Abstract
Intermittent claudication is the primary symptom of peripheral arterial disease (PAD), affecting between 1 and 3 million Americans. Symptomatic improvement can be achieved by endovascular revascularization, but such procedures are invasive, expensive and may be associated with procedural adverse events. Medical treatment options, including claudication medications and supervised exercise training, are also known to be effective, albeit also with associated limitations. The CLEVER Study (Claudication: Exercise Vs. Endoluminal Revascularization), funded by the National Institutes of Health Heart, Lung, and Blood Institute, is a prospective, multicenter, randomized, controlled clinical trial evaluating the relative efficacy, safety, and health economic impact of four treatment strategies for people with aortoiliac PAD and claudication. The treatment arms are: 1. optimal medical care (claudication pharmacotherapy); 2. primary stent placement; 3. supervised exercise rehabilitation; and 4. combined stenting with supervised exercise rehabilitation. CLEVER is a five-year randomized controlled clinical trial to be conducted at approximately 25 centers in the U.S. that will follow 252 patients and their responses to treatment over an 18 month follow-up period. The primary endpoint is change in maximum walking duration (MWD) on a graded treadmill test. Secondary endpoints include the change in MWD from baseline at 18 months, comparisons of free-living daily activity levels assessed by pedometer, health-related quality of life, and cost-effectiveness. Other analyses include the effect of these treatment strategies on anthropomorphic and physiologic variables (e.g., body mass index (BMI), waist circumference, blood pressure, pulse pressure, and resting pulse), and biochemical markers of cardiovascular health (e.g., fasting lipids, fibrinogen, c-reactive protein, and hemoglobin A1c values).
Introduction
Claudication is the most frequent symptom of peripheral arterial disease (P.A.D.)1, and is usually experienced as a reproducible fatigue, discomfort, or pain in the thigh or calf muscles that is provoked by walking and 2,2,3 relieved by rest . All individuals with PAD suffer functional impairment, but symptoms of moderate to severe claudication profoundly limit physical functioning4. Physical activity is known to be inversely associated with incident CVD ischemic events5, and a lack of physical activity is known to contribute to obesity, which may further increase CVD risk6. Obesity in adults is associated with walking disability7, and walking disability caused by claudication results in a sedentary lifestyle8, self-perceived ambulatory dysfunction9, and lower health-related quality of life10. Moderate to severe claudication usually precludes an active, healthy lifestyle.
Management of Intermittent Claudication
Treatment of claudication varies dependent on the severity of presenting symptoms and the associated impact of this physicial disability on individual lifestyle. Patients with mild to moderate disablility from claudication have traditionally been treated by a regimen of risk factor modification, smoking cessation and physician-derived exhortations to exercise. The de facto past standard of care has been for the physician to advise patients to “stop smoking and keep walking”11.
The role of revascularization for those with intermittent claudication has been controversial12,13. Vascular surgeons have traditionally reserved lower extremity surgical bypass for patients with moderate to severe symptoms of claudication, unresponsive to medical therapy who are at low operative risk and who are likely to enjoy good long term functional survival. This approach has been based on the low but significant perioperative cardiovascular morbidity and mortality associated with surgical bypass and the observation that the majority of patients with claudication do not progress to limb threatening ischemia. This therapeutic paradigm probably reduces procedure-related deaths, but offers minimal symptom relief to the majority of patients. In recent years, stenting has become one preferred method to achieve revascularization of occlusive lesions of the aortoiliac segment and has demonstrated clinical improvement in symptoms of claudication in uncontrolled series14. Recent advances in percutaneous treatment of lower extremity peripheral arterial disease has prompted many physicians who treat claudication to consider more liberal indications for percutaneous intervention. At the same time, it has been demonstrated that excellent improvement in maximum walking distance can also be achieved with supervised exercise therapy15, with a low risk of disease progression or amputation16 without revascularization12,13,13. Pharmacologic agents (e.g., cilostazol) have been shown to improve the symptoms of claudication in multiple randomized placebo-controlled clinical trials. The efficacy of supervised exercise and pharmacotherapy and a lack of evidence that either angioplasty or bypass surgery are more effective at preventing amputation17 in patients with claudication has offered a strong rationale that supports the use of medical management (claudication pharmacotherapy and/or supervised exercise) as the first line of therapy over any form of revascularization in patients with claudication from peripheral arterial disease. This evidence base and associated treatment algorithms have recently been encompassed in an intersocietal lower extremity PAD treatment guideline18.
