and aerobic growth (Table 2). In E. coli, the increase in torCAD promoter activity in response to TMAO is controlled by the TorTSR regulatory system (4, 19, 32) where TorR binds to specific DNA sequences upstream of the torCAD operon called tor boxes (2). In the absence of TorR, TorCAD is not expressed (26). Similar to those in E. coli, TorRS in S. oneidensis are involved in positive regulation of TorECAD expression (9), and tor boxes are present upstream of torECAD (8). Interestingly, canonical tor boxes were not identified upstream of torECA in V. fischeri (data not shown), possibly contributing to the observed differences in promoter activity patterns between these organisms in relation to the presence of TMAO. These differences may be related to the different ecosystems the organisms inhabit, and it is possible that marine host-associated V. fischeri encounters TMAO on a more regular basis than E. coli, and TMAO reductase activity is required more frequently for optimal growth.
Another notable difference in promoter activity patterns between V. fischeri and E. coli involves PtorYZ. The torYZ operon has previously been characterized only in E. coli, where it was determined to encode a third TMAO reductase (16). Unlike the torECA operon, the torYZ operon was determined to be constitutively expressed at low levels under the conditions tested and expression was not induced by TMAO (16). These experiments were conducted using a lacZ reporter on a multicopy plasmid (16). In V. fischeri, when measured using a multicopy reporter, PtorYZ activity appears to be induced in the presence of TMAO when cells are grown anaerobically to the stationary phase in LBS medium (Table 2 and Fig. 4B). However, when PtorYZ activity is measured using a chromosomal-based reporter (strain AKD807), TMAO-based induction is not observed (data not shown), suggesting that that the increased copy number either affects the activity of an unknown regulatory protein or enhances overall expression such that subtle differences in LacZ activity are easier to detect. In addition, data from the anaerobic growth curves suggest that TorYZ can contribute to enhanced growth levels in the stationary phase in the presence of TMAO in LBS medium but not in MS medium or in MS medium containing glucose (Fig. 5). Previous work with R. capsulata indicated a role for TMAO as an electron sink during fermentative growth (22). LBS medium contains tryptone and yeast extract as carbon and nitrogen sources, and V. fischeri is able to grow fermentatively in this medium, as well as in MS containing glucose. It is possible that in V. fischeri, TorYZ could be involved in maintaining a redox balance while fermenting compounds other than glucose, contributing to enhanced growth as observed in Fig. 5C; however, this hypothesis will require further testing.
Interestingly, although TMAO reductase activity is not required for early host colonization, the putative TMAO reductase promoters were active during colonization in relative activity levels corresponding to PtorECA > PtorYZ ≥ PdmsABC (Fig. 2). At the time points when the juvenile animals were visualized using epifluorescence microscopy, the bacterial cells are densely packed, which could correspond to the stationary phase in laboratory culture. The promoter activity data for stationary growth in laboratory culture (Table 2) indicate that in most cases, the promoter activity levels fall into a similar pattern of PtorECA > PtorYZ ≥ PdmsABC. This pattern of activity levels can be observed in both the presence and absence of TMAO (Table 2) and suggests that although TMAO concentration in the juvenile light organ tissue cannot be determined from these data, no symbiosis-specific pattern of promoter activity is detected in our assays. Future studies analyzing TMAO abundance in E. scolopes light organ tissue will be necessary to determine whether this compound is readily available to V. fischeri in the light organ.
This study represents the first genetics-based investigation of the contribution of TMAO reductase operons to the utilization of TMAO in the Vibrionaceae, and the first in-depth study of TMAO-based respiration in marine bacteria other than Shewanella spp., laying the foundation for increasing our understanding of how TMAO reduction contributes to the ecology of marine bacteria. We demonstrate that V. fischeri has significant differences from well-studied bacteria in the operon structure and regulation of promoter activity of these reductases. Although the presence of each of the three operons varies between different Vibrio species (Fig. 1), the regulatory proteins appear similar and the results from this study may be applicable to other medically and economically important marine-associated Vibrio species.
Acknowledgments
We thank Robert Maier for helpful discussions and access to equipment used in this study and Jeffrey Bose for contributing to Fig. 2.
This work was funded by a National Science Foundation CAREER award (MCB-0347317) to E.V.S. and by funds provided by the University of Oklahoma to A.K.D.
