Abstract
The human T-cell leukemia virus type 1 (HTLV-1) Tax transactivator is known to induce or repress various cellular genes, several of them encoding transcription factors. As Tax is known to deregulate various basic bHLH factors, we looked more specifically at its effect on TAL1 (T-cell acute lymphoblastic leukemia 1), also known as SCL (stem cell leukemia). Indeed, TAL1 is deregulated in a high percentage of T-cell acute lymphoblastic leukemia cells, and its oncogenic properties are well-established. Here we show that Tax induces transcription of this proto-oncogene by stimulating the activity of the TAL1 gene promoter 1b, through both the CREB and NF-κB pathways. It was also observed that TAL1 upregulates HTLV-1 promoter activity, in either the presence or the absence of Tax. The viral promoter is inhibited in trans by expression of the E2A protein E47, and TAL1 is able to abrogate this inhibition. These data show the existence of a positive feedback loop between Tax and TAL1 expression and support the notion that this proto-oncogene participates in generation of adult T-cell leukemia/lymphoma by increasing the amount of the Tax oncoprotein but also possibly by its own transforming activities.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia/lymphoma (ATL) and tropical spastic paraparesis/HTLV-1-associated myelopathy (14, 38, 41, 46). ATL develops in approximately 1% to 2% of HTLV-1-infected individuals, after a long period of latency, suggesting a multistep process of T-lymphocyte transformation (15, 32). HTLV-1 encodes the 40-kDa oncoprotein Tax, which potently transactivates the HTLV-1 long terminal repeat (LTR) promoter and which plays a key role in T-lymphocyte transformation. Activation of the viral promoter involves molecular contacts between Tax and the cyclic AMP response element binding protein (CREB), the p300/CBP coactivators, and general transcription factors, including the TATA box binding protein (TBP) and the TBP-associated factor 28 (2, 5, 8, 9, 21, 47). The transforming properties of Tax have been well established in various cellular and animal models, and they are likely to result from pleiotropic effects of the viral protein on various transcription factors, including members of the CREB, SRF, and Ets families; on signal transduction pathways such as NF-κB or those involving PDZ proteins; on cell cycle progression regulatory factors, on factors of the DNA damage response; and also on members of the cell division control machinery (15, 16, 19-22, 28, 31-34). Among these various activities, Tax is also able to deregulate transcription mediated by E proteins (40).
E proteins are transcription factors of the basic helix-loop-helix (bHLH) family and include the E2A (E47 and E12), HEB, and E2-2 genes products. The HLH domain is implicated in protein dimerization, while the basic region is responsible for DNA binding on the E-box consensus motif (CANNTG). These class I bHLH proteins are widely expressed, whereas class II bHLH proteins, such as TAL1 (T-cell acute lymphoblastic leukemia 1) or MyoD, represent tissue-specific transcription factors. TAL1 is expressed in early hematopoiesis and is essential for generation of the erythroid and myeloid lineage (for a review, see reference 26). It also plays a key role in angiogenesis. After various observations, TAL1 can be considered an essential factor of a putative hemangioblast viewed as a precursor leading to formation of both hematopoietic and endothelial cells. In the lymphoid lineage, TAL1 expression is normally lost early in the differentiation process. However, it is now well characterized that its expression is maintained in many T-cell acute lymphoblastic leukemia (T-ALL) cells, either due to chromosomal rearrangement, commonly placing the TAL1 gene under control of the SIL promoter (1, 30), or due to epigenetic activation (13).
Like most tissue-specific bHLH factors, TAL1 binds to E-box motifs as an heterodimer with E proteins, and it can nucleate various protein complexes which can be either activating or inhibitory (37). TAL1 binds the LIM-only proteins LMO1 and LMO2 (43), and the LMO-TAL1-E-protein complex can associate with GATA factors, leading to transcription activation (23, 36, 43). In these multiprotein complexes TAL1 can establish functional interactions with various transcription factors, including Sp1. TAL1 has also been shown to interact with the p300 coactivator through its basic domain (18). In the case of repression, TAL1 interacts through the same domain with mSin3A (17). Using mouse models, O'Neil et al. have shown that TAL1 induces leukemia by repressing E47/HEB activity and that this effect involves recruitment of the mSin3A/HDAC1 corepressor complex (35).
HTLV-1 Tax has been shown to downregulate bHLH-mediated transcription by inhibiting recruitment of p300/CBP coactivators with which it interacts (40). Wencker et al. recently reported that Tax suppresses the E47-mediated activation of the pTα promoter in immature thymocytes by the same mechanism (45). Considering the effect of Tax on bHLH factors, we further wondered whether it could act on TAL1, which is a key factor in T-cell leukemogenesis. Here we provide evidence that HTLV-1 Tax is able to transactivate the TAL1 gene promoter 1b through both NF-κB and CREB binding sites. Moreover, TAL1 activation upregulates the activity of the HTLV-1 promoter by hindering a negative effect of E47.
MATERIALS AND METHODS
Constructs.
pSGF-TAL1 was generated by amplifying with appropriate primers the TAL1-coding sequence from a plasmid including the complete human TAL1 open reading frame, kindly provided by D. Mathieu. The PCR product was inserted in the BamHI restriction site of the pSGF vector, which is a pSG5 derivative including the FLAG epitope (12).
Based on the published sequence (GenBank accession no. NM_003189), the TAL1 promoter 1b was cloned from Jurkat genomic DNA using Pfu polymerase with appropriate primers. The purified PCR product was inserted into pGL3-Basic vector (Promega) to obtain pGL3-TAL1b. Mutations in the Sp1 and Ets binding sites were introduced using a site-directed mutagenesis kit (Clontech) and primers 5′-CGCCGCCCCACGCGCCTCGGAGAC-3′ and 5′-TCTCTTCCGAGCTCCCCCCTTTTCCTTA-3′, respectively. pCMV-Flag-E47 was kindly provided by C. Gallego (29). pSG5-Tax and pSG-Tat have been described previously (9, 44). The HTLV1-LTR-luc was kindly provided by A. Rabson (27). pJFE-Tax WT, M22, M47, and K88A were previously described (45). Plasmid pSUPER-TAL1 was generated by annealing the oligonucleotides 5′-GATCCCCGAAGCTCAGCAAGAATGAGCTCTTCCTCCTCATTCTTGCTGAGCTTCTTTTT-3′ and 5′-AGCTAAAAAGAAGCTCAGCAAGAATGAGGAGGAAGAGCTCATTCTTGCTGAGCTTCGGG-3′ and inserting the resulting DNA fragment between the BglII and HindIII restriction sites of pSUPER (6).
Cell culture, transfection, and infection.
