Abstract
The high toxicity of potent chemotherapeutic drugs like Doxorubicin (Dox) limits the therapeutic window in which they can be applied. This window can be expanded by controlling the drug delivery in both space and time such that non-targeted tissues are not adversely affected. Recent research has shown that ultrasound (US) can be used to control the release of Dox and other hydrophobic drugs from polymeric micelles in both time and space. It has also been shown using an in vivo rat tumor model that Dox activity can be enhanced by ultrasound in one region, while in an adjacent region there is little or no effect of the drug. In this article, we review the in vivo and in vitro research being conducted in the area of micellar drug delivery and ultrasound to cancerous tissues. Additionally, we summarize our previously published mathematical models that attempt to represent the release and re-encapsulation phenomena of Dox from Pluronic® P105 micelles upon the application of ultrasound. The potential benefits of such controlled chemotherapy compels a thorough investigation of role of ultrasound (US) and the mechanisms by which US accomplishes drug release and/or enhances drug potency. Therefore we will summarize our findings related the mechanism involved in acoustically activated micellar drug delivery to tumors.
Keywords: targeted delivery, polymeric micelles, thermo-responsive polymers, ultrasound, drug delivery, liposomes
1. Introduction
This review article pertains to the use of ultrasound (US) to release chemotherapeutic drugs from nanometer-sized carriers, thus targeting the release drugs to the tissue insonated by the ultrasound. We will start our review with a background on the nature of ultrasound and its use in therapy.
2.1. Ultrasonic Drug Delivery
2.1.1 Ultrasound
Ultrasound consists of pressure waves with frequencies greater that 20 kHz usually generated by piezoelectric transducers that change an applied voltage waveform into mechanical translation from the face of the transducer. Like optical or audio waves, ultrasonic waves can be focused, reflected and refracted, and propagated through a medium, thus allowing the waves to be directed to and/or focused on a particular volume of tissue. The technology for ultrasonic wave control and delivery are well advanced and pervasive in the areas of biomedical imaging and flow measurement. Ultrasound is also used in hyperthermic cancer therapy for breast and other cancers (1-4) and in physical therapy for warming tissues (5). The main advantage of ultrasound is its non-invasive nature: the transducer is placed in contact with a water-based gel on the skin, and no insertion or surgery is required.
2.1.2 Mechanisms of bio-interactions
The interactions of ultrasound with biological tissues are divided into two broad categories: thermal and non-thermal effects. Thermal effects are associated with the absorption of acoustic energy by the fluids or tissues, and are reviewed by Nyborg (6). Non-thermal bio-effects are generally associated with oscillating or cavitating bubbles, but also include non-cavitation effects such as radiation pressure, radiation torque, and acoustic streaming (6). With respect to drug delivery, these latter effects are probably not involved except to the degree that fluid or particle motion (via acoustic streaming or radiation pressure) increases convection and transport of drug. Bio-effects related to cavitation can produce strong stresses on cells, which may increase drug interactions with the cell, including increased transport toward and into the cell.
Cavitation occurs as gas bubbles oscillate in size in response to the oscillating pressure. Ultrasound excites all sizes of bubbles, but those bubbles whose size imparts a natural resonance frequency near or matching the frequency of the acoustic field will achieve the highest amplitude of oscillation. As the acoustic pressure increases, or as the size of the bubble approaches the resonance size, the oscillations become non-linear, and eventually can result in the total collapse of the bubble as the inertia of the inward-moving water surface overcomes the internal pressure of the bubble. This collapse event, known as inertial cavitation, causes shock waves, creates pressures on the order of 100 atm, and produces temperatures on the order of several thousand degrees K. These violent events, and the radicals generated by the high temperatures, can damage and even destroy cells (6). However, even the non-inertial (stable) cavitation, in which bubbles oscillate without collapsing, can cause significant bio-effects. The rapidly oscillating surfaces of the bubbles create high fluid shear stresses that can shear cell membranes, making them more permeable to small molecules or even disrupting their membranes (7-9). The existence of cavitation can be detected by several techniques, including measurement of sub and ultraharmonic oscillations (10-12), trapping free radicals, sonoluminescence, and more (13). A decrease in acoustic-related effects as ambient pressure increases is also used to verify the occurrence of cavitation because the increased pressure compresses the bubbles and suppresses the amplitude of oscillation (10,11,13,14).
2.1.3 Ultrasound-assisted drug delivery
During the past decade, ultrasound has been investigated by several groups as a potential facilitator of the delivery and absorption of drugs (15,16). Early studies on transdermal drug delivery using higher frequencies available in diagnostic equipment had limited success (17-20), but by using lower frequencies (20 kHz) Mitragotri achieved transdermal delivery of medium molecular weight proteins (insulin, interferon, and erythropoeitin) (19). His hypothesis is that cavitation events disrupted the stratum corneum and that such cavitation was more prevalent at lower frequencies. In our work, we believe that cavitation disrupts micelles, leading to drug release (21-24). Kruskal et al. reported that higher frequency ultrasound (imaging frequencies) increased the permeability of blood vessels and increased the quantity of Dox delivered by stable liposomes to hepatic colorectal metastases in a mouse model (25). Thus US may increase even further the enhanced permeability of tumor capillaries which already allow passive targeting of tumors by drugs (26-28). Kwok et al. demonstrated ultrasonic-activated release of insulin from a monolithic drug reservoir with an impermeable surface coating that is disrupted by the action of ultrasound (29). After insonation is stopped, the coating reforms and blocks further release of drug. Ultrasound is credited with causing or enhancing chemical reactions that can be chemotherapeutic (30-35). Additionally, ultrasound has been shown to aid in the delivery of therapeutic drugs to the brain, by causing transient disruptions in the blood brain barrier (BBB) (36). The effect on the BBB was increased further when microbubbles were used in conjunction with US.
2.2 Micellar Drug Delivery
Several molecular vehicles have been used to delivery therapeutic drug to the body. These include liposomes, micelles, shelled vesicles, solid lipid particles and others (37). This review will focus on the use of polymeric micelles and their role in acoustically activated drug delivery to cancerous tissues.
2.2.1 Micelles
A micelle consists of an assembly of amphiphilic molecules arranged to form a hydrophobic core and a hydrophilic corona. The hydrophobic/hydrophilic interactions of the molecules control the structure of the micelle. Thus, hydrophobic drugs are able to penetrate and accumulate inside the hydrophobic core of these micelles. Their sequestration minimizes the drug interactions with the outer aqueous environment. Polymeric micelles have an average diameter of 20 nm and are considered to have several advantages over other types of drug carriers (38-50) (38,39,41,42,44-48,50-55)including
Structural stability: some polymeric micelles dissociate slowly at levels below their critical micelle concentration (CMC) (hours to days instead of milliseconds for dissociation of low molecular weight) (38,39,42,44-48,50,53-55);
Prolonged shelf life;
Long circulation time in blood and stability in biological fluids;
An appropriate size to escape renal excretion;
An appropriate size to allow extravasation at the tumor site;
Simplicity in drug incorporation, in comparison with covalent bonding of the drug to the polymeric carrier;
Drug delivery independent of drug character (56).
