Abstract
Unmethylated CpG islands are known to keep adjacent promoters transcriptionally active. In the CpG island adjacent to the adenosine phosphoribosyltransferase gene, the protection against transcriptional silencing can be attributed to the short CpG-rich core element containing Sp1 binding sites. We report here the insertion of this CpG island core element, IE, into the long terminal repeat of a retroviral vector derived from Rous sarcoma virus, which normally suffers from progressive transcriptional silencing in mammalian cells. IE insertion into a specific position between enhancer and promoter sequences led to efficient protection of the integrated vector from silencing and gradual CpG methylation in rodent and human cells. Individual cell clones with IE-modified reporter vectors display high levels of reporter expression for a sustained period and without substantial variegation in the cell culture. The presence of Sp1 binding sites is important for the protective effect of IE, but at least some part of the entire antisilencing capacity is maintained in IE with mutated Sp1 sites. We suggest that this strategy of antisilencing protection by the CpG island core element may prove generally useful in retroviral vectors.
Among retroviral vectors used for gene transfer, transgenesis, or even gene therapy, the avian sarcoma and leukosis virus-based vectors (ASLV vectors) are of special importance. They have been used for infection of both avian and mammalian cells expressing specific surface receptors. Alternatively, these vectors have been pantropized by vesicular stomatitis virus glycoprotein. The replication-competent ASLV vector with a splice acceptor (RCAS) was developed to facilitate the production of high-titer virus in avian cells (11) and efficient infection of mammalian cells when the avian envelope gene is replaced with murine envelope genes, amphotropic or ecotropic (1, 2). A transgenic mouse expressing the chicken receptor for the ASLV-A subgroup was established as a platform for in vivo infection of mammalian hosts with ASLV (13), and a similar system with the ASLV-C subgroup receptor (8) is in preparation. Successful transduction of hematopoietic progenitor cells in rhesus monkeys (26) has brought the RCAS vector system and other ASLV-based vectors closer to use as a tool for gene therapy.
Replication of retroviruses requires cellular cofactors for proper entry, uncoating, transcription, splicing, and assembly. ASLVs cannot replicate in mammalian cells because of the lack of specific avian cofactors required for correct splicing, polyprotein cleavage, virus assembly, etc. (48). Therefore, ASLV-derived vectors such as RCAS do not produce any infectious progeny in mammalian cells in vitro (12) or in vivo (26, 39). In addition, mammalian cells do not contain any endogenous retroviruses, which are capable of recombination with ASLVs, but a high-titer virus can be produced in the ev-locus-free chicken cell line DF-1 (23). The ASLV-based vectors are thus very stable and safe for gene therapy. Another advantage of ASLV-based vectors is their integration pattern, which differs from those of murine leukemia virus (MLV) and human immunodeficiency virus type 1 (HIV-1). Genome-wide analyses of retrovirus integration showed that HIV-1-based vectors integrate preferentially into gene-rich regions (9) and particularly into open chromatin of highly transcribed genes (44). MLV also integrates with a high preference for expressed genes, particularly into the transcriptional start sites of genes (51), and such integration might transcriptionally activate adjacent proto-oncogenes, as shown in the SCID-X1 gene therapy trial (17). In comparison with MLV and HIV-1, ASLVs display the weakest preference for integration into genes and have no bias for promoter regions (35, 38, 42). Hence, ASLV-based vectors might be safer than the widely used lentiviral or MLV-based vectors.
Lentiviral vectors have recently been used predominantly due to their ability to transduce differentiated nondividing cells. However, the presence of at least a weak nuclear localization signal was shown in ASLV (30). Active nuclear import of the preintegration complex and efficient transduction of nondividing cells and terminally differentiated neurons were shown in vitro (14, 18, 28). Increased transduction times improved the efficiency of gene transfer into various types of hematopoietic cells in vivo (26). Optimization of transduction protocols might further increase this efficiency and broaden the use of ASLV-derived vectors in mammalian cells, particularly in hematopoietic cell gene therapy.
Another obstacle to the use of ASLV-derived vectors in mammalian cells is the transcriptional silencing of integrated proviruses. In general, the expression of retrovirus-driven gene reporters is not stable in long-term in vitro cultures, and gradual silencing of transduced vectors correlates with epigenetic changes of retroviral long terminal repeats (LTRs). CpG methylation of DNA and/or modifications of histones in nucleosomes linked to the promoter region were found in silenced proviruses in vitro. In particular, the silencing of MLV and HIV-1 has been characterized in detail (4, 25, 31, 36, 40). It was found that the chromatin environment at the site of retrovirus integration determines the transcriptional activity of the integrated provirus (27). Rous sarcoma virus (RSV)- and ASLV-derived vectors are not progressively silenced in chicken cells; however, in the cells of heterologous mammalian hosts, they are efficiently methylated and transcriptionally silenced (45, 22). Protection from silencing and position-dependent suppressive effects of surrounding chromatin is, therefore, highly needed for the ASLV-based vectors. Various antimethylation and insulation strategies have been applied to increase the stability and position independence of expression of lentiviral and MLV-based vectors (6, 43, 49, 52, 53). We observed previously that RSV proviruses are methylation and silencing resistant when flanked with the CpG island from the mouse adenine phosphoribosyl-transferase (aprt) gene (20). In the present study, we analyzed the effect of the short island element (IE), the core sequence of the Syrian hamster aprt CpG island (46), inserted within the RSV LTR. We show here the stable and position-independent expression of the reporter vector, which suggests that this type of LTR modification might be used in construction of vectors with protracted transcriptional activity and for the improvement of ALV-based vectors for therapeutic application.
MATERIALS AND METHODS
Construction of retroviral vectors.
We used the plasmids pLPCX, pLXRN, pIRES2-EGFP (all from Clontech, Mountain View, CA), and pH19KE (20) to generate the series of constructs used in this study. We inserted the neomycin resistance (Neor) gene as a 1,419-bp BglII-EcoRV fragment from pLXRN into the multiple cloning site of pIRES2-EGFP cut with BglII and SmaI to form pN-IRES-G. The pRMR construct was made by ligation of the 3,445-bp BstEII-BalI RSV LTR-bearing fragment of pH19KE and the 2,548-bp PshAI-PvuII inner fragment of pLPCX. The cassette containing the Neor gene, internal ribosomal entry site (IRES), and enhanced green fluorescent protein (EGFP) gene, the 2,864-bp Eco47III-HpaI fragment of pN-IRES-G, was then ligated into the 4,579-bp pRMR backbone fragment cut with SmaI and XhoI. The resulting retrovirus vector pRNIG (Fig. 1A) was used for the construction of all insertion variants of the RSV LTR. The pMNIG vector was constructed by ligation of the 3,509-bp BstEII-ClaI Neor, IRES, and EGFP cassette from pRNIG and the 4,458-bp BstEII-ClaI LTR fragment of pLPCX. The 5′ and 3′ LTR sequences in pLPCX come from Moloney MLV and its replication-defective derivative Moloney murine sarcoma virus, respectively. Minor sequence differences between these LTRs enabled discrimination of the cloned plasmid retrovirus DNA from the proviral DNA that went through reverse transcription.