Jurkat cells were cultured in RPMI 1640 medium with 10% fetal calf serum (FCS) (Sigma), 100 units/ml penicillin, and 100 μg/ml streptomycin (Gibco) at 37°C in a 5% CO2 humidified atmosphere. HeLa and 293T cells were cultured in Dulbecco's modified Eagle medium with 10% FCS (Gibco), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified atmosphere. Immature thymocytes were cultured in α-minimal essential medium with 20% FCS, 10 μg/ml of interleukin-7, and 10 μg/ml of stem cell factor. For transfection, the FCS concentration was reduced to 5%. Expression vectors were transfected using the calcium phosphate precipitation method as previously described (9). Jurkat cells were transfected using the Nucleofector kit (Amaxa). Cellular extracts were normalized with respect to protein concentrations, which were quantified with the DC protein assay kit (Bio-Rad). Infection of Jurkat cells with the Semliki forest virus (SFV)-glutathione S-transferase (GST) and SFV-Tax recombinant viruses was performed as previously described (4).
Transfection of immature human thymocytes.
cDNA from Tax-expressing immature thymocytes was obtained and analyzed as described previously (45). Briefly, total thymocytes were extracted from thymus fragments removed during cardiac surgery of patients aged 1 week to 2 years. Immature thymocytes were isolated using magnetic separation (StemCell Technologies Inc., Vancouver, British Columbia, Canada) based on the expression of CD3, CD8, and CD4 markers. The population obtained is composed mostly of CD4ISP cells (namely, CD3−, CD34+/−, CD4+, and CD8−). Cells were transfected with either a cytomegalovirus (CMV)-Tax-green fluorescent protein (GFP)- or a CMV-GFP-expressing vector, and GFP positive cells were sorted from both cultures using a FACSVantage (Becton Dickinson). Total RNA was extracted from thymocytes using TRIzol reagent (Invitrogen) according to the manufacturer's instructions, reverse transcribed, and used as template for quantitative reverse transcription PCR (qRT-PCR) analyses.
Luciferase assays.
HeLa and 293T cells (1.4 × 105 cells) were transfected with 900 ng to 1.5 μg of firefly luciferase constructs (HTLV-1-LTR promoter and promoter 1b of TAL1) and 15 ng of thymidine kinase Renilla luciferase (pRLTK) by calcium phosphate precipitation. Reporter gene analysis was performed 48 h after transfection by using a dual-luciferase reporter assay system (Promega). The luciferase activity associated with each construct was normalized on the basis of pRLTK activity. The values presented correspond to the means from three independent points of transfection (with standard deviations) from one representative experiment (out of three experiments).
Immunoblotting.
After separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Amersham Biosciences). For immunoblotting, primary antibodies were diluted 1:1,000 or as indicated by the manufacturer, and detection was performed by chemiluminescence using ECL or ECL+ kits.
Monoclonal antibody against TAL1 (3BTL73) was kindly provided by D. Mathieu (39). Antibody against HTLV-1 Tax was obtained from the AIDS Research and Reference Reagent program catalogue. The following antibodies were purchased: FLAG (clone M2; Sigma), β-actin (Sigma), and hemagglutinin (clone 12CA5; Roche).
Real-time qRT-PCR.
Total RNAs were extracted from frozen cells using the RNeasy minikit (Qiagen). One step RT-PCRs were performed using the QuantiTect Sybr green RT-PCR kit (Qiagen) and the LightCycler apparatus (Roche). The sequences of sense and antisense primers (designed using the Primer3 software) used for quantitative PCR are described in Fig. S3 in the supplemental material.
RESULTS
Activation of TAL1 expression by Tax.
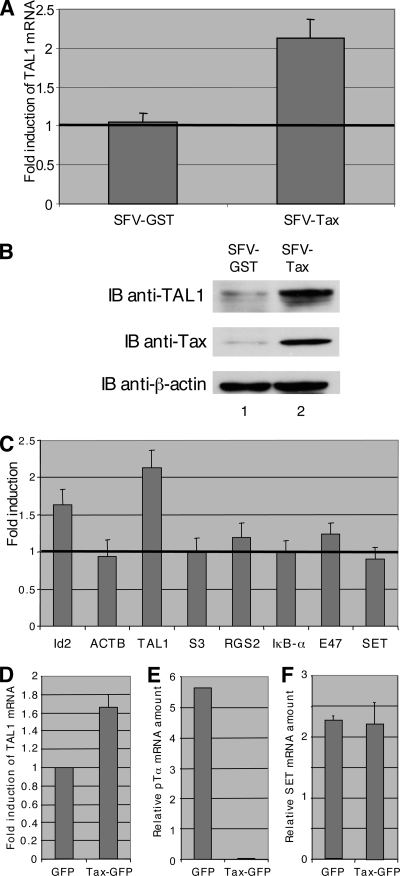
To test whether the Tax transactivator acts on TAL1 expression, Jurkat cells were infected with an SFV producing the viral oncoprotein. Previous studies have established that this system allows rapid and efficient production of Tax, as shown here by immunoblot analysis (5) (Fig. 1B, middle panel). As control, Jurkat cells were infected with a GST-producing SFV. Quantification of the TAL1 mRNA by qRT-PCR showed that Tax production is associated with a twofold increase in the amount of TAL1 mRNA (Fig. 1A). Immunoblot analysis of extracts of these cells showed that when Tax is expressed, the amount of TAL1 protein is clearly increased (Fig. 1B, upper panel). This increase appears stronger than that of the mRNA, likely due to an amplification effect of translation. It was further checked whether this effect is specific of TAL1 or not. To this end, expression of various genes was tested by qRT-PCR using the RNAs of SFV-infected cells. Among the eight tested genes, only TAL1 was clearly activated, and Id2 was activated to a lesser extent (Fig. 1C). Another bHLH transcription factor, E47, was insensitive to Tax expression (Fig. 1C). This indicates that the effect on TAL1 was specific and did not result from a general increase in transcription. We also tested whether this activation can be seen in primary T cells. To this end, immature thymocytes (CD34+/−, CD4+, CD8−) were purified from human thymic lobes and nucleofected with a vector encoding a Tax-GFP fusion protein. Compared with expression of GFP alone under similar conditions, the presence of Tax-GFP raised expression of TAL1 by 60% (Fig. 1D). In agreement with a previous study (45), the amount of pTα mRNA was strongly reduced under similar conditions (Fig. 1E). Under the same conditions, the amount of either the E47 mRNA (45) or the SET mRNA (Fig. 1F) was not affected by Tax-GFP expression. These observations showed that Tax was able to activate expression of TAL1 in T-cell lines and also in primary thymocytes.
FIG. 1.