Polymeric micelles are considered to be much more structurally stable than micelles formed by low molecular weight compounds. Examples of polymeric micelles are those formed by Pluronic® block copolymers, which are triblock copolymers of poly (ethylene oxide) (PEO) and poly (propylene oxide) (PPO), often denoted by PEO-PPO-PEO. Pluronic® compounds have gained special attention in cancer drug delivery because of their ability, at low concentrations, to sensitize multi-drug resistant (MDR) cancer cells (57). Pluronic® compounds have low in vivo toxicity (57).
2.2.2 Liposomes
There are many publications of Dox and other drugs delivery from liposomes, which can be larger (∼1 um) than micelles (∼0.05 um), and which posses a lipid bilayer encapsulating water soluble drugs to prevent unwanted release (26). Liposomes that are masked with PEO remain in circulation longer than those without PEO (58). There are some reports on the use of ultrasound to release drugs from liposomes by disrupting and spilling their contents (59-61). A disadvantage of using liposomes is that they are more difficult to prepare, and the drug is not re-encapsulated when the insonation is stopped, as it is with our micellar system. Liposomal drug delivery is beyond the scope of this article.
3.1 Role of Ultrasound in Producing Drug Release from Micelles
3.1.1. Micellar Drug Carrier
As mentioned above micelles are currently being investigated as drug delivery vehicles. The most important micelles used in drug delivery are made of a hydrophilic part (poly-ethylene-oxide-PEO) and a hydrophobic portion (poly-propylene-oxide-PPO), which allows for their spontaneous assemble in water eventually forming a spherical micelle with a hydrophobic core. The most common copolymers used in acoustically activated drug delivery belong to the Pluronic® family of triblock copolymers, e.g. P105, F127, P85, L61, etc. In P105, the most commonly used Pluronic®; the number of monomer units of PEO and PPO are 37 and 56, respectively, which creates a weight fraction of approximately 50% PEO and 50% PPO. This surfactant was found to be an ideal drug carrier for ultrasonic-activated drug release for several reasons:
It forms micelles quickly upon simple dissolution in water;
The core of PPO is sufficiently hydrophobic to stabilize the micelle and sequester hydrophobic drugs (62);
The micelles can be perturbed by low frequency ultrasound to release the drug (22);
The drug is quickly re-encapsulated in the carrier when insonation is stopped (21);
At low concentrations, Pluronic® compounds are non-toxic and can be cleared by the kidneys (63).
Other Pluronic® compounds have been investigated as drug delivery vehicles, but were found to be unsatisfactory when used as pure Pluronic® compounds (not mixed Pluronic® compounds) because those with longer PEO blocks had too high of a critical micelle concentration, and those with longer PPO blocks could not dissolve easily in water (64). Thus the composition of Pluronic® P105 appears to be close to optimal for drug sequestration and ultrasonic release.
3.1.2. Ultrasonic Drug Release
Proper quantification of the amount of drug release from micelles is essential in these studies. To this end, a laser fluorescence detection system was developed to quantify the amount and the kinetics of Dox release from these micelles (21-24,65). The system consists of an argon-ion laser at 488 nm directed into a glass cuvette containing the trial solution to be insonated. A fiber optic probe is used to collect the fluorescence emission from the cuvette. The collected light passes through a bandpass filter centered at 535 nm to a sensitive silicon detector, whose signal is digitized and stored on a computer. The temperature of the ultrasonic exposure chamber is maintained at 37°C by a recirculating thermostatic bath. A decrease in fluorescence is attributed to Dox being released from the micelle core to the aqueous phase, and the release was quantified using a calibration with free Dox (22,23), in which Dox dissolved in PBS simulated 100% release. Because the emission from Dox is quenched by water, the measured fluorescence decreased as Dox is transferred from the hydrophobic core of the micelle to the aqueous phase. Results using this system revealed that up to 10% of the Dox is released, depending upon the insonation intensity and frequency (21-24,65). Drug release was also observed at 20 kHz (0.05 W/cm2). Pulsed insonation resulted in pulsed drug release and re-encapsulation (22).
The release of Dox from Pluronic® micelles was studied as a function of frequency and intensity. There are several aspects of Dox release and cell lysis at 20 kHz and 70 kHz that merit discussion. First, the same level of release could be attained at both frequencies, but much less acoustic intensity was required at the lower frequency to produce the same amount of release. This is consistent with the hypothesized cavitation mechanism since bubble amplitude and cavitation activity in general increases as frequency decreases (66). Alternatively, the observation could also be attributed to a greater population of bubbles of near-resonant size at the lower frequency.
In an attempt to study the mechanism of ultrasonic drug release from Pluronic® micelles, Husseini et al. (23) improved the detection technique and allowed for coaxial cable that is capable of both directing the laser light into the sample and collecting the emissions. The group also collected acoustic spectra at the different power densities investigated. The results of these experiments showed the existence of a drug release threshold (ranging between 0.35 and 0.4 W/cm2) suggesting that collapse cavitation is a required element in this release phenomena. Furthermore, the onset of drug release corresponded to the emergence of subharmonic peak in the acoustic spectra which is indicative of the onset of collapse cavitation. The authors hypothesized that shock waves caused by the collapse of cavitating bubbles are capable of perturbing the micelle structure enough for the drug to be released. The same group found a similar release threshold when they studied two other micellar systems, namely Plurogel™ (stabilized micelles) and PNHL (a copolymer consisting of a PEO block covalently bonded to NIPAAm and polyactate esters of hydroxyl-ethyl methacrylate. While the observed amount of release was lower than for non-stabilized micelles, the first measurable release was correlated with the appearance of a subharmonic peak.
Recently, Stevenson-AbuoelNasr et al. developed a new kinetic model to account for the triphasic nature of the release profile (67). The new model introduced 5 different constants in an attempt to capture the complex behavior of release, namely the rate of micelle destruction, micelle assembly, drug re-encapsulation, nuclei destruction and the maximum amount of Dox that micelles can hold. Additionally, the model used a size distribution of micelles and it predicted the larger micelles to be destroyed first, which would in turn cause a fast release phase. This phase is followed by a slower phase where smaller micelles are sheared. Finally a third phase was modeled in which the smaller fragments and smaller micelles coalesce into larger micelles. The advantage of this model is that it incorporates the various phases of cavitation phenomena into kinetic calculations, thus introducing a more accurate representation of the physical mechanism involved in drug release.
