Abstract
Biofilm formation by the gram-positive, motile, food-borne pathogen Listeria monocytogenes was demonstrated to occur by an ordered series of stages. Biofilm development involves flagellum-based motility, which when blocked decreases initial bacterial surface attachment but subsequently leads to the formation of hyperbiofilms, surface-attached communities reaching high density.
Listeria monocytogenes has emerged as a major food-borne pathogen posing a major public health concern because listeriosis has a fatality rate of 25% (13). Therefore, efforts to limit human exposure to L. monocytogenes that focus on defining mechanisms affecting its entry into the food supply have substantial public health value. The survival of L. monocytogenes in the food processing environment is prolonged because of its ability to establish biofilms on the surfaces of equipment. This is postulated to be a major reservoir contributing to food contamination and disease transmission (6, 19). Biofilms formed by both gram-negative and gram-positive bacteria that have been studied in detail include Pseudomonas fluorescens, Escherichia coli, Vibrio cholerae, Staphylococcus epidermidis, Staphylococcus aureus, and numerous other species. These studies indicate that biofilm development, maturation, and dissociation pro-ceed through recognizable stages (12, 18). Despite the wealth of knowledge that has come from these and other studies, it has also become clear that each species retains unique genetic, physiological, and structural attributes that are optimized to its ecological niche.
L. monocytogenes is currently an understudied organism, considering its importance to human health and the goal of the food industry to limit its entry into human consumables. Abiotic surfaces on which L. monocytogenes grows as an attached community include borosilicate glass, stainless steel, rubber, and various plastics (3, 4, 14). Biotic surfaces of food materials known to sustain L. monocytogenes biofilms include those of plant and animal origin (7). A few specific determinants for biofilm formation have been proposed, including flagellum-dependent motility and quorum sensing (11, 17). Thus, while there is tantalizing evidence that L. monocytogenes biofilm development is a complex phenomenon (13), the genetic and molecular processes involved in the transition to a surface-attached lifestyle remain obscure. We examined L. monocytogenes biofilm development using both static-culture-based assays and flow cells. This analysis revealed that defects in motility have a more complex effect on biofilm development than previously recognized.
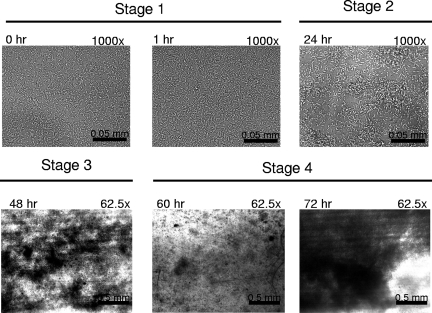
Initial experiments evaluated culture media that consistently promoted the formation of L. monocytogenes 10403s, serotype 1/2a, biofilms on polyvinyl chloride using a static-microtiter-plate-based assay combined with crystal violet staining of surface-attached biomass (5). From this survey modified Welshimer's broth (MWB) (16) resulted in the greatest amount of biomass attachment (data not shown). A flow cell apparatus (9) combined with bright-field microscopy was then utilized as an alternative approach to confirm MWB as an appropriate biofilm-promoting medium (Fig. 1). This analysis revealed that bacterial adherence to the surface and subsequent outgrowth of the surface-attached bacterial community occurred with recognizable stages similar to those that have been previously documented for other bacteria. Initial bacterial attachment (stage 1) was followed by microcolony formation within the first 24 h (stage 2), and tertiary structure maturation of the biofilm was complete by 48 h (stage 3). These events were then followed by cycles of dissociation events (stage 4) with subsequent regrowth of the biofilm at intervals of approximately 12 h.
FIG. 1.
Bright-field microscopy of flow cells inoculated with L. monocytogenes 10403s. The flow cell at ambient temperature was inoculated and allowed to remain static for 1 h. Medium flow was then restored, and images of surface-attached cells were captured at a magnification of either ×1,000 to image individual surface-attached cells or ×62.5 to visualize masses of surface-attached cells (dark regions against light background). The time points and level of magnification for each image are indicted in the upper left and right, respectively. Stage 1, surface attachment of individual cells; stage 2, microcolony formation; stage 3, biofilm maturation; stage 4, community dissociation (60 h) followed by regeneration (72 h).