Induction of TAL1 synthesis by HTLV-1 Tax. (A) Expression of Tax in Jurkat cells increases TAL1 mRNA. Jurkat cells were infected with 1 × 108 particles of SFV-Tax or the control SFV-GST. Total RNAs were prepared at 24 h postinfection, and TAL1 mRNA was analyzed by real-time qRT-PCR. Quantification was performed with respect to RNA from uninfected Jurkat cells. Means of three measurements are shown with standard deviations. (B) Tax expression in Jurkat cells increases TAL1 protein level. At 24 h after infection with SFV-Tax (lane 1) or SFV-GST (lane 2), cell extracts were analyzed by immunoblotting (IB) using antibodies to TAL1 (top panel), to Tax (middle panel), or to β-actin (bottom panel). (C) Specificity of TAL1 induction by Tax. Jurkat cells were infected with 1 × 108 particles of SFV-Tax. Total RNAs were prepared at 24 h postinfection and analyzed by real-time qRT-PCR for expression of various genes as indicated. Quantification was performed with respect to RNA from uninfected Jurkat cells. Means of three measurements are shown with standard deviations. (D) Induction of TAL1 mRNA in Tax-expressing human immature thymocytes. Human immature thymocytes were isolated and nucleofected with a pCMV-GFP control vector or a pCMV-TaxGFP vector. After 24 h of incubation with interleukin-7 and stem cell factor, the GFP-positive cells were sorted from both cultures, and total RNA were isolated and reverse transcribed. cDNA samples from GFP cells and from TaxGFP cells were subjected to qPCR using primers specific for TAL1 and β-actin. The induction of TAL1 mRNA expression is expressed relative to β-actin expression. (E and F) Analysis of pTα (E) and SET (F) mRNAs in Tax-expressing thymocytes. These mRNA were used as internal controls for the experiment on human immature thymocytes.
Tax activates promoter 1b of TAL1 through NF-κB and CREB sites.
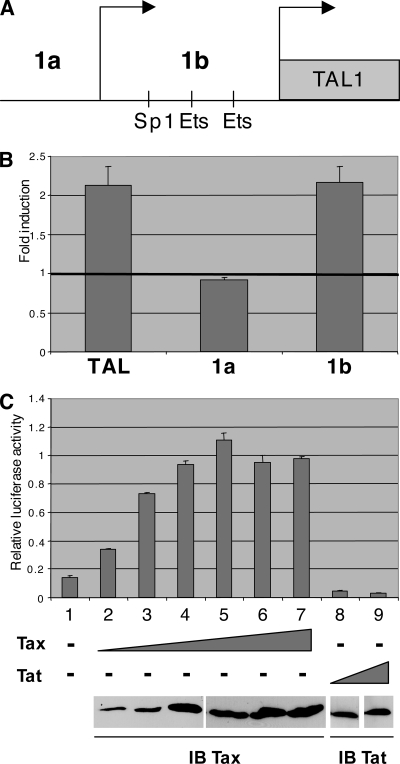
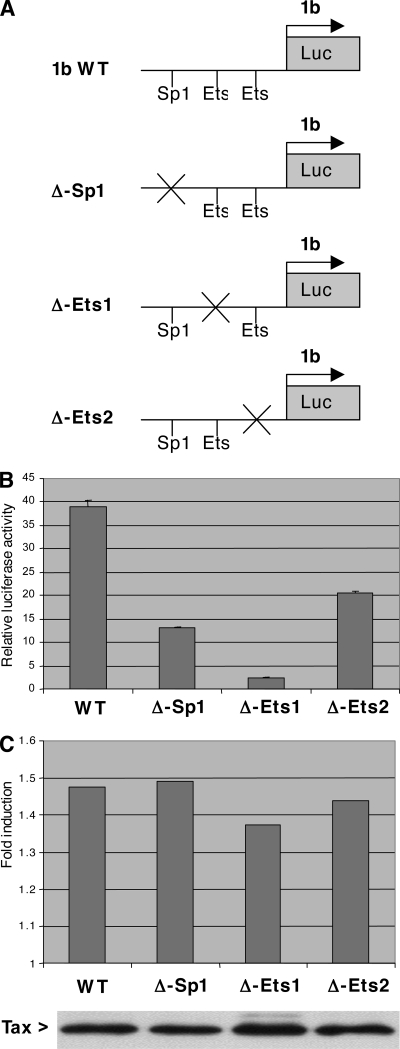
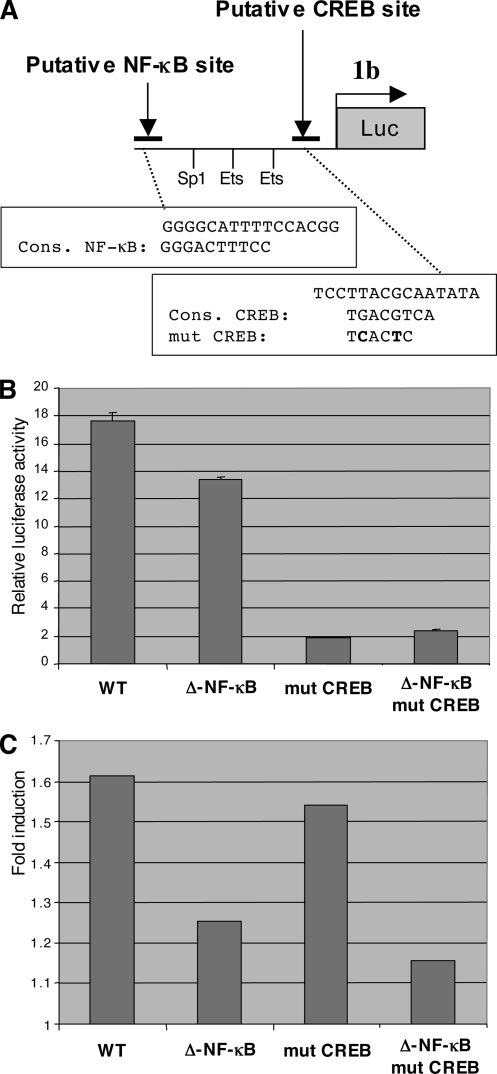
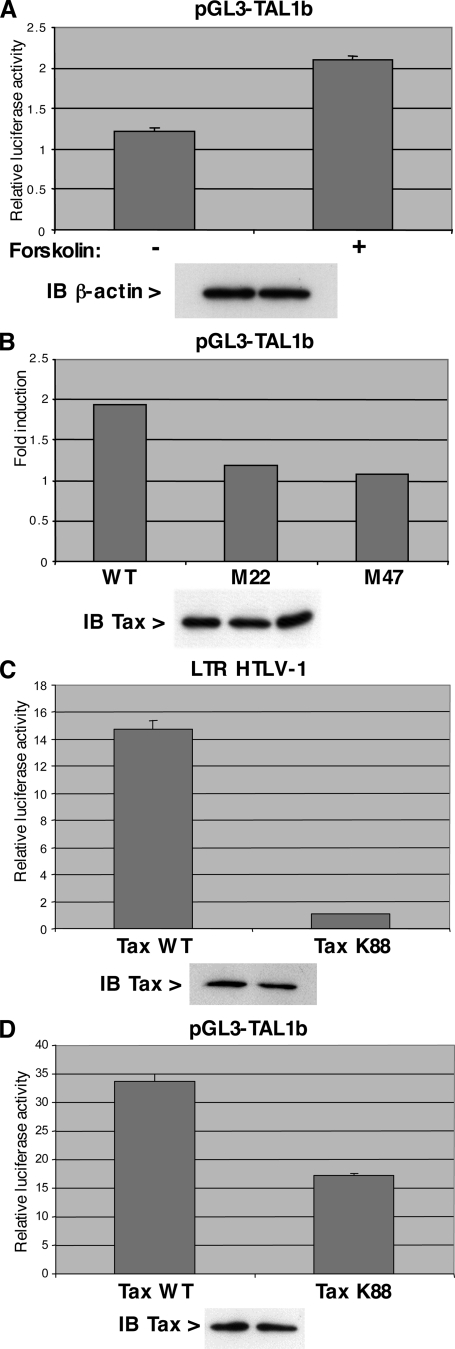
It has been shown in mammals that transcription of the TAL1 gene is driven by two promoters, 1a and 1b (Fig. 2A). To determine which promoter is transactivated by Tax, TAL1 mRNAs produced by both promoters were quantified with specific primers. Using SFV-infected Jurkat cells, we observed that endogenous promoter 1b was activated twofold but that promoter 1a was insensitive to the Tax transactivator (Fig. 2B). Accordingly, a reporter construct with the luciferase gene under the control of the TAL1 promoter 1b is activated by Tax in a dose-dependent manner (Fig. 2C, lanes 2 to 7), but is insensitive to the HIV-1 Tat transactivator (Fig. 2C, lanes 8 and 9). These observations clearly show that Tax can specifically activate promoter 1b of the TAL1 gene. It has been shown that this promoter is regulated by Ets and Sp1 transcription factors (25). It contains one Sp1 binding site and two Ets binding sites, designated Ets-BS1 and Ets-BS2 starting from the distal part of the promoter (Fig. 3A). Mutation of these three binding sites (Sp1-BS, Ets-BS1, and Ets-BS2) reduced the activity of the TAL1 promoter 1b driving expression of the luciferase reporter gene (Fig. 3B). The most powerful effect was due to mutation of Ets-BS1. However, when activation of these various mutated constructs by Tax was tested, none of these mutations markedly affected the Tax effect (Fig. 3C). This indicates that Tax transactivation of the TAL1 promoter 1b does not rely on Sp1 or Ets transcription factors. Bioinformatics analysis of the promoter also revealed degenerate NF-κB and CREB binding sites in the distal and proximal parts of the promoter, respectively (Fig. 4A). In the absence of the Tax transactivator, mutation of these sites in the TAL1 promoter 1b showed that they both contribute to the activity, especially the proximal CREB binding site (Fig. 4B). In