Abstract
Peptidoglycan fragments released by Neisseria gonorrhoeae contribute to the inflammation and ciliated cell death associated with gonorrhea and pelvic inflammatory disease. However, little is known about the production and release of these fragments during bacterial growth. Previous studies demonstrated that one lytic transglycosylase, LtgA, was responsible for the production of approximately half of the released peptidoglycan monomers. Systematic mutational analysis of other putative lytic transglycosylase genes identified lytic transglycosylase D (LtgD) as responsible for release of peptidoglycan monomers from gonococci. An ltgA ltgD double mutant was found not to release peptidoglycan monomers and instead released large, soluble peptidoglycan fragments. In pulse-chase experiments, recycled peptidoglycan was not found in cytoplasmic extracts from the ltgA ltgD mutant as it was for the wild-type strain, indicating that generation of anhydro peptidoglycan monomers by lytic transglycosylases facilitates peptidoglycan recycling. The ltgA ltgD double mutant showed no growth abnormalities or cell separation defects, suggesting that these enzymes are involved in pathogenesis but not necessary for normal growth.
Peptidoglycan (PG) fragments released during growth contribute to the pathogenesis of multiple bacterial infections, including those of Bordetella pertussis, Helicobacter pylori, and Neisseria gonorrhoeae (6, 23, 34). PG fragments induce the production of inflammatory cytokines, cause ciliated cell damage and fluid efflux, and trigger the Nod signaling cascade (reviewed in reference 5). Although PG fragments have been studied biochemically and for immunologic effects in multiple systems, the repertoire of genes and enzymes involved in PG fragment production and release from growing bacteria is unknown.
PG fragments released from gram-negative bacterial pathogens are predicted to be produced by the action of lytic transglycosylases. Lytic transglycosylases cleave the N-acetylmuramic acid-β-1,4-N-acetylglucosamine linkage in PG and catalyze the formation of a 1,6-anhydro bond on the N-acetylmuramic acid (16). PG monomers released from N. gonorrhoeae and B. pertussis were shown to have the 1,6-anhydro bond, indicating that they were generated by lytic transglycosylases (26, 30). To identify genes for PG monomer production, we systematically mutated the genes for lytic transglycosylase homologues in N. gonorrhoeae. Mutation of lytic transglycosylase A (ltgA) resulted in a substantial decrease in PG monomers released (3). Mutations in lytic transglycosylase B (ltgB) or lytic transglycosylase C (ltgC) genes had no effect on PG monomer release (4, 19), although the ltgC mutant showed a severe defect in cell separation. These findings suggested the presence of other lytic transglycosylases in N. gonorrhoeae involved in release of PG monomers.
Here we show that lytic transglycosylase LtgD is involved in the release of PG monomers. Additionally, we generated and characterized an N. gonorrhoeae strain deleted for both ltgA and ltgD and found that this strain does not release PG monomers. Our studies demonstrate that lytic transglycosylases in N. gonorrhoeae have specific functions and that LtgA and LtgD are responsible for the production of 1,6-anhydro PG monomers, a virulence factor in gonococcal infections.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains and plasmids used in this study are listed in Table 1. All gonococcal strains are derivatives of MS11 (31), and all experiments except transformations were performed with nonpiliated variants. Gonococci were grown with aeration in GC base liquid (GCBL) medium (1.5% proteose peptone no. 3, 0.4% K2HPO4, 0.1% KH2PO4, 0.1% NaCl; pH 7.2) containing Kellogg's supplements and 0.042% NaHCO3 or on GCB agar plates (Difco) in the presence of 5% CO2 at 37°C (18, 24). Escherichia coli was grown in Luria broth or on Luria agar plates (27). Antibiotics were used at the following concentrations: for N. gonorrhoeae, 10 μg of erythromycin (Erm) per ml and 100 μg of streptomycin (Str) per ml; for E. coli, 500 μg of Erm per ml, 40 μg of kanamycin (Kan) per ml, and 100 μg of Str per ml.
TABLE 1.