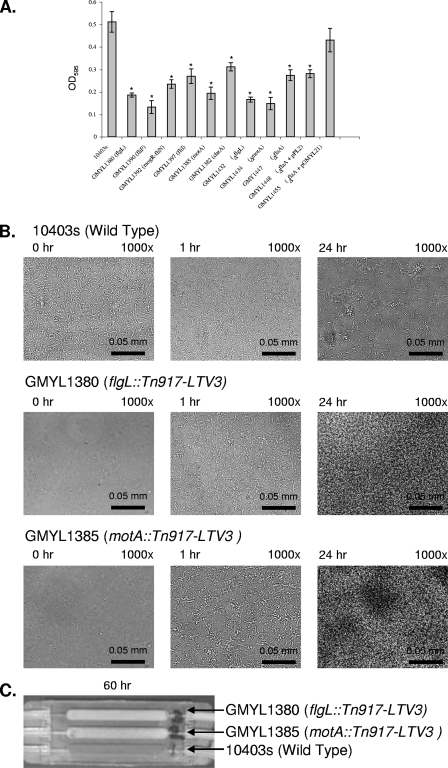
To define genes important for biofilm formation, individual strains of a previously constructed library of transposon Tn917-LTV3 mutants were screened using the static-microtiter-plate assay (2). This effort defined two genetic lesions affecting biofilm formation, which mapped to flgL and the intergenic region between the divergently transcribed flagellar genes mogR and lmo0675, with similarity to fliN (Fig. 2A). To gain a broader perspective on the link between flagellum-based motility and biofilm development, additional motility mutants were isolated (cheA, fliF, fliI, and motA) and found to display similar phenotypes (Fig. 2A). To gain insight as to how motility affects biofilm development, selected mutants were examined by bright-field microscopy when cultured in a flow cell (Fig. 2B). This examination included the flgL and motA mutants, representing strains defective for flagellum formation and those producing paralyzed flagella, respectively. Each mutant displayed a reduction in initial surface attachment during the period following inoculation of the flow cell and prior to the resumption of medium flow. This result was consistent with the static-microtiter-plate assay and with other studies that used similar static-culture conditions (11). Strikingly, the flow cell revealed other characteristics that were not reflected by the static assays. Within 1 h following the start of feeding medium into the flow cell chamber, bacteria progressively colonized the surface, surpassing strain 10403s to form biofilms by 24 h (Fig. 2B). Extended observation of the biofilms formed by the motility mutants revealed that they continued to increase in biomass to become readily visible by macroscopic observation (Fig. 2C). This distinct hyperbiofilm (HB) phenotype is unlike that of the wild-type strain, which displays periods of population dissociation followed by biofilm regeneration. The HB phenotype did not depend on the use of MWB since it occurred in flow cells fed Luria-Bertani broth or brain heart infusion broth (data not shown). A trivial explanation for the HB phenotype is that the mutants are altered such that there is a change in their surface property which then causes them to aggregate. This does not appear to be the case, as macroscopic and microscopic observation revealed no bacterial aggregates when the mutants were cultured as planktonic cells in liquid medium with mild aeration or as static samples (data not shown). Thus, it appears that the HB phenotype is an inducible phenomenon of motility mutants.
FIG. 2.
Loss of flagellar motility alters the dynamics of L. monocytogenes biofilm development. (A) Quantification of crystal violet staining (optical density at 595 nm [OD595]) to indirectly measure the amount of surface-attached biomass for selected strains following 24 h of cultivation in static 96-well polyvinyl chloride microtiter plates at 30°C. Each strain examined is indicated along with the relevant genotype. Each assay was completed in triplicate. The data were statistically analyzed by a two-tailed t test to compare each mutant to the wild-type strain. Significant differences are indicated by an asterisk when P is <0.05. (B) Examination of motility mutants cultured in a flow cell at ambient temperature. Mutants display an initial lag in surface attachment (compare at 0 h). Subsequently, the mutants progressively form surface-attached communities to a greater extent than the wild-type strain. The flow cells were treated as described in the legend of Fig. 1. The time points and level of magnification for each image are indicted in the upper left and right, respectively. (C) Motility mutants form HBs, an accumulation of surface-attached cells that impede the flow of medium to the flow cell. Bacterial strains were cultivated as described in the legend of Fig. 1 but were allowed to grow for 60 h. The image was captured with a standard digital camera to show a macroscopic view. Each chamber of the flow cell shown was inoculated with a different L. monocytogenes strain as indicated.