agreement with a regulation by CREB, treatment of cells with forskolin, which activates this transcription factor via its effect on cyclic AMP, led to a stimulation of the TAL1 promoter 1b activity (Fig. 5B). Analysis of the mutated constructs also showed that these sites contribute to Tax transactivation. Mutation of the distal NF-κB binding site had a clear effect (Fig. 4C), and that of the proximal CREB motif led to a smaller drop in transactivation. Mutation of both sites led an almost complete loss of the Tax effect on the TAL1 promoter 1b (Fig. 4C). Interestingly, analysis of the effect of TAL1 overexpression in HeLa cells stably transformed with a episomic vector derived from Epstein-Barr virus showed that this factor inhibits IκB-α expression. This effect is likely to amplify NF-κB activation and hence TAL1 expression, thereby establishing a positive feedback loop (see Fig. S1 in the supplemental material).
FIG. 2.
Transactivation of the TAL1 promoter by HTLV-1 Tax. (A) Schematic representation of TAL1 promoters 1a and 1b. The Sp1 and Ets binding sites of promoter 1b are shown. (B) Specific induction of TAL1 promoter 1b by Tax. Jurkat cells were infected with 1 × 108 particles. Total RNAs were prepared at 24 h postinfection, and TAL1 mRNA generated from promoter 1a or 1b was analyzed by real-time qRT-PCR. Quantification was performed with respect to RNA from uninfected Jurkat cells, and means of three measurements are shown with standard deviations. (C) Dose-dependent induction of TAL1b promoter by Tax. HeLa cells were transfected with 1.5 μg of pGL3-TAL1b together with increasing amounts (100, 200, 300, 400, 500, and 600 ng) of pSG5-Tax (lanes 2 to 7) or pSG-Tat (300 and 600 ng) (lanes 8 and 9) as a negative control. Luciferase activities were normalized using TK-luc plasmid. As control for expression of Tax and Tat, cell extracts were analyzed by immunoblotting (IB) using antibodies to Tax and Tat.
FIG. 3.
Ets and Sp1 binding sites do not mediate TAL1 promoter 1b transactivation by Tax. (A) Schematic representation of the pGL3-TAL1b promoter. Derivatives of this construct were generated with mutation of the Sp1, Ets1, or Ets2 binding site. (B) Effect of Ets and Sp1 binding site mutations on TAL1 promoter 1b activity. HeLa cells were transfected with 1.5 μg of pGL3-TAL1b, either wild type (WT) or with mutation of the Sp1, Ets1, or Ets2 binding site. Cell extracts were prepared 48 h after transfection and used for luciferase activity measurement. (C) Effect of Ets and Sp1 binding site mutations on TAL1 promoter 1b transactivation by Tax. The experiment was performed as for panel B, except that the reporter constructs were cotransfected with 300 ng of either pSG5 or pSG-Tax. The graph represents the fold induction by Tax for each construct. Tax expression was monitored by immunoblotting for points including pSG-Tax (panel below the graph). Error bars standard deviations.
FIG. 4.
Characterization of NF-κB and CREB binding sites in TAL1 promoter 1b. (A) Schematic representation of TAL1 promoter 1b, showing positions of the putative NF-κB and CREB binding sites. Boxes depict the exact sequences of these sites with respect to the NF-κB and CREB consensus. For CREB the sequence of the mutated site is also given. (B) Effect of putative NF-κB and CREB binding sites on TAL1 promoter 1b activity. The experiment was performed as described for Fig. 3B by transfecting HeLa cells with pGL3-TAL1b, with wild type (WT), deleted of the putative NF-κB binding site, mutated in the CREB binding site, or bearing modifications of both sites. (C) Effect of putative NF-κB and CREB binding sites on TAL1 promoter 1b transactivation by Tax. The experiment was performed as for panel B with or without cotransfection with 300 ng of pSG-Tax. The graph represents the fold induction by Tax for each construct. Error bars indicate standard deviations.
FIG. 5.
