Abstract
Human immunodeficiency virus type 1 (HIV-1)-infected cells transmit viral products to uninfected CD4+ cells very rapidly. However, the natures of the transmitted viral products and the mechanism of transmission, as well as the relative virological consequences, have not yet been fully clarified. We studied the virological events occurring a few hours after contact between HIV-1-infected and uninfected CD4+ cells using a coculture cell system in which the virus expression in target cells could be monitored through the induction of a green fluorescent protein reporter gene driven by HIV-1 long terminal repeats. Within 16 h of coculture, we observed two phenomena not related to the cell-free virus infection, i.e., the formation of donor-target cell fusions and a fusion-independent internalization of viral particles likely occurring at least in part through intercellular connections. Both events depended on the expression of Env and CD4 in donor and target cells, respectively, whereas the HIV-1 internalization required clathrin activity in target cells. Importantly, both phenomena were also observed in cocultures of primary CD4+ lymphocytes, while primary macrophages supported only HIV-1 endocytosis. By investigating the virological consequences of these events, we noticed that while fused cells released infectious HIV-1 particles, albeit with reduced efficiency compared with donor cells, no virus expression was detectable upon HIV-1 endocytosis in target cells. In sum, the HIV-1 transmission following contact between an HIV-1-infected and an uninfected CD4+ cell can occur through different mechanisms, leading to distinguishable virological outcomes.
Human immunodeficiency virus type 1 (HIV-1) propagates through both cell-free virus and cell-cell intercellular connections. The mechanisms underlying the entry of cell-free HIV-1 have been thoroughly investigated (for a recent review, see reference 24). In addition to the typical pH-independent cell entry mediated by the interaction between HIV Env receptors and CD4 and CXCR4 or CCR5 cell membrane receptors, HIV-1 can enter target cells through either CD4-independent (34) or -dependent (35) endocytosis. In both lymphocytes and macrophages, endocytic HIV-1 entry leads to poor viral replication due to the degradation of viral particles in the low-pH endosome/lysosome intracellular compartments, as proven by the increase in the endocytosis-mediated HIV-1 infection efficiency induced by drugs that raise the endosomal pH (14). Alternatively, HIV-1 can enter dendritic cells (DCs) upon Env/DC-SIGN interaction, thereby accumulating in intracellular vacuolar structures before transmission to lymphocytes (40). HIV-1 virions bound to the DC cell membrane also contribute to this process, known as trans-infection (5).
On the other hand, when an infected cell contacts a susceptible target cell, the virus can be transmitted through intercellular connections protected from the extracellular milieu. Within retroviruses, this has been described for both human T-lymphotropic virus type 1 (19) and HIV-1 (4, 8, 10, 20, 29, 33). The cell-to-cell virus transmission relies on the formation of virological synapses, i.e., zones of tight cell-cell contact stabilized by the interaction among the receptors of juxtaposed cells, including, in the case of HIV-1, Env, CD4, inflammatory cell adhesion molecule 1, and lymphocyte function-associated antigen 1. This process leads to the clustering of HIV-1 coreceptors in target cells and accumulation of viral products in the zones of cell-cell contact. The formation of virological synapses has been described in both the trans-infection process (25) and cell-to-cell HIV-1 transmission between lymphocytes (21).
Cell-to-cell virus transmission, as expected, plays a relevant role in HIV primary infection (17), as well as in the spread of virus in regions like lymph nodes, where cells are densely packed. However, this mechanism has been proposed to be the predominant mode of HIV-1 transmission within lymphocytes. In this case, it was reported that the efficiency of cell-to-cell virus spread overcomes that of the cell-free viral particles by several orders of magnitude (6). However, several aspects of the events occurring early after the contact between HIV-1-infected and uninfected CD4+ target cells deserve further investigation. In this regard, cell-free virus infection, authentic cell-to-cell virus transmission, cell fusion, virion endocytosis, and internalization of shed viral proteins could each potentially contribute to the transmission of HIV-1 products to target cells. Thus, it would be of interest to evaluate the relative involvement of these events in the effects observed in target cells. Also, it is still unclear whether and through which mechanisms the viral products transmitted to target cells can induce active virus replication. Using lymphocytic target cells expressing either fluorescent or selectable marker genes regulated by HIV-1 Tat transactivation, we found that the contact between HIV-1-infected and CD4+ cells basically leads to donor-to-target cell fusion coupling with viral release dictated by the donor cells, and to a CD4-dependent, fusion-independent HIV-1 internalization partly occurring through cell-to-cell connections but unable to induce detectable virus expression.
MATERIALS AND METHODS
Cell lines, cell purification, and cocultivations.
U937HIV-1, U937purorHIV-1, and H9HIV-1 are cell lines derived from the U937, U937puror (27), and H9 cell lines, respectively, upon chronic infection with the human T-lymphotropic virus (IIIB) HIV-1 isolate. These cells, as well as the CEMGFP (15), 8E5 (12), CEMHN (26), and C8166 cell lines, were grown in RPMI medium supplemented with 10% decomplemented fetal calf serum (dFCS). Human embryonic kidney epithelial 293T cells were grown in Dulbecco's modified Eagle's medium plus 10% dFCS. Human primary CD4 lymphocytes were negatively selected from peripheral blood mononuclear cells (PBMCs) using the appropriate immunomagnetism-based selection kit from Miltenyi Biotec (Auburn, CA), activated with 5 μg/ml of phytohemagglutinin, and cultivated in RPMI containing 20% dFCS and 100 units/ml of recombinant interleukin 2. Human primary monocytes were isolated by an immunomagnetism-based procedure as previously described (11). Briefly, PBMCs were recovered from the buffy coat obtained from 20- to 40-year-old healthy male donors. Monocytes were isolated by 1 h of adherence of PBMCs, followed by immunodepletion carried out using an immunomagnetic monocyte selection kit (Miltenyi Biotec). The purity of recovered cell populations was assayed by fluorescence-activated cell sorter (FACS) analysis by means of phycoerythrin (PE)- conjugated anti-CD14 monoclonal antibody (MAb) (Becton Dickinson, Mountain View, CA), and cell preparations staining below 95% positive for CD14 were discarded. Monocytes were cultured in 48-well plates in RPMI 1640 supplemented with 20% dFCS.
Cocultivations were typically set up in 0.5 ml of RPMI-10% dFCS in 48-well plates by seeding 4 × 105 donor cells with 2 × 105 target cells. Infected CEMHN cells were separated from HIV-1-infected donor cells upon incubation with the anti-human nerve growth factor receptor (NGFr) 20.2 MAb, followed by incubation with anti-mouse immunoglobulin G (IgG)-coupled microbeads (Miltenyi Biotec). The trans-well cocultures were carried out in six-well plates (Becton Dickinson) with Cell Culture Insert Falcon membranes (25-mm diameter; 0.4-μm pore size). Soluble CD4, anti-HIV-1 Env gp120 b12 MAbs, zidovudine (AZT), T-20, ritonavir, and AMD3001 were obtained from the NIH AIDS Research and Reference Reagent Program. Amiloride, chlorampromazine, cytochalasin D, filipin complex, and bafilomycin A1 were obtained from Sigma-Aldrich (St. Louis, MO).
HIV-1 preparations, infections, and titration.
The recovery of both Δenv NL4-3 and NL4-3/NefF12 HIV-1 molecular clones has already been described (11). The (AD8env) NL4-3 R5 HIV-1 molecular clone was a generous gift from O. Schwartz (Institut Pasteur, Paris, France). Preparations of HIV-1 strains pseudotyped with glycoprotein G of vesicular stomatitis virus (VSV-G) were obtained from the supernatants of 293T cells cotransfected by the Lipofectamine 2000 (Invitrogen, Carlsbad, CA)-based method with the respective HIV-1 molecular clone and an immediate-early cytomegalovirus promoter-regulated VSV-G-expressing vector in a 5:1 molar ratio. Supernatants recovered 48 and 72 h later were clarified and concentrated by ultracentrifugation on a 20% sucrose cushion. Virus preparations were titrated by measuring the CAp24 contents by quantitative enzyme-linked immunosorbent assay (Innogenetics, Gent, Belgium) and by the reverse transcriptase assay. The cell infections were carried out by spinoculation at 400 × g for 30 min at room temperature using 50 to 500 ng/105 cells of wild-type (wt) or VSV-G-pseudotyped HIV-1. Then, the virus adsorption was prolonged for an additional 2 h at 37°C, and finally, the cells were washed and refed with the complete medium.
The infectivities of supernatants were evaluated by scoring the syncytium number of C8166 cells 5 days after infection. For shaken cocultures, the plates were placed on a rocker and gently shaken at 40 movements per minute as previously described (37).
FACS analysis.