Footnotes
Published ahead of print on 7 July 2008.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 2.Ansaldi, M., G. Simon, M. Lepelletier, and V. Mejean. 2000. The TorR high-affinity binding site plays a key role in both torR autoregulation and torCAD operon expression in Escherichia coli. J. Bacteriol. 182961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansaldi, M., L. Theraulaz, C. Baraquet, G. Panis, and V. Mejean. 2007. Aerobic TMAO respiration in Escherichia coli. Mol. Microbiol. 66484-494. [DOI] [PubMed] [Google Scholar]
- 4.Baraquet, C., L. Theraulaz, M. Guiral, D. Lafitte, V. Mejean, and C. Jourlin-Castelli. 2006. TorT, a member of a new periplasmic binding protein family, triggers induction of the Tor respiratory system upon trimethylamine N-oxide electron-acceptor binding in Escherichia coli. J. Biol. Chem. 28138189-38199. [DOI] [PubMed] [Google Scholar]
- 5.Barrett, E. L., and H. S. Kwan. 1985. Bacterial reduction of trimethylamine oxide. Annu. Rev. Microbiol. 39131-149. [DOI] [PubMed] [Google Scholar]
- 6.Bearson, S. M., J. A. Albrecht, and R. P. Gunsalus. 2002. Oxygen and nitrate-dependent regulation of dmsABC operon expression in Escherichia coli: sites for Fnr and NarL protein interactions. BMC Microbiol. 213-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boettcher, K. J., and E. G. Ruby. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 1723701-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordi, C., M. Ansaldi, S. Gon, C. Jourlin-Castelli, C. Iobbi-Nivol, and V. Mejean. 2004. Genes regulated by TorR, the trimethylamine oxide response regulator of Shewanella oneidensis. J. Bacteriol. 1864502-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bordi, C., C. Iobbi-Nivol, V. Mejean, and J. C. Patte. 2003. Effects of ISSo2 insertions in structural and regulatory genes of the trimethylamine oxide reductase of Shewanella oneidensis. J. Bacteriol. 1852042-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose, J. L., U. Kim, W. Bartkowski, R. P. Gunsalus, A. M. Overley, N. L. Lyell, K. L. Visick, and E. V. Stabb. 2007. Bioluminescence in Vibrio fischeri is controlled by the redox-responsive regulator ArcA. Mol. Microbiol. 62538-553. [DOI] [PubMed] [Google Scholar]
- 11.Cotter, P. A., and R. P. Gunsalus. 1989. Oxygen, nitrate, and molybdenum regulation of dmsABC gene expression in Escherichia coli. J. Bacteriol. 1713817-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dos Santos, J. P., C. Iobbi-Nivol, C. Couillault, G. Giordano, and V. Mejean. 1998. Molecular analysis of the trimethylamine N-oxide (TMAO) reductase respiratory system from a Shewanella species. J. Mol. Biol. 284421-433. [DOI] [PubMed] [Google Scholar]
- 13.Dunn, A. K., M. O. Martin, and E. V. Stabb. 2005. Characterization of pES213, a small mobilizable plasmid from Vibrio fischeri. Plasmid 54114-134. [DOI] [PubMed] [Google Scholar]
- 14.Dunn, A. K., D. S. Millikan, D. M. Adin, J. L. Bose, and E. V. Stabb. 2006. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 72802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gon, S., J. C. Patte, J. P. Dos Santos, and V. Mejean. 2002. Reconstitution of the trimethylamine oxide reductase regulatory elements of Shewanella oneidensis in Escherichia coli. J. Bacteriol. 1841262-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gon, S., J. C. Patte, V. Mejean, and C. Iobbi-Nivol. 2000. The torYZ (yecK bisZ) operon encodes a third respiratory trimethylamine N-oxide reductase in Escherichia coli. J. Bacteriol. 1825779-5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 18.Hussa, E. A., T. M. O'Shea, C. L. Darnell, E. G. Ruby, and K. L. Visick. 2007. Two-component response regulators of Vibrio fischeri: identification, mutagenesis, and characterization. J. Bacteriol. 1895825-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jourlin, C., A. Bengrine, M. Chippaux, and V. Mejean. 1996. An unorthodox sensor protein (TorS) mediates the induction of the tor structural genes in response to trimethylamine N-oxide in Escherichia coli. Mol. Microbiol. 201297-1306. [DOI] [PubMed] [Google Scholar]
- 20.Karl, H. 1998. Nitrate and nitrite content in the edible part of fishes, crustaceans, and molluscans. Dtsch. Lebensm.-Rundsch. 9417-20. [Google Scholar]