Contribution of both CREB and NF-κB to Tax transactivation of TAL1 promoter 1b. (A) Forskolin treatment activates TAL1 promoter 1b. HeLa cells were transfected with 1.5 μg of pGL3-TAL1b and either mock treated or treated for 1 h with 20 μM forskolin, and luciferase activity was measured. Use of equal amounts of extract was monitored by immunoblotting (IB) with an antibody to β-actin. (B) Effect of Tax M22 and M47 mutants. Cells were transfected with 1.5 μg of pGL3-TAL1b and 300 ng of vectors expressing Tax or its M22 and M47 mutants. The amount of Tax was monitored by immunoblotting using an antibody directed against Tax, and luciferase activity was determined. (C) Loss of induction of HTLV1-LTR promoter transactivation by the Tax K88A mutant. HeLa cells were transfected with 1.5 μg of HTLV1-LTR-luc vector together with 300 ng of pJFE-Tax, wild type (WT) or including a K88A mutation. Cell extracts were prepared 48 h after transfection, and an aliquot of each extract was used for either luciferase activity measurement or immunoblot analysis using an antibody to Tax. (D) The experiment was performed as for panel C, except that the reporter construct was pGL3-TAL1b. Error bars indicate standard deviations.
To confirm an effect of both CREB and NF-κB in Tax transactivation of TAL1 promoter 1b, we tested the effect of Tax mutants M22 and M47 (42). These mutants have been characterized to be defective for the NF-κB and CREB pathways, respectively. Both mutants, which were expressed in similar amounts compared to the wild type as evaluated by immunoblot analysis, were ineffective in fully stimulating TAL1 promoter 1b (Fig. 5B). Moreover, the K88 mutant of Tax, which impairs association with the CBP coactivator, is less active for transactivation of the TAL1 promoter 1b than the wild type at equal intracellular concentrations (Fig. 5D). This mutation did not lead to a complete loss of activation by Tax, as is the case for the HTLV-1 promoter (Fig. 5C), likely because it affects only CREB activity and not the effect of Tax on the NF-κB pathway. Collectively, these data establish that Tax transactivates the TAL1 promoter 1b via degenerate NF-κB and CREB binding sites but not via the Sp1 and Ets motifs.
TAL1 activates both constitutive and Tax-induced activity of the HTLV-1 promoter.
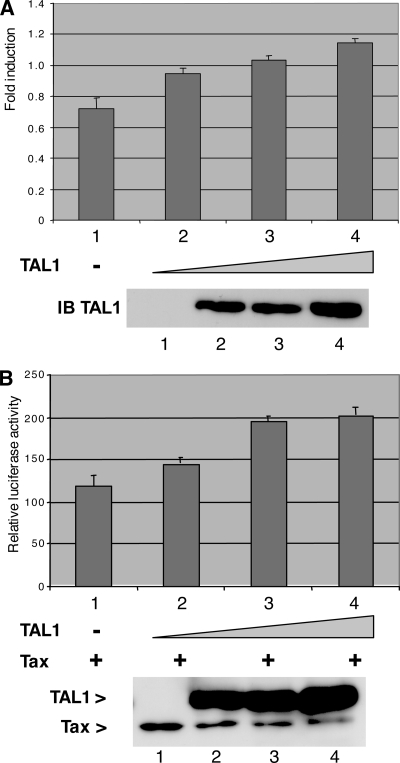
It has been previously shown that the bovine leukemia virus promoter was repressed by bHLH factors such as E47 (6). Considering the possibility that the HTLV-1 promoter might also be regulated by bHLH factors, we examined whether TAL1 modifies its activity. A reporter construct including the HTLV-1 LTR upstream of the luciferase-coding sequence was cotransfected with increasing amounts of a TAL1 expression vector in the absence and presence of Tax coexpression. Under both conditions, the increase in the expression of TAL1 was able to increase the activity of the promoter up to approximately 60% (Fig. 6A and B). In similar experiments performed with promoters repressed by TAL1, a clear reduction in transcriptional activity was observed, showing the specificity of the HTLV-1 promoter response (data not shown). As TAL1 has been reported to interact with HDAC1, we checked whether it might alleviate a potential negative effect of this histone deacetylase on the HTLV-1 promoter. HDAC1 was indeed able to inhibit the HTLV-1 promoter activity, but this effect was not modified by coexpression of TAL1 (see Fig. S2 in the supplemental material). To understand further this effect of TAL1, we examined whether it relates to reversion of a negative effect of bHLH factors such as E47. Overexpression of this factor indeed caused a strong inhibition of the activity of the HTLV-1 promoter in both the presence (Fig. 7A, lanes 2 to 7) and the absence (Fig. 7B, compare lanes 1 and 2) of Tax. TAL1 counteracted this inhibitory effect of E47 in a dose-dependent manner (Fig. 7B, lanes 3 to 5). This also occurred when Tax was coexpressed (Fig. 7C, compare lanes 2 and 3). Immunoblot analyses of the protein levels showed that this effect was not related to a decrease in the amount of E47. These observations shows that E47 has a negative effect on the expression of the HTLV-1 promoter which is reversed by TAL1.
FIG. 6.
Effect of TAL1 on the HTLV-1 promoter. (A) Effect of TAL1 on constitutive HTLV-1 promoter activity. 293T cells were transfected with 1.5 μg of pHTLV-LTR with increasing amounts (75, 150, and 300 ng) (lanes 2 to 4) of pSG5-FLAG-TAL. Luciferase activities were normalized with TK-luc activities. Cell extracts were prepared 48 h after transfection, and an aliquot of each extract was used for either luciferase activity measurement (graph) or immunoblot (IB) analysis using an antibody to TAL1 (bottom panel). (B) Effect of TAL1 on LTR transactivation by Tax. The experiment was performed as for panel A except that 300 ng of pSG-Tax was cotransfected. The immunoblot analysis of the extracts (bottom panel) was performed with a mix of antibodies directed against Tax and TAL1. Error bars indicate standard deviations.
FIG. 7.
TAL1 reverses inhibition of HTLV-1 promoter activity by E47. (A) E47 dose-dependent inhibition of HTLV-1 promoter transactivation by Tax. 293T cells were transfected with 1.5 μg of pHTLV-LTR together with 300 ng of pSG5-Tax and increasing amounts of pCMV-Flag-E47 (25, 50, 75, 100, 200, and 300 ng) (lanes 2 to 7). Cell extracts were prepared 48 h after transfection, and an aliquot of each extract was used for either luciferase activity measurement (graph) or immunoblot analysis using an antibody to E47 (bottom panel). Luciferase activities were normalized with TK-luc activities. (B) Inhibition of constitutive HTLV-1 promoter activity by E47 and reversion by TAL1. 293T cells were transfected with 1.5 μg of pHTLV-LTR, without (lane 1) or with (lanes 2 to 5) 300 ng of pCMV-Flag-E47 and increasing amounts (75, 150, and 300 ng) (lanes 3 to 5) of pSG5-Flag-TAL. Results are depicted as for panel A, except that immunoblot analysis was performed with a mix of antibodies directed against E47 and TAL1. (C) TAL1 reverses the repressive effect of E47 also in the presence of Tax. 293T cells were transfected with 1.5 μg of pHTLV-LTR and 300 ng of pSG5-Tax, without (lane 1) or with (lanes 2 and 3) 50 ng of pCMV-FLAG-E47 and without (lanes 1 and 2) or with (lane 3) 300 ng of pSG5-FLAG-TAL. Results are depicted as for panel A, except that immunoblot analysis was performed with an antibody to E47, along with a mix of antibodies directed against TAL1 and Tax. Error bars indicate standard deviations.