Numerous clinical trials have demonstrated that supervised exercise therapy9,19–23 and claudication medications24 can significantly improve walking performance and quality of life in patients with intermittent claudication15. The first randomized controlled trial of exercise therapy in PAD patients demonstrating improvement was completed in 196625. On average, individuals enrolled in supervised exercise programs improve maximum walking duration between 74% and 240% on a continuous-load treadmill protocol26. Most comparisons of supervised exercise therapy and unsupervised exercise show no improvement15, or at best modest improvement19 in MWD for unsupervised exercise. Despite robust proof of therapeutic efficacy, one of the major factors that now severely limits access to supervised exercise rehabilitation for individuals with intermittent claudication is lack of reimbursement by healthcare payors.
The relative roles of angioplasty and exercise for patients with stable PAD and intermittent claudication have been the subject of a Cochrane Database Review27. This review presented data from two studies that randomized claudication patients to angioplasty or other treatment. In these series, most patients were treated for femoropopliteal PAD with balloon angioplasty alone, and stents were not used28,29. Results of the two randomized trials were discordant at 6 months; the Edinburgh study showed most improvement in walking distance in the angioplasty group29, while the Oxford experience showed better results with supervised exercise30. Notably, the Oxford group used supervised exercise, which offers better outcomes than home exercise31. A subgroup analysis of the Oxford experience, showed better exercise performance with angioplasty for those with aortoiliac PAD at baseline, as opposed to femoropopliteal PAD30.
The CLEVER study thus challenges the popular management paradigm for individuals with intermittent claudication by directly comparing four differing treatment strategies: 1. a primary medical therapeutic approach (e.g., home exercise with claudication pharmacotherapy, defined as “optimal medical care” or “OMC”); 2. OMC plus interventional revascularization; 3. OMC plus supervised exercise; and 4. a previously untested treatment strategy that can provide insight into the potential synergy of these approaches, OMC plus intervention plus supervised exercise therapy).
Population
The CLEVER study eligibility criteria (Table 1) target individuals with intermittent claudication resulting from ipsilateral lower extremity PAD in the aortoiliac or above-knee femoropopliteal artery segments (or both). Patients whose comorbid conditions significantly limit their walking ability are excluded from the CLEVER study as the study targets patients whose primary walking impairment is due to claudication resulting from arterial insufficiency. The only anatomic contraindications for randomization will be patients who are randomized to stent revascularization by noninvasive tests and are found at angiography to have total aortoiliac obstruction from the renal arteries to the inguinal ligaments at angiography, those with common femoral artery stenoses or occlusions, or those with femoropopliteal arterial insufficiency that includes the origin of the superficial femoral artery or the popliteal artery at or below the knee joint or doesn’t run off into at least a single patent calf artery. For those enrolled due to femoropopliteal artery insufficiency, many will be enrolled after imaging tests like magnetic resonance angiography (MRA) or computed tomographic angiography (CTA). However, any patients with these criteria identified after randomization (for example, those with aortoiliac disease identified by noninvasive tests alone) will be retained in their assigned treatment group and will be analyzed in their intention-to-treat cohort.
Table 1.
CLEVER Study Eligibility Criteria. Scoring of the San Diego Claudication Questionnaire for CLEVER eligibility is unique to this study and explained in the protocol. All duplex ultrasound, MRA and CTA images are submitted to the duplex core lab or Clinical Coordinating Center for over read.