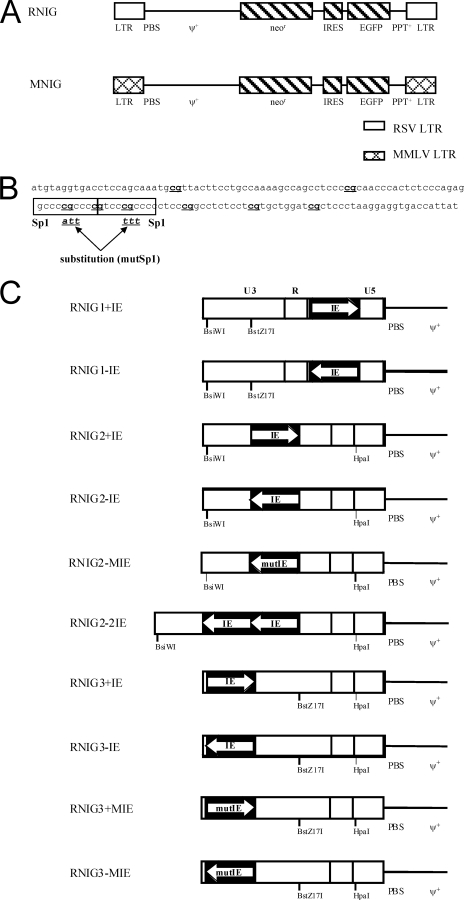
FIG. 1.
Schematic representation of retroviral vectors used in this study. (A) The basic vectors RNIG and MNIG containing the Neor and EGFP genes driven from the 5′ LTR of RSV or MLV, respectively. PBS, primer-binding site; ψ+, encapsidation signal from MLV; PPT, polypurine tract. (B) Nucleotide sequence of the IE of the hamster aprt CpG island. The Sp1 binding sites are in frames, and CpG dinucleotides are in bold. The substitution of three bases in each Sp1 binding site that abolished the Sp1 binding capacity is depicted. (C) Modifications of the RNIG vector LTRs. Single or duplicated IEs with intact or mutated Sp1 binding sites were inserted in the depicted unique restriction sites in sense and/or antisense orientation. The orientation of IE is indicated with an arrow; the bicistronic coding regions and 3′ LTR are not shown.
Three unique restriction sites were introduced into the LTRs of the pRNIG by in vitro mutagenesis (see below). The BsiW I site at position −226 upstream from the transcription start site and the BstZ17I site at position −89 were introduced into the 3′ LTR and HpaI site at position +27 in the 5′ LTR. The unique restriction sites were used for the insertion of the IE element. pRNIG1+IE and pRNIG1-IE were constructed by insertion of the IE element into the HpaI restriction site in the sense and antisense orientations, respectively. The pRNIG2 and pRNIG3 variants were formed analogically by insertion of the IE element into the BstZ17I and BsiWI restriction sites, respectively. The pRNIG2-2IE variant was created analogically by inserting two copies of the IE element into the BstZ17I restriction site in antisense orientation (Fig. 1C). Vectors with insertion of the IE with mutated Sp1 binding sites (see below) were designated RNIG3+MIE, RNIG3-MIE, and RNIG2-MIE. The correct insertion and orientation of IEs was confirmed by DNA sequencing.
Assembly of the IE.
The core element of the CpG island of the aprt gene (46) was constructed by gene assembly PCR using eight oligonucleotides: 1F, 5′-AGTCGTATACTCCAGCAAATGCGTTACTTCCTGCC-3′; 2F, 5′-AAAAGCCAGCCTCCCCGCAACCCAC-3′; 3F, 5′-TCTCCCAGAGGCCCCGCCCCGTCCC-3′; 4F, 5′-GCCCCCTCCCGGCCTCTCCTCGTGCTGG-3′; 1R, 5′-TGCGGGGAGGCTGGCTTTTGGCAGG-3′; 2R, 5′-GGGCGGGGCCTCTGGGAGAGTGGGT-3′; 3R, 5′-CGAGGAGAGGCCGGGAGGGGGCGGGACG-3′; and 4R, 5′-ATGCGTATACTCCTTAGGGAGCGATCCAGCA-3′. These oligonucleotides were combined in pairs (1F + 1R, 2F + 2R, 3F + 3R, and 4F + 4R) in four assembly reactions (reactions 1 to 4, respectively). The cycling conditions were as follows: 96°C for 2 min; 4 cycles of 95°C for 40 s, 70°C for 10 s, a 0.3°C/s ramp to 25°C, and 72°C for 1 min; and 35 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 30 s; the final extension was at 72°C for 3 min. Products of reactions 1 and 2 were mixed, and PCR was set up with primers 1F and 2R. Reactions 3 and 4 were processed analogically with primers 3F and 4R. The PCR conditions were as follows: 96°C for 2 min and 35 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 40 s; the final extension was at 72°C for 5 min. The products of these two reactions were mixed and PCR, under the conditions used for the secondary PCR, was performed using primers 1F and 4R, which provide a 142-bp product with BstZ17I restriction sites on both ends. To prepare the IE with BsiWI restriction sites, we performed a PCR of the final product with primers 1Fb (5′-AGTCCGTACGTCCAGCAAATGCGTTACTTCCTGC-3′) and 4Rb (5′-AGTCCGTACGTCCTTAGGGAGCGATCCAGC-3′).
Site-directed mutagenesis.
All site-directed mutagenesis experiments were performed with a Transformer site-directed mutagenesis kit (Clontech, Mountain View, CA) according to the manufacturer's protocol. The assembled IE was cloned into the pGEM-T Easy vector (Promega, Madison, WI). For mutation of Sp1 binding sites, we used a mutagenic primer (5′-CTCCCAGAGGCCCATTCCCGTCCTTTCCCCTCCCGGC-3′), which also served as a selection primer. The selection was performed with the restriction enzyme DraII. To introduce unique cloning sites into the pRNIG construct, we used the mutagenic primer 5′-GGAAATGTAGTCGTACGCAATACTCCTG-3′ for introduction of the BsiWI cloning site, the mutagenic primer 5′-CTTATTAGGAAGGTATACAGACGGGTC-3′ for introduction of the BstZ17I site, and the mutagenic primer 5′-ACATTGGTGTTAACCTGGGTTG-3′ for introduction of the HpaI site. The selection of the vectors with the new sites was performed with alternate use of two selection primers, the selection ScaI/BglII primer 5′-GTGACTGGTGAGATCTCAACCAAG-3′ and the reselection BglII/ScaI primer 5′-GTGACTGGTGAGTACTCAACCAAG-3′.