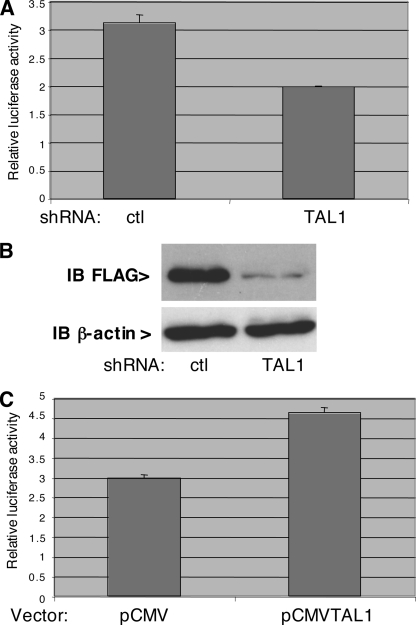
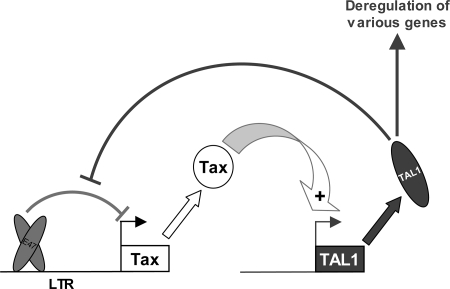
Following these transient-expression experiments, we sought to confirm that TAL1 can indeed regulate the HTLV-1 promoter at the endogenous level. To this end we used a short hairpin RNA (shRNA) developed in the laboratory which efficiently lowers the amount of TAL1 (Fig. 8B). Analysis of the HTLV-1 promoter in Jurkat cells cotransfected with a vector expressing this shRNA showed that suppression of TAL1 indeed caused a decrease in its activity (Fig. 8A). By contrast, if the HTLV-1 reporter construct was cotransfected with a TAL1 expression vector, the activity was increased, similarly to what was observed previously in other cells (Fig. 8C). Collectively these results show that TAL1 is a physiologic regulator of the HTLV-1 promoter and support the notion of a positive feedback loop where Tax activates TAL1, which in turn acts positively on HTLV-1 transcription (Fig. 9).
FIG. 8.
Suppression of endogenous TAL1 reduces HTLV-1 promoter activity. (A) Jurkat cells were transfected with 3 μg of pHTLV-LTR and 2 μg of either pSUPER-HSP (an shRNA targeting HSPC021 as a control) or pSUPER-TAL1. At 24 h after transfection, the luciferase activity was measured. (B) Efficiency of the shRNA directed against TAL1. Cells were transfected with pSG5-FLAG-TAL and either pSUPER-HSP or pSUPER-TAL1. Protein extracts of these cells were analyzed by immunoblotting (IB) using antibodies to FLAG (top panel) or β-actin (bottom panel). (C) The experiment was performed as for panel A, except that pHTLV-LTR was cotransfected with either pCMV or pCMV-TAL1 instead of pSUPER derivatives. Error bars indicate standard deviations.
FIG. 9.
Positive feedback loop between Tax and TAL1. Expression of the viral protein Tax is able to increase TAL1 expression through activation of promoter 1b. TAL1 is able to reverse inhibition of the HTLV-1 proviral promoter by the bHLH factor E47. This mutual positive effect strengthens expression of both oncoproteins, which can act on their specific targets.
DISCUSSION
Activation of TAL1 expression by Tax.
Most T-ALL cells exhibit TAL1 overexpression (13). This has been shown to result either from a (1;14)(p34;q11) chromosomal translocation, which causes juxtaposition of the TAL1 gene with sequences of the T-cell receptor α/δ chain locus, or from upstream sequence deletion, an event known as the Tald recombination (3). However, such DNA rearrangements have been characterized in only a fraction of T-ALL cells with increased TAL1 expression. Hence, it is likely that modification of transcriptional regulatory pathways or epigenetic events can also lead to TAL1 overexpression in these hematological malignancies. In this report we show that this event can be driven by a viral transactivator: the HTLV-1 Tax protein. Tax expression can indeed increase the amount of TAL1 mRNA in a T-lymphocyte cell line. Accordingly, TAL1 expression is observed in all tested cell lines derived from HTLV-1-infected lymphocytes which express Tax and also in cellular clones isolated from HTLV-1-infected patients (J.-M. Terme, unpublished data). Finally, Tax activates reporter constructs including the promoter of the TAL1 gene. It has been shown in both mice and humans that transcription of the gene is driven by two promoters, 1a and 1b. Our data show that the Tax effect is specific for promoter 1b, which includes binding sites for the ETS and Sp1 transcription factors. Although these motifs clearly participate in the activity of the promoter, as shown by the negative effect of their mutation, they do not mediate the Tax effect. In fact, activation by Tax relies on NF-κB and CREB binding sites. The former appears to contribute the most clearly to Tax activation, but its complete loss requires the proximal CREB degenerate binding site. Tax is known to act on many cellular genes. Our data shows that TAL1 has to be added to this list. As TAL1 expression appears to be a key event in T-cell leukemogenesis, this effect might contribute importantly in the generation of ATL. Our observations also indicate that NF-κB can drive expression of TAL1. This might be important in ALL with TAL1 overexpression without gene rearrangement.
TAL1 modulates transcription of the HTLV-1 provirus.
TAL1 is known to repress or to activate other genes (37). Hence, we analyzed whether TAL1 overexpression can modify transcription driven by the HTLV-1 promoter. It was observed that E2A bHLH protein E47 acts as a strong repressor of HTLV-1 promoter activity, both in the absence and the presence of Tax. A bioinformatics analysis indicates a putative E box in the promoter 28 bp upstream of the TATA box. It is possible that E47, by binding to this site, impedes recruitment of p300 at the CREB binding sites within the repeated upstream 21-bp Tax-responsive elements. Such a competition could act negatively on the promoter activity. TAL1 can reverse this negative effect of E47 on both the constitutive and Tax-induced activities of the HTLV-1 promoter. This could be the consequence of an impaired ability of the E47-TAL1 complex to recruit p300, resulting in the full availability of the coactivator to act in combination with CREB and Tax to induce viral transcription. It has been reported that in bovine leukemia virus, an E box overlaps the CREB binding sites and acts negatively on promoter activity by competition with CREB (7). In the HTLV-1 promoter no E box is clearly apparent in the three 21-bp elements, but such a model remains possible and would require functional testing. Whatever the molecular details, our data show that TAL1 is able to counteract the negative effect of E47 on the HTLV-1 promoter and hence acts as a positive regulator of it. Moreover, we show that this activity is exerted by the endogenous TAL1 in Jurkat cells. These activities indicate the existence of a positive feedback loop between HTLV-1 and the TAL1 gene via the Tax transactivator. These interleaved effects of Tax on TAL1 promoter 1b and of TAL1 on the HTLV-1 promoter stimulate expression of both factors in T lymphocytes. This also suggests that HTLV-1 replication might be favored in early cells of the T lineage which expresses TAL1. It is also interesting to consider this point in light of newborns infection that occurs by natural breast-feeding. Cells expressing TAL1 are likely to be good hosts for viral replication.