Artificial neural networks (ANN) were also used to capture the non-linear nature of release (68). Previously collected release data were compiled and used to train, validate, and test an ANN model. Sensitivity analysis was then performed on the following operating conditions: ultrasonic frequency, power density, Pluronic® P105 concentration and temperature. The model predicted that drug release was most efficient at lower frequencies. As expected the release increased as the power density increased. Sensitivity plots of ultrasound intensity reveal a drug release threshold of 0.015 W/cm2 and 1 W/cm2 at 20 and 70 kHz, respectively. The presence of a power density threshold provides strong evidence that cavitation plays an important role in acoustically activated drug release from polymeric micelles. Based on the developed model, Dox release is not a strong function of temperature suggesting that thermal effects do not play a major role in the physical mechanism involved. Finally, sensitivity plots of P105 concentration indicates that higher release was observed at lower copolymer concentrations. Current research using ANN is now to develop a controller that can optimize the above mentioned acoustic parameters in a clinical setting.
3.1.3. Stabilized Plurogel™ Drug Carrier
The difficulty with using Pluronic® micelles in vivo is that the micelles are diluted below their CMC when injected into the blood stream, thus dissolving and prematurely dropping their load of drug. Therefore, micelles used in tumor targeting were stabilized by polymerizing an interpenetrating network (IPN) of thermally sensitive acrylamide in the hydrophobic core. In this synthesis, a 10 wt% solution of P105 was placed in a round-bottomed flask, and 0.5 wt% N,N-diethyl acrylamide (NNDEA) was added, along with azobisisobutyronitrile (AIBN) as an initiator (0.001 wt%), and bis-acryloyl cystamine (BAC) as the crosslinker (0.1 wt%). The solution was heated to 65°C under nitrogen for 24 hrs, resulting in polymerization of a crosslinked interpenetrating network entangling the PPO core of the micelle that prevents the micelle from dissolving upon dilution (64).
These stabilized micelles, called Plurogels™, have many advantages as ultrasonic-activated drug delivery vehicles. First, the Plurogels™ still sequester Dox and release it upon insonation (21). The only difference between Pluronic® and Plurogel™ ultrasonic release noted to date is that at similar intensities, Plurogels™ released less drug than did Pluronic®, possibly due to the stronger forces of the covalent network holding the hydrophobic core together (24). Secondly, because the NNDEA interpenetrating network itself is a thermally reversible hydrogel, the Plurogel™ core expands below 31°C (thus enabling drug loading at room temperature) and collapses into a tight hydrophobic core above 31°C (thus retaining the drug in the hydrophobic core). Dynamic light scattering shows the size of a Plurogel™ to be about 60 nm at 37°C, small enough to pass easily through capillaries and be extravasated in leaky tumor capillary networks, and yet large enough to avoid renal clearance.
Plurogels™ are completely biodegradable. Because the non-toxic Pluronic® chains are not crosslinked to the interpenetrating network, they slowly diffuse away and are cleared by the kidneys. The remaining interpenetrating network will degrade slowly by the reduction of the disulfide bonds, thus releasing non-toxic polyNNDEA chains of about 22,000 Daltons. These chains are short enough to be cleared by the kidneys (69).
Dissolution of Plurogel™ is required to prevent its accumulation in the body. However, the Plurogel™ must remain stable long enough to deliver their drug to the target tissue, and to avoid rapid release of drug into the system, producing unwanted high concentrations and side effects in other tissues. For this reason, Plurogel™ stability was studied with a luminescence spectrometer, using diphenyl hexatriene (DPH) as a fluorescent probe (64).
The emission spectrum of DPH is highly dependent upon the hydrophobicity of the local environment, and DPH has almost no fluorescence in aqueous solutions while it is very fluorescent in hydrophobic environments (70). This makes DPH very useful for determining if a hydrophobic environment is present. A stock solution of DPH in tetrahydrofuran (THF) was added to one mL of the undiluted (10 wt% Plurogel™ polymerization sample. The sample was then diluted to 0.01 wt%, leaving 0.1 μg/ml DPH and 0.05 μl THF/ml. The samples were excited at 360 nm, while the emission was measured at 430 nm. Preliminary experiments demonstrated that the low concentration of THF present did not affect the emission intensity.
The 0.01 wt% P105 sample showed very low emission intensity while the 0.01 wt% Plurogel™ sample showed a higher emission intensity. The DPH emission intensity was monitored over time, and a decline in the emission intensity from the Plurogel™ sample was observed over hours. The emission intensity appears to decline exponentially with a half-life of about 17 hours. It was hypothesized that the decline in emission intensity is due to disentanglement of Pluronic® P105 molecules from the interpenetrating network of poly (NNDEA). Residual emission is attributed to the residual polyNNDEA network that did not dissolve in water. It does, however, dissolve upon addition of β-mercapto ethanol (69).
By changing the crosslink density of the interpenetrating network, the hypothesis that Plurogel™ stability and its rate of dissolution are controlled by the rate of diffusion of Pluronic® chains from the core was tested. A series of Plurogels™ were made with increasing amounts of the BAC crosslinker. In theory, as crosslink density increases, the size of the closed rings entrapping Pluronic® chains will decrease, thus reducing the rate of diffusion out of the stabilized Plurogel™ structure. Plurogels™ were made with BAC ranging from 0.001 to 0.167 wt% in the polymerization mixture with 0.5 wt% NNDEA (69). As the amount of crosslinker increased, micellar stability (as measured by DPH emission) also increased. However, at the highest concentration the resulting solution was cloudy, indicating that some crosslinking was occurring in the aqueous phase, not solely the micelle core (69). The Plurogel™ made from 0.1 wt% BAC was used in the subsequent in vivo rat experiments.
Recently, Rapoport et al. (71-73) combined the use of polymeric micelles (poly(ethylene oxide) block-poly lactide (PEG-PLLA)) with a perfluoropentane (PFC5) solution to generate biodegradable microbubbles. At an PFC5 volume concentration of 0.1%, micelles still existed in solution with an average size of 21.5 nm while at higher PFC5 concentrations (1% vol) no micelles were detectable since all the polymeric chains were expended in stabilizing the PFC5 droplet-emulsion. At the higher PFC5 concentration, two sizes of droplets were observed, one at 256 nm and the other at 811 nm. The authors concluded that the 256 nm particles will be able to penetrate tumor capillaries while the larger particles, too large to accumulate in cancerous tissues, can aid in drug delivery from smaller droplets by increasing the tumor intracellular uptake upon their collapse.
3.2 In Vitro Research and Mechanism of Ultrasound-Cell Interaction
HL-60 cells were exposed to Dox and Dox-encapsulated in either Pluronic® micelles or Plurogel™ (74-76). Cells exposed to encapsulated Dox (both Pluronic® and Plurogel™) had much longer cell survival than cells exposed to free Dox. When 70 kHz ultrasound was applied to the cells, those exposed to encapsulated Dox died much more rapidly than cells exposed to free Dox. These results indicated that both Pluronic® and Plurogel™ sequestered Dox from the HL-60 cells, and increase cell survival compared to survival with free Dox. However, insonation appeared to release the Dox and quickly kill the cells. The protective effect of Plurogels™ lasted about 20 hours, similar to the half-life of the Plurogel™.