| Inclusion Criteria | ||||
| 1. | Subject has symptoms suggestive of intermittent claudication, such as exercise-induced pain, cramps, fatigue, or other equivalent discomfort, involving large muscle groups of the leg(s) (calf, thigh, buttocks), relieved by rest. | |||
| 2. | Subject is ≥ 40 years old. | |||
| 3. | Subject has resting ipsilateral ankle-brachial index (ABI) <0.9. | |||
| 4. | Claudication score consistent with “Rose”, “atypical”, or “noncalf” claudication by San Diego Claudication Questionnaire (see Appendix A for acceptable responses) | |||
| 5. | Significant lower extremity artery PAD on the most symptomatic side(s) (bilaterally if symptoms are equal): | |||
| 5.1 | For Aortoiliac Insufficiency | |||
| a. | Contrast Arteriography: Contrast arteriogram showing at least 50% stenosis in the aorta, common iliac artery, or external iliac artery, OR | |||
| b. | CTA or MRA: At least 60% stenosis in the aorta, common iliac artery, external iliac artery, accompanied by a biphasic or monophasic Doppler wave form at the common femoral artery (loss of early diastolic flow reversal or loss of forward flow during diastole), OR | |||
| c. | Duplex Ultrasound: Occlusion of focal doubling of peak systolic velocity in the aorta, common iliac artery, or external iliac artery, accompanied by a biphasic or monophasic Doppler wave form at the common femoral artery (loss of early diastolic flow reversal or loss of forward flow during diastole), OR | |||
| d. | Vascular Noninvasive Physiologic Tests: Resting thigh-brachial index (thigh-BI) < 1.1, and common femoral artery Doppler systolic acceleration time >140 msec [these tests may be ordered for study screening]. | |||
| Note: MRA/CTA, and contrast arteriogram images images must be submitted to the Clinical Coordinating Center and Doppler waveform tracings to the Noninvasive Test Committee for over read pre- or post-randomization. | ||||
| 5.2 | For Femoropopliteal Artery Insufficiency | |||
| Contrast Arteriography or CTA or MRA: At least 50% stenosis in the femoropopliteal segment between 5 mm past the origin of the superficial femoral artery to the popliteal artery at least 3 cm cephalad to the knee joint. Cumulative lesion length (adding up the total lengths of disease segments) must be less than 25 cm long. There must be no significant arterial obstruction caudal to 3 cm above the knee joint, and there must be at least one calf artery patent to at least the ankle level (peroneal artery to its bifurcation qualifies). | ||||
| 6. | Highest ankle pressure reduced by at least 25 mm Hg after exercise compared to resting pressure. | |||
| Note: The highest ankle pressure result is determined by using the higher result of either the dorsalis pedis or posterior tibial artery measurement. | ||||
| 7. | Subject has moderate to severe claudication symptoms, defined as less than 9 minutes MWD at baseline (initial) Gardner treadmill test (see Appendix B). | |||
| 8. | Performance on a second Gardner treadmill test within 25% of the initial baseline MWD test result. | |||
| 3.3.2. | Exclusion Criteria | |||
| 1. | Presence of critical limb ischemia (Rutherford Grade II or III PAD, defined as pain at rest, ischemic ulceration, gangrene) or acute limb ischemia (pain, pallor, pulselessness, paresthesias, paralysis) in either leg. | |||
| 2. | Common femoral artery (CFA) occlusion or >=50% stenosis by angiography, MRA, CTA, or duplex ultrasound or doubling of systolic velocity in the ipsilateral common femoral artery by duplex ultrasound, or 50% diameter stenosis by visual estimate in the CFA by angiography, MRA, or CTA, (inadequate outflow for iliac stent intervention), if available pre-randomization | |||
| 3. | Known total aortic occlusion from the renal arteries to the bifurcation of the aorta. | |||
| 4. | Patient has bilateral aortoiliac arterial insufficiency but one side is anatomically ineligible (e.g., not amenable to stenting due to common femoral artery stenosis or occlusion). | |||
| 5. | If aortoiliac and femoropopliteal arterial insufficiency coexist on the same side, and aortoiliac insufficiency is not revascularizable due to total aortoiliac occlusion from the renal arteries through the external iliac arteries, or due to common femoral artery significant (>=50%) stenosis or occlusion, patients are ineligible. [Patients with combined aortoiliac and femoropopliteal insufficiency can be enrolled if aortoiliac insufficiency meets eligibility criteria even if femoropopliteal insufficiency doesn’t meet anatomic eligibility criteria. Therefore, if a patient has significant (at least 50% by diameter) stenosis in the aorta or iliac arteries, and has ipsilateral femoropopliteal artery disease which is anatomically ineligible, they can be enrolled under the “Aortoiliac Insufficiency” Criteria above (section 3.3.1, number 5).] Patients with extensive femoropopliteal disease exceeding inclusion criteria and qualifying aortoiliac disease can be enrolled but intervention should not be done in the femoropopliteal segment. | |||
| 6. | Participant has bilateral claudication symptoms and the limb that is more symptomatic does not show evidence of aortoiliac insufficiency or femoropopliteal artery insufficiency as described in inclusion criterion number 5. | |||
| 7. | Participant has bilateral claudication symptoms, but both limbs are equally symptomatic but one side does not show evidence of aortoiliac insufficiency or femoropopliteal artery insufficiency as described in inclusion criterion number 5. | |||
| 8. | Subject meets the following exclusions based upon modified American College of Sports Medicine criteria for exercise training: | |||
| i. | Ambulation limited by co-morbid condition other than claudication, for example: | |||
| 1. | severe coronary artery disease | |||