Contribution of TAL1 to ATL.
In line with this effect, it is possible that the Tax TAL1 cross talk plays a role in transformation. The increase in TAL1 expression could drive cell transformation additively to the pleiotropic effects of Tax. Moreover, it is known that in vivo after the early phase of infection, Tax expression is repressed in vivo, likely as a consequence of immune surveillance. It is possible that TAL1 induction persists after initial Tax activation, maintaining cells in an intermediately differentiated state favoring their transformation. TAL1 affects important genes for the control of cell proliferation or apoptosis. Along this line, we have observed in this work that IκB-α expression is decreased by TAL1. Similarly, Chang et al. have shown that TAL1 represses transcription of the NF-κB1 gene, thereby decreasing the amount of p50 and favoring the p65·cRel dimer, leading to elevated NF-κB activity (10). Thus, TAL1 acts on multiple components of the NF-κB pathway to cause an elevated constitutive NF-κB activity in the cell, with a consequent protective activity on apoptosis. Another interesting point is the role of TAL1 in endothelial cells. Deleuze et al. and Lazrak et al. have shown that TAL1 modulates angiogenesis and that its ectopic expression enhances vascularization (11, 24). As Tax can be secreted by infected lymphocytes, it is possible that Tax locally stimulates angiogenesis by acting on TAL1. Such an effect might participate in infiltration of infected lymphocytes through blood vessels.
In conclusion our data show that TAL1 expression is stimulated by the HTLV-1 Tax transactivator and hence might be an important mediator of the T-cell transformation resulting from viral infection. Consequently, inhibition of the TAL1 activity might be interesting from a therapeutic perspective for many T-ALL cases but also for ATL.
Supplementary Material
Acknowledgments
We are grateful to A. Rabson and C. Gallego for gifts of plasmids and to D. Mathieu for generously providing us with the TAL1 cDNA along with a monoclonal antibody directed against this protein. We also thank A. Roisin for assistance with cell culture.
This work was supported by the Comité du Rhône de la Ligue Nationale contre le Cancer (fellowship to J.-M.T.) and by the Institut National du Cancer.
Footnotes
Published ahead of print on 21 May 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aplan, P. D., D. P. Lombardi, G. H. Reaman, H. N. Sather, G. D. Hammond, and I. R. Kirsch. 1992. Involvement of the putative hematopoietic transcription factor SCL in T-cell acute lymphoblastic leukemia. Blood 791327-1333. [PubMed] [Google Scholar]
- 2.Bantignies, F., R. Rousset, C. Desbois, and P. Jalinot. 1996. Genetic characterization of transactivation of the human T-cell leukemia virus type 1 promoter: binding of Tax to Tax-responsive element 1 is mediated by the cyclic AMP-responsive members of the CREB/ATF family of transcription factors. Mol. Cell. Biol. 162174-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bash, R. O., W. M. Crist, J. J. Shuster, M. P. Link, M. Amylon, J. Pullen, A. J. Carroll, G. R. Buchanan, R. G. Smith, and R. Baer. 1993. Clinical features and outcome of T-cell acute lymphoblastic leukemia in childhood with respect to alterations at the TAL1 locus: a Pediatric Oncology Group study. Blood 812110-2117. [PubMed] [Google Scholar]
- 4.Bex, F., K. Murphy, R. Wattiez, A. Burny, and R. B. Gaynor. 1999. Phosphorylation of the human T-cell leukemia virus type 1 transactivator Tax on adjacent serine residues is critical for tax activation. J. Virol. 73738-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bex, F., M. J. Yin, A. Burny, and R. B. Gaynor. 1998. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol. Cell. Biol. 182392-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296550-553. [DOI] [PubMed] [Google Scholar]
- 7.Calomme, C., A. Dekoninck, S. Nizet, E. Adam, T. L. Nguyen, A. Van Den Broeke, L. Willems, R. Kettmann, A. Burny, and C. Van Lint. 2004. Overlapping CRE and E box motifs in the enhancer sequences of the bovine leukemia virus 5′ long terminal repeat are critical for basal and acetylation-dependent transcriptional activity of the viral promoter: implications for viral latency. J. Virol. 7813848-13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caron, C., G. Mengus, V. Dubrowskaya, A. Roisin, I. Davidson, and P. Jalinot. 1997. Human TAF(II)28 interacts with the human T cell leukemia virus type I Tax transactivator and promotes its transcriptional activity. Proc. Natl. Acad. Sci. USA 943662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caron, C., R. Rousset, C. Beraud, V. Moncollin, J. M. Egly, and P. Jalinot. 1993. Functional and biochemical interaction of the HTLV-I Tax1 transactivator with TBP. EMBO J. 124269-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, P. Y., K. Draheim, M. A. Kelliher, and S. Miyamoto. 2006. NFKB1 is a direct target of the TAL1 oncoprotein in human T leukemia cells. Cancer Res. 666008-6013. [DOI] [PubMed] [Google Scholar]
- 11.Deleuze, V., E. Chalhoub, R. El-Hajj, C. Dohet, M. Le Clech, P. O. Couraud, P. Huber, and D. Mathieu. 2007. TAL-1/SCL and its partners E47 and LMO2 up-regulate VE-cadherin expression in endothelial cells. Mol. Cell. Biol. 272687-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desbois, C., R. Rousset, F. Bantignies, and P. Jalinot. 1996. Exclusion of Int-6 from PML nuclear bodies by binding to the HTLV-I Tax oncoprotein. Science 273951-953. [DOI] [PubMed] [Google Scholar]
- 13.Ferrando, A. A., D. S. Neuberg, J. Staunton, M. L. Loh, C. Huard, S. C. Raimondi, F. G. Behm, C. H. Pui, J. R. Downing, D. G. Gilliland, E. S. Lander, T. R. Golub, and A. T. Look. 2002. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 175-87. [DOI] [PubMed] [Google Scholar]
- 14.Gessain, A., H. Francis, T. Sonan, C. Giordano, F. Akani, M. Piquemal, C. Caudie, G. Malone, M. Essex, and G. de The. 1986. HTLV-I and tropical spastic paraparesis in Africa. Lancet ii698. [DOI] [PubMed] [Google Scholar]
- 15.Grassmann, R., M. Aboud, and K. T. Jeang. 2005. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 245976-5985. [DOI] [PubMed] [Google Scholar]