Cell culture.
The packaging GP293 cell line (Clontech, Mountain View, CA) was maintained in Dulbecco's modified essential medium-F-12 Eagle's modified medium (Sigma, St. Louis, MO) supplemented with 5% newborn calf serum, 5% fetal calf serum (both from Gibco-BRL, Gaithersburg, MD), and penicillin-streptomycin (100 mg/ml each). HEK 293 human embryo kidney cells, NIL-2 hamster fibroblastoid cells (7), QT6 quail methylcholanthrene-transformed cells (37), and DF1 chicken fibroblastic cells (23) were maintained in Dulbecco's modified essential medium-F-12 Eagle's modified medium supplemented with 5% newborn calf serum, 2% fetal calf serum, 1% chicken serum (Gibco BRL, Gaithersburg, MD), and penicillin-streptomycin (100 mg/ml each). The tissue cultures were cultivated at 37°C in a 3% CO2 atmosphere. In reactivation experiments, 5-azacytidine (5-AzaC) and trichostatin A (TSA) treatment was performed with 4 μM 5-AzaC (Sigma) and with 0.5 μM or 1 μM TSA (Sigma) for 4 days.
Vector propagation.
The MNIG and RNIG vectors and their modified forms were propagated by transfection of plasmid DNA containing the proviral forms of reporter vectors together with the plasmid pVSV-G (Clontech, Mountain View, CA) into GP293 cells. GP293 cells (1.5 × 107) were seeded on 140-mm petri dishes. After 24 h, the cells were cotransfected with 10 μg of pVSV-G and 50 μg of the vector plasmid. Cotransfection was performed by calcium phosphate precipitation. The culture medium containing the vector particles was collected 1, 2, and 3 days posttransfection. The collected viral stocks were clarified by centrifugation at 200 × g for 10 min at 4°C. The supernatant was collected and centrifuged at 24,000 rpm for 2 h 30 min at 4°C in an SW28 rotor, Beckman Optima100 (Beckman, Fullerton, CA). The pellet was resuspended in a culture medium with 10% newborn calf serum, frozen, and stored in −80°C. The titration of infectious virus particles was performed by serial dilution of the virus stock and subsequent infection of DF1 cells. In repeated experiments, vectors with modified and unmodified LTRs reached similar titers within the range of 2 × 104 to 1 × 105 IU/ml. Twenty-four hours postinfection, 400 μg/ml of G418 (Sigma, St. Louis, MO) was introduced, and the cells were selected for 15 days. The number of G418-resistant colonies was counted after the selection.
Transduction of cells and FACS analysis.
Cells were seeded at 5 × 105 per 60-mm petri dish, and after 5 h, 200 μl of viral suspension with 15 μg/ml Polybrene was applied to the cell culture and allowed to adsorb for 40 min at room temperature. After the adsorption, fresh medium was added up to 4 ml and the cells were placed at 37°C and 3% CO2. Twenty-four hours postinfection, selection with 400 μg/ml G418 (Sigma) was introduced, and medium with fresh G418 was changed every 2 or 3 days. After 15 days of selection (6 days in the reactivation experiment), G418 was removed. At 1-week intervals, the cell cultures were analyzed with an LSR II cytometer (Becton-Dickinson, San Jose, CA), and the frequencies of GFP-positive cells were assessed. At specific intervals, the cultures were sorted with a FACSVantage SE (Becton-Dickinson) device according to the presence or absence of GFP expression. In the case of clonal fluorescence-activated cell sorting (FACS) analysis, the cell clones were obtained by limiting dilution of transduced cell cultures.
Methylation analysis.
The genomic DNA isolated from infected cells was treated with sodium bisulfite using an EpiTect bisulfite kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Seminested PCR of the upper strand was performed with primers complementary to the U3 region of the RSV LTR and the leader region (see Fig. 5B) comprising all but one CpG within the LTR. The sequences of the primers were 5′-GTTTTATAAGGAAAGAAAAG-3′ (upper), 5′-AACCCCCAAATAAAAAACCCC-3′ (lower-inner), and 5′-AAACAAAAATCTCCAAATCC-3′ (lower-outer). PCRs were carried out with 200 ng of DNA at 25 cycles of 95°C for 1 min, 58°C for 2 min, and 72°C for 90 s. The PCR products were cloned into pGEM-T Easy vector (Promega) and sequenced by using the universal pUC/M13 forward primer.
FIG. 5.
DNA methylation of integrated vectors. The methylation status of CpGs within the LTR of integrated vectors was assayed by the bisulfite technique. (A) CpG methylation status of the 5′ LTR sequence of silencing-prone vectors RNIG and RNIG3-IE in three cell clones with different levels of GFP silencing. NIL-2 cells transduced with vectors were sampled 9 weeks after antibiotic removal and subjected to bisulfite sequencing. (B) CpG methylation status of the 5′ LTR sequence of the RNIG2-2IE vector protected from CpG methylation in two cell clones. NIL-2 cells transduced with RNIG2-2IE vector were sampled 9 weeks after G418 removal and subjected to bisulfite sequencing. (C) CpG methylation status of the 5′ LTR sequence of silencing-prone vectors RNIG and RNIG3-MIE in two cell cultures enriched with GFP-negative cells early after vector infection. NIL-2 cells transduced with vectors and enriched with GFP-negative cells by FACS 3 weeks after G418 removal were sampled and subjected to bisulfite sequencing. Each line with circles represents one independent LTR sequence of the PCR product obtained from the bisulfite-treated DNA. Methylated CpGs are depicted by solid circles; nonmethylated CpGs are indicated by open circles. The locations of primers used for seminested PCR after the sodium bisulfite treatment and the sites of insertion of the IE in the RNIG3-IE vector are depicted by horizontal and vertical arrows, respectively.
RESULTS
The core element of the CpG island stabilizes long-term expression of the RSV LTR-driven retroviral vector.
We constructed retroviral vectors RNIG and MNIG with Neor and EGFP genes under the control of the RSV and MLV LTRs, respectively. Both genes are expressed from a single bicistronic mRNA by virtue of the IRES from the encephalomyocarditis virus (Fig. 1A). The RSV LTR contains a highly efficient promoter but is prone to CpG methylation and transcriptional silencing upon infection of mammalian cells (21, 22). For the antisilencing protection of RSV LTR, we chose the IE of the hamster aprt CpG island, characterized previously as an efficient protective motif (46). The IE comprises a 120-bp sequence that includes eight CpG dinucleotides and two high-affinity Sp1 binding sites (Fig. 1B) and is responsible for the majority of the CpG island properties. A single IE was inserted in both orientations into three positions within the RSV LTR: in the U5 region downstream of the promoter and transcription start sites; in the middle of the U3 region between the enhancer and the promoter; and at the beginning of the U3 region, upstream of the enhancer. Furthermore, two IEs were inserted in an antisense orientation between the enhancer and the promoter. The U5 region was targeted in the 5′ LTR and U3 in the 3′ LTR, so that after reverse transcription of the corresponding RNA, all modifications appeared within both LTRs of the resulting provirus. The complete set of modified RSV LTRs with the names of corresponding retroviral vectors is shown in Fig. 1C.