For the detection of intracellular HIV-1 Gag-related products, cocultures were incubated with trypsin for 15 min at 37°C and then permeabilized with Cytoperm-Cytofix reagents (Pharmingen, San Diego, CA). Afterwards, the cells were labeled for 30 min at 4°C with 1:100-diluted PE-conjugated anti-HIV-1 CAp24 KC57-RD1 MAb (Coulter Corp., Hialeah, FL). For the detection of intracellular HIV-1 Env gp120, the cells were incubated with trypsin, permeabilized, and labeled with 1:100-diluted 4G10 anti-gp120 MAb for 30 min at 4°C. Finally, the cells were incubated with PE-conjugated goat-anti mouse IgG for one additional hour at 4°C. Cell membrane CD4 receptors were detected by incubating the cells at 4°C with a 1:100 dilution of a PE-conjugated anti-CD4 MAb from EuroBioSciences (Friesoythe, Germany) or, as a control, with isotype-matched PE-conjugated IgG. The expression of cell membrane ΔNGFr was revealed through indirect FACS labeling using the anti-human NGFr 20.4 MAb.
HIV-1 35S metabolic labeling and radioimmunoprecipitation assay.
CEMHN cells were cocultivated with U937purorHIV-1 cells for 16 h, separated as described above, and labeled for 6 h with 1.85 MBq of both [35S]methionine and [35S]cysteine in methionine/cysteine-free medium in the presence of 10% dialyzed dFCS and 2.5 μg/ml of puromycin. The supernatants were then clarified and ultracentrifuged on a 20% sucrose cushion. Finally, the viral pellets were lysed in 10 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1 mM EDTA (TNE)-0.1% Triton X-100, and equal volumes of the lysates were immunoprecipitated with a pool of strongly reactive anti-HIV-1 antibodies. Immunoprecipitations were carried out by incubating protein A-G agarose beads (Pierce, Rockford, IL) for 2 h at room temperature with either anti-HIV-1 or control sera. Afterwards, the labeled virions were added in the presence of antiproteolytic agents. After an overnight incubation, immunocomplexed proteins were resolved in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and finally revealed by autoradiography.
RESULTS
Different mechanisms of HIV-1 transfer upon cell-cell contact.
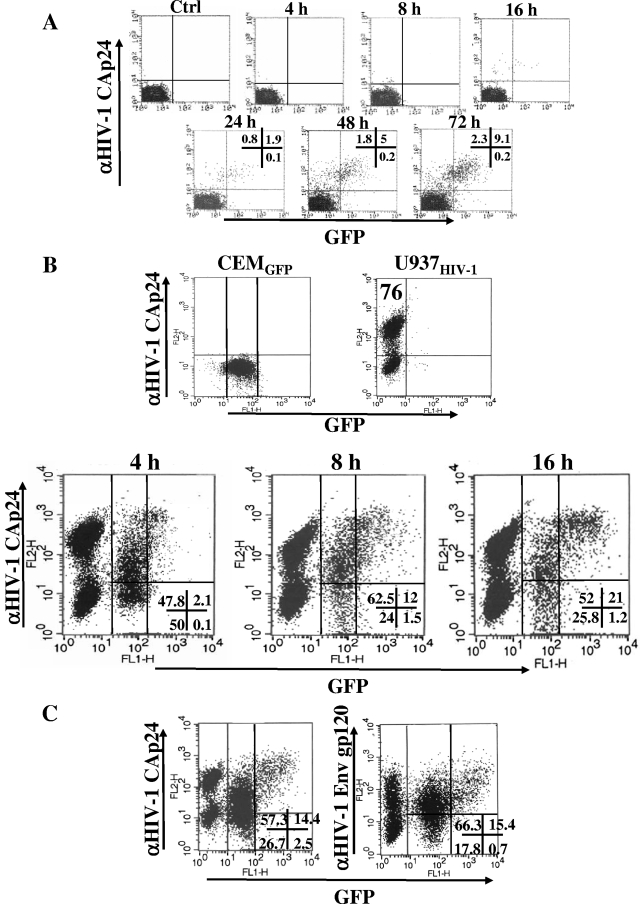
The HIV-1 transmission in target cells following contact with HIV-1-infected cells has been documented by antibody-mediated detection of viral products (37), direct visualization of fluorescent HIV-1 Gag products (6), and electron microscope analysis (20, 29). However, some aspects of the mechanisms of virus transfer deserve further clarification. Moreover, the intrinsic infectivity of the viral material transferred to target cells remains undetermined. We sought to shed light on these points by setting up a coculture assay in which both the transfer efficiencies of the HIV-1 products and viral expression in target cells could be simultaneously monitored. This was carried out using CEMGFP cells (15) as target cells, i.e., a CD4+ human cell line expressing the green fluorescent protein (gfp) gene under the control of the HIV-1 long terminal repeat (LTR) promoter. Meanwhile, the presence of HIV-1 Gag-related products was detected through intracellular FACs analysis. We preventively monitored these cells in terms of the timing of detection of both GFP and HIV-1 Gag-related products by FACS. This was done by infecting the cells with 200 ng/105 cells of cell-free HIV-1. The cells were then cultivated in the presence of 1 μM of ritonavir to guarantee a single-cycle infection, harvested at different times, treated with trypsin to remove virion nonspecifically bound to the cell membrane, and FACS analyzed for the expression of both GFP- and HIV-1 Gag-related products (Fig. 1A). Very few positive cells were detectable until 16 h postinfection, whereas an easily distinguishable double-positive subpopulation appeared starting at the 48-h time point. Of note, no significant percentages of Gag−/GFP+ cells were found at any time point, indicating that the GFP induction was not sensitive to soluble cellular or viral factors released in the supernatants.
FIG. 1.
Analysis of the expression of both GFP and HIV-1 products in CEMGFP cells upon HIV-1 infection or cocultivation with U937HIV-1 cells. (A) FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP cells at different times after infection with the NL4-3 HIV-1 strain. The percentages of cells positive for either fluorescence are indicated at the late time points. (B) FACS analyses for the detection of both GFP and HIV-1 Gag products in U937HIV-1/CEMGFP cocultures carried out for 4, 8, and 16 h. In the top row, the analyses of the single-cell populations are shown, together with the percentages of HIV-1 Gag-positive U937HIV-1 cells. In the bottom row, the coculture analyses, including the percentages of events scored in the CEMGFP-related quadrants, are shown. (C) FACS analyses for the presence of GFP and HIV-1 Gag (left) or Env gp120 (right) in CEMGFP cells cocultured for 16 h with U937HIV-1 cells. The percentages of events scored in the CEMGFP-related quadrants are reported. The results are representative of two (A), four (B), and two (C) independent experiments.
Next, we cocultivated HIV-1-infected donor cells (i.e., U937HIV-1) with CEMGFP cells in a 2:1 donor/target cell ratio, and the cocultures were FACs analyzed 4, 8, and 16 h later after treatment with trypsin. Within the resulting HIV-1 Gag-positive CEMGFP cell population, we distinguished a Gag+/GFP− and a Gagbright/GFP+ subpopulation, with the former the most represented and appearing most rapidly (Fig. 1B). Similar results were obtained by monitoring the transfer of HIV-1 Env gp120 (Fig. 1C), as well as using H9HIV-1 cells as donor cells (data not shown).
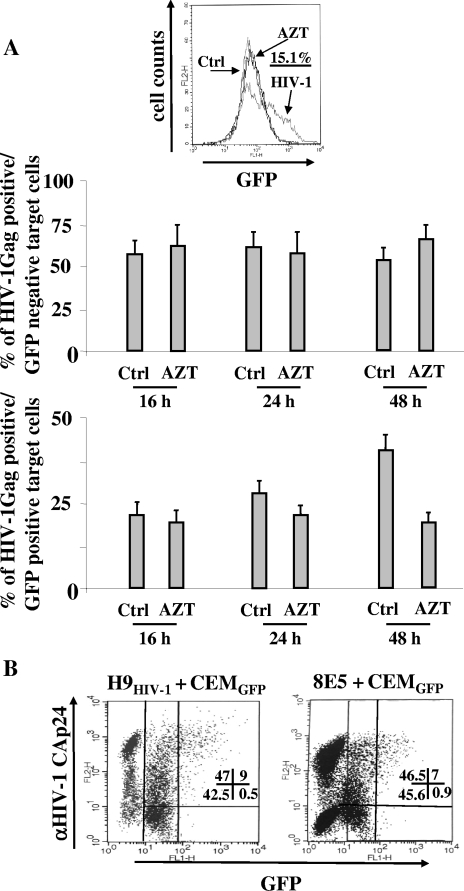
To interpret the biologic significance of these results, we first evaluated the contribution of de novo viral synthesis to the appearance of HIV-1 products in target cells. This was done by repeating the cocultivation experiment, but in the presence of the reverse transcriptase inhibitor AZT. To this end, CEMGFP target cells were pretreated for 6 h with 10 μM of AZT, and the cocultures were then set up in the presence of the AZT concentration that efficiently blocked CEMGFP cell infection with a high dose of cell-free HIV-1 (Fig. 2A). The cocultures were sampled starting at the 16-h time point and FACS analyzed for both GFP and HIV-1 Gag contents. We noticed that AZT did not significantly affect the formation of the two distinct HIV-1 Gag-positive CEMGFP subpopulations until the 24-h time point (Fig. 2A), strongly indicating that de novo viral synthesis was not significantly involved in the early events we detected. Conversely, the AZT treatment correlated with a significant reduction in the percentages of GFP+ target cells 48 h after the coculture setup (Fig. 2A), most likely as a consequence of the inhibition of the de novo infection of target cells. Similar results were obtained by monitoring the transfer of HIV-1 Env gp120 (data not shown).