Strains and plasmids used in the study
| Strain or plasmid | Properties | Reference |
|---|---|---|
| Plasmids | ||
| pHSS6 | E. coli cloning vector (Kanr) | 28 |
| pIDN1 | N. gonorrhoeae insertion/duplication plasmid (Ermr) | 15 |
| pIDN3 | N. gonorrhoeae insertion/duplication plasmid (Ermr) | 15 |
| pKC1 | N. gonorrhoeae insertion/duplication, positive/negative selection plasmid (Ermr/Strs) | 3 |
| pKC3 | ltgA in pHSS6 (Kanr) | 3 |
| pKH61 | ltgA deletion plasmid (Kanr) | This work |
| pKH82 | ltgA deletion in pIDN1 (Ermr) | This work |
| pKH84 | pKH82 plus flanking sequence (Ermr) | This work |
| pKH75 | ltgE insertion/duplication plasmid (Ermr) | This work |
| pKC4 | ltgA disruption plasmid (Ermr/Strs/Kanr) | 3 |
| pKC12 | ltgD PCR product digested with EcoRV/BclI cloned into BamHI, EcoRV sites of pKC1 (Ermr/Strs) | This work |
| pKC14 | ltgD internal deletion plasmid, formed by SmaI digestion of pKC12 (Ermr/Strs) | This work |
| pKC16 | ltgD internal deletion plasmid, ltgD region subcloned into HindIII/XbaI sites of pHSS6 (Kanr) | This work |
| pKC18 | ltgD disruption plasmid, ermC/rpsL cloned into BglI site of pKC16 (Kanr/Ermr/Strs) | This work |
| N. gonorrhoeae strains | ||
| MS11 | Wild type (Strr) | 31 |
| KC119 | MS11 ltgD | This work |
| KH560 | MS11 ltgA ltgD | This work |
| KH571 | MS11 ltgE | This work |
| ND500 | MS11 with the GGI deleted | 14 |
Growth rate comparisons.
To evaluate the effects of the ltgA and ltgD mutations on growth, the ltgA ltgD double mutant KH560 and its wild-type parent strain MS11 were grown in liquid culture in either complex medium (GCBL) as described above or in defined medium (Graver-Wade medium) (35). N. gonorrhoeae strains were grown on GCB agar plates for 20 h and then the bacteria were inoculated into the liquid medium at a density of approximately 108 CFU/ml (optical density at 540 nm [OD540], 0.2). The cultures were grown at 37°C with aeration, and optical density readings (OD540) were taken at 0, 1, 2, 4, 6, 8, 10, 12, and 24 h postinoculation. The numbers of viable bacteria were also determined at 10 h postinoculation by plating serial dilutions of the cultures on GCB agar plates.
Plasmid construction.
For cloning of ltgD, the following specific primers were designed based on the sequence of N. gonorrhoeae strain FA1090 (GenBank accession no. AE004969): 5′-AAACCCTCGCCACGGAATACACTT-3′ and 5′-CCCTTCAATCCCTTGCTGCGTAAAA-3′. ltgD was amplified from MS11 chromosomal DNA using an annealing temperature of 60°C. The ltgD PCR product was digested with EcoRV and BclI and ligated into the BamHI and EcoRV sites of pKC1 (3), forming pKC12. An internal deletion in ltgD was formed by digesting pKC12 with SmaI, removing 1 kb of coding sequence (pKC14). The ltgD deletion region from pKC14 was excised by digestion with HindIII and SpeI and then subcloned into the HindIII and XbaI sites of pHSS6 (28), forming pKC16. pKC1 was digested with NheI and SmaI, and then the ermC/rpsL region was blunted with T4 DNA polymerase and ligated into the BglI site (blunted) of pKC16, forming pKC18. An insertion/duplication construct for mutation of ltgE was constructed by PCR amplification. Primers 5′-CTGAAGCTTGCAGCAACCATGCGTTTGAC-3′ and 5′-GCACTAGTAACGGGAGGCAGATACAACA-3′ were used to amplify a fragment of ltgE. The ltgE PCR product was restriction endonuclease digested with BspEI and FspI and then ligated into the Ecl136II and XmaI sites of pIDN3 (15), forming plasmid pKH75. Plasmid construction was verified by restriction endonuclease mapping.
Construction of gonococcal mutants.