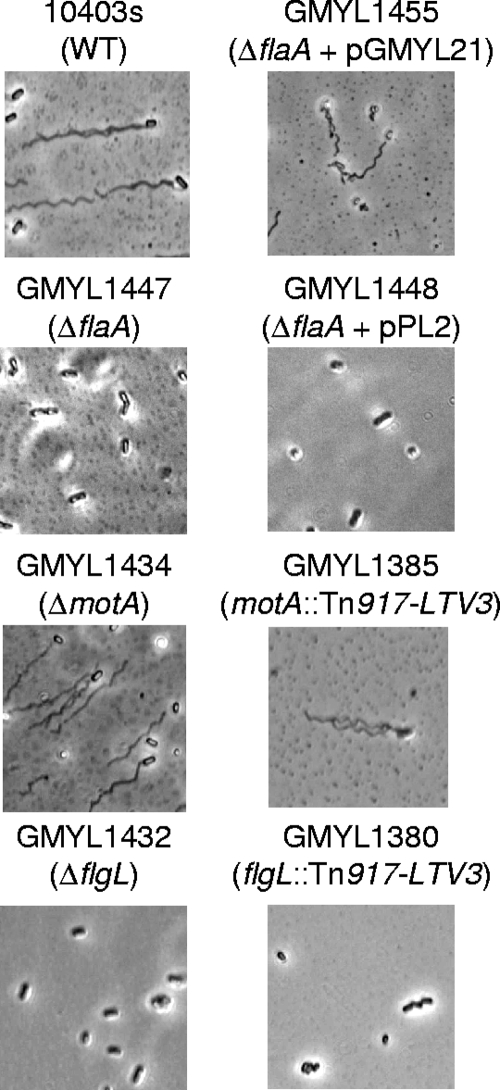
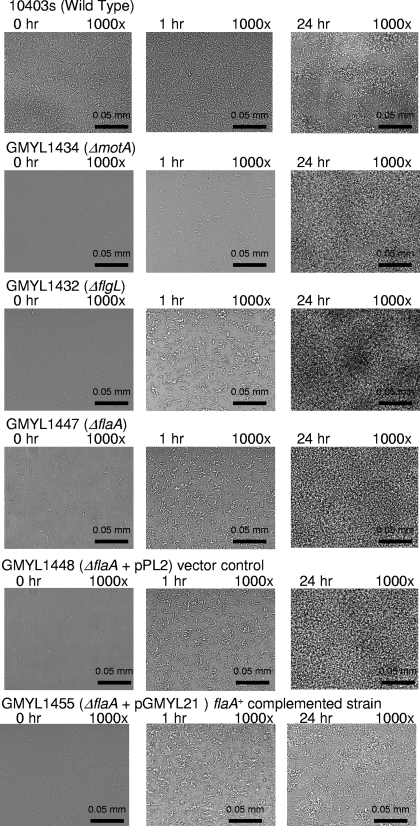
By constructing strains harboring deletion mutations of flgL and motA, it was established that the behavior of the motility mutants was not due to unforeseen secondary effects caused by Tn917-LTV3. In addition, a strain was constructed that contained a deletion of flaA, which encodes the main subunit of the flagellar filament. Cells of each strain were stained to visualize flagella and examined by bright-field microscopy (Fig. 3). As expected, GMY1434 (ΔmotA) produced flagella but was not motile when examined by soft agar assays or when examined by microscopy (Fig. 3 and data not shown). Strains GMYL1447 (ΔflaA) and GMYL1432 (ΔflgL) lacked flagella as did the corresponding transposon insertion mutants (Fig. 3). Consistent with the analysis of the transposon insertion mutants, each strain affected for motility displayed a reduced capacity to form a biofilm when assessed by the static-microtiter-plate assay (Fig. 2A). Assays with flow cells further confirmed that each motility mutant was affected for initial bacterial attachment and subsequently displayed an HB phenotype (Fig. 4 and data not shown). Genetic complementation of the ΔflaA mutation with a functional copy of flaA partially suppressed the initial surface attachment defect and abolished the HB phenotype to restore a cyclic pattern of bacterial dissociation-biofilm regeneration as displayed by strain 10403s (Fig. 4 and data not shown). Similar initial surface attachment and HB phenotypes were observed for motA and flaA mutants derived from strains RM2387 (serotype 4b) and RM2992 (serotype 4b/4e) (our unpublished results). This suggests that the observed phenomena are not restricted to serotype 1/2a represented by strain 10403s.
FIG. 3.
Microscopic examination of selected motility mutants and the wild-type strain. Each strain was cultivated in liquid medium as static planktonic cultures. Bacteria were stained as previously described (8). As expected, GMY1447, GMYL1448, GMY1432, and GMY1380 did not produce flagella. The wild-type (WT) strain 10403s and GMYL1455 produced flagella and were motile. Strain characteristics are indicated on the figure; plasmid pGMY21 is a flaA+ derivative of the integration vector pPL2 (10). Strains GMYL1434 and GMYL1385 produced flagella but were not motile, which is consistent with loss of motor function resulting in paralyzed flagella.
FIG. 4.
Deletion of flagellar genes affects biofilm development. The experimental conditions are as described in the legend of Fig. 1. Each chamber of the flow cell was inoculated with a different L. monocytogenes strain, as indicated above the images.
Conclusions.