FIG. 2.
Analysis of both HIV-1 Gag and GFP accumulation in CEMGFP cells cocultivated for 16 to 48 h with HIV-1-infected cells in the presence of reverse transcriptase inhibition. (A) FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP cells cocultured with U937HIV-1 cells in the presence of 10 μM AZT. At the top, the antiviral efficiency of AZT was tested by FACS analysis of GFP+ expression within the CEMGFP cell population infected 2 days before with 500 ng of CAp24/105 cells of the NL4-3 HIV-1 strain. The percentage of infected cells in the absence of AZT is indicated. Ctrl, noninfected cells. Below, the mean values plus standard deviations of the percentages of both Gag+/GFP− and Gag+/GFP+ CEMGFP cells as calculated from the data for four independent experiments are shown. (B) FACS analysis for the detection of both GFP and HIV-1 Gag products in CEMGFP cells after 16 h of cocultivation with either H9HIV-1 or 8E5 cells. The percentages of events scored in the CEMGFP-related quadrants are reported. The results are representative of two independent experiments.
The lack of AZT effects we observed 16 h after the coculture setup appeared consistent with what we observed upon infection with cell-free virus and implied that both the GFP induction and the HIV-1 Gag products detected in target cells relied on the HIV-1 expression in donor cells. Importantly, we exclude the possibility that gfp was transactivated through a paracrine mechanism, since (i) no significant percentages of Gag−/GFP+ CEMGFP cells were detectable in FACS analyses (Fig. 1 and 2) and (ii) no induction of GFP was observed by setting the cocultures in trans-well plates (data not shown).
Since the possible contribution of de novo-synthesized viral products was a critical point, we also addressed it using a human lymphoblastoid cell line chronically infected with a reverse transcriptase-defective HIV-1 variant (8E5 cells) as donor cells. The fact that by FACs analysis we noticed a fluorescence pattern similar to that detected in cocultures with donor cells infected with wt HIV-1 (Fig. 2B) reinforced the idea that de novo viral expression in target cells did not play a major role in the phenomena we observed after 16 h of coculture.
We conclude that the contact between CD4+ and HIV-1-infected cells results in at least two apparently different modalities of virus transmission, both independent of de novo viral synthesis in target cells.
Cell fusion partly accounts for the passage of HIV-1 products in target cells.
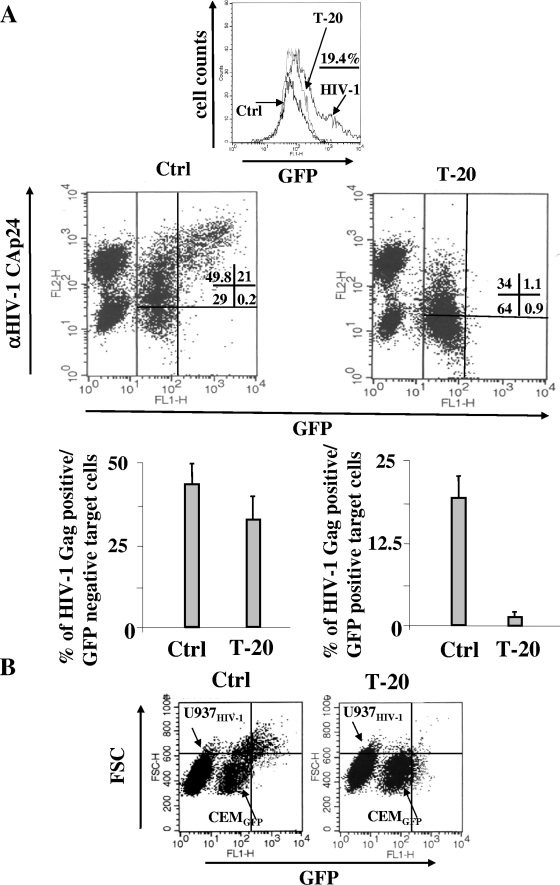
To characterize the mechanisms of the observed HIV-1 transmission, we first tried to establish the contribution of cell fusion. To this end, we reproduced the coculture experiments, but in the presence of both AZT and T-20; the latter is a potent inhibitor of the fusion mediated by the HIV-1 Env receptors (22). It is especially interesting that we noticed that the block in cell fusion correlated with the disappearance of the Gagbright/GFP+ subpopulation (Fig. 3A) while only a few Gag+/GFP− cells disappeared, most likely including fused cells that accumulated insufficient amounts of GFP to be detected by FACs. The evidence that the GFP induction in target cells was the consequence of cell fusion was also confirmed by the forward-scatter FACs analysis of cocultures, where the GFP+ target cells appeared significantly enlarged compared with the GFP-negative ones (Fig. 3B). Notably, the presence of an enlarged but GFP-negative cell subpopulation that disappeared upon T-20 treatment further supports the idea that, within the 16-h time frame considered, some of the fused cells did not accumulate amounts of GFP sufficient to be detected by FACS.
FIG. 3.
The inhibition of cell fusion leads to the disappearance of GFP+ cells within the CEMGFP target cell population. (A) FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP cells cocultured for 16 h with U937HIV-1 cells in the presence of 1 μg/ml T-20. At the top, the antiviral efficiency of T-20 was tested by FACS analysis of the GFP+ expression within the CEMGFP population infected 2 days before with 500 ng of CAp24/105 cells of the NL4-3 HIV-1 strain. The percentage of infected cells in the absence of T-20 is indicated. Ctrl, noninfected cells. In the middle row, the results from a representative coculture experiment are shown. The percentages of events scored in the CEMGFP-related quadrants are indicated. In the bottom row, the mean values plus standard deviations of the percentages of both Gag+/GFP− and Gag+/GFP+ CEMGFP cells as calculated from the data from seven independent experiments are shown. (B) Forward-scatter (FSC) and GFP FACS analysis of CEMGFP/U937HIV-1 cocultures carried out for 16 h. The single-cell populations are indicated. The quadrants are set on the basis of uninfected CEMGFP cells. The results are representative of at least 11 independent experiments.
These data highlight cell fusion as a mechanism of transfer of HIV-1 products upon cell-cell contact.
Fusion-independent, Env-CD4-dependent HIV-1 cell-to-cell transmission.
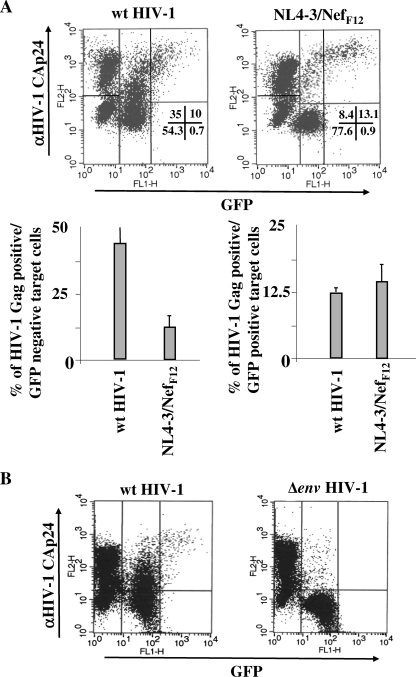
We were next interested in characterizing the mechanism underlying the fusion-independent onset of the HIV-1 Gag+/GFP− subpopulation. For this, we first sought to establish whether the detection of HIV-1 Gag products reflected the internalization of viral particles or, alternatively, of viral products shed from donor cells. To this end, we exploited the unique property of the NL4-3-NefF12 HIV-1 strain, which expresses levels of viral proteins similar to those of the wt counterpart but in the absence of viral-particle release (28). Importantly, the amounts of shed viral proteins were also similar to those measured in the virus-free supernatants of cells infected with wt HIV-1 (not shown). Thus, U937 cells previously infected with (VSV-G) wt HIV-1 or with the (VSV-G) NL4-3-NefF12 HIV-1 variant were cocultivated with CEMGFP cells in the presence of AZT and FACS analyzed 16 h later. The strong decrease in the Gag+/GFP− cells in NL4-3-NefF12 compared with wt HIV-1 cocultures (Fig. 4A) clearly indicated that the formation of this CEMGFP subpopulation primarily relied on the release of intact viral particles from donor cells.
FIG. 4.