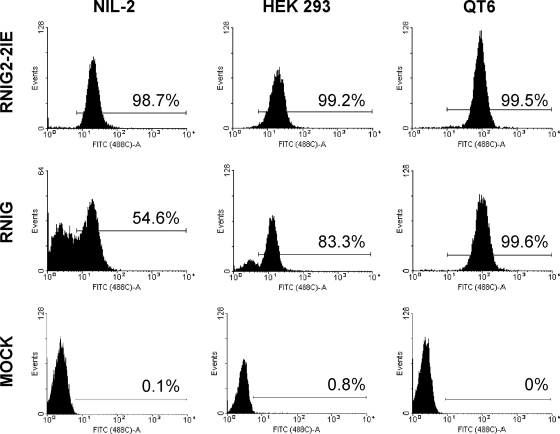
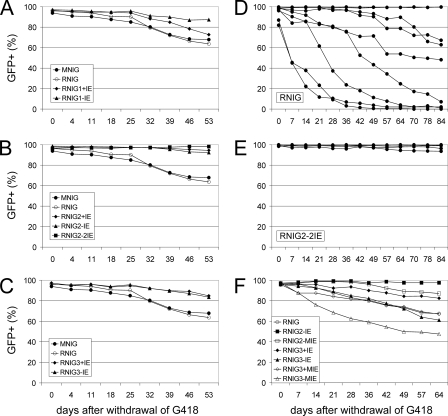
To study the stability of the long-term expression, we packaged the wild-type and modified vectors in GP293 cells and transduced them into two avian cell lines, chicken DF1 and quail QT6, and two mammalian cell lines, hamster NIL-2 and human HEK 293. The cells that contained transcriptionally active transduced genes were selected with G418, and the expression of GFP was monitored at regular intervals after G418 removal by microscopic inspection of GFP variegation and FACS. All vectors with either wild-type or modified LTRs exhibited very stable expression in DF1 cells during in vitro cultivation. Only few GFP-negative cells appeared after 2 months of cultivation without selective pressure (data not shown). A similar lack of variegation was also observed in QT6 cells transduced with RNIG, RNIG2-2IE, and MNIG vectors. We isolated six monocellular clones of the G418-resistant cells after transduction with each of these three vectors and cultivated them separately for more than 2 months. All clones exhibited very stable expression, often without any sign of silencing (data not shown). There was only one clone from the QT6 cell line transduced with the vector with an unmodified RSV LTR which was progressively silenced, and after 70 days of cultivation (without selection), 22% of the cells were GFP negative.
Different behavior of integrated vectors was observed after the transduction of mammalian cells. The vectors RNIG and MNIG were progressively silenced in NIL-2 cells, whereas the vectors with inserted IE elements exhibited various degrees of protection from transcriptional silencing (Fig. 2 and 3A to C). The insertion of a single or duplicated IE between the promoter and the enhancer nearly completely stabilized the long-term expression of RNIG vectors, irrespective of the orientation (Fig. 2 and 3B). Because of the significant variability among cell cultures transduced with the same vector modification, we isolated several G418-resistant NIL-2 clones after infection with individual vectors and observed GFP expression over time. Various numbers of clones were inspected, from six clones with the RNIG2+IE vector up to 19 clones with the RNIG2-2IE vector. The data are shown individually for both the wild-type RNIG and RNIG2-2IE vectors and cumulatively for the remaining vectors. The RNIG vector was progressively silenced in most cell clones. Out of nine clones isolated, only two were without variegation and exhibited stable GFP expression (Fig. 3D), probably due to insertion into a particular site in the host cell genome, which supported efficient transcription of the integrated retrovirus. In contrast, almost no silencing was observed in clones bearing the vector RNIG2-2IE modified by insertion of double IE in the −89 position. We analyzed 19 clones of NIL-2 cells transduced with this vector and observed weak silencing of GFP expression only in two of them. They exhibited 2% and 8% GFP-negative cells after 91 days of cultivation. The remaining 17 clones did not exhibit any sign of silencing (Fig. 3E).
FIG. 2.
FACS analysis of GFP expression from modified and unmodified vector LTRs. NIL-2, HEK 293, and QT6 cell lines transduced with RNIG and RNIG2-2IE vectors were selected with G418 and FACS analyzed 57 days after G418 removal. One clone from each cell line with each of the two vectors is shown. In histograms, the relative GFP fluorescence is plotted against the cell count, and the range of GFP-positive cells is indicated with a horizontal bar. As a negative control, the autofluorescence of mock-infected NIL-2, HEK 293, and QT6 cells is shown.
FIG. 3.
Stability of GFP expression from retroviral vectors with modified and unmodified LTRs during long-term cultivation of transduced cells. NIL-2 cells were infected with retroviral vectors modified by IE inserted into the U5 region of LTR (A), between the LTR promoter and enhancer (B), and at the beginning of the U3 region (C). Transduced cells were selected with G418 for 15 days, the percent GFP-positive cells was quantified by FACS in regular intervals after G418 was removed, and the cells were cultivated without selection. Each point represents the mean from three culture dishes. (D and E) NIL-2 cells were infected with the modified and unmodified vectors, and individual clones of G418-resistant cells were isolated after 7 days of selection and cultivated separately without selection. The time course of vector silencing was followed by FACS at regular intervals. (E) Stability of GFP expression after G418 removal in 19 individual cell clones transduced with the RNIG2-2IE vector. The diagrams of 17 nonsilenced cell clones were merged into one. (D) Stability of the GFP expression of nine individual cell clones transduced with the control RNIG vector. (F) Stability of expression of vectors modified by insertion of IE with mutated Sp1 binding sites. The population of NIL-2 cells was transduced with the vectors and selected with G418, and individual clones were isolated and cultivated separately. Each curve represents the mean for all cell clones transduced with the same vector construct.