A construct containing ltgD disrupted with the ermC/rpsL cassette in pKC18 was used to transform MS11. To replace the positive-negative selection cassette with an internal deletion of ltgD, integrants were transformed with pKC16 linearized by EcoRI. Transformants were selected for streptomycin resistance and screened for erythromycin sensitivity. Mutation of ltgD in the MS11 background was confirmed by PCR, and the strain was named KC119. To construct MS11 ltgA ltgD, an in-frame start-to-stop deletion of ltgA was created in plasmid pKC3 (3) by amplification with primers 5′-TGAACGGGTCTCAGTCACATCGGATTTCCTTAAGAATCGGAAC-3′ and 5′-TCTAGCGGTCTCATGACGTGCCGATGCCGTCTG-3′ (restriction sites are underlined) followed by digestion with BsaI and ligation to form pKH61. The deletion construct region was excised from pKH61 with NotI (blunted) and EcoRI and was ligated into pIDN1 (15) digested with EcoRI and EcoRV to generate pKH82. To increase the homologous recombination frequency of the ltgA deletion construct with the gonococcal chromosome, a 181-bp region 3′ to ltgA was amplified from MS11 chromosomal DNA with primers 5′-GCGAATTCAACCATAAATATAAGACAATC-3′ and 5′-GACTGCGGCCGCCCATCATATCGGTGGAAAGGGTA-3′ and ligated into pKH82 at the EcoRI and NotI sites to generate pKH84. ltgD deletion strain KC119 was transformed with pKC4 to insert an ermC-rpsL marker into ltgA as previously described (3). Erythromycin-resistant transformants were selected and screened for streptomycin sensitivity. One such transformant was subsequently transformed with NsiI-digested pKH84, and Strr transformants were selected. One Strr Erms transformant, KH560, was selected for further study. Southern blotting demonstrated that KH560 was deleted for ltgA and ltgD as expected (see Fig. S1 in the supplemental material). An ltgE mutation was introduced into MS11 by transformation with pKH75 and selection for Ermr colonies. The interruption in ltgE was confirmed by PCR.
Characterization of released peptidoglycan fragments.
Gonococcal PG was purified and characterized following the methods of Rosenthal and Dziarski (25) as described by Cloud and Dillard (3). Briefly, log-phase gonococci were suspended at an OD540 of 0.2 in GCBL medium lacking glucose and containing 0.4% pyruvate, 0.1% glutamine, 0.0002% thiamine pyrophosphate, 0.0005% ferric nitrate, and 0.042% NaHCO3. [6-3H]glucosamine was added at a concentration of 2 μCi/ml, and the cells were grown for 2 hours. Cells were washed in GCBL medium and grown for 2.5 h in GCBL medium containing glucose and without label. Supernatants were harvested by centrifugation and applied to 350-ml Bio-Gel P6 and Bio-Gel P30 size exclusion columns connected in tandem. The columns were eluted with 0.1 M LiCl and 3-ml fractions collected. 3H content was determined by liquid scintillation counting with 300 μl of each fraction.
Characterization of cytoplasmic peptidoglycan fragments.
To examine PG fragments in the cytoplasm generated through PG recycling, the cell wall was pulse-labeled with [6-3H]glucosamine as described above, and hot water extracts were produced as described by Garcia and Dillard (13). PG fragments in the extracts were separated by size exclusion chromatography and detected by scintillation counting.
RESULTS
Multiple lytic transglycosylases are present in N. gonorrhoeae.
N. gonorrhoeae encodes seven lytic transglycosylase homologues (Table 2). To determine which of these enzymes act in the production of cytotoxic PG fragments, we systematically mutated the gonococcal genes for the lytic transglycosylases and tested the mutants for PG fragment release. Previous work demonstrated that LtgA, the homologue of E. coli Slt70, was responsible for approximately half of the PG monomers released (3). Mutation of ltgB or ltgC did not affect monomer release (4, 19). A search of the gonococcal genome sequence of strain FA1090 identified two additional putative lytic transglycosylases, which we named LtgD and LtgE. The gonococcal genetic island (GGI), present in 80% of gonococcal strains but not in sequenced strain FA1090 (9), contains two more lytic transglycosylase homologues, AtlA and LtgX (14). We recently demonstrated that mutation of atlA does not affect PG fragment release (20).
TABLE 2.
Seven lytic transglycosylases are encoded in the gonococcal genome
| Gonococcal lytic transglycosylase | Function | E. coli homologue | % Identity/range (amino acids) | Meningococcal homologue |
|---|---|---|---|---|
| LtgA (NGO2135) | PG monomer production | Slt70 | 25/556 | +a |
| LtgB (NGO1033) | Unknown | MltC | 33/199 | + |
| LtgC (NGO2048) | Cell separation | MltA | 21/441 | GNA33 |
| LtgD (NGO0626) | PG monomer production | MltB | 48/123 | MltB |
| LtgE (NGO0608) | Unknown | MltD | 34/341 | + |
| LtgXb (NGO5004) | Type IV secretion | F-plasmid ORF169 | 33/129 | +/−c |
| AtlAb (NGO5025) | Type IV secretion | Lambda endolysin | 38/158 | −d |
Homologue present.
GGI-encoded lytic transglycosylase.