- 21.Kelly, R. H., and P. H. Yancey. 1999. High contents of trimethylamine oxide correlating with depth in deep-sea teleost fishes, skates, and decapod crustaceans. Biol. Bull. 19618-25. [DOI] [PubMed] [Google Scholar]
- 22.Madigan, M. T., J. C. Cox, and H. Gest. 1980. Physiology of dark fermentative growth of Rhodopseudomonas capsulata. J. Bacteriol. 142908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCann, J., E. V. Stabb, D. S. Millikan, and E. G. Ruby. 2003. Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl. Environ. Microbiol. 695928-5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCrindle, S. L., U. Kappler, and A. G. McEwan. 2005. Microbial dimethylsulfoxide and trimethylamine-N-oxide respiration. Adv. Microb. Physiol. 50147-198. [DOI] [PubMed] [Google Scholar]
- 25.Nealson, K. H., and J. W. Hastings. 1979. Bacterial bioluminescence: its control and ecological significance. Microbiol. Rev. 43496-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascal, M. C., M. Lepelletier, G. Giordano, and M. Chippaux. 1991. A regulatory mutant of the trimethylamine N-oxide reductase of Escherichia coli K12. FEMS Microbiol. Lett. 62297-300. [DOI] [PubMed] [Google Scholar]
- 27.Proctor, L. M., and R. P. Gunsalus. 2000. Anaerobic respiratory growth of Vibrio harveyi, Vibrio fischeri and Photobacterium leiognathi with trimethylamine N-oxide, nitrate and fumarate: ecological implications. Environ. Microbiol. 2399-406. [DOI] [PubMed] [Google Scholar]
- 28.Ravcheev, D. A., A. V. Gerasimova, A. A. Mironov, and M. S. Gelfand. 2007. Comparative genomic analysis of regulation of anaerobic respiration in ten genomes from three families of gamma-proteobacteria (Enterobacteriaceae, Pasteurellaceae, Vibrionaceae). BMC Genomics 854-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruby, E. G. 1996. Lessons from a cooperative, bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 50591-624. [DOI] [PubMed] [Google Scholar]
- 30.Ruby, E. G., and M. J. McFall-Ngai. 1999. Oxygen-utilizing reactions and symbiotic colonization of the squid light organ by Vibrio fischeri. Trends Microbiol. 7414-420. [DOI] [PubMed] [Google Scholar]
- 31.Ruby, E. G., M. Urbanowski, J. Campbell, A. Dunn, M. Faini, R. Gunsalus, P. Lostroh, C. Lupp, J. McCann, D. Millikan, A. Schaefer, E. Stabb, A. Stevens, K. Visick, C. Whistler, and E. P. Greenberg. 2005. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. USA 1023004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon, G., V. Mejean, C. Jourlin, M. Chippaux, and M. C. Pascal. 1994. The torR gene of Escherichia coli encodes a response regulator protein involved in the expression of the trimethylamine N-oxide reductase genes. J. Bacteriol. 1765601-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon, R., M. O'Connell, M. Labes, and A. Puhler. 1986. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 118640-659. [DOI] [PubMed] [Google Scholar]
- 34.Spiro, S., and J. R. Guest. 1990. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol. Rev. 6399-428. [DOI] [PubMed] [Google Scholar]
- 35.Stabb, E. V., K. A. Reich, and E. G. Ruby. 2001. Vibrio fischeri genes hvnA and hvnB encode secreted NAD+-glycohydrolases. J. Bacteriol. 183309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stabb, E. V., and E. G. Ruby. 2003. Contribution of pilA to competitive colonization of the squid Euprymna scolopes by Vibrio fischeri. Appl. Environ. Microbiol. 69820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stabb, E. V., and E. G. Ruby. 2002. RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol. 358413-426. [DOI] [PubMed] [Google Scholar]
- 38.Thauer, R. K., K. Jungermann, and K. Decker. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visick, K. L., and E. G. Ruby. 1998. The periplasmic, group III catalase of Vibrio fischeri is required for normal symbiotic competence and is induced both by oxidative stress and by approach to stationary phase. J. Bacteriol. 1802087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visick, K. L., and E. G. Ruby. 2006. Vibrio fischeri and its host: it takes two to tango. Curr. Opin. Microbiol. 9632-638. [DOI] [PubMed] [Google Scholar]
- 41.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 2171214-1222. [DOI] [PubMed] [Google Scholar]