The variability in the rate of GFP silencing among individual cell clones corresponded with the protective effect of the IE insertion. There was vast variability in the case of poorly protective LTR modifications, but even here we also observed rare unsilenced clones with stable GFP expression. On the other hand, the clones bearing very stable vectors were rather uniform, with weak silencing in only a few clones, as in the case of the RNIG2-2 vector (Fig. 3E). All experiments mentioned were confirmed in the human HEK 293 cell line. Data concerning silencing and antisilencing protection correspond to the data obtained in NIL-2 cells. The only difference was generally slower progression of silencing in the HEK 293 cell line. The intensity of GFP fluorescence was similar in NIL-2 and HEK 293 cells and strikingly higher in QT6 cells. This corresponds with the higher level of transcription driven by the RSV LTR in avian cells (21). Insertion of IE, even in tandem between the enhancer and the promoter, did not significantly increase the mean intensity of GFP fluorescence, which means that the transcription and titers of modified vectors were not affected. Much more striking differences in mean fluorescence intensity (MFI) were found among individual clones with vectors integrated into various genomic positions (data not shown).
The Sp1 binding sites are important for the protective role of the CpG island core element.
The IE used in this study comprises two Sp1 binding sites. We introduced point mutations into the Sp1 binding sites that abolished the protein binding capacity of the DNA sequence. RNIG2-IE, RNIG3+IE, and RNIG3-IE vectors were mutagenized in this manner, and the resulting vectors were named RNIG2-MIE, RNIG3+MIE, and RNIG3-MIE, respectively. All Sp1-mutated vectors exhibited a significantly decreased protective effect on GFP expression in NIL-2 cells compared to their nonmutated counterparts. RNIG3+MIE and RNIG3-MIE were silenced more rapidly than the original vector without any element inserted (Fig. 3F). In avian DF-1 and QT6 cells, GFP expression from RNIG3-MIE was stable and no silencing was observed (data not shown).
Reactivation of the silenced vectors with 5-AzaC and TSA.
We assessed the possibility of reverting the transcriptional suppression of integrated proviruses by the DNA methyltransferase inhibitor 5-AzaC and/or the histone deacetylase inhibitor TSA. We sorted the GFP-negative cells from silencing-prone NIL-2 clones transduced with RNIG and RNIG3-MIE 11 days after neomycin removal and treated the cell cultures at regular intervals with the drugs, either alone or in combination. An increase in the percentage of GFP-positive cells was assessed by FACS analysis. Both 5-AzaC and TSA applied separately reactivated GFP expression, but the combination of both inhibitors provided the strongest effect (Fig. 4). The majority of silenced vectors were reactivated by 5-AzaC and TSA 21 days after G418 removal. However, the effect of these drugs gradually decreased, and after 112 days of cultivation without selection, only 2% of the vectors were reactivated by the treatment (Fig. 4).
FIG. 4.
Reactivation of silenced vectors. NIL-2 cells were infected with silencing-prone vectors RNIG and RNIG3-MIE with mutated Sp1 binding sites. The cells were selected with G418, and after 6 days of selection, the antibiotic was removed. After an 18-day cultivation, the GFP-negative cells were sorted by FACS. Each GFP-negative population was then divided into two subpopulations 6 days after the sorting: one was treated with 5-AzaC and/or TSA for 4 days, and the proportion of GFP-expressing cells was assessed. The nontreated GFP-negative cell population was cultivated further, and the treatment was repeated at regular intervals. Each experiment was done in triplicate.
DNA methylation analysis of the integrated vectors.
Because transcriptional silencing of genes or proviruses is usually caused by DNA methylation of promoters, we analyzed and compared the CpG methylation patterns of the vector LTRs that were either silenced or transcriptionally active. For this analysis, we chose two NIL-2 clonal cell cultures transduced by the RNIG vector, one with a high and the other with a very low percentage of GFP-positive cells, and one clonal cell culture transduced by the RNIG3-IE vector with 30% GFP-positive cells. These clones were sampled approximately 9 weeks after G418 selection of transduced cells. Bisulfite sequencing of the vectors displayed almost fully methylated 5′ LTR sequences in the cell clone with silent RNIG proviruses. In contrast, we found almost no CpG methylation in the cell clone without RNIG vector silencing. The cell clone with 30% GFP-positive cells was intermediate with regard to the methylation of 5′ LTR of the RNIG3-IE vector (Fig. 5A). There was no obvious methylation pattern, and the methylated CpG dinucleotides were observed throughout the entire LTR. To show that the tandem of two IEs inserted in the LTR of RNIG2-2IE protects itself and the adjacent DNA sequence from CpG methylation, we analyzed the methylation in two clones from the experiment shown in Fig. 3E. The bisulfite sequencing confirmed the presence of two IEs and the nonmethylated status of the whole LTR (Fig. 5B). The level of CpG methylation was extremely low here, 1.6 and 4.0%.
In order to see the early phase of vector silencing, we examined CpG methylation in the rare GFP-negative cells that appeared soon after the selection of vector-transduced cells. We selected these cells from two NIL-2 cell cultures transduced with RNIG and RNIG3-MIE vectors by FACS after 21 days of cultivation without selective pressure. As it is difficult to separate weakly expressing cells, the resulting cell cultures were enriched for GFP-negative cells but also contained 15% and 28% positive cells, respectively (Fig. 5C). The density of CpG methylation within LTR sequences was 28% and 19%, respectively, much lower than that of the 9-week-old cell culture transduced with RNIG3IE, which contained a comparable percentage of silenced cells (Fig. 5C). We therefore conclude that the silencing of integrated proviruses is associated with CpG methylation of promoter sequence; however, there are other mechanisms of silencing that precede the onset of heavy methylation of proviral LTRs.
DISCUSSION
In this study, we report the capacity of the CpG island core element from the hamster aprt gene, the IE, to stabilize long-term expression of a retroviral vector and protect it from transcriptional silencing. Our data show that insertion of the 120-bp IE into a specific site of the LTR ensures efficient transcription of ASLV-derived vectors and overcomes the repressive chromosomal position effects in nonpermissive mammalian cells. We have found the best protective effect in LTRs with two IEs inserted in tandem between the promoter and enhancer sequences. None of the 19 cell clones analyzed transduced with this modification of the reporter vector exhibited substantial vector silencing after 9 weeks of cultivation. We suggest our optimized vector design as a new strategy for improving retroviral vectors for efficient gene transfer and therapy.
There are two basic strategies to counteract the silencing and variegation of retroviral vectors, based on both gammaretroviruses and lentiviruses (10). The first is the elimination of silencers defined, e.g., in the LTR and primer binding site of MLV (5). Multiple-point mutation of all CpGs in the LTR region also stabilizes the expression of MLV vectors in embryonic stem cells (49). In a complementary approach, matrix attachment regions or insulators have been employed to prevent position effects of adjacent cellular silencers (6, 24, 43, 52). Our previous experiments with the mouse aprt CpG island (20) adjacent to the RSV reporter provirus indicated that the antisilencing and antimethylation protection might also have been caused by some insulative effect. We have found that insertion of the CpG island protects the downstream enhancer/promoter in an orientation-dependent manner without any increase of the promoter strength. The range of the antimethylation effect was relatively narrow, covering just the RSV LTR.