Homologue present in some strains.
Homologue not detected.
Factors encoded in the gonococcal genetic island are not required for PG fragment release.
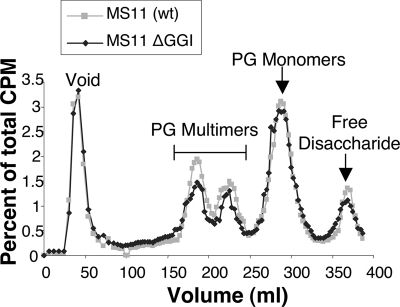
The gonococcal genetic island carries 61 open reading frames and encodes a type IV secretion system and two lytic transglycosylase homologues as well as other proteins (14). Viala and coworkers identified a link between type IV secretion function and PG monomer release in H. pylori (34), leading to the possible conclusion that PG fragments might be transported by the type IV secretion system (7). However, comparison of wild-type gonococcal strain MS11 and its isogenic GGI deletion mutant (ND500) showed that the GGI deletion mutant was not deficient in PG monomer release (Fig. 1). The two strains were pulse-labeled by growth in medium containing [6-3H]glucosamine to label the PG. PG fragments released into the supernatant were separated by size exclusion chromatography. Radioactivity in the samples was detected by scintillation counting and was normalized to total radioactivity released to correct for differences in the amount of label incorporated in the different cultures. No differences were seen in the profiles of released PG fragments (Fig. 1). This result indicates that neither the type IV secretion system components nor the GGI-encoded lytic transglycosylases AtlA and LtgX are necessary for PG fragment release in culture.
FIG. 1.
Profile of PG fragments released by GGI deletion strain ND500 and wild-type parent strain MS11. 3H-labeled PG fragments released into the culture medium were separated by size exclusion chromatography and detected by scintillation counting.
Mutation of ltgE does not decrease PG monomer release.
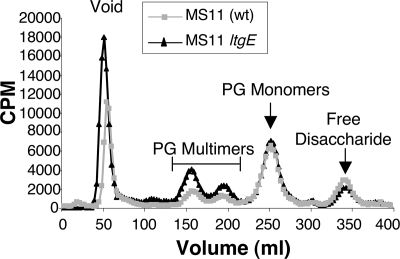
A putative lytic transglycosylase gene (ltgE) encoding a protein similar to E. coli MltD was identified in the gonococcal chromosome by sequence comparisons (Table 2). An analysis of H. pylori mutants deficient in the LtgE homologue (MltD) indicated that the H. pylori enzyme acts as an endo-type lytic transglycosylase, shortening chain length and increasing anhydro ends in intact cell walls (2). Such an activity might generate more substrates for exo-type lytic transglycosylases and increase anhydro PG monomer production. An ltgE insertion mutation was constructed in gonococcal strain MS11 by transformation with a nonreplicating plasmid containing an internal fragment of ltgE. The PG of the wild-type and ltgE strains was pulse-labeled as before. For a direct quantitative comparison of PG fragments released, radioactivity in the cell pellet was determined after the labeling step and the cells were diluted to an equivalent amount of radioactivity for each culture for the chase period. Released PG fragments were analyzed by size exclusion chromatography (Fig. 2). Mutation of ltgE did not alter PG monomer release. However, PG multimer release appeared slightly increased in the mutant. These results suggest that LtgE is not significantly involved in PG monomer production.
FIG. 2.
Profile of PG fragments released by ltgE insertion mutant KH571 or wild-type parent strain MS11. Released 3H-labeled PG fragments were separated by size exclusion chromatography and detected by scintillation counting.
Lytic transglycosylase D acts in PG monomer production.
N. gonorrhoeae also encodes a putative lytic transglycosylase similar to E. coli membrane-bound lytic transglycosylase B (MltB) (Table 2). We designated this protein lytic transglycosylase D (LtgD). LtgD is predicted to be a lipoprotein of 363 amino acids. The catalytic residues (Glu162, Ser216, and Asn339), PG binding residues (Arg187, Phe226, Tyr259, and Tyr338), and amino acids lining the hydrophobic pocket (Ile158, Gln225, Tyr338, and Tyr344) of E. coli MltB, determined in biochemical and crystallographic studies (11, 33), are conserved in the LtgD sequence (data not shown).
In order to determine if LtgD acts in the release of PG monomers, an ltgD deletion mutation was made. The positive/negative selection method of Johnston and Cannon was used to generate an internal deletion in ltgD (17). In the first transformation, ltgD was interrupted with an ermC/rpsL cassette. In a subsequent transformation, an allele of ltgD containing a 1,000-bp deletion was introduced, generating an MS11 ltgD mutant (KC119) containing no antibiotic resistance markers.