Our research group has measured the uptake of Dox by cancer cells in vitro (56,77-79). Uptake was measured both directly and indirectly. In the latter method, Dox absorption by cells was calculated by measuring drug depletion from the incubating medium (using a spectrofluorometer). In the former method, fluorescence was measured in cell lysates. The cells were lysed in 2% SDS at 37°C for 2-3 days with periodical stirring, which results in Dox transfer from the cells to the SDS micelles. Calibration experiments showed linear dependence of Dox fluorescence on Dox concentration in PBS, Pluronic®, or SDS micelles. The cell concentration in the lysates was measured by BCA assay (77) or by counting cells before lysis using a hemacytometer. These experiments have quantified Dox uptake kinetics and isotherms in several studies (56,77,78). Ultrasound and the presence of Pluronic® has a large influence on the uptake of Dox by drug sensitive and drug resistant breast cancer cells (A2780 and A2780/ADR) (80). Insonation increased uptake in both cell lines; Pluronic® at 10% reduced the amount of uptake compared to 0.1% Pluronic® (81).
The uptake of Dox by HL-60 cells was studied by pulsing 70 kHz US in tone bursts ranging from 0.1 sec to 2.0 sec in duration, while the time between tone bursts varied from 0.1 to 2 sec in duration. The results showed that with constant “inter-burst” time, and with constant total insonation time, the amount of uptake by the cells increased with insonation length up to about 3 seconds, which was the same uptake observed under CW insonation. However, the amount of uptake did not depend upon the length of the “inter-burst” period (77). From these experiments the time to achieve 90% of full uptake was calculated to be about 2.5 sec of insonation.
Therefore, uptake into the cell proceeds at a slightly slower rate than release, but both are the same order of magnitude. The important observation is that the amount of cellular uptake was independent of the length of inter-burst time. This indicated that the cancer cells did not allow any Dox to go back to the micelles (beyond what might have gone back faster than the shortest inter-burst time of 0.1 sec). Although Dox returns to the micelles in the absence of cancer cells, it apparently does not happen in the presence of cells, which indicates that the cells are very effective at competing with the micelles for the Dox.
The fluorescence of Dox makes it ideal for uptake studies using flow cytometry and fluorescence microscopy. For fluorescence microscopy and confocal microscopy, cells were first fixed with 3% formalin, then washed with PBS containing 3% formalin, and then sealed on glass slides. Confocal microscopy showed different distributions of Dox within the cell, with Dox mostly in the nucleus (80). Pluronic® P105 was then labeled with 5,6-carboxy-2′7′-dichlorofluorescein, a pH-sensitive fluor which fluoresces brighter at pH 7.4 than in acidic conditions (81). HL-60 cells exposed to these labeled Pluronic® micelles, with or without insonation, took up the label and were subsequently studied with confocal microscopy and flow cytometry. Fluorescence micrographs and confocal microscopy showed distribution of Pluronic® to membrane, vesicles, and the cytosol.
Husseini et al. (82) examined the hypothesis that US increases the rate of endocytosis of micelles into HL-60 cells. In their experiments, they used a label (Lysosensor Green) that fluoresces more strongly in a lysosome or an endosome, where the pH is more acidic (about 4.8), compared to a pH of 7.1 outside these compartments (83,84). Flow cytometry was then used to analyze cells exposed to US and P105 micelles with Lysosensor Green. Results showed no significant difference in fluorescence between cells incubated and ultrasonicated for 1 hour at 70 kHz. Their mechanistic study concluded that since ultrasound did not cause the fluorescent probe to partition to a more acidic environment more than it did without ultrasound, endocytosis is not likely to be one of the mechanisms involved in US-assisted micellar drug delivery.
While the study above did not show any evidence of receptor mediated endocytosis or pinocytosis, Rapoport et al. (85) reported that acoustic stimulus enhanced the rate of endocytosis of micelles into two human cell lines. Sheikov et al. later showed that US was capable of promoting pinocytosis in the endothelial cells lining brain arterioles and capillaries (86). Thus the mechanism of this acoustic enhancement of drugs from micelles is still under debate and more research was undertaken by our research group to answer this mechanistic question. These studies are summarized below.
Stringham et al. (87) reported that collapse cavitation was implicated in colon cancer cell membrane disruption. Their study showed that calcein uptake was reduced by increasing the hydrostatic pressure (up to 3 atm) at constant ultrasonic intensity and frequency. Increasing hydrostatic pressure reduced acoustic bubble collapse associated with collapse cavitation, although stable cavitation still occurs. In these experiments lower membrane permeability correlated directly with higher hydrostatic pressure and cavitation suppression.
Husseini et al. (23,74) investigated the mode of death associated with cells exposed to a combination of Pluronic micelles, US and Dox. Using the comet assay, the group studied the electrophoretic pattern of the nuclear DNA from HL-60 cells sonicated in the presence of 10% P105 and a Dox concentration of 10 μg/ml for 2 hours at 70 kHz. The gradual damage observed after two hours of acoustic exposure were consistent with apoptosis as a mode of cell death rather than necrosis. The fact that apoptosis is the primary mechanism of cell death in acoustically triggered micellar drug delivery is an important evidence that the Dox released by US is causing gradual DNA disintegration as opposed to the severe cell membrane damage caused by ultrasound resulting in necrosis.
Howard el al. (88) studied the effect of ultrasound on paclitaxel in micelles of methyl capped poly(ethylene oxide)-co-poly-(L-lactide)-tocopherol on a breast cancer drug-resistant cell line. Their study used 1 MHz Ultrasound at a power density of 1.7 W/cm2 and duty cycle of 33%. Without ultrasonication, less encapsulated Paclitaxel accumulated inside the cells when compared to Paclitaxel administered from a clinical formulation (free or non-encapsulated Paclitaxel), confirming previous reports of cell protection from the action of chemotherapeutic agents by encapsulation using polymeric micelles. However, upon the application of ultrasound, drug accumulation from encapsulated Paclitaxel drastically increased and surpassed the amount of drug found in non-insonated cells. The same study also showed that using the above mentioned polymeric micelles in conjunction with ultrasound was effective in complete tumor regression in nu/nu mice inoculated with the same drug resistant cell line.
All of the above in vitro studies convincingly indicate that ultrasonic cavitation is producing stress on the cell membrane in a manner to allow greater drug uptake than would occur without ultrasound. This data, coupled with other studies showing how US creates repairable holes in cell membranes, leads to the conclusion that in vitro, US both releases drug from some types of carriers and creates transient holes in cell membranes through which free or released drug, or even micelles, can enter into the cell cytosol, thus bypassing the endocytotic pathway.