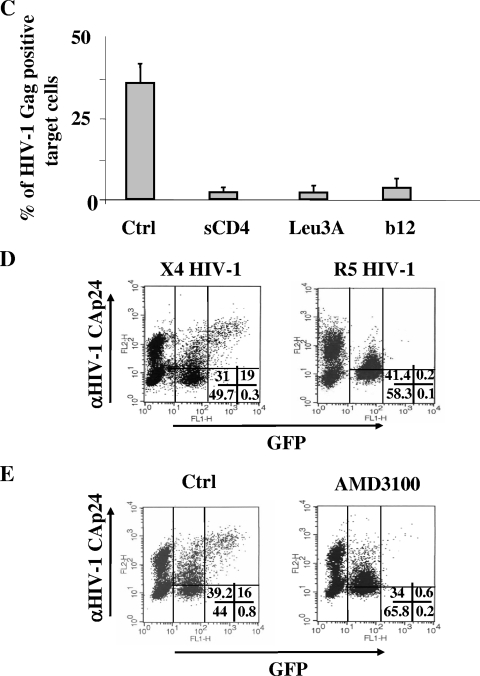
Consequences of the release of viral particles from donor cells and of the Env/CD4 interaction in the transfer of HIV-1 products to CEMGFP target cells. (A) The release of viral particles from donor cells is largely correlated with the appearance of a Gag+/GFP− subpopulation in CEMGFP target cells. Shown are FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP cells cocultured for 16 h with U937 cells acutely infected 2 days before with 100 ng of CAp24/105 cells of (VSV-G) NL4-3 or (VSV-G) NL4-3/NefF12 HIV-1 strains. At the top, data from a representative experiment are shown. The percentages of events scored in the CEMGFP-related quadrants are indicated. The percentages of HIV-1 Gag-positive cells were 81% and 82.5% for U937 cells infected with wt or NL4-3/NefF12 HIV-1 strains, respectively. At the bottom, the mean values plus standard deviations of the percentages of both Gag+/GFP− and Gag+/GFP+ CEMGFP cells as calculated from the data from four independent experiments are shown. (B) The Env/CD4 interaction is required for the internalization of HIV-1 products. Shown are FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP cells cocultured for 16 h with U937 cells acutely infected 2 days before with 100 ng of CAp24/105 cells of (VSV-G) NL4-3 or (VSV-G) Δenv NL4-3 HIV-1 strains. The percentages of HIV-1 Gag-positive cells were 74% and 89% for U937 cells infected with the wt or Δenv NL4-3 HIV-1 strain, respectively. The quadrants are set on the basis of uninfected CEMGFP cells. The data are representative of three independent experiments. (C) Mean values plus standard deviations of the percentages of Gag+ CEMGFP cells after coculture for 16 h with U937HIV-1 cells upon treatment with either 50 μg/ml of soluble CD4, 0.5 μg/ml of anti-CD4 Leu3A MAb, or 5 μg/ml of anti-HIV-1 Env gp120 b12 MAb, as calculated from the data from three independent experiments. (D) HIV-1 coreceptors are not involved in HIV-1 endocytosis. Shown are FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP cells cocultured for 16 h in the presence of AZT with U937 cells acutely infected 2 days before with 100 ng of CAp24/105 cells of a (VSV-G) NL4-3 or (VSV-G) AD8 NL4-3 HIV-1 strain. (E) Effects of the AMD3100 CXCR4 inhibitor. Shown are FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP cells cocultured for 16 h with U937HIV-1 cells in the presence of AZT alone (Ctrl) or together with 10 μg/ml of AMD3100. For panels D and E, data representative of two experiments are shown. The percentages of events scored in the CEMGFP-related quadrants are indicated.
On the other hand, we gained evidence that the cell-cell contact strongly improves HIV-1 endocytosis activity. In fact, no HIV-1 Gag-positive target cells were detectable by FACS upon trans-well cocultures carried out for 16 h with HIV-1-expressing donor cells, although in the target cell chamber, we detected numbers of HIV-1 particles largely comparable to those measurable in donor cell supernatants (data not shown).
HIV-1 can be internalized through either CD4-independent (34) or -dependent (35) endocytosis. To establish the role of the Env/CD4 interaction in the HIV-1 endocytosis we observed, U937 cells infected with either a (VSV-G) HIV-1 variant with the env gene deleted or with the wt counterpart were cocultivated with CEMGFP target cells in the presence of AZT and FACS analyzed after 16 h. Clearly, the absence of Env expression in donor cells led to the lack of both HIV-1 endocytosis and, as expected, cell fusion (Fig. 4B). This was not due to a defect in virus release, since Δenv HIV-1-infected cells produced numbers of viral particles comparable to those released by the wt counterpart (not shown). Thus, we conclude that the Env expression in donor cells is critically involved in HIV-1 internalization. Notably, these results also confirm that trypsin treatment adequately removes the virions nonspecifically adsorbed on the cell membranes of target cells.
Next, to confirm the critical role of the Env/CD4 interaction in the HIV-1 endocytosis detectable in target cells, we evaluated the effects of either soluble CD4 or MAbs recognizing the binding site of either HIV-1 Env gp120 (anti-CD4 Leu3A) or CD4 (anti-HIV-1 Env gp120 b12) (2). To this end, U937HIV-1 cells were pretreated for 2 h at 4°C with anti-Env reagents and, under parallel conditions, CEMGFP cells were incubated with anti-CD4 MAbs. Thereafter, cocultures were carried out for 8 h in the presence of the respective Env or CD4 ligands. Under all conditions, we noticed a strong inhibition of HIV-1 endocytosis in target cells (Fig. 4C) coupled with a block in cell fusion (not shown). The inhibitory effect gradually regressed unless the anti-Env or -CD4 reagents were readded (not shown).
Next, to establish whether the HIV-1 coreceptors in target cells are involved in HIV-1 endocytosis, CEMGFP target cells (which express the CXCR4 but not the CCR5 HIV coreceptor) were cocultivated for 16 h in the presence of AZT with donor cells previously infected with (VSV-G) HIV-1 strains expressing either X4- or R5-Env receptors. The comparable percentages of Gag+/GFP− cells we detected (Fig. 4D) strongly supported the idea that HIV-1 coreceptors are not involved in the observed HIV-1 endocytosis. This conclusion was further reinforced by the inhibition of cell fusion we observed by adding the AMD3001 CXCR4 ligand in cocultures including X4 HIV-1-infected donor cells (Fig. 4E).
Taken together, these results indicate that neither the internalization of viral proteins shed from infected cells nor the CD4-independent endocytosis of extracellular viral particles contributes to the overall HIV-1 endocytosis in target cells. This, conversely, strictly depends on the Env-CD4 interaction that likely primes both the cell-cell contact and the CD4-dependent endocytosis of HIV-1 particles.
HIV-1 endocytosis in cocultures depends on clathrin activity in target cells.
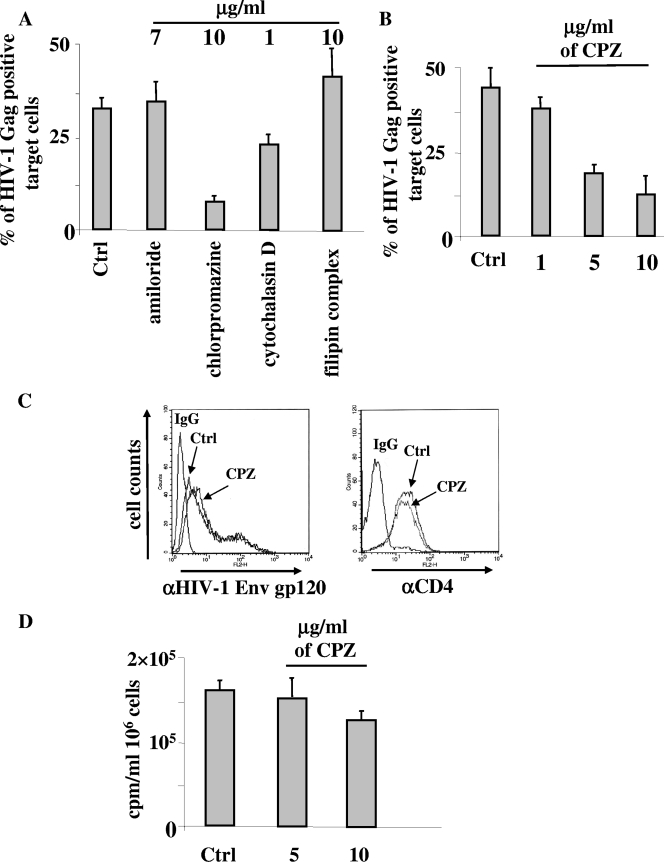
Next, to identify the mechanism involved in the CD4-dependent endocytosis in target cells, cocultures were treated for 16 h with T-20, together with inhibitors targeting different mechanisms of endocytosis. In particular, either amiloride, chlorpromazine (CPZ), cytochalasin D, or filipin complex was used to inhibit macropinocytosis, receptor-mediated endocytosis, phagocytosis, or caveola-mediated endocytosis, respectively. We used the highest nontoxic concentration of each drug, whose functionality was tested in advance by monitoring the inhibition of the uptake of either fluorescein isothiocyanate-bovine serum albumin or fluorescein isothiocyanate-dextran (not shown). We noticed that the HIV-1 endocytosis barely decreased upon cytochalasin D treatment, while, conversely, it was efficiently inhibited by CPZ (Fig. 5A) up to a concentration of 5 μg/ml (Fig. 5B). The effect seemed specific, since CPZ did not affect the expression of Env gp120 and CD4 on the cell membranes of donor and target cells, respectively (Fig. 5C), and, similarly, did not influence HIV-1 production from donor cells (Fig. 5D).
FIG. 5.