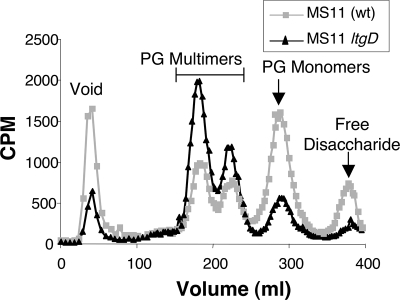
Analysis of PG fragments released by ltgD deletion mutant KC119 demonstrated that ltgD is required for a substantial portion of PG monomer production. KC119 was metabolically labeled with [6-3H]glucosamine, and released PG fragments were characterized by size exclusion chromatography. The resulting profile differed significantly from that of wild-type strain MS11. The ltgD mutant released less PG monomer and more PG multimers than wild type (Fig. 3). These data demonstrate that LtgD acts in the production of PG monomers and suggest that LtgD may function to degrade larger PG fragments liberated from the cell wall by other enzymes.
FIG. 3.
Profile of PG fragments released by ltgD deletion mutant KC119 or wild-type parent strain MS11. 3H-labeled PG fragments released into the culture medium were separated by size exclusion chromatography and detected by scintillation counting.
An ltgA ltgD double mutant does not release PG monomers.
The previous results with an ltgA mutant (3) and the results shown here for the ltgD mutant (Fig. 3) indicate that each of these two putative lytic transglycosylases contributes substantially to PG monomer production. To determine if these are the only lytic transglycosylases required for PG monomer release, we introduced an ltgA mutation into the ltgD mutant. An in-frame deletion of the ltgA coding sequence was created, and this construct was introduced into ltgD mutant KC119. The presence of the two deletions in the resulting mutant KH560 was confirmed by Southern blotting (see Fig. S1 in the supplemental material). Although mutation of some gonococcal peptidoglycanases alters cell separation and growth characteristics (4, 12), mutation of ltgA and ltgD had no significant effects on in vitro growth of N. gonorrhoeae in either complex or defined medium (data not shown).
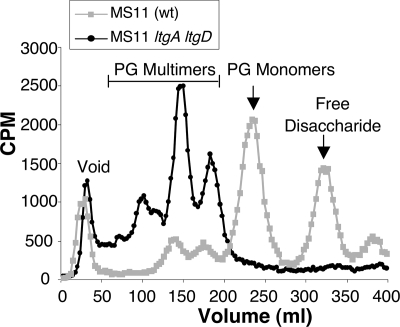
PG monomer release was evaluated in the ltgA ltgD double mutant with the pulse-chase labeling method and characterization of the released fragments by size exclusion chromatography. The profile of the released fragments showed no peak for PG monomers, suggesting that the ltgA ltgD mutant does not produce these cytotoxic PG fragments (Fig. 4). The profile for the mutant also showed an increase in release of larger, soluble PG fragments. The increased release of PG multimers is similar to that of the single ltgA or ltgD mutants, but more pronounced. This result suggests that the ltgA ltgD mutant is able to degrade macromolecular PG to soluble fragments during growth but unable to break them down into monomers.
FIG. 4.
Loss of PG monomer release by the MS11 ltgA ltgD double mutant. 3H-labeled PG fragments released into the culture medium were separated by size exclusion chromatography.
In addition to not releasing PG monomers, the ltgA ltgD mutant also showed no release of free disaccharide. Free disaccharide production requires the action of the amidase AmiC and is predicted to also require the function of a lytic transglycosylase (4, 12). The loss of free disaccharide release by the ltgA ltgD mutant as well as the reduced release of free disaccharide by the individual ltgA and ltgD mutants suggest that these two lytic transglycosylases may act in the generation of free disaccharide.
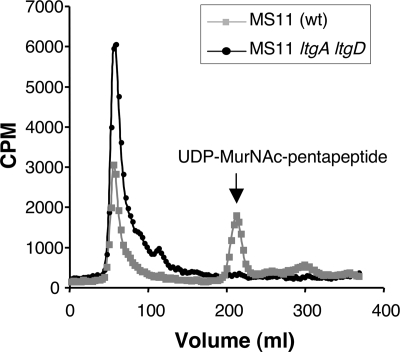
An alternative explanation for the absence of free disaccharide release in the ltgA ltgD mutant is that the mutant is not able to recycle the larger PG fragments and has increased metabolism of free disaccharide, as was demonstrated for a gonococcal ampD mutant (13). To test this hypothesis we repeated the pulse-chase experiment and looked for PG fragments in the cytoplasm (Fig. 5). The wild-type strain showed a single peak in the included volume corresponding to UDP-MurNAc-pentapeptide. The presence of labeled material in this PG biosynthesis precursor indicates that PG fragments were liberated from the cell wall and brought back into the cytoplasm for reuse, by the normal PG turnover and recycling processes. The absence of this peak in the ltgA ltgD mutant suggests that the mutant does not recycle liberated PG fragments.