Here, we employed the short core element of the hamster aprt CpG island as defined by Siegfried et al. (46), which can be inserted into the retroviral LTR. This IE comprises the protective effect of the whole CpG island but differs from it in several respects. The observed stabilization of reporter expression was strongly dependent on the position of the IE within the LTR. The IE inserted at the beginning of the U3 region had only a minor effect, but insertion between viral enhancers and promoter ensured efficient antisilencing protection. We do not possess exact data on the promoter strength of IE-modified LTRs, but an estimate of the MFI of noncloned cell cultures infected with individual vectors demonstrates that there are only mild differences in comparison with the wild-type RSV LTR (data not shown). Furthermore, these differences do not correlate with the antisilencing effect. For example, the silencing-prone vector RNIG3-MIE resulted in a slightly higher MFI than the RNIG vector, whereas the well-protected vector RNIG2+IE resulted in a slightly decreased MFI. Substantial differences in MFI were found among individual clones irrespective of the transduced vector (data not shown), which points to the strong influence of the integration site. In our previous study (32), we showed the increased expression driven by the RSV LTR enriched in Sp1 sites without the context of the CpG island. Together, these data contradict the effect of insulator elements defined as sequences capable of obstructing outside enhancers to influence promoters. The range of antimethylation effect is limited to approximately 150 bp from the IE (20, 46). This might explain the weak- or no-protection effect of the IE inserted upstream of the enhancer and the best protection from the IE inserted between the enhancer and the promoter. Both transcriptional regulators are in this way within the range of IE antisilencing influence. The position 89 bp upstream of the transcription start site best corresponds to the position of the element within the hamster aprt gene, 107 bp upstream of the transcription start site. This distance is probably favorable for keeping the promoter transcriptionally active. In contrast to the full-length mouse CpG island, the effect of the IE orientation is only weak.
Transcriptional silencing of retroviruses in two experimental settings has been described. First, complete transcriptional silencing occurs shortly after infection, most probably during the process of integration. Second, the selected reporter-positive cells variegate during the time course of cultivation. Even cell clones that inherited initially active and uniformly integrated reporter proviruses vary and eventually lose expression of the transduced reporter. In our case, we describe just the second type of provirus silencing. To assess early peri-integration silencing, we performed a colony-forming assay that, in a preliminary experiment, displayed a lower number of reporter-positive cells or selected cell colonies that did not correspond to the virus dose determined independently (data not shown). Furthermore, the rate of silencing observed in our experiments might be underestimated. We start our GFP monitoring with completely GFP-positive clonal cell cultures after 2 weeks of G418 selection. The cells in which the silencing occurred most rapidly could have been already selected against at that time. If we monitored the loss of GFP-expressing cells a few days after the infection, we would not obtain reliable data due to the background from noninfected cells as well as from cells transiently expressing the unintegrated forms of retrovirus.
Several studies have described the important role of Sp1 binding sites in the antimethylation capacity of CpG islands (3, 20, 33). We tested the effect of Sp1 mutations in three of our modified LTRs. In all three vectors, mutation of the Sp1 sites significantly increased the provirus silencing during prolonged cultivation, abrogating the protective effect of the inserted IE. In particular, the vector RNIG3-MIE was silenced even more efficiently than the vector with an unmodified LTR. This is probably due to the accumulation of CpG dinucleotides that, without the context of Sp1 sites, become a target of DNA methyltransferases (3). The most stable vector, RNIG2-IE, retains a part of this antisilencing capacity even after mutation of Sp1 sites. This indicates that Sp1 binding sites are involved in the antisilencing capacity of IE, but these sites are not the only cis-acting elements involved. We do not know whether the binding of Sp1 factor to the IE is necessary for its antisilencing activity. Nevertheless, the CpG islands in cells derived from Sp1 knockout mouse embryos are kept methylation free (34), suggesting that Sp1 binding sites act in cis. Finally, it turns out that the methylation status of CpG islands is established in early embryonic cells and then is maintained in differentiated cells. Based on aberrant methylation of CpG islands in the modified β-actin allele, Strathdee et al. (47) proposed a two-step process to define a CpG island, and the role of Sp1 binding sites might be different in pluripotent and differentiated cells. It will be very interesting to check the antimethylation and antisilencing capacity of our IE-modified LTRs in vivo in transgenes passing the germ line and early embryo stages.
The reactivation experiments showed additive effects of 5-AzaC and TSA and revealed that vector silencing was associated with DNA methylation and/or histone deacetylation. The interdependence of both mechanisms of provirus silencing was described in detail in reports of experiments with lentiviral vectors (19). Katz et al. (29) also found silenced ASLV-based vectors to be reactivatable by TSA alone. We observed that this capability strongly depends on the time elapsed from transduction, with a gradual loss of reactivation. This can be explained by the slow and multistep process of heterochromatinization, which requires further histone and chromatin modifiers, finally locking the provirus in a constitutively inactive form (16).
The efficient silencing of ASLV-based vectors in rodent cells has been described (22, 45; for a review, see reference 48). To be sure that this phenomenon and our approach to antisilencing protection of vectors can be applied to other systems, we performed these experiments in parallel with the human HEK 293 cell line. The results obtained were basically the same as those results seen in NIL-2 cells for all vectors with modified 5′ LTRs, but the silencing of the wild-type RNIG vector was slower and, correspondingly, the antisilencing effects of IE insertions were less pronounced. Because the silencing can be associated with distributive DNA methylation by Dnmt1 (50) during the S phase of the cell cycle, it is probable that the slower silencing in HEK 293 cells correlates with slower proliferation and longer doubling times in comparison to NIL-2. Also, other cell-specific factors may play a role in the different rate of silencing (15, 41).
Transcriptional silencing is a major obstacle to the use of retroviral and lentiviral vectors in gene transfer and gene therapy. Our strategy of antimethylation protection of ASLV-based vectors by the CpG island core element indicates that retroviral vectors completely resistant to silencing can be constructed. This modification may prove immediately useful whenever, e.g., RCAS vectors are used in mammalian cells, and it could be part of a general optimized vector design if also proven to be effective in gammaretroviral and lentiviral vectors.
Acknowledgments
We thank Jan Svoboda and Jasper Manning (both at the Institute of Molecular Genetics, Prague, Czech Republic) for their helpful comments, encouragement, and careful reading of the manuscript and Dana Kučerová, Věra Hoserová, and Helena Burešová for excellent technical assistance.
This research was supported by the Grant Agency of the Czech Republic (grants 204/05/0939 and 523/07/1171 to J.H.) and by the Academy of Sciences of the Czech Republic (grant AV0Z50529514).
Footnotes
Published ahead of print on 11 June 2008.