We exploited two different approaches, the use of flow cells and the static-microtiter-plate assay, to gain insight into L. monocytogenes biofilm development. Consistent with previous studies, the use of the static-microtiter-plate assay revealed that flagellar motility contributes to biofilm formation (11, 17). However, the use of flow cells combined with microscopy provided an additional perspective by revealing that L. monocytogenes biofilm formation, as in numerous bacterial species, displays stages of development. We are not aware of another study that has clearly established a defined sequence of biofilm development for L. monocytogenes. These results therefore help to bring clarity to the general idea that L. monocytogenes retains the ability to robustly form a single-species sessile community. The comparison of the results obtained from the microtiter plate and flow cell assays suggests that future investigation of L. monocytogenes may benefit from a multifaceted approach. In particular, the inability of the microtiter plate assay to reveal the HB phenotype raises questions about the utility of the microtiter plate assay to provide clear predictions about how L. monocytogenes will behave in natural and industrial settings. On the basis of this methodology, a number of reports have drawn conclusions about the potential for different clinical and environmental isolates to form biofilms (1, 5, 15). It may be that the same strains would be scored differently if they were assessed in a flow cell system. We speculate that differences observed between these experimental approaches are due to the numerous changes that occur as bacteria reach a high density in static cultures, including changes in pH, oxygen tension, and nutrient availability. We propose that, at least under some conditions, flagellum-based motility is not necessary for biofilm formation, as evidenced by the HB phenotype of motility mutants cultivated in flow cells. This speculative conclusion has practical implications because it suggests that flagellar motility is not necessarily an optimal target for the development of methods to reduce or eliminate biofilms from surfaces. Such strategies may actually induce the HB phenotype and exacerbate situations where contamination is problematic.
Acknowledgments
The members of the Young laboratory are gratefully acknowledged for their insightful feedback and review of this study. Daniel Portnoy (University of California, Berkeley, CA) is gratefully acknowledged for providing strain 10403s, serotype 1/2a, and the transposon mutant library. Lisa Gorski (USDA) kindly provided RM2387, serotype 4b, and RM2992, serotype 4b/4e, and the corresponding mutants derived from these strains.
This work was support by grants to G.M.Y. from the USDA (NRI, 2003-03082, and National Alliance for Food Safety and Security) and the U.C.D., Western Institute for Food Safety and Security in cooperation with the California Department of Food and Agriculture.
Footnotes
Published ahead of print on 27 June 2008.
REFERENCES
- 1.Borucki, M. K., J. D. Peppin, D. White, F. Loge, and D. R. Call. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 697336-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilli, A., A. Portnoy, and P. Youngman. 1990. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J. Bacteriol. 1723738-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chae, M. S. 2001. Cell viability of Listeria monocytogenes biofilms. Food Microbiol. 18103-112. [Google Scholar]
- 4.Chae, M. S., and H. Schraft. 2000. Comparative evaluation of adhesion and biofilm formation of different Listeria monocytogenes strains. Int. J. Food Microbiol. 62103-111. [DOI] [PubMed] [Google Scholar]
- 5.Djordjevic, D., M. Wiedmann, and L. A. McLandsborough. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 682950-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi, M., and M. L. Chikindas. 2007. Listeria: a foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 1131-15. [DOI] [PubMed] [Google Scholar]
- 7.Gorski, L., J. D. Palumbo, and R. E. Mandrell. 2003. Attachment of Listeria monocytogenes to radish tissue is dependent upon temperature and flagellar motility. Appl. Environ. Microbiol. 69258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heimbrook, M. E., W. L. Wang, and G. Campbell. 1989. Stining bacterial flagella easily. J. Clin. Microbiol. 272612-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klausen, M., A. Heydorn, P. Ragas, L. Lambertsen, A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 481511-1524. [DOI] [PubMed] [Google Scholar]
- 10.Lauer, P., M. Y. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 1844177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemon, K. P., D. E. Higgins, and R. Kolter. 2007. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J. Bacteriol. 1894418-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 5449-79. [DOI] [PubMed] [Google Scholar]
- 13.Rocourt, J., and P. Cossart. 1997. Listeria monocytogenes, p. 337-352. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers. ASM Press, Washington, DC.
- 14.Stepanovic, S., I. Cirkovic, L. Ranin, and M. Svabic-Vlahovic. 2004. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 38428-432. [DOI] [PubMed] [Google Scholar]
- 15.Tresse, O., K. Shannon, A. Pinon, P. Malle, M. Vialette, and G. Midelet-Bourdin. 2007. Variable adhesion of Listeria monocytogenes isolates from food-processing facilities and clinical cases to inert surfaces. J. Food Prot. 701569-1578. [DOI] [PubMed] [Google Scholar]
- 16.Tsai, H. N., and D. A. Hodgson. 2003. Development of a synthetic minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 696943-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vatanyoopaisarn, S., A. Nazli, C. E. Dodd, C. E. Rees, and W. M. Waites. 2000. Effect of flagella on initial attachment of Listeria monocytogenes to stainless steel. Appl. Environ. Microbiol. 66860-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 1822675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong, A. 1998. Biofilms in food processing environments. J. Dairy Sci. 812765-2770. [DOI] [PubMed] [Google Scholar]