FIG. 5.
Detection of recycled PG fragments in the cytoplasm of ltgA ltgD mutant KH560 or wild-type parent strain MS11. Size exclusion chromatography of hot water extracts shows the presence of the biosynthesis intermediate UDP-MurNAc-pentapeptide in the wild-type strain but its absence from the ltgA ltgD mutant.
DISCUSSION
The data presented here show LtgA and LtgD are responsible for PG monomer release by N. gonorrhoeae. These are two putative lipoproteins predicted to be inserted into the inner leaflet of the outer membrane (32). Gonococci have five other lytic transglycosylases that are not involved in PG monomer production (Table 2). It has been suggested that the lytic transglycosylases of E. coli may have overlapping functions and that one may substitute for the loss of another (21). That is not the case in N. gonorrhoeae. Gonococcal lytic transglycosylase C, LtgC, is the only lytic transglycosylase required for normal growth characteristics, being required for cell separation after division (4). The lytic transglycosylases encoded in the GGI are required for type IV secretion of DNA but do not affect PG monomer production or cell growth (20). The functions of LtgB and LtgE remain a mystery (19), although mutation of ltgE has a slight effect on PG dimer release (Fig. 2), a result consistent with its possible role as an endolytic transglycosylase as predicted from H. pylori studies (2).
LtgA and LtgD homologues may also be required for PG monomer release in other bacterial species and thus affect infection phenotypes. Mutation of the ltgA homologue has been shown to reduce PG monomer production in several other bacterial species. An H. pylori ltgA homologue mutant is reduced 40% in monomer release and induces less interleukin-8 (IL-8) production in human (HEK293) cells (34). Likewise, Shigella flexneri requires an ltgA homologue for full virulence, with the mutant showing reduced inflammation in the guinea pig conjunctivitis model (1). LtgD homologues are found in species known to release PG monomers or where PG monomers have been demonstrated to affect infection phenotypes, including S. flexneri (GenBank accession no. ABF 04888.1), B. pertussis (NP 879760.1), and Vibrio fischeri (AAW86197.1).
It is not too surprising that the GGI-encoded lytic transglycosylases AtlA and LtgX are not required for PG monomer release into the medium, since these enzymes were shown to function in type IV secretion of DNA (20). Secretion system lytic transglycosylases are thought to create a small opening in the cell wall in order to allow for assembly of the secretion apparatus and would thus be expected to have a localized and limited function (8). However, it was shown that H. pylori mutants defective in the type IV secretion system induced less IL-8 production and less Nod1 signaling (34). It was suggested that PG fragments might pass through or be transported through the type IV secretion system (7). It is not known how PG fragments traverse the outer membrane in N. gonorrhoeae, but it is clear from our results that the type IV secretion system is not necessary for the transport. As PG monomers are small (921 Da for the anhydro disaccharide tetrapeptide monomer), they may simply diffuse across the outer membrane. This scenario does not exclude the possibility that the type IV secretion system could enhance transport of PG fragments into a host cell during infection.
It is not clear why gonococci would need two different enzymes, LtgA and LtgD, to perform what is apparently the same function. One possibility is that the functions they perform are not exactly the same. LtgA and LtgD may have different substrate specificities or act in coordination with other enzymes. For example, maybe LtgA degrades PG strands that are not cross-linked, while LtgD might act on cross-linked PG and require an endopeptidase for monomer liberation. Maybe the two enzymes perform the same reaction but at different places in the cell. One might degrade PG at the poles and the other in the expanding side wall. Further characterizations will be necessary to identify differences in LtgA and LtgD functions.