3.3 In Vivo Experiments for Ultrasonically Assisted Drug Delivery
Once the mechanism of ultrasonically activated drug release in vitro was understood, it was time to examine the phenomenon in vivo. A convenient model is that of tumor bearing rats and mice. The Pitt group uses a rat model (89-91), while a mouse model is used by the Rapoport group (92,93) (73) and the Myhr group (94).
In the rat model, a colon carcinogen cell line DHD/K12/TRb was grown in RPMI containing 2 mM nystatin, 0.2 mM Gentamicin, 2 mM L-glutamine, and 20% fetal bovine serum. Forty-six 6-wk-old BDIX rats were anesthetized with a combination of ketamine HCl and medetomidine HCl, inoculated in each upper hind leg with a subcutaneous injection of the tumor cell suspension (2×106 cells/mL) and allowed to recover (90). Tumor volume was estimated by making two perpendicular measurements (a and b, where a>b) with a caliper and by using the formula TV = ab2. The rats were randomly divided into ten groups including a control group. Selection of right or left tumor for US treatment was randomized. Five weeks following tumor inoculation, rats were preanesthetized with an ip injection of ketamine and pretreated with sq injections of dexamethasone and diphenhydramine to reduce incidence of anaphylactic shock (95). Intravenous administration of free Dox or Dox encapsulated in Plurogel™ occurred via the tail vein. Immediately following injection, the anesthetic regimen was completed and the rats were placed in a special restraint device to exposed one depilated leg and its tumor to unfocused 20 kHz or 70 kHz ultrasound. The rest of the body including the other leg was not exposed to US. The variables investigated in these experiment included applied power density (1 and 2 W/cm2), ultrasonic frequency (20 and 70 kHz), Dox concentration (1.33, 2.67, and 8 mg/kg), power train (continuous and pulsed), and treatment regimen (once and twice weekly). US was applied for 1 hr to one leg of the animal and treatment was repeated once weekly for 4 weeks on the same leg.
Dox concentration of 8 mg/kg was lethal within two weeks of the first ultrasound/Dox treatment (91). Subsequent studies in later months showed that doses of 5.33 and 4.0 mg/kg produced death within 6 weeks. Lower concentrations of 1.33 and 2.67 mg/kg were not fatal. The growth of the bilateral tumors in the negative control groups was relatively similar, increasing approximately exponentially over time.
The tumors that were exposed to ultrasound and encapsulated Dox, however, did not generally grow as much as the non-insonated tumors. In fact, the US-treated tumor generally slowed in growth and sometimes even regressed. A paired comparison of insonated vs. non-insonated tumor size in rats receiving encapsulated Dox (any concentration) showed that insonated tumors were significantly smaller than untreated tumors (p=0.0062). A similar paired analysis of all rats that received encapsulated Dox at 2.67 mg/kg revealed that insonated tumors were again significantly smaller than when not insonated (p=0.017, n). All of the tumors exposed to 70 kHz US and encapsulated Dox during their treatment were significantly smaller than the non-treated tumors (p=0.029). The positive control group receiving free Dox showed no statistical difference, but then neither did any other individual treatment group because of the scatter in tumor growth patterns.
As a continuation of the above work, Staples et al. investigated the exposure of the same tumor cell line to Plurogel™ encapsulated Dox at two US frequencies, namely 20 kHz and 476 kHz for 15 minutes (96). The exposed tumors grew more slowly than the non-sonicated controls (p = 0.0047). However, both frequencies produced the same reduction in tumor growth (p = 0.93).
Staples also compared Dox bio-distribution in several organs at different times after the rats were treated with a combination of Dox/ Plurogel™/US (89,96). In spite of the slower growth of tumors receiving US/Dox/ compared to Dox/Plurogel™ without US, their study found that shortly after injection and ultrasonic exposure, the insonated tumors contained slightly more Dox than the contra lateral control tumors. Dox concentrations deceased with time in all other tissues investigated (the heart, kidneys, liver, and muscle) within a week of drug administration.
Using a mouse model, Gao et al. (97) studied the intracellular distribution of two fluorescently labeled carriers. They insonated ovarian cancer tumors inoculated in nu-nu mice in the presence of unstabilized Pluronic P105 and Pluronic P105 stabilized using PEG-diacylphospholipid at 1 MHz. The study showed that insonation for 30 s or more was capable of increasing the concentration these two labeled micelle formulations at the tumor site. Rapoport et al. (98) showed that the accumulation of the micelle was significantly higher in the sonicated tumor than in the non-sonicated mice model mentioned above. The study also reported that encapsulated Dox did not accumulate in the heart, which would alleviate the cardiotoxicity of Dox.
Myhr et al. (94) used Plurogel to encapsulate a chemotherapy drug (fluorouracil). The authors then applied ultrasound to mice inoculated with a human colon cancer cell line. Ultrasound significantly reduced the tumor volume compared to the control group. The authors also concluded that more significant tumor reductions were observed when higher drug concentrations were administered.
4. Conclusion
Ultrasound has great potential as a mediator of chemotherapy because it can be non-invasively focused on a tissue volume within the body, and thus can mediate or activate drug delivery to that site only. Thus the drug delivery can be controlled in spatial position and in the timing of the delivery. Polymeric micelles are ideal for drug delivery because of their size. In this review, we report on the application of ultrasound in the area of chemotherapy using a micelle-sized carrier that sequesters the chemotherapeutic agent and prevents its interaction with the rest of the body, but then can release the agent at the desired place and time upon application of ultrasound.
Numerous mechanistic studies have shown that ultrasonic cavitation is responsible for US-activated drug delivery. In vitro, cavitation releases drug from some types of micelles, particularly those that are Pluronic-based. Also cells in vitro are rendered more permeable to the drugs by cavitation events. It is most like that shear stresses from collapse cavitation events are rupturing micelles and permeabilizing cell membranes. In vivo ultrasonic-activated chemotherapeutic delivery from micelles reduces tumors in rats and mice. There is more drug present in the insonated tumors, most probably the result of cavitation-induced shear of micelles. It is highly likely that the tumor cell membrane is also permeabilized, resulting in greater drug uptake.
The advantages of such a delivery system are numerous. Since harmful drug is sequestered until the desired release place and time, the side effects of chemotherapy can be minimized. The drug loading in the body could be increased without detriment, and the drug loading will be concentrated at the targeted site to produce the maximum effect. This technology could also be combined with other novel targeting techniques, such as attachment of tumor-targeting molecules to the outside of the micelles. Such a technology would provide the oncologist with a very effective weapon in the fight against cancer.