| 2. | angina pectoris | |||
| 3. | chronic lung disease | |||
| 4. | neurological disorder such as hemiparesis | |||
| 5. | arthritis, or other musculoskeletal conditions including amputation | |||
| ii | Poorly-controlled hypertension (SBP>180 mm Hg) | |||
| iii | Poorly-controlled diabetes mellitus | |||
| iv | Other active significant medical problems such as cancer, known chronic renal disease (serum creatinine >2.0 mg/dl within 90 days or renal replacement therapy), known chronic liver disease or anemia, active substance abuse, or known history of dementia. | |||
| 9. | Contraindication to exercise testing according to AHA/ACC guideline, specifically: Acute myocardial infarction (within 3-5 days), unstable angina, uncontrolled cardiac arrhythmias causing symptoms or hemodynamic compromise, active endocarditis, symptomatic severe aortic stenosis, acute pulmonary embolus or pulmonary infarction, acute noncardiac disorder that may affect exercise performance or be aggravated by exercise such as infection, thyrotoxicosis, acute myocarditis or pericarditis, known physical disability that would preclude safe and adequate test performance, known thrombosis of the lower extremity, known left main coronary stenosis or its equivalent, moderate stenotic valvular heart disease, electrolyte abnormalities, known pulmonary hypertension, tachyarrhythmias or bradyarrhythmias, hypertrophic cardiomyopathy, mental impairment leading to inability to cooperate, or high degree atrioventricular block | |||
| 10. | Subject is eligible due to only aortoiliac insufficiency and has had previous ipsilateral aortic or iliac artery revascularization (surgery or intervention). | |||
| 11. | Subject is eligible due to only femoropopliteal artery insufficiency and has had previous ipsilateral femoropopliteal artery revascularization (surgery or intervention) [if qualified by both aortoiliac and femoropopliteal artery insufficiency and prior femoropopliteal artery intervention has been done the participant can be eligible and femoropopliteal intervention or surgery can be done at the operator’s discretion if restenosis is present]. | |||
| 12. | Recent major surgery in the last 3 months. | |||
| 13. | Abdominal aortic aneurysm > 4 cm or iliac artery aneurysm >1.5 cm is present. | |||
| 14. | Patients who are pregnant, planning to become pregnant, or lactating. | |||
| 15. | Unwilling or unable to attend regular (3 times a week) supervised exercise sessions. {Please review this commitment carefully with each prospective participant} | |||
| 16. | Weight >350 lbs or 159 kg (may exceed treadmill and angiography table limits). | |||
| 17. | Language barrier exists for primary QoL instruments (available in English and Spanish). | |||
| 18. | Inability to understand and sign informed consent forms due to cognitive or language barriers (interpreter permitted). | |||
| 19. | Absolute contraindication to iodinated contrast due to prior near-fatal anaphylactoid reaction (laryngospasm, bronchospasm, cardiorespiratory collapse, or equivalent) and which would preclude patient from participation in angiographic procedures. | |||
| 20. | Allergy to stainless steel or nitinol. | |||
| 21. | Allergy or other intolerance to cilostazol (bleeding history) or history of congestive heart failure [if ejection fraction is shown to be >=50% patient may be enrolled] | |||
| 22. | Nonatherosclerotic cause of PAD (fibromuscular dysplasia, dissection, trauma, etc). | |||
| 23. | Inability to walk on a treadmill without grade at a speed of at least 2 mph for at least 2 minutes on the first treadmill test. | |||
| 24. | ST-segment depression >1 mm in any of the standard 12 ECG leads or sustained (>30 seconds) arrhythmia other than tachycardia or occasional premature atrial or ventricular contractions during exercise testing. | |||
| 25. | Post-exercise systolic blood pressure within the first five minutes after eligibility treadmill test lower than pre-exercise systolic blood pressure. | |||
| 26. | A peak heart rate ≥80% of maximum (calculated by subtracting age from 220) while reporting ”onset” of claudication symptoms (level 3 or 4) during the second baseline examination. | |||
| 27. | Repeat treadmill test shows a MWD result that is >25% different than the subject’s initial Gardner treadmill test result. | |||
| 28. | Current active involved in a supervised exercise program (e.g., cardiac rehabilitation) for more than 2 weeks within the prior 6 weeks. | |||
Treatment groups
There are four treatment groups in the CLEVER Study: Optimal medical care (OMC), intervention/stent (ST), supervised exercise (SE), and combined ST + SE. All CLEVER study participants will receive (a) PAD-specific recommendations for risk factor management, including advice on diet and home-based exercise and (b) claudication medication (cilostazol as Pletal®, Otsuka America Pharmaceuticals) without charge for the duration of the study. The SE group will enroll in a supervised exercise training program for 26 weeks, consisting of 3 sessions per week at a designated training center, with sessions one hour long. The baseline workload and subsequent increases in workload are protocol-driven using methods that have been previously described33. Supervised exercise performance including improvement in performance will be centrally monitored with triggers identified to indicate advancement of exercise prescription and to optimize treatment delivery. An Exercise Training Committee consisting of 7 members will interact with sites directly prior to and during training of each participant enrolled in and exercise training treatment group. Furthermore, a personal health educator will deliver a telephone-based behavioral intervention designed to maintain exercise from months 7 through 18 after the supervised exercise ends for this group.