CPZ inhibits HIV-1 endocytosis in CEMGFP target cells. (A) Effects of a panel of endocytosis inhibitors on the internalization of HIV-1 particles in CEMGFP cells cocultivated for 16 h with U937HIV-1 cells. CEMGFP target cells were pretreated for 2 h with the indicated inhibitors, which were thereafter maintained in the cocultures in the presence of 1 μg/ml of T-20. The concentrations of the inhibitors used are indicated. Finally, the percentages of Gag+ CEMGFP target cells were scored by FACS. The mean values from three independent experiments plus standard deviations are reported. (B) Dose-response effects of CPZ on HIV-1 endocytosis. Cocultures carried out as described for panel A were treated with different CPZ concentrations. The results are given as mean values plus standard deviations of the percentages of Gag+ CEMGFP target cells from three independent experiments. (C) Anti-Env gp120 (left) and anti-CD4 (right) FACS analyses of U937HIV-1 and CEMGFP cells, respectively, treated with 10 μg/ml of CPZ for 16 h or left untreated (Ctrl). The histograms of cells labeled with the IgG isotype controls are also reported (IgG). (D) Reverse transcriptase activities in the supernatants of U937HIV-1 cells treated for 16 h with different doses of CPZ or left untreated (Ctrl). Cells (106) were seeded in 1 ml in the presence or absence of the inhibitor, and after 16 h, the supernatants were collected, ultracentrifuged at 150,000 × g for 15 min, lysed in 100 μl of TNE-Triton X-100 0.1% buffer, and assayed for reverse transcriptase activity. The viability of all cell cultures at the time of supernatant collection was >90%. The results are given as mean values plus standard deviations for 106 cells calculated from three independent experiments.
CPZ is a well-known inhibitor of clathrin-mediated endocytosis and acts by relocating clathrin molecules and the adaptor protein AP-2 from the cell surface (41). Hence, our data suggest that cell-cell contact efficiently induces strong CD4-dependent, clathrin-mediated HIV-1 endocytosis.
Extracellular versus cell-to-cell HIV-1 internalization in cocultures.
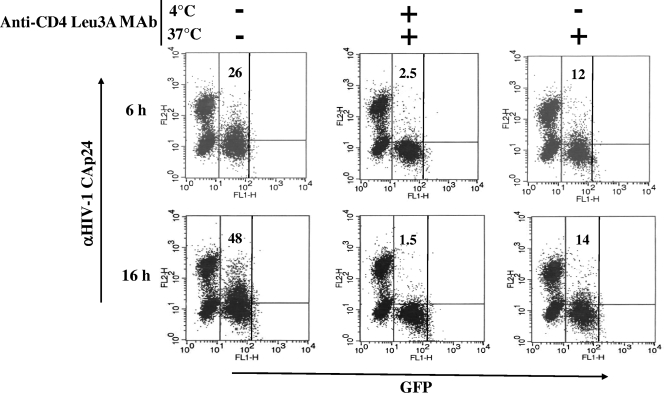
Here, we provide evidence that cell-cell contact induces a massive transfer of virions to target cells by endocytosis. However, HIV-1 would be transferred either through a bona fide cell-to-cell mechanism, i.e., without exposure to the extracellular milieu, or by the endocytosis of extracellular virions whose efficiency can be increased by donor-target cell contiguity. To evaluate the relative contribution of each mechanism, we set up an assay in which cell-to-cell HIV-1 endocytosis was monitored under conditions in which the cell-cell contact occurred in the presence of a block in both cell fusion and HIV-1 endocytosis. This was achieved by pelleting CEMGFP cells, together with U937HIV-1, in the same well and incubating the coculture at 4°C for 3 h to allow cell-cell contact, and in the presence of T-20 to block escaping cell fusion. Afterwards, at the time of the shift to 37°C, the cocultures were treated with anti-CD4 Leu3A MAbs to block both further CD4-mediated cell-cell adhesion and the endocytosis of extracellular particles. Under these conditions, the resulting HIV-1 endocytosis activity was expected to be the exclusive consequence of the cell-cell contact that occurred during the 3-h incubation at 4°C. As a control, CEMGFP cells were also pretreated with anti-CD4 Leu3A MAbs or left untreated over time. The FACS analyses carried out 6 and 16 h later (Fig. 6) showed an HIV-1 endocytosis activity that, even if clearly reduced compared with control cocultures, appeared significantly higher than in the cocultures where the cell-cell contact was totally hindered by treatment with anti-CD4 MAbs over time. Importantly, the HIV-1 Gag-specific signal we detected was not the consequence of escaping HIV-1 endocytosis that occurred during the 3 h of incubation at 4°C, which was in fact undetectable under any conditions (not shown). In addition, it should be noted that anti-CD4 MAb treatment at 37°C basically blocked an increase in the Gag+ cell percentage over time, most likely as a consequence of the inhibition of CD4-dependent cell-cell contact, as well as of further endocytosis of extracellular HIV-1.
FIG. 6.
HIV-1 endocytosis in CEMGFP target cells can occur independently of the internalization of cell-free viral particles. Shown are FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP cells cocultured for 6 and 16 h with U937HIV-1 cells after incubation for 3 h at 4°C in the presence of 0.5 μg/ml of Leu3A anti-CD4 MAbs. CEMGFP cells (5 × 104) were pelleted with 105 U937HIV-1 in a U-bottom 96-well plate and suspended in 20 μl of complete medium in the presence of both 1 μg/ml of T-20 and the anti-CD4 MAbs. After 3 h at 4°C, 80 μl of complete medium complemented with the anti-CD4 MAbs was added, and the plate was immediately incubated at 37°C. As controls, parallel cocultures were run in the absence of anti-CD4 MAbs or upon pretreatment of CEMGFP cells for 30 min at 4°C with the anti-CD4 MAbs, which were afterwards maintained over time. The cells were then harvested at the indicated times and analyzed by FACS. The quadrants are set on the basis of uninfected CEMGFP cells. The percentages of Gag+ cells are indicated. The results are representative of two independent experiments.
Taken together, these results suggest that cell-cell contact induces a massive HIV-1 endocytosis that is the result of the internalization of extracellular viral particles, together with the influx of virions through a structure impermeable to antibodies, likely virological synapses.
HIV-1 cell-to-cell transmission in ex vivo cell cocultures.
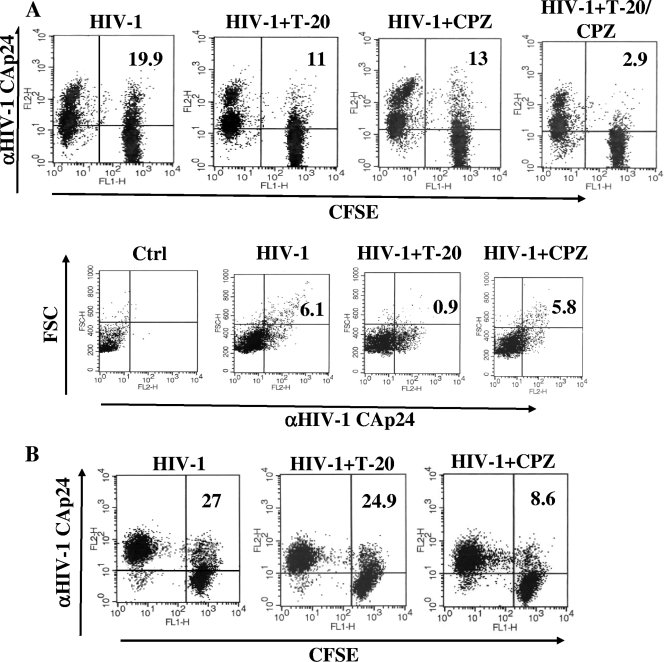
To add relevance to our data, we reproduced the cocultivation experiments, but using primary CD4+ lymphocytes or macrophages. To this end, activated CD4+ lymphocytes were labeled with carboxyfluorescein succinimidyl ester (CFSE) and used as target cells in cocultures with autologous CD4+ lymphocytes acutely infected 2 days before with (VSV-G) HIV-1. After 16 h of coculture in the presence of AZT, T-20, and/or CPZ, we observed significantly reduced percentages of CFSE+/Gag+ lymphocytes in both T-20- and CPZ-treated cocultures, and the inhibitory effects were additive (Fig. 7A). This strongly suggests that both cell fusion and HIV-1 endocytosis also occur in primary CD4+ lymphocyte cocultures. The lymphocyte fusion was further documented by the Gag/forward-scatter analysis of the CFSE+ subpopulation (Fig. 7A), showing that, consistent with what was already noticed in cell line cocultures, the T-20 treatment specifically affected the most enlarged Gag-positive cells.
FIG. 7.
Analysis of cell fusion and HIV-1 endocytosis in cocultures of primary cells. (A) FACS analysis of CD4+ primary lymphocytes infected 2 days before with 200 ng of CAp24/105 cells of (VSV-G) wt HIV-1 and cocultivated for 16 h with CFSE-labeled autologous CD4+ lymphocytes. The cocultures were carried out in the presence of 10 μΜ ΑΖΤ plus 1 μg/ml of T-20 and/or 5 μg/ml of CPZ. At the top are shown the HIV-1 CFSE/Gag FACS results, representative of three independent experiments. The percentages of Gag+/CFSE+ cells out of the total CFSE+ lymphocytes are reported. The quadrants are set on the basis of uninfected cocultures. At the bottom are shown the forward-scatter (FSC) and HIV-1 Gag FACS analyses of the CFSE+ subpopulation. The quadrants are set on the basis of the size and background fluorescence of uninfected cells labeled with the anti-HIV-1 CAp24 MAbs. The percentages of Gag+ cells showing a size increase compared with uninfected cells are indicated. (B) CFSE/HIV-1 Gag FACS analysis of MDMs infected 2 days before with 50 ng of CAp24/105 cells of (VSV-G) AD8 HIV-1 and cocultivated for 16 h with CFSE-labeled autologous MDMs. The cocultures were carried out in the presence of 10 μΜ ΑΖΤ and of either 1 μg/ml of T-20 or 5 μg/ml of CPZ. The percentages of Gag+/CFSE+ cells out of the whole CFSE+ subpopulation are reported. The quadrants are set on the basis of uninfected cocultures. The results shown in both panels are representative of two independent experiments.