Why do gonococci have these lytic transglycosylases at all? The ltgA ltgD double mutant showed no abnormalities in growth or cell separation. Thus, it appears that gonococci do not need these enzymes. What then is their purpose in the cell, and why do gonococci have not just one, but two genes for this function? One possibility is that they contribute to infection processes. Clearly, released PG fragments modulate the host immune response and kill ciliated cells of the fallopian tubes (10, 23). Killing of ciliated cells could help provide a niche for gonococcal growth and proliferation, eliminating cilial beating and allowing access for the bacteria to subepithelial tissue. Also, it has been suggested that sterility in women, such as tubal factor infertility caused by N. gonorrhoeae, at least in ancient times, may have led to increased numbers of sexual encounters and thus increased the spread of sexually transmitted diseases (22). Spread of N. gonorrhoeae may also be enhanced by a strong inflammatory response in men and women. Induction of IL-8 by PG fragments would lead to a large influx of neutrophils into the site of infection. Gonococci can survive in neutrophils (29) and may be transmitted to the next individual in these cells in the purulent discharge typical of gonococcal infection.
The absence of free disaccharide release by the ltgA ltgD double mutant suggests another reason for the presence of these enzymes, i.e., for PG recycling. Gonococci have an efficient PG recycling system that takes up and reuses 85% of the PG fragments generated during growth in vitro (13). The PG fragments taken up into the cytoplasm are the monomers and, to a lesser extent, free disaccharide, i.e., those generated by the action of lytic transglycosylases. A mutant deficient in PG monomer recycling, as demonstrated by the gonococcal ampD mutant, shows virtually no release of free disaccharides even though it produces the same amount as the wild type. The ampD mutant was shown to have increased uptake and metabolism of free disaccharide in response to the recycling deficiency, using up nearly all the free disaccharide instead of releasing it (13). Since the ltgA ltgD mutant does not produce PG monomers (Fig. 4), then the decreased PG fragment recycling may similarly result in increased metabolism of free disaccharide. The function of lytic transglycosylases may be to provide PG fragments that can be recycled. The lack of recycled PG fragments in the ltgA ltgD cytosol indicates that this mutant is deficient in PG recycling (Fig. 5). PG recycling would allow for recovery of nutrients, the ability to sense the growth state of the cell wall, and the possible ability to regulate the release of PG fragments.
In summary, systematic mutagenesis has demonstrated that N. gonorrhoeae uses two of its seven lytic transglycosylases for production of cytotoxic PG monomers. These enzymes are not necessary for normal growth but do affect the ability of the bacterium to recover liberated PG fragments for PG recycling.
Supplementary Material
Acknowledgments
This work was supported by NIH grant AI047958 to J.P.D. and NRSA AI054325 to D.L.G.
We acknowledge the assistance of the Gonococcal Genome Sequencing Project, supported by USPHS/NIH grant AI38399, and B. A. Roe, L. Song, S. P. Lin, X. Yuan, S. Clifton, T. Ducey, L. Lewis, and D. W. Dyer of the University of Oklahoma.
Footnotes
Published ahead of print on 20 June 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Bartoleschi, C., M. C. Pardini, C. Scaringi, M. C. Martino, C. Pazzani, and M. L. Bernardini. 2002. Selection of Shigella flexneri candidate virulence genes specifically induced in bacteria resident in host cell cytoplasm. Cell. Microbiol. 4613-626. [DOI] [PubMed] [Google Scholar]
- 2.Chaput, C., A. Labigne, and I. G. Boneca. 2007. Characterization of Helicobacter pylori lytic transglycosylases Slt and MltD. J. Bacteriol. 189422-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloud, K. A., and J. P. Dillard. 2002. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 702752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cloud, K. A., and J. P. Dillard. 2004. Mutation of a single lytic transglycosylase causes aberrant septation and inhibits cell separation of Neisseria gonorrhoeae. J. Bacteriol. 1867811-7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloud-Hansen, K. A., S. B. Peterson, E. V. Stabb, W. E. Goldman, M. J. McFall-Ngai, and J. Handelsman. 2006. Breaching the great wall: peptidoglycan and microbial interactions. Nat. Rev. Microbiol. 4710-716. [DOI] [PubMed] [Google Scholar]
- 6.Cookson, B. T., A. N. Tyler, and W. E. Goldman. 1989. Primary structure of the peptidoglycan-derived tracheal cytotoxin of Bordetella pertussis. Biochemistry 281744-1749. [DOI] [PubMed] [Google Scholar]
- 7.De Gregorio, E., and R. Rappuoli. 2004. Inside sensors detecting outside pathogens. Nat. Immunol. 51099-1100. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra, A. J., and W. Keck. 1996. Peptidoglycan as a barrier to transenvelope transport. J. Bacteriol. 1785555-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillard, J. P., and H. S. Seifert. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41263-278. [DOI] [PubMed] [Google Scholar]