Patients randomized to intervention/stenting will receive OMC care and stent revascularization of aortoiliac and above-knee femoropopliteal atherosclerotic stenoses.
The ST+SE group is designed to explore the relative additive benefit of supervised exercise program on physical functioning for individuals who also undergo stent placement. This strategy has intuitive appeal although no data regarding this combined approach have been published. Analysis of this group will be “hypothesis-generating” as this study is not powered to evaluate the potential synergy between supervised exercise therapy and stent placement in the primary analysis.
Cardiovascular Risk Factor Management and Background Therapy
In CLEVER, all treatment arms will receive specific recommendations to achieve risk factor modification to specific guideline-derived targets in order to reduce their risk of cardiovascular ischemic events. CLEVER investigators have adopted current treatment recommendations to facilitate success for each randomized subject, regardless of treatment assignment, to achieve smoking cessation, lipid and blood pressure management, and diabetes control. Additionally, all patients will receive educational materials designed to foster community-based exercise and, if renal function is normal, about the DASH Eating Plan34. Active engagement of risk factor therapy and background medical therapy for each subject at every study site has rarely been a component of prior claudication clinical trials.
Endpoints
The primary endpoint of the CLEVER study is change in maximum walking duration (MWD) on a graded treadmill test (Gardner Protocol) from baseline to 6 months, which is the total duration of the supervised exercise treatment. After supervised exercise ends, a behavioral medicine program will start and continue for twelve more months. Then, comparisons of change in MWD from baseline will be repeated at 18 months to evaluate the durability of both stent and supervised exercise results. Other secondary endpoints to be evaluated at both six and eighteen months include: comparisons of free-living daily activity levels using pedometer readings, health-related quality of life (as assessed by the SF-12, the Walking Impairment Questionnaire, and the Peripheral Artery Questionnaire), cost-effectiveness, effect of treatments on diverse cardiovascular risk factors such as body mass index (BMI), waist circumference, blood pressure, pulse pressure, resting pulse, and biochemical markers such as fasting lipids, fibrinogen, c-reactive protein, and hemoglobin A1c; and examination of subgroup interactions.
Hypothesis Testing and Randomization
The primary aim of the study is to compare the relative effect of the three arms (Optimal Medical Care, OMC plus Intervention/Stent, and OMC plus Supervised Exercise) on improvement of maximal walking duration (MWD), measured as the change in MWD between baseline and six months after randomization. The study is powered to detect a 30% difference in the change in MWD for stent versus supervised exercise treatments14. Although the stent versus supervised exercise comparison is of primary interest, there are limited data demonstrating that either of these treatments is superior to optimal medical care. Therefore, CLEVER is designed to test whether each of these interventional therapies is superior to Optimal Medical Care before comparing them to each other. This sequential analysis plan will preserve the desired 5% alpha for the primary comparison of interest.
Assuming a baseline mean MWD of five minutes and 125% increase in MWD after supervised exercise9,31,33, a sample size of 63 patients for each of the ST and SE groups yields 80% power to detect a 30% treatment difference in mean change from baseline using a two-sided 0.05 level of significance. CLEVER will enroll 84 patients in each of these group to account for subject loss to follow-up. Assuming a 60% improvement in MWD between baseline and six months for the Optimal Medical Care group, 37 Optimal Medical Care patients provide 99% and 98% power to detect a 30% relative improvement for each of OMC plus Stent and OMC plus Supervised Exercise groups, respectively, using a one-sided 0.025 level of significance for each comparison. CLEVER will enroll 42 patients to provide 37 for analysis.