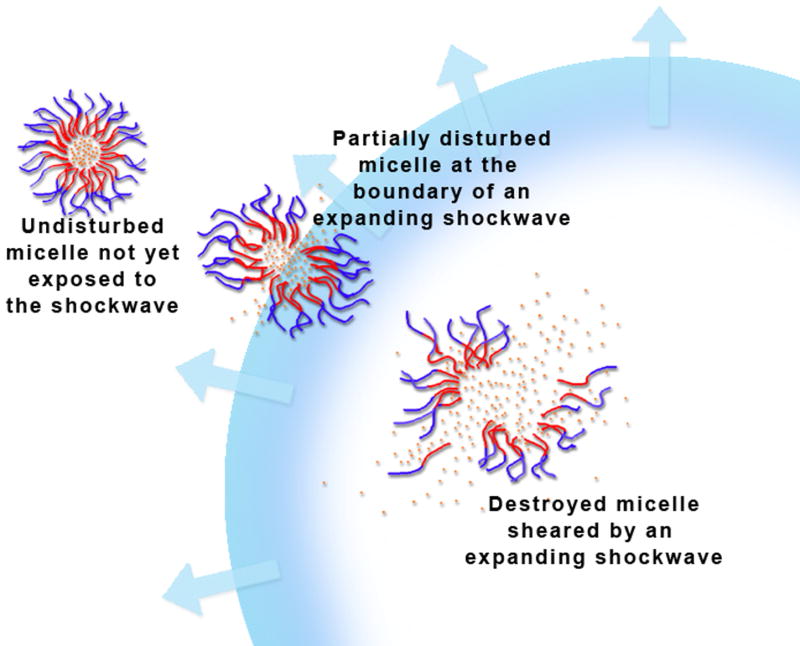
Figure 1.
A schematic showing the proposal mechanism of ultrasonic release of Dox from Pluronic micelles.
Figure 2.
5. Nomenclature
- AIBN
Azobisisobutyronitrile
- ANN
Artificial Neural Networks
- BAC
Bis-acryloyl cystamine
- BBB
Blood Brain Barrier
- BCA
Bicinchoninic Acid
- CMC
Critical Micellar Concentration
- Dox
Doxorubicin
- DPH
Diphenylhexatriene
- EPR
Electron Paramagnetic Resonance
- IPN
Interpenetrating Network
- MDR
Multidrug Resistance
- NIPAAm
N-isopropylacrylamide
- NNDEA
N,N′-diethylacrylamide
- PEO
Poly(ethyleneoxide)
- PEG-PLLA
Poly(ethylene oxide) block-poly(L-lactide)
- PEG-PLC
Poly(ethylene oxide) block-poly lactide
- PFC5
Perfluoropentane
- Pluronic®
A triblock copolymer of PEO-PPO-PEO
- Plurogel™
A Pluronic P105 micelle stabilized with an IPN of NNDEA
- P105
Pluronic P105 (PEO37-PPO56-PEO37)
- PPO
Poly(propyleneoxide)
- SDS
Sodium dodecyl sulfate
- US
Ultrasound
Biography
Brief Biography of Authors

Name: Ghaleb A. Husseini
Affiliation: Assistant Professor of Chemical Engineering – American University of Sharjah
Address: PO Box 26666, Chemical Engineering Department, American University of Sharjah, Sharjah, United Arab Emirates
Brief Biographical History: Dr. Ghaleb Husseini has been an Assistant Professor of Chemical Engineering at the American University of Sharjah since January 2004. Dr. Husseini graduated with a PhD in Chemical Engineering (biomedical engineering emphasis) from Brigham Young University, Provo, UT, in August 2001. His PhD research involved sequestering chemotherapeutic agents (namely Doxorubicin and Ruboxyl) in stabilized Pluronic micelles. After graduation, he conducted research in the area of photochemical lithography and soft lithography. His projects aimed at creating acid chloride and epoxide functionalized surfaces by exposure to UV light. He also developed a novel method of micro-printing on silicon surfaces using scribed silicon wafers (soft lithography). He has started research in the area of non-viral gene delivery using ultrasonic power.
Membership in Learned Societies: American Institute of Chemical Engineers, Society For Biomaterials, Controlled Release Society

Name: Dr. William G. Pitt
Affiliation: Department of Chemical Engineering
Address: 350 Clyde Bldg., Brigham Young University, Provo, UT 84602 USA
Brief Biographical History: BS, 1983, Brigham Young University, Chemical Engineering, PhD, 1987, University of Wisconsin-Madison, Chemical Engineering, Teaching and research at Brigham Young University (Chemical Engineering Department) since 1987
Membership in Learned Societies: American Institute of Chemical Engineers, Society for Biomaterials, Controlled Release Society, American Association for Cancer Research
References
- 1.Huber PE, Debus J. Radiation Research. 2001;156:301. doi: 10.1667/0033-7587(2001)156[0301:tcivar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Huber PE, Jenne JW, Rastert R, Simiantonakis I, Sinn HP, Strittmatter HJ, von Fournier D, Wannenmacher MF, Debus J. Cancer Res. 2001;61:8441. [PubMed] [Google Scholar]
- 3.Lu XQ, Burdette EC, Bornstein BA, Hansen JL, Svensson GK. Int J Hyperthermia. 1996;12:375. doi: 10.3109/02656739609022526. [DOI] [PubMed] [Google Scholar]
- 4.Underwood HR, Burdette EC, Ocheltree KB, Magin RL. Int J Hyperthermia. 1987;3:257. doi: 10.3109/02656738709140392. [DOI] [PubMed] [Google Scholar]
- 5.Draper DO, Castel JC, Castel D. J Orthop Sports Phys Ther. 1995;22:142. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]
- 6.Nyborg WL. Ultrasound Med Biol. 2001;27:301. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 7.Williams AR, Miller DL. Ultrasound Med Biol. 1980;6:251. doi: 10.1016/0301-5629(80)90020-4. [DOI] [PubMed] [Google Scholar]
- 8.Rooney JA. In: Ultrasound Its Chemical, Physical, and Biological Effects. Suslick KS, editor. VCH; New York: 1988. p. 65. [DOI] [PubMed] [Google Scholar]
- 9.Rooney JA. Science. 1970;169:869. doi: 10.1126/science.169.3948.869. [DOI] [PubMed] [Google Scholar]
- 10.Morton KI, Ter Haar GR, Stratford IJ, Hill CR. Br J Cancer. 1982;45:147. [PMC free article] [PubMed] [Google Scholar]
- 11.Morton KI, Haar GRt, Stratford IJ, Hill CR. Ultrasound Med Biol. 1983;9:629. doi: 10.1016/0301-5629(83)90008-x. [DOI] [PubMed] [Google Scholar]
- 12.Everbach EC, Makin IRS, Azadniv M, Meltzer RS. Ultrasound Med Biol. 1997;23:619. doi: 10.1016/s0301-5629(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 13.Atchley AA, Crum LA. In: Ultrasound Its Chemical, Physical, and Biological Effects. Suslick KS, editor. VCH Publishers; New York: 1988. p. 1. [Google Scholar]
- 14.Delius M. Ultrasound Med Biol. 1997;23:611. doi: 10.1016/s0301-5629(97)00038-0. [DOI] [PubMed] [Google Scholar]
- 15.Pitt WG. Am J Drug Deliv. 2003;1:27. [Google Scholar]
- 16.Pitt WG, Husseini GA, Staples BJ. Expert Opin Drug Delivery. 2004;1:37. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena J, Sharma N, Makiod MC, Banakar UV. J Biomat Appl. 1993;7:227. doi: 10.1177/088532829300700306. [DOI] [PubMed] [Google Scholar]
- 18.Mitragotri S, Blankschtein D, Langer R. Science. 1995;269:850. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 19.Mitragotri S, Edwards DA, Blankschtein D, Langer R. Journal of Pharmaceutical Sciences. 1995;84:697. doi: 10.1002/jps.2600840607. [DOI] [PubMed] [Google Scholar]
- 20.Kassan DG, Lynch AM, S MJ. J Amer Acad Dermatology. 1996;34:657. doi: 10.1016/s0190-9622(96)80069-7. [DOI] [PubMed] [Google Scholar]