- 10.Dokter, W. H. A., A. J. Dijkstra, S. B. Koopmans, B. K. Stulp, W. Keck, M. R. Halie, and E. Vellenga. 1994. G(Anh)MTetra, a natural bacterial cell wall breakdown product induces interleukin-1β and interleukin-6 expression in human monocytes. J. Biol. Chem. 2694201-4206. [PubMed] [Google Scholar]
- 11.Engel, H., A. J. Smink, L. van Wijngaarden, and W. Keck. 1992. Murein-metabolizing enzymes from Escherichia coli: existence of a second lytic transglycosylase. J. Bacteriol. 1746394-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia, D. L., and J. P. Dillard. 2006. AmiC functions as an N-acetylmuramyl-l-alanine amidase necessary for cell separation and can promote autolysis in Neisseria gonorrhoeae. J. Bacteriol. 1887211-7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia, D. L., and J. P. Dillard. 2008. Mutations in ampG or ampD affect peptidoglycan fragment release from Neisseria gonorrhoeae. J. Bacteriol. 1903799-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton, H. L., N. M. Domínguez, K. J. Schwartz, K. T. Hackett, and J. P. Dillard. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol. Microbiol. 551704-1721. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton, H. L., K. J. Schwartz, and J. P. Dillard. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 1834718-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höltje, J.-V., D. Mirelman, N. Sharon, and U. Schwartz. 1975. Novel type of murein transglycosylase in Escherichia coli. J. Bacteriol. 1241067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston, D. M., and J. G. Cannon. 1999. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic markers using a two gene cassette with positive and negative selection. Gene 236179-184. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. L. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 851274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohler, P. L., K. A. Cloud, K. T. Hackett, E. T. Beck, and J. P. Dillard. 2005. Characterization of the role of LtgB, a putative lytic transglycosylase in Neisseria gonorrhoeae. Microbiology 1513081-3088. [DOI] [PubMed] [Google Scholar]
- 20.Kohler, P. L., H. L. Hamilton, K. Cloud-Hansen, and J. P. Dillard. 2007. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. J. Bacteriol. 1895421-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lommatzsch, J., M. F. Templin, A. R. Kraft, W. Vollmer, and J.-V. Höltje. 1997. Outer membrane localization of murein hydrolases: MltA, a third lipoprotein lytic transglycosylase in Escherichia coli. J. Bacteriol. 1795465-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackey, W. C., and R. S. Immerman. 2003. A proposed feedback loop of sexually transmitted diseases and sexual behavior: the Red Queen's dilemma. Soc. Biol. 50281-299. [PubMed] [Google Scholar]
- 23.Melly, M. A., Z. A. McGee, and R. S. Rosenthal. 1984. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 149378-386. [DOI] [PubMed] [Google Scholar]
- 24.Morse, S. A., and L. Bartenstein. 1974. Factors affecting autolysis of Neisseria gonorrhoeae. Proc. Soc. Exp. Biol. Med. 1451418-1421. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal, R. S., and R. Dziarski. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 235235-285. [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal, R. S., W. Nogami, B. T. Cookson, W. E. Goldman, and W. J. Folkening. 1987. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect. Immun. 552117-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 28.Seifert, H. S., E. Y. Chen, M. So, and F. Heffron. 1986. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cervisiae. Proc. Natl. Acad. Sci. USA 83735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simons, M. P., W. M. Nauseef, and M. A. Apicella. 2005. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect. Immun. 731971-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha, R. K., and R. S. Rosenthal. 1980. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 29914-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson, J., S. J. Kraus, and E. C. Gotschlich. 1971. Studies on gonococcus infection I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J. Exp. Med. 134886-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terada, M., T. Kuroda, S. I. Matsuyama, and H. Tokuda. 2001. Lipoprotein sorting signals evaluated as the LolA-dependent release of lipoproteins from the cytoplasmic membrane of Escherichia coli. J. Biol. Chem. 27647690-47694. [DOI] [PubMed] [Google Scholar]
- 33.van Asselt, E. J., K. H. Kalk, and B. W. Dijkstra. 2000. Crystallographic studies of the interactions of Escherichia coli lytic transglycosylase Slt35 with peptidoglycan. Biochemistry 391924-1934. [DOI] [PubMed] [Google Scholar]
- 34.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Giardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 51166-1174. [DOI] [PubMed] [Google Scholar]
- 35.Wade, J. J., and M. A. Graver. 2007. A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol. Lett. 27335-37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.