In addition to CD4+ lymphocytes, macrophages also are key cells for in vivo HIV-1 replication and AIDS pathogenesis (38). To establish how efficiently macrophages act as HIV-1 donor/target cells, monocyte-derived macrophages (MDMs) were infected with (VSV-G) AD8 Env HIV-1 and, 2 days later, cocultivated with CFSE-labeled autologous MDMs in the presence of AZT and T-20 or CPZ. As shown in Fig. 7B, the T-20 treatment had no effect, while CPZ significantly reduced the percentages of Gag+ MDMs, indicating that macrophages support HIV-1 endocytosis, but not cell fusion.
We conclude that the mechanisms described in cell lines accurately reflect the events occurring in cocultures of primary cells.
Virological consequences of cell fusion.
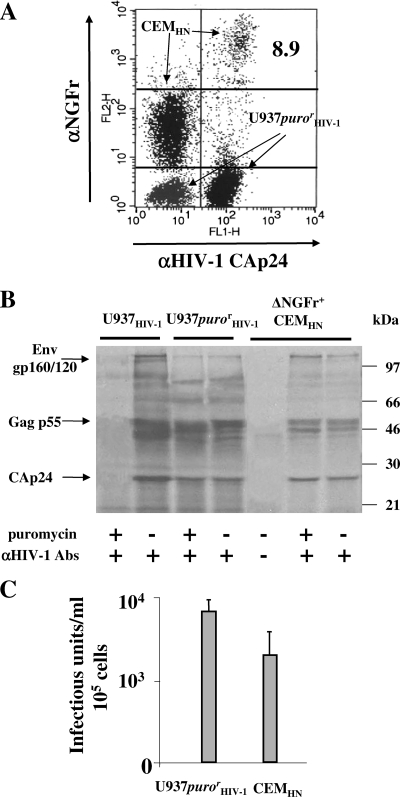
Next, we were interested in establishing the virological consequences of the mechanisms of HIV-1 transfer described above. To evaluate the virological impact of the donor-target cell fusion, a CEM cell line (CEMHN) expressing NGFr truncated in its cytoplasmic domain (ΔNGFr) under the control of HIV-1 LTRs (26) was used as a target. This allowed a simple and reliable selection of target cells switching the ΔNGFr expression in response to Tat. In addition, puromycin-resistant U937HIV-1 cells (U937purorHIV-1) were used as donor cells to evaluate the correlation between cell fusion and virus production. In fact, in the presence of the antibiotic, target cells were expected to express the virus only upon fusion with donor cells. Thus, CEMHN cells were cocultivated for 16 h with U937purorHIV-1 cells in the presence of AZT. Afterwards, the strongly ΔNGFr-positive CEMHN subpopulation (Fig. 8A) was selected and metabolically labeled for 6 h in the presence or absence of puromycin. Thereafter, the 35S-labeled virions were harvested, purified on a 20% sucrose cushion, and analyzed by an immunoprecipitation assay. We observed that in the presence of puromycin, the ΔNGFr-positive cells also released viral particles showing an unmodified protein pattern compared with donor cells (Fig. 8B). It is noteworthy that the puromycin resistance clearly showed that the vast majority of selected ΔNGFr-positive cells originated from cell fusion events.
FIG. 8.
Fused cells express HIV-1 and release infectious HIV-1 particles. (A) FACS analysis for the expression of both HIV-1 Gag and ΔNGFr in 16-h cocultures of U937purorHIV-1 and CEMHN cells. The single-cell populations are indicated, as well as the percentage of the Gag+/ΔNGFr+ subpopulation within the CEMHN cell population. (B) RIPA assay of viral particles purified from the supernatants of 2 × 105 CEMHN cells 6 h after separation from U937purorHIV-1 donor cells. These cells or, as controls, equal numbers of U937HIV-1 or U937purorHIV-1 cells were metabolically labeled with both [35S]cysteine and [35S] methionine in 1 ml of both cysteine- and methionine-free medium plus 10% dialyzed FCS and in the presence or absence of 2.5 μg/ml of puromycin. After 6 h of incubation, the supernatants were concentrated by ultracentrifugation on a 20% sucrose cushion and lysed in 100 μl of TNE-Triton X-100 1% buffer. Thereafter, 50 μl of each sample was immunoprecipitated with 5 μl of a pool of strongly positive anti-HIV-1 human sera (+) or with the same volume of a HIV-1-negative human serum (−), and the resulting products were resolved in a 10% polyacrylamide gel electrophoresis. Abs, antibodies. (C) Infectious units measured in the supernatants from either U937purorHIV-1 cells or CEMHN cells isolated from the coculture as described for panel B. The titrations were carried out on C8166 cells by the end dilution method. For all panels, the results are representative of three independent experiments. In panel C, the mean values plus standard deviations were also calculated.
Next, we were interested in determining how efficiently fused cells produced HIV-1 infectious particles. To this end, ΔNGFr+ CEMHN cells purified from the cocultures with U937purorHIV-1 cells were cultivated for 6 h, and the supernatants were harvested and titrated in terms of HIV-1 infectious units. As shown in Fig. 8C, ΔNGFr+ CEMHN cells released numbers of infectious HIV-1 particles only about twofold reduced compared with donor cells. Of note, the HIV-1 release decreased over time, consistently with the decrease in cell viability, which dropped below 10% within 24 h of culture (not shown).
Taken together, these findings further support the idea that cell fusion is indeed an effective method of virus spread, even in the absence of viral neo synthesis in target cells.
Virological consequences of HIV-1 endocytosis.
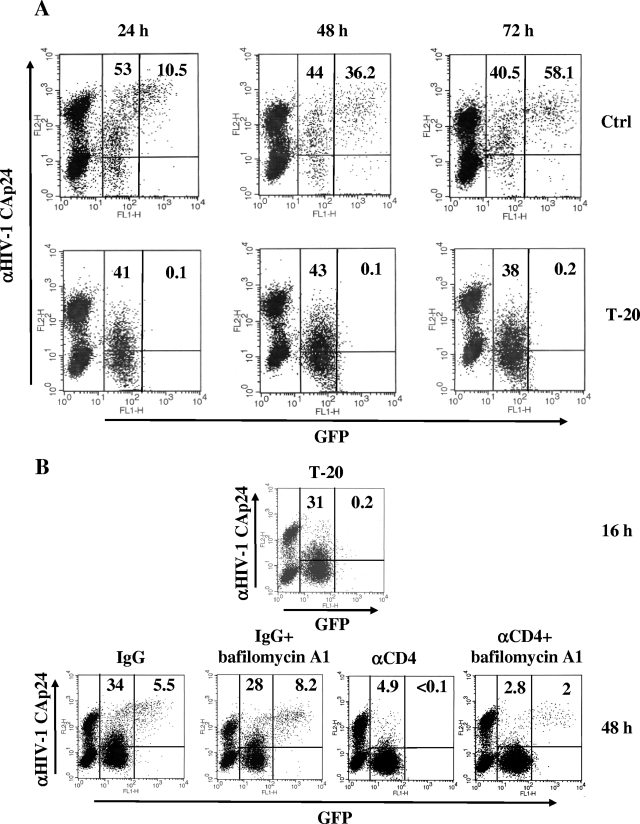
To evaluate the virological effects of HIV-1 endocytosis, the analysis of U937HIV-1/CEMGFP cocultures in the presence of T-20 was extended to 3 days after the coculture setup. As shown in Fig. 9A, no GFP signal over the background was detectable at any time point analyzed in the presence of constant percentages of HIV-1 Gag-positive target cells. To further increase the sensitivity of the assay, and by exploiting the resistance to the G418 antibiotic of CEMGFP cells (15), the cocultures were carried out for an additional 7 days in the presence of high concentrations of G418 (i.e., 2 mg/ml) to rapidly kill the donor cells. Again, no increase in the percentages of GFP+ cells over the control conditions was detected (not shown). The lack of GFP activation in target cells in the presence of T-20 strongly suggests that the internalized HIV-1 virions undergo efficient intracellular degradation.
FIG. 9.