- 16.Haller, K., Y. Wu, E. Derow, I. Schmitt, K. T. Jeang, and R. Grassmann. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 223327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, S., and S. J. Brandt. 2000. mSin3A regulates murine erythroleukemia cell differentiation through association with the TAL1 (or SCL) transcription factor. Mol. Cell. Biol. 202248-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, S., Y. Qiu, R. W. Stein, and S. J. Brandt. 1999. p300 functions as a transcriptional coactivator for the TAL1/SCL oncoprotein. Oncogene 184958-4967. [DOI] [PubMed] [Google Scholar]
- 19.Jeang, K. T., S. G. Widen, O. J. t. Semmes, and S. H. Wilson. 1990. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 2471082-1084. [DOI] [PubMed] [Google Scholar]
- 20.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 9381-91. [DOI] [PubMed] [Google Scholar]
- 21.Kashanchi, F., and J. N. Brady. 2005. Transcriptional and post-transcriptional gene regulation of HTLV-1. Oncogene 245938-5951. [DOI] [PubMed] [Google Scholar]
- 22.Kehn, K., L. Fuente Cde, K. Strouss, R. Berro, H. Jiang, J. Brady, R. Mahieux, A. Pumfery, M. E. Bottazzi, and F. Kashanchi. 2005. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene 24525-540. [DOI] [PubMed] [Google Scholar]
- 23.Lahlil, R., E. Lecuyer, S. Herblot, and T. Hoang. 2004. SCL assembles a multifactorial complex that determines glycophorin A expression. Mol. Cell. Biol. 241439-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazrak, M., V. Deleuze, D. Noel, D. Haouzi, E. Chalhoub, C. Dohet, I. Robbins, and D. Mathieu. 2004. The bHLH TAL-1/SCL regulates endothelial cell migration and morphogenesis. J. Cell Sci. 1171161-1171. [DOI] [PubMed] [Google Scholar]
- 25.Le Clech, M., E. Chalhoub, C. Dohet, V. Roure, S. Fichelson, F. Moreau-Gachelin, and D. Mathieu. 2006. PU.1/Spi-1 binds to the human TAL-1 silencer to mediate its activity. J. Mol. Biol. 3559-19. [DOI] [PubMed] [Google Scholar]
- 26.Lecuyer, E., and T. Hoang. 2004. SCL: from the origin of hematopoiesis to stem cells and leukemia. Exp. Hematol. 3211-24. [DOI] [PubMed] [Google Scholar]
- 27.Lin, H. C., M. Hickey, L. Hsu, D. Medina, and A. B. Rabson. 2005. Activation of human T cell leukemia virus type 1 LTR promoter and cellular promoter elements by T cell receptor signaling and HTLV-1 Tax expression. Virology 3391-11. [DOI] [PubMed] [Google Scholar]
- 28.Liu, B., M. H. Liang, Y. L. Kuo, W. Liao, I. Boros, T. Kleinberger, J. Blancato, and C. Z. Giam. 2003. Human T-lymphotropic virus type 1 oncoprotein Tax promotes unscheduled degradation of Pds1p/securin and Clb2p/cyclin B1 and causes chromosomal instability. Mol. Cell. Biol. 235269-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Y., M. Encinas, J. X. Comella, M. Aldea, and C. Gallego. 2004. Basic helix-loop-helix proteins bind to TrkB and p21(Cip1) promoters linking differentiation and cell cycle arrest in neuroblastoma cells. Mol. Cell. Biol. 242662-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macintyre, E. A., L. Smit, J. Ritz, I. R. Kirsch, and J. L. Strominger. 1992. Disruption of the SCL locus in T-lymphoid malignancies correlates with commitment to the T-cell receptor alpha beta lineage. Blood 801511-1520. [PubMed] [Google Scholar]
- 31.Marriott, S. J., and O. J. Semmes. 2005. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 245986-5995. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka, M., and K. T. Jeang. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7270-280. [DOI] [PubMed] [Google Scholar]
- 33.Miyazato, A., S. Sheleg, H. Iha, Y. Li, and K. T. Jeang. 2005. Evidence for NF-κB- and CBP-independent repression of p53's transcriptional activity by human T-cell leukemia virus type 1 Tax in mouse embryo and primary human fibroblasts. J. Virol. 799346-9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuveut, C., K. G. Low, F. Maldarelli, I. Schmitt, F. Majone, R. Grassmann, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 183620-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Neil, J., J. Shank, N. Cusson, C. Murre, and M. Kelliher. 2004. TAL1/SCL induces leukemia by inhibiting the transcriptional activity of E47/HEB. Cancer Cell 5587-596. [DOI] [PubMed] [Google Scholar]
- 36.Osada, H., G. Grutz, H. Axelson, A. Forster, and T. H. Rabbitts. 1995. Association of erythroid transcription factors: complexes involving the LIM protein RBTN2 and the zinc-finger protein GATA1. Proc. Natl. Acad. Sci. USA 929585-9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palomero, T., D. T. Odom, J. O'Neil, A. A. Ferrando, A. Margolin, D. S. Neuberg, S. S. Winter, R. S. Larson, W. Li, X. S. Liu, R. A. Young, and A. T. Look. 2006. Transcriptional regulatory networks downstream of TAL1/SCL in T-cell acute lymphoblastic leukemia. Blood 108986-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 777415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulford, K., N. Lecointe, K. Leroy-Viard, M. Jones, D. Mathieu-Mahul, and D. Y. Mason. 1995. Expression of TAL-1 proteins in human tissues. Blood 85675-684. [PubMed] [Google Scholar]
- 40.Riou, P., F. Bex, and L. Gazzolo. 2000. The human T cell leukemia/lymphotropic virus type 1 Tax protein represses MyoD-dependent transcription by inhibiting MyoD-binding to the KIX domain of p300. A potential mechanism for Tax-mediated repression of the transcriptional activity of basic helix-loop-helix factors. J. Biol. Chem. 27510551-10560. [DOI] [PubMed] [Google Scholar]
- 41.Seiki, M., S. Hattori, Y. Hirayama, and M. Yoshida. 1983. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA 803618-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 41875-1885. [DOI] [PubMed] [Google Scholar]
- 43.Valge-Archer, V. E., H. Osada, A. J. Warren, A. Forster, J. Li, R. Baer, and T. H. Rabbitts. 1994. The LIM protein RBTN2 and the basic helix-loop-helix protein TAL1 are present in a complex in erythroid cells. Proc. Natl. Acad. Sci. USA 918617-8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veschambre, P., P. Simard, and P. Jalinot. 1995. Evidence for functional interaction between the HIV-1 Tat transactivator and the TATA box binding protein in vivo. J. Mol. Biol. 250169-180. [DOI] [PubMed] [Google Scholar]
- 45.Wencker, M., C. Sausse, D. Derse, L. Gazzolo, and M. Duc Dodon. 2007. Human T-cell leukemia virus type 1 Tax protein down-regulates pre-T-cell receptor alpha gene transcription in human immature thymocytes. J. Virol. 81301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 792031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, W., J. W. Nisbet, B. Albrecht, W. Ding, F. Kashanchi, J. T. Bartoe, and M. D. Lairmore. 2001. Human T-lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J. Virol. 759885-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.