REFERENCES
- 1.Barsov, E. V., and S. H. Hughes. 1996. Gene transfer into mammalian cells by a Rous sarcoma virus-based retroviral vector with the host range of the amphotropic murine leukemia virus. J. Virol. 703922-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsov, E. V., W. S. Payne, and S. H. Hughes. 2001. Adaptation of chimeric retroviruses in vitro and in vivo: isolation of avian retroviral vectors with extended host range. J. Virol. 754973-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandeis, M., D. Frank, I. Keshet, Z. Siegfried, M. Mendelsohn, A. Nemes, V. Temper, A. Razin, and H. Cedar. 1994. Sp1 elements protect a CpG island from de novo methylation. Nature 371435-438. [DOI] [PubMed] [Google Scholar]
- 4.Challita, P. M., and D. B. Kohn. 1994. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc. Natl. Acad. Sci. USA 912567-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Challita, P. M., D. Skelton, A. El-Khoueiry, X. J. Yu, K. Weinberg, and D. B. Kohn. 1995. Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J. Virol. 69748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang, Q., J. Auten, and I. Plavec. 2000. Human beta interferon scaffold attachment region inhibits de novo methylation and confers long-term, copy number-dependent expression to a retroviral vector. J. Virol. 742671-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, L. 1967. Two spontaneously transformed cell lines derived from the same hamster embryo culture. Int. J. Cancer 2143-152. [DOI] [PubMed] [Google Scholar]
- 8.Elleder, D., V. Stepanets, D. C. Melder, F. Šenigl, J. Geryk, P. Pajer, J. Plachý, J. Hejnar, J. Svoboda, and M. J. Federspiel. 2005. The receptor for the subgroup C avian sarcoma and leukosis viruses, Tvc, is related to mammalian butyrophilins, members of the immunoglobulin superfamily. J. Virol. 7910408-10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elleder, D., A. Pavlíček, J. Pačes, and J. Hejnar. 2002. Preferential integration of human immunodeficiency virus type 1 into genes, cytogenetic R bands and GC-rich DNA regions: insight from the human genome sequence. FEBS Lett. 517285-286. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, J. 2005. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 161241-1246. [DOI] [PubMed] [Google Scholar]
- 11.Federspiel, M. J., and S. H. Hughes. 1994. Effects of the gag region on genome stability: avian retroviral vectors that contain sequences from the Bryan strain of Rous sarcoma virus. Virology 203211-220. [DOI] [PubMed] [Google Scholar]
- 12.Federspiel, M. J., D. A. Swing, B. Eagleson, S. W. Reid, and S. H. Hughes. 1996. Expression of transduced genes in mice generated by infecting blastocysts with avian leukosis virus-based retroviral vectors. Proc. Natl. Acad. Sci. USA 934931-4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federspiel, M. J., P. Bates, J. A. Young, H. E. Varmus, and S. H. Hughes. 1994. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc. Natl. Acad. Sci. USA 9111241-11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greger, J. G., R. A. Katz, K. Taganov, G. F. Rall, and A. M. Skalka. 2004. Transduction of terminally differentiated neurons by avian sarcoma virus. J. Virol. 784902-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greger, J. G., R. A. Katz, A. M. Ishov, G. G. Maul, and A. M. Skalka. 2005. The cellular protein Daxx interacts with avian sarcoma virus integrase and viral DNA to repress viral transcription. J. Virol. 794610-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groth, A., W. Rocha, A. Verreault, and G. Almouzni. 2007. Chromatin challenges during DNA replication and repair. Cell 12821-733. [DOI] [PubMed] [Google Scholar]
- 17.Hacein-Bey-Abina, S., C. Von Kalle, M. Schmidt, M. P. McCormack, N. Wulffraat, P. Leboulch, A. Lim, C. S. Osborne, R. Pawliuk, E. Morillon, R. Sorensen, A. Forster, P. Fraser, J. I. Cohen, G. de Saint Basile, I. Alexander, U. Wintergerst, T. Frebourg, A. Aurias, D. Stoppa-Lyonnet, S. Romana, I. Radford-Weiss, F. Gross, F. Valensi, E. Delabesse, E. MacIntyre, F. Sigaux, J. Soulier, L. E. Leiva, M. Wissler, C. Prinz, T. H. Rabbitts, F. Le Deist, A. Fischer, and M. Cavazzana-Calvo. 2003. 2003. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302415-419. (Erratum, 302:568.) [DOI] [PubMed] [Google Scholar]
- 18.Hatziioannou, T., and S. P. Goff. 2001. Infection of nondividing cells by Rous sarcoma virus. J. Virol. 759526-9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, J., Q. Yang, and L. J. Chang. 2005. Dynamic DNA methylation and histone modifications contribute to lentiviral transgene silencing in murine embryonic carcinoma cells. J. Virol. 7913497-13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hejnar, J., P. Hájková, J. Plachý, D. Elleder, V. Stepanets, and J. Svoboda. 2001. CpG island protects Rous sarcoma virus-derived vectors integrated into nonpermissive cells from DNA methylation and transcriptional suppression. Proc. Natl. Acad. Sci. USA 98565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hejnar, J., J. Plachý, J. Geryk, O. Machoň, K. Trejbalová, R. V. Gunataka, and J. Svoboda. 1999. Inhibition of the Rous sarcoma virus long terminal repeat-driven transcription by in vitro methylation: different sensitivity in permissive chicken cells versus mammalian cells. Virology 255171-181. [DOI] [PubMed] [Google Scholar]
- 22.Hejnar, J., J. Svoboda, J. Geryk, V. J. Fincham, and R. Hák. 1994. High rate of morphological reversion in tumor cell line H-19 associated with permanent transcriptional suppression of the LTR, v-src, LTR provirus. Cell Growth Differ. 5277-285. [PubMed] [Google Scholar]
- 23.Himly, M., D. N. Foster, I. Bottoli, J. S. Iacovoni, and P. K. Vogt. 1998. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 248295-304. [DOI] [PubMed] [Google Scholar]
- 24.Hino, S., J. Fan, S. Taguwa, K. Akasaka, and M. Matsuoka. 2004. Sea urchin insulator protects lentiviral vector from silencing by maintaining active chromatin structure. Gene Ther. 11819-828. [DOI] [PubMed] [Google Scholar]
- 25.Hoeben, R. C., A. A. Migchielsen, R. C. van der Jagt, H. van Ormondt, and A. J. van der Eb. 1991. Inactivation of the Moloney murine leukemia virus long terminal repeat in murine fibroblast cell lines is associated with methylation and dependent on its chromosomal position. J. Virol. 65904-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, J., A. Ferris, A. Larochelle, A. E. Krouse, M. E. Metzger, R. E. Donahue, S. H. Hughes, and C. E. Dunbar. 2007. Transduction of Rhesus macaque hematopoietic stem and progenitor cells with avian sarcoma and leukosis viral vectors. Hum. Gene Ther. 18691-700. [DOI] [PubMed] [Google Scholar]