Endocytosis in CEMGFP target cells does not lead to the expression of infectious HIV-1. (A) Shown are FACS analyses for the detection of both GFP and HIV-1 Gag products in CEMGFP/U937HIV-1 cocultures carried out for 24, 48, and 72 h in the presence or absence of 1 μg/ml of T-20. The quadrants are set on the basis of uninfected CEMGFP cells. The results are representative of three independent experiments. (B) Target cells endocytose infectious HIV-1 particles. CEMGFP/U937HIV-1 cocultures were carried out for 16 h in the presence of 1 μg/ml of T-20 and then washed and incubated for 30 min at 4°C with either the Leu3A anti-CD4 MAb or a nonspecific isotype. Afterwards, cocultures were replated in the presence of the respective antibodies, either alone or with 100 nM bafilomycin A1. After an additional 24 h, the cocultures were analyzed by FACS. The results are representative of two independent experiments. For both panels, the percentages of both Gag+/GFP− and Gag+/GFP+ subpopulations are reported in the respective quadrants of each plot.
Finally, we tested the infectivity of endocytosed HIV-1 particles by treating cocultures with bafilomycin A1, an inhibitor of the endosomal ATPase, whose ultimate effect is to increase the endosomal pH. This treatment is expected to allow the endocytosed HIV-1 to fuse with the endosomal membranes, leading to viral expression and replication (14). To this end, cocultures were carried out in the presence of T-20 for 16 h. At this time, as expected, the appearance of an easily recognizable Gag+ subpopulation (Fig. 9B) mirrored the HIV-1 endocytosis that occurred in target cells. To test whether at least some of the endocytosed HIV-1 particles retained their infectivity, the block in fusion imposed by T-20 was removed to allow intracellular virion fusion. Meanwhile, the cocultures were treated with the Leu3A anti-CD4 MAbs to inhibit both further virion endocytosis and cell fusion without, however, interfering with intracellular virion fusion, since antibodies are expected to access the endosomal compartment with very low efficiency. Thus, cocultures were washed and treated for 30 min at 4°C with anti-CD4 MAbs, which were readded when the cocultures were shifted to 37°C in the presence or absence of 100 nM bafilomycin A1. The FACS analysis carried out after an additional 24 h clearly showed the appearance of a Gagbright/GFP+ subpopulation in the presence of bafilomycin A1 (Fig. 9B), strongly suggesting that the neutralization of the acidic compartment at least partly rescued the infectivity of the endocytosed virions. A bafilomycin A1-dependent increase in the Gagbright/GFP+ subpopulation was also detectable under conditions lacking treatment with anti-CD4 MAbs, but in this case, overcoming cell fusion events might interfere with the overall outcome. More significantly, these conditions proved that treatment with bafilomycin A1 did not grossly influence cell fusion and HIV-1 endocytosis. On the other hand, we also noticed that treatment of uninfected CEMGFP cells with bafilomycin A1 did not increase GFP expression (not shown), indicating that the drug has no effects on LTR-regulated gene expression, as reported previously (14).
Taken together, these results indicate that the endocytosis of infectious HIV-1 particles occurring upon cell-cell contact does not generate detectable virus replication.
DISCUSSION
Here, we provide evidence that the overall HIV-1 transfer in target cells soon after cell-cell contact essentially consists of both cell fusion and clathrin-dependent HIV-1 endocytosis. Of note, the early time points we primarily considered, as well as the use of antivirals, such as AZT and T-20, precluded analysis of the consequences of the fusion-mediated entry of HIV-1, either cell free or occurring through virological synapses.
We noticed that both cell fusion and virion endocytosis depend on Env-CD4 interaction, but not on de novo viral expression, in target cells. This was observed in cocultures of either cell lines or primary cells, as well as using either AZT or the 118-D-24 HIV-1 integrase inhibitor (reference 39 and data not shown), and also by testing the reverse transcriptase-defective 8E5 cell line as donor cells. These results are in full agreement with those reported in a seminal paper on HIV-1 cell-to-cell transmission (16), but also with the more recent observations of virus passage from HIV-1-infected MOLT-4 cells to either CD4+ primary lymphocytes or CD4+ HeLa cells (1). Conversely, it was also reported that the HIV-1 transfer from infected to uninfected Jurkat cells is at least partly sensitive to nevirapine, an alternative inhibitor of viral retrotranscription, as detected starting 16 h after the coculture setup (36, 37). Considering that, conversely, no effects of nevirapine were detected in this coculture system for 6 h, we explain this apparent discrepancy essentially as the consequence of the faster kinetics of the events induced by cell-cell contact within Jurkat cells compared to those occurring in CEMGFP or primary lymphocyte-based cocultures.
Inconsistent results can also be found in the literature regarding the relevance of cell fusion events in overall cell-to-cell HIV-1 transmission. In fact, while on the one hand Jurkat cells infected with an HIV-1 variant expressing a nonfusogenic Env receptor were found to be unable to transmit HIV-1 to target cells (37), on the other hand, the inhibition of cell fusion was shown to be unable to inhibit HIV-1 passage from either MOLT-4 (1) or Jurkat (6) lymphocyte lines to CD4+ lymphocytes. For the first time, we found that the block in cell fusion reduces but does not abolish HIV-1 transmission to target cells.
The remarkable levels of cell fusion we detected are not surprising, since, for instance, relevant cell fusion activity (i.e., 7 to 10% of the total HIV-1 Gag-positive target cells) was also observed in Jurkat cocultures (37). The same authors reported that infection by either cell-free virus or HIV-1-infected cells was severely impaired by gently shaking the cultures or cocultures (37). In an effort to establish whether the early events we detected after the coculture setup were sensitive to culture shaking, we ran parallel cocultures of U937HIV-1 with CEMGFP cells in the presence or absence of continuous, gentle shaking. The results we obtained showed that culture shaking was correlated with a decrease in Gag+/GFP− cells from 45.9 ± 4.1 to 41 ± 5.9 and in Gagbright/GFP+ cells from 18 ± 3.4 to 15 ± 4.1, representing statistically nonsignificant variations. This indicates that the mechanisms underlying both HIV-1 endocytosis and cell fusion are scarcely influenced by cell movement.
We found that primary CD4+ lymphocytes also undergo cell fusion, while conversely, this process was not detectable within primary macrophages. In addition to the pathogenetic significance correlated with the events occurring in AIDS patients' lymph nodes, where viral spread can be strongly enhanced by cell-cell contact, the productive infection resulting from cell fusion may be seen as a privileged mechanism of HIV-1 transmission in primary infection (for a review, see reference 23). In this case, the elusion of the antiviral activities of factors present in extracellular fluids surrounding the mucosae, like proteases, complement, antibodies, and soluble CD4, can significantly favor HIV-1 transmission.
Interestingly, we noticed a relevant increase in Gag−/GFP− target cells upon T-20 treatment of cocultures, suggesting that no HIV-1 endocytosis occurs in cells where the block in cell fusion is operative. Since the endocytosis we detected in cocultures depends on Env/CD4 interaction, we hypothesize that the CD4 clustering occurring in target cells upon contact with the Env molecules of donor cells (20) reduces the CD4 density to under the threshold required to initiate HIV-1 endocytosis upon interaction with either another donor cell or extracellular virions.
The nonproductiveness of the NL4-3-NefF12 variant served to establish that the Gag+/GFP− CEMGFP subpopulation we detected in cocultures primarily originated from HIV-1 endocytosis. This phenomenon could be part of the previously described coreceptor-independent HIV-1 transfer (1), as well as of the recently reported Env-dependent HIV-1 transmission resistant to HIV-1-neutralizing antibodies (6). For the first time, we have evidence that the overall HIV-1 endocytosis in target cells is strongly affected by the inhibition of clathrin functions. In this regard, considering that the physiologic CD4 cell internalization occurs through the formation of clathrin-coated vesicles (13), it would be of interest to investigate whether in cocultures HIV-1 can usurp the CD4 internalization machinery to enter target cells.
The authentic cell-to-cell HIV-1 transfer we found to be part of the overall HIV-1 endocytosis activity could rely on the passage of intact virions through the extracellular space generated within two or more points of cell junction, as already observed by electron microscope analysis (20, 30). Alternatively, and similarly to the mechanism of vesicle trafficking through cell membrane gap junctions recently described (31), it is also conceivable that the viral budding occurs directly into the target cells at the virological synapse. Interestingly, this form of endocytosis was also found to be strictly dependent on clathrin activity in target cells (31).
Importantly, we also detected massive HIV-1 endocytosis in cocultures of both primary lymphocytes and macrophages. In this regard, extending the investigations of DCs contacting HIV-1 infected cells will be of pathogenetic relevance, since high levels of HIV-1 endocytosis in DCs may contribute significantly to virus spread in the infected host through the trans-infection mechanism. On the other hand, strong HIV-1 endocytosis in professional antigen-presenting cells would also have important immunologic consequences, as it could generate a strong presentation in major histocompatibility complex class II of virus degradation products, thereby inducing a potent activation of HIV-1-specific CD4+ lymphocytes. This, in turn, may have beneficial effects on virus spread, considering that HIV-1 replicates best in HIV-1-specific CD4+ lymphocytes (7, 9, 18).