- 21.Husseini GA, Christensen DA, Rapoport NY, Pitt WG. J Controlled Rel. 2002;83:302. doi: 10.1016/s0168-3659(02)00203-1. [DOI] [PubMed] [Google Scholar]
- 22.Husseini GA, Myrup GD, Pitt WG, Christensen DA, Rapoport NY. J Controlled Release. 2000;69:43. doi: 10.1016/s0168-3659(00)00278-9. [DOI] [PubMed] [Google Scholar]
- 23.Husseini GA, Diaz MA, Richardson ES, Christensen DA, Pitt WG. J Controlled Release. 2005;107:253. doi: 10.1016/j.jconrel.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husseini GA, Diaz MA, Zeng Y, Christensen DA, Pitt WG. Journal of Nanoscience and Nanotechnology. 2007;7:1. doi: 10.1166/jnn.2007.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruskal J, Miner B, Goldberg SN, Kane RA. Radiology. 2002;225:587. [Google Scholar]
- 26.Maeda H. Adv Enzyme Regul. 2001;41:189. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 27.Greish K, Sawa T, Fang J, Akaike T, Maeda H. Journal of Controlled Release. 2004;97:219. doi: 10.1016/j.jconrel.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Cancer Res. 1994;54:987. [PubMed] [Google Scholar]
- 29.Kwok CS, Mourad PD, Crum LA, Ratner BD. J Biomed Mater Res. 2001;57:151. doi: 10.1002/1097-4636(200111)57:2<151::aid-jbm1154>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Takada E, Sunagawa M, Ohdaira E, Ido M. Ultrasound Med Biol. 1997;23:S132. [Google Scholar]
- 31.Tata DB, Hahn G, Dunn F. Ultrasonics. 1993;31:447. doi: 10.1016/0041-624x(93)90054-4. [DOI] [PubMed] [Google Scholar]
- 32.Tata DB, Biglow J, Wu JR, Tritton TR, Dunn F. Ultrasonics Sonochem. 1996;3:39. [Google Scholar]
- 33.Saad AH, Hahn GM. Cancer Res. 1989;49:5931. [PubMed] [Google Scholar]
- 34.Saad AH, Hahn GM. Ultrasound Med Biol. 1992;18:715. doi: 10.1016/0301-5629(92)90122-q. [DOI] [PubMed] [Google Scholar]
- 35.Saad AH, Hahn GM. In: Heat transfer in bioengineering and medicine. Chato JC, Diller TE, Diller KR, Roemer RB, editors. Am. Soc. Mech. Engr. Press; New York: 1987. p. 28. [Google Scholar]
- 36.Stephen M, Alonso A. Prog Biophys Molec Biol. 2006;93:354. [Google Scholar]
- 37.Torchilin VP. Adv Drug Delivery Rev. 2006;58:1532. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Kataoka K, Kwon GS, Yokoyama M, Okano T, Sakurai Y. J Controlled Release. 1993;24:119. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 39.Kataoka K, Matumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS. Journal of Controlled Release. 2000;64:143. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 40.Kim YH, Kwon IC, Bae YH, Kim SW. Macromolecules. 1995;28:939. [Google Scholar]
- 41.Kwon G, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K. J Control Release. 1997;48:195. [Google Scholar]
- 42.Kwon G, Naito M, Yokoyama M, Sakurai Y, Okano T, Kataoka K. Langmuir. 1993;9 [Google Scholar]
- 43.Kwon GS, Kataoka K. Advanced Drug Delivery Reviews. 1995;16:295. doi: 10.1016/j.addr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Kwon GS, Naito M, Kataoka K, Yokoyama M, Sakurai Y, Okano T. Colloids and Surfaces B: Biointerfaces. 1994;2:429. [Google Scholar]
- 45.Kwon GS, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Pharm Res. 1995;12:192. doi: 10.1023/a:1016266523505. [DOI] [PubMed] [Google Scholar]
- 46.Kwon GS, Suwa S, Yokoyama M, Okano T, Sakurai Y, Kataoka K. J Contr Rel. 1994;29:17. [Google Scholar]
- 47.Kwon GS, Suwa S, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Pharm Res. 1995;12:192. doi: 10.1023/a:1016266523505. [DOI] [PubMed] [Google Scholar]
- 48.Kwon GS, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Pharmac Res. 1993;10:970. doi: 10.1023/a:1018998203127. [DOI] [PubMed] [Google Scholar]
- 49.Kwon OH, Kikuchi A, Yamato M, Sakurai Y, Okano t. J Biomed Mater Res. 2000;50:82. doi: 10.1002/(sici)1097-4636(200004)50:1<82::aid-jbm12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama M, Kwon GS, Naito M, Okano T, sakurai Y, Seto T, Kataoka K. Bioconj Chem. 1992;3:295. doi: 10.1021/bc00016a007. [DOI] [PubMed] [Google Scholar]
- 51.Chung JE, Yokoyama M, Yamato M, Aohagi T, Sakurai Y, Okano T. J Control Rel. 1999;62:115. doi: 10.1016/s0168-3659(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama M. In: Advances in Polymeric Systems for Drug Delivery. Okano T, editor. Gordon and Breach Science Publishers; Iverdon, Switzerland: 1994. p. 24. [Google Scholar]
- 53.Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Cancer Res. 1991;51:3229. [PubMed] [Google Scholar]
- 54.Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kataoka K. J Drug Targetting. 1999;7:171. doi: 10.3109/10611869909085500. [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama M, Sugiyama T, Okano T, Sakurai Y, Naito M, Kataoka K. Pharmac Res. 1993;10:895. doi: 10.1023/a:1018921513605. [DOI] [PubMed] [Google Scholar]
- 56.Rapoport NY, Herron JN, Pitt WG, Pitina L. J Controlled Rel. 1999;58:153. doi: 10.1016/s0168-3659(98)00149-7. [DOI] [PubMed] [Google Scholar]
- 57.Batrakova E, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Pharm Res. 1999;16:1373. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- 58.Woodle MC. Adv Drug Deliv Rev. 1998;32:139. doi: 10.1016/s0169-409x(97)00136-1. [DOI] [PubMed] [Google Scholar]
- 59.Ning S, Macleod K, Abra R, Huang AH, Hahn GM. Int J Radiation Oncology Phys. 1994;29:827. doi: 10.1016/0360-3016(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 60.Vyas SP, Singh R, Asati RK. J Microencapsul. 1995;12:149. doi: 10.3109/02652049509015285. [DOI] [PubMed] [Google Scholar]