- 27.Jordan, A., P. Defechereux, and E. Verdin. 2001. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 201726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz, R. A., J. G. Greger, K. Darby, P. Boimel, G. F. Rall, and A. M. Skalka. 2002. Transduction of interphase cells by avian sarcoma virus. J. Virol. 765422-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz, R. A., E. Jack-Scott, A. Narezkina, I. Palagin, P. Boimel, J. Kulkosky, E. Nicolas, J. G. Greger, and A. M. Skalka. 2007. High-frequency epigenetic repression and silencing of retroviruses can be antagonized by histone deacetylase inhibitors and transcriptional activators, but uniform reactivation in cell clones is restricted by additional mechanisms. J. Virol. 812592-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kukolj, G., R. A. Katz, and A. M. Skalka. 1998. Characterization of the nuclear localization signal in the avian sarcoma virus integrase. Gene 26157-163. [DOI] [PubMed] [Google Scholar]
- 31.Lorincz, M. C., D. Schuebeler, S. C. Goeke, M. Walters, M. Groudine, and D. I. K. Martin. 2000. Dynamic analysis of proviral induction and De Novo methylation: implications for a histone deacetylase-independent, methylation density-dependent mechanism of transcriptional repression. Mol. Cell. Biol. 20842-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machoň, O., V. Strmen, J. Hejnar, J. Geryk, and J. Svoboda. 1998. Sp1 binding sites inserted into the Rous sarcoma virus long terminal repeat enhance LTR-driven gene expression. Gene 20873-82. [DOI] [PubMed] [Google Scholar]
- 33.Macleod, D., J. Charlton, J. Mullins, and A. P. Bird. 1994. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 82282-2292. [DOI] [PubMed] [Google Scholar]
- 34.Marin, M., A. Karis, P. Visser, F. Grosveld, and S. Philipsen. 1997. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell 89619-628. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell, R. S., B. F. Beitzel, A. F. Schroeder, P. Shinn, H. Chen, C. C. Berry, J. R. Ecker, and F. D. Bushman. 2004. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2E234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mok, H. P., S. Javed, and A. Lever. 2007. Stable gene expression occurs from a minority of integrated HIV-1-based vectors: transcriptional silencing is present in the majority. Gene Ther. 14741-751. [DOI] [PubMed] [Google Scholar]
- 37.Moscovici, C., M. G. Moscovici, and H. Jimenez. 1977. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell 1195-103. [DOI] [PubMed] [Google Scholar]
- 38.Narezkina, A., K. D. Taganov, S. Litwin, R. Stoyanova, J. Hayashi, C. Seeger, A. M. Skalka, and R. A. Katz. 2004. Genome-wide analyses of avian sarcoma virus integration sites. J. Virol. 7811656-11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pao, W., D. S. Klimstra, G. H. Fisher, and H. E. Varmus. 2003. Use of avian retroviral vectors to introduce transcriptional regulators into mammalian cells for analyses of tumor maintenance. Proc. Natl. Acad. Sci. USA 1008764-8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pawliuk, R., K. A. Westerman, M. E. Fabry, E. Payen, R. Tighe, E. E. Bouhassira, S. A. Acharya, J. Ellis, I. M. London, C. J. Eaves, R. K. Humphries, Y. Beuzard, R. L. Nagel, and P. Leboulch. 2001. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science 2942368-2371. [DOI] [PubMed] [Google Scholar]
- 41.Poleshko, A., I. Pelagin, R. Zhang, P. Boimel, C. Castagna, P. D. Adams, A. M. Skalka, and R. A. Katz. 2008. Identification of cellular proteins that maintain retroviral epigenetic silencing: evidence for an antiviral response. J. Virol. 822313-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinišová, M., A. Pavlíček, P. Divina, J. Geryk, J. Plachý, and J. Hejnar. 2008. Target site preferences of subgroup C Rous sarcoma virus integration into the chicken DNA. Open Genomics J. 16-12. [Google Scholar]
- 43.Rivella, S., J. A. Callegari, C. May, C. W. Tan, and M. Sadelain. 2000. The cHS4 insulator increases the probability of retroviral expression at random chromosomal integration sites. J. Virol. 744679-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroeder, A. R., P. Shinn, H. Chen, C. C. Berry, J. R. Ecker, and F. Bushman. 2002. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 110521-529. [DOI] [PubMed] [Google Scholar]
- 45.Searle, S., D. A. Gillespie, D. J. Chiswell, and J. A. Wyke. 1984. Analysis of the variations in proviral cytosine methylation that accompany transformation and morphological reversion in a line of Rous sarcoma virus-infected Rat-1 cells. Nucleic Acids Res. 125193-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegfried, Z., S. Eden, M. Mendelsohn, X. Feng, B. Z. Tsuberi, and H. Cedar. 1999. DNA methylation represses transcription in vivo. Nat. Genet. 22203-206. [DOI] [PubMed] [Google Scholar]
- 47.Strathdee, D., C. B. A. Whitelaw, and A. J. Clark. 2008. Distal transgene insertion affects CpG island maintenance during differentiation. J. Biol. Chem. 28311509-11515. [DOI] [PubMed] [Google Scholar]
- 48.Svoboda, J., J. Hejnar, J. Geryk, D. Elleder, and Z. Vernerová. 2000. Retroviruses in foreign species and the problem of provirus silencing. Gene 261181-188. [DOI] [PubMed] [Google Scholar]
- 49.Swindle, C. S., H. G. Kim, and C. A. Klug. 2004. Mutation of CpGs in the murine stem cell virus retroviral vector long terminal repeat represses silencing in embryonic stem cells. J. Biol. Chem. 27934-41. [DOI] [PubMed] [Google Scholar]
- 50.Vilkaitis, G., I. Suetake, S. Klimašauskas, and S. Tajima. 2005. Processive methylation of hemimethylated CpG sites by mouse Dnmt1 methyltransferase. J. Biol. Chem. 28064-72. [DOI] [PubMed] [Google Scholar]
- 51.Wu, X., Y. Li, B. Crise, and S. M. Burgess. 2003. Transcription start regions in the human genome are favored targets for MLV integration. Science 3001749-1751. [DOI] [PubMed] [Google Scholar]
- 52.Yannaki, E., J. Tubb, M. Aker, G. Stamatoyannopoulos, and D. W. Emery. 2002. Topological constraints governing the use of the chicken HS4 chromatin insulator in oncoretrovirus vectors. Mol. Ther. 5589-598. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, F., S. I. Thornhill, S. J. Howe, M. Ulaganathan, A. Schambach, J. Sinclair, C. Kinnon, H. B. Gaspar, M. Antoniou, and A. J. Thrasher. 2007. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in haematopoietic cells. Blood 1101448-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]