Virological synapses are defined as stable contact zones between donor and target cells, ultimately favoring cell-to-cell virus transmission (32), and were first described in cocultures between DCs and lymphocytes (3). We believe that in the process of cell fusion, the cell-cell contact zones cannot be considered virological synapses, since they rapidly evolve toward complete fusion between two or more cells. On the contrary, at least part of the HIV-1 endocytosis occurring in cocultures likely takes place through the formation of stable cell-cell contacts, likely virological synapses, which are expected to also be privileged platforms for the virus-cell fusion process initiating the productive viral life cycle. The identification of the discriminatory molecular events moving cell-cell contact toward cell fusion rather than the formation of stable virological synapses awaits clarification and represents a relevant field of future investigation.
In HIV-infected patients, both the frequency of infected cells and the HIV-1 cell expression levels may be significantly lower than those reproduced in our experimental setting. Therefore, while our results can be helpful for defining some mechanistic aspects of the events following cell-cell contact, how much the phenomena described here can contribute to HIV spread in infected hosts remains to be clarified. Hence, further advances in the description of the mechanisms of cell-to-cell HIV-1 transmission and of its role in virus spread in infected individuals will be of utmost importance for the identification of innovative therapeutics against AIDS.
Acknowledgments
This work was supported by grants from the AIDS project of the Ministry of Health, Rome, Italy.
We are indebted to Federica M. Regini and Paola Sergiampietri for excellent editorial assistance. AZT, sCD4, and ritonavir, as well as the b12 and 4G10 anti-HIV-1 Env gp120 MAbs, were obtained from the NIH AIDS Research and Reference Reagent Program. We thank Laura Fantuzzi, Department of Cell Biology and Neurosciences, Istituto Superiore di Sanità, Rome, Italy, for kindly providing the AMD3100 inhibitor.
Footnotes
Published ahead of print on 28 May 2008.
REFERENCES
- 1.Blanco, J., B. Bosch, M. T. Fernandez-Figueras, J. Barretina, B. Clotet, and J. A. Este. 2004. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J. Biol. Chem. 27951305-51314. [DOI] [PubMed] [Google Scholar]
- 2.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, and P. L. Nara. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 2661024-1027. [DOI] [PubMed] [Google Scholar]
- 3.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257383-387. [DOI] [PubMed] [Google Scholar]
- 4.Carr, J. M., H. Hocking, P. Li, and C. J. Burrell. 1999. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265319-329. [DOI] [PubMed] [Google Scholar]
- 5.Cavrois, M., J. Neidleman, J. F. Kreisberg, and W. C. Greene. 2007. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface bound HIV-1 virions. PLoS Pathog. 3e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, P., W. Hubner, M. A. Spinelli, and B. K. Chen. 2007. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J. Virol. 8112582-12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demoustier, A., B. Gubler, O. Lambotte, M. G. de Goer, C. Wallon, C. Goujard, J. F. Delfraissy, and Y. Taoufik. 2002. In patients on prolonged HAART, a significant pool of HIV infected CD4 T cells are HIV-specific. AIDS 161749-1754. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 672182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 41795-98. [DOI] [PubMed] [Google Scholar]
- 10.Dustin, M. 2003. Viral spread through protoplasmic kiss. Nat. Cell Biol. 5271-272. [DOI] [PubMed] [Google Scholar]
- 11.Federico, M., Z. Percario, E. Olivetta, G. Fiorucci, C. Muratori, A. Micheli, G. Romeo, and E. Affabris. 2001. HIV-1 Nef activates STAT1 in human monocytes/macrophages through the release of soluble factors. Blood 982752-2761. [DOI] [PubMed] [Google Scholar]
- 12.Folks, T. M., D. Powell, M. Lightfoote, S. Koenig, A. S. Fauci, S. Benn, A. Rabson, D. Daugherty, H. E. Gendelman, and M. D. Hoggan. 1986. Biological and biochemical characterization of a cloned Leu-3− cell surviving infection with the acquired immune deficiency syndrome retrovirus. J. Exp. Med. 164280-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foti, M., J. L. Carpentier, C. Aiken, D. Trono, D. P. Lew, and K. H. Krause. 1997. Second-messenger regulation of receptor association with clathrin-coated pits: a novel and selective mechanism in the control of CD4 endocytosis. Mol. Biol. Cell 81377-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredericksen, B. L., B. L. Wei, J. Yao, T. Luo, and J. V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 7611440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gervaix, A., D. West, L. M. Leoni, D. D. Richman, F. Wong-Staal, and J. Corbeil. 1997. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc. Natl. Acad. Sci. USA 944653-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta, P., R. Balachandran, M. Ho, A. Enrico, and C. Rinaldo. 1989. Cell-to-cell transmission of human immunodeficiency virus type 1 in the presence of azidothymidine and neutralizing antibody. J. Virol. 632361-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5783-792. [DOI] [PubMed] [Google Scholar]
- 18.Harari, A., G. P. Rizzardi, K. Ellefsen, D. Ciuffreda, P. Champagne, P. A. Bart, D. Kaufmann, A. Telenti, R. Sahli, G. Tambussi, L. Kaiser, A. Lazzarin, L. Perrin, and G. Pantaleo. 2002. Analysis of HIV-1- and CMV-specific memory CD4 T-cell responses during primary and chronic infection. Blood 1001381-1387. [DOI] [PubMed] [Google Scholar]
- 19.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 2991713-1716. [DOI] [PubMed] [Google Scholar]
- 20.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolly, C., and Q. J. Sattentau. 2004. Retroviral spread by induction of virological synapses. Traffic 5643-650. [DOI] [PubMed] [Google Scholar]
- 22.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 41302-1307. [DOI] [PubMed] [Google Scholar]
- 23.Margolis, L., and R. Shattock. 2006. Selective transmission of CCR5-utilizing HIV-1: the ‘gatekeeper’ problem resolved? Nat. Rev. Microbiol. 4312-317. [DOI] [PubMed] [Google Scholar]
- 24.Markovic, I., and K. A. Clouse. 2004. Recent advances in understanding the molecular mechanisms of HIV-1 entry and fusion: revisiting current targets and considering new options for therapeutic intervention. Curr. HIV Res. 2223-234. [DOI] [PubMed] [Google Scholar]
- 25.McDonald, D., L. Wu, S. M. Bohks, V. N. Kewalramani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 3001295-1297. [DOI] [PubMed] [Google Scholar]
- 26.Muratori, C., I. Schiavoni, G. Melucci-Vigo, E. Olivetta, A. C. Santarcangelo, K. Pugliese, P. Verani, and M. Federico. 2002. Inducible expression of the δNGFr/F12Nef fusion protein as a new tool for anti-human immunodeficiency virus type 1 gene therapy. Hum. Gene Ther. 131751-1766. [DOI] [PubMed] [Google Scholar]
- 27.Olivetta, E., D. Pietraforte, I. Schiavoni, M. Minetti, M. Federico, and M. Sanchez. 2005. HIV-1 Nef regulates the release of superoxide anions from human macrophages. Biochem. J. 390591-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivetta, E., K. Pugliese, R. Bona, P. D'Aloja, F. Ferrantelli, A. C. Santarcangelo, G. Mattia, P. Verani, and M. Federico. 2000. cis expression of the F12 human immunodeficiency virus (HIV) Nef allele transforms the highly productive NL4-3 HIV type 1 to a replication-defective strain: involvement of both Env gp41 and CD4 intracytoplasmic tails. J. Virol. 74483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips, D. M. 1994. The role of cell-to-cell transmission in HIV infection. AIDS 8719-731. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, D. M., and A. S. Bourinbaiar. 1992. Mechanism of HIV spread from lymphocytes to epithelia. Virology 186261-273. [DOI] [PubMed] [Google Scholar]
- 31.Piehl, M., C. Lehmann, A. Gumpert, J. P. Denizot, D. Segretain, and M. M. Falk. 2007. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol. Biol. Cell 18337-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piguet, V., and Q. Sattentau. 2004. Dangerous liaisons at the virological synapse. J. Clin. Investig. 114605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato, H., J. Orenstein, D. Dimitrov, and M. Martin. 1992. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology 186712-724. [DOI] [PubMed] [Google Scholar]
- 34.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 752993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaeffer, E., V. B. Soros, and W. C. Greene. 2004. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J. Virol. 781375-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sol-Foulon, N., M. Sourisseau, F. Porrot, M. I. Thoulouze, C. Trouillet, C. Nobile, F. Blanchet, V. do Bartp;p, N. Noraz, N. Taylor, A. Alcover, C. Hivroz, and O. Schwartz. 2007. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. EMBO J. 26516-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sourisseau, M., N. Sol-Foulon, F. Porrot, F. Blanchet, and O. Schwartz. 2007. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 811000-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson, M. 2003. HIV-1 pathogenesis. Nat. Med. 9853-860. [DOI] [PubMed] [Google Scholar]
- 39.Svarovskaia, E. S., R. Barr, X. Zhang, G. C. Pais, C. Marchand, Y. Pommier, T. R. Burke, Jr., and V. K. Pathak. 2004. Azido-containing diketo acid derivatives inhibit human immunodeficiency virus type 1 integrase in vivo and influence the frequency of deletions at two-long-terminal-repeat-circle junctions. J. Virol. 783210-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turville, S., J. Wilkinson, P. Cameron, J. Dable, and A. L. Cunningham. 2003. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J. Leukoc. Biol. 74710-718. [DOI] [PubMed] [Google Scholar]
- 41.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1231107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]