- 61.Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Invest Radiol. 1998;33:886. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Munshi N, Rapoport N, Pitt WG. Cancer Letters. 1997;117:1. doi: 10.1016/s0304-3835(97)00218-8. [DOI] [PubMed] [Google Scholar]
- 63.Venne A, Li S, Mandeville R, Kabanov A, Alakhov V. Cancer Res. 1996;56:3626. [PubMed] [Google Scholar]
- 64.Pruitt JD, Husseini G, Rapoport N, Pitt WG. Macromolecules. 2000;33:9306. [Google Scholar]
- 65.Husseini GA, Rapoport NY, Christensen DA, Pruitt JD, Pitt WG. Coll Surf B: Biointerfaces. 2002;24:253. [Google Scholar]
- 66.Brennen CE. Cavitation and Bubble Dynamics. Oxford University Press; New York: 1995. [Google Scholar]
- 67.Stevenson-Abouelnasr D, Husseini GA, Pitt WG. Colloids and Surfaces B. 2007;55:59. doi: 10.1016/j.colsurfb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Husseini GA, Abdel-Jabbar NM, Mjalli FS, Pitt WG. Technology in Cancer Research & Treatment. 2007;6:49. doi: 10.1177/153303460700600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pruitt J. Ph.D. Dissertation. Brigham Young University; Provo, Utah: 2001. Stabilization of Pluronic P-105 for Targeted Nanoparticle Drug Delivery. [Google Scholar]
- 70.Chattopadhyay A, London E. Analytical Biochemistry. 1984;139:408. doi: 10.1016/0003-2697(84)90026-5. [DOI] [PubMed] [Google Scholar]
- 71.Rapoport N, Gao Z. Polymer Reprints. 2006;47:49. [Google Scholar]
- 72.Rapoport N. Progress in Polymer Science. 2007;32:962. [Google Scholar]
- 73.Rapoport N, Gao ZG, Kennedy A. Journal of the National Cancer Institute. 2007;99:1095. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 74.Husseini GA, El-Fayoumi RI, O'Neill KL, Rapoport NY, Pitt WG. Cancer Letters. 2000;154:211. doi: 10.1016/s0304-3835(00)00399-2. [DOI] [PubMed] [Google Scholar]
- 75.Husseini GA, O'Neill KL, Pitt WG. Technology in Cancer Research & Treatment. 2005;4:707. doi: 10.1177/153303460500400616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pruitt JD, Pitt WG. Drug Delivery. 2002;9:253. doi: 10.1080/10717540260397873. [DOI] [PubMed] [Google Scholar]
- 77.Marin A, Muniruzzaman M, Rapoport N. J Control Rel. 2001;75:69. doi: 10.1016/s0168-3659(01)00363-7. [DOI] [PubMed] [Google Scholar]
- 78.Marin A, Sun H, Husseini GA, Pitt WG, Christensen DA, Rapoport NY. J Controlled Rel. 2002;84:39. doi: 10.1016/s0168-3659(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 79.Marin A, Muniruzzaman M, Rapoport N. J Controlled Rel. 2001;71:239. doi: 10.1016/s0168-3659(01)00216-4. [DOI] [PubMed] [Google Scholar]
- 80.Rapoport N, Marin A, Luo Y, Prestwich GD, Munirzzaman M. J Pharm Sci. 2002;91:157. doi: 10.1002/jps.10006. [DOI] [PubMed] [Google Scholar]
- 81.Muniruzzaman MD, Marin A, Luo Y, Prestwich GD, Pitt WG, Husseini G, Rapoport NY. Colloids and Surfaces B: Biointerfaces. 2002;25:233. [Google Scholar]
- 82.Husseini GA, Runyan CM, Pitt WG. BMC Cancer. 2002;2:1. doi: 10.1186/1471-2407-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin HH, Cheng YL. Macromolecules. 2001;34:3710. [Google Scholar]
- 84.Lin HJ, Herman P, Kang JS, Lakowicz JR. Analytical Biochemistry. 2001;294:118. doi: 10.1006/abio.2001.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rapoport N. International Journal of Pharmaceutics. 2004;227:155. doi: 10.1016/j.ijpharm.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 86.Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Ultrasound in Medicine and Biology. 2006;32:1399. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 87.Stringham SB, Viskovska MA, Richardson ES, Ohmine S, GA H, Murray BK, Pitt WG. Ultrasound in medicine and biology. 2007 doi: 10.1016/j.ultrasmedbio.2008.09.004. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howard B, Gao A, Lee SW, Seo MH, Rapoport N. Am J Drug Deliv. 2006;4:97. [Google Scholar]
- 89.Staples BJ. M.S. Thesis. Brigham Young University; Provo, UT: 2007. Pharmacokinetics of Ultrasonically-Released, Micelle-Encapsulated Doxorubicin in the Rat Model and its Effect on Tumor Growth. [Google Scholar]
- 90.Nelson JL. M.S. Brigham Young University; Provo, UT: 2002. Ultrasonically Enhanced Drug Delivery of Doxorubicin in vivo from Stabilized Pluronic Micelle Carriers. [Google Scholar]
- 91.Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Cancer Res. 2002;62:7280. [PubMed] [Google Scholar]
- 92.Gao ZG, Fain HD, Rapoport N. Journal of Controlled Release. 2005;102:203. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 93.Gao ZG, Lee DH, Kim DI, Bae YH. Journal of Drug Targeting. 2005;13:391. doi: 10.1080/10611860500376741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Myhr G, Moan J. Cancer Letters. 2006;232:206. doi: 10.1016/j.canlet.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 95.Lucroy MD. Compendium. 2002;24:140. [Google Scholar]
- 96.Staples BJ, Roeder BL, Husseini GA, Badamjav O, Schaalje GB, Pitt WG. Role of Frequency and Mechanical INdex in Ultrasound-Enhanced Chemotherapy of Tumors in Rats. Cancer Research. 2007 doi: 10.1007/s00280-008-0910-8. submitted. [DOI] [PubMed] [Google Scholar]
- 97.Gao Z, Fain HD, Rapoport N. Molecular Pharmaceutics. 2004 doi: 10.1021/mp049958h. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rapoport N, Christensen DA, Fain HD, Barrows L, Gao Z. Ultrasoincs. 2004;42:943. doi: 10.1016/j.ultras.2004.01.087. [DOI] [PubMed] [Google Scholar]