In addition to the three study arm comparisons, there is also interest in a possible additive effect of combined therapy with stenting and supervised exercise compared with stenting alone. CLEVER will randomize 42 such patients to evaluate this secondary hypothesis.
To achieve the desired unequal sample sizes, randomization will be performed according to the scheme in Figure 1. Pairwise analyses of covariance will be used to assess the significance of the difference between (a) stent versus medical care, (b) supervised exercise versus medical care, and (c) supervised exercise versus stent on the change from baseline MWD at 6-months (Figure 2). Each pairwise contrast will be carried out one at a time in the order above, using analysis of covariance, adjusting for baseline MWD, baseline cilostazol use, and study center.
Figure 1.
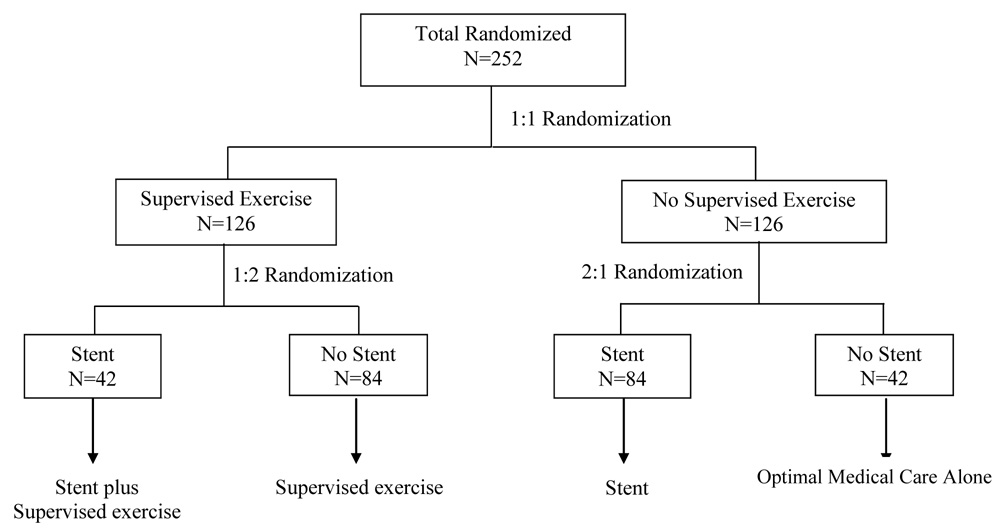
Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) trial subject treatment allocation plan. (All treatment groups included optimal medical care).
Figure 2.

Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) trial statistical analysis plan.
A Novel Approach to Claudication Clinical Investigation
CLEVER should provide a database that can inform many aspects that underpin the choice of treatment strategy for individuals with claudication. First, the relative efficacy of the three major current treatments will be defined in a “real world” multicenter clinical trial, as well as assessment of a new combined therapy, stent plus supervised exercise, which could be the optimal treatment for this disease by providing local as well as systemic therapy. Second, these data should, beyond defining the impact of these therapies on treadmill-based exercise performance, also define the impact of these treatments on subjective quality of life,community-based walking, and potentially other surrogate markers of ischemic cardiovascular disease risk. CLEVER may elucidate differential impacts among treatments in important population subgroups (e.g., by age, gender, or ethnicity). Claudication treatment decisions are complex, and offer distinct balances of effects on short- and long-term individual and public health, and the CLEVER database should permit these decisions to be made with greater precision to optimize patient outcomes.
Conclusions
New cardiovascular therapeutic technologies and medications may be approved and then rapidly disseminate into clinical practice without rigorous evaluation of their relative benefit and risk. For individuals with PAD and claudication, small single-center randomized clinical trials have offered inconsistent results and do not fully inform clinicians and patients regarding the relative efficacy and safety of invasive, pharmacologic, and exercise claudication treatments. The current high prevalence of claudication and its anticipated increase in our aging population mandate that prospective clinical trials continue to measure the potential benefit, risk, and health economic impact of each potential strategy on management of this common cardiovascular disease. The CLEVER trial, sponsored by the National Heart, Lung, and Blood Institute of the National Institutes of Health, is currently enrolling subjects and provides a unique opportunity to test both established and emerging strategies of care for individuals with PAD and claudication.
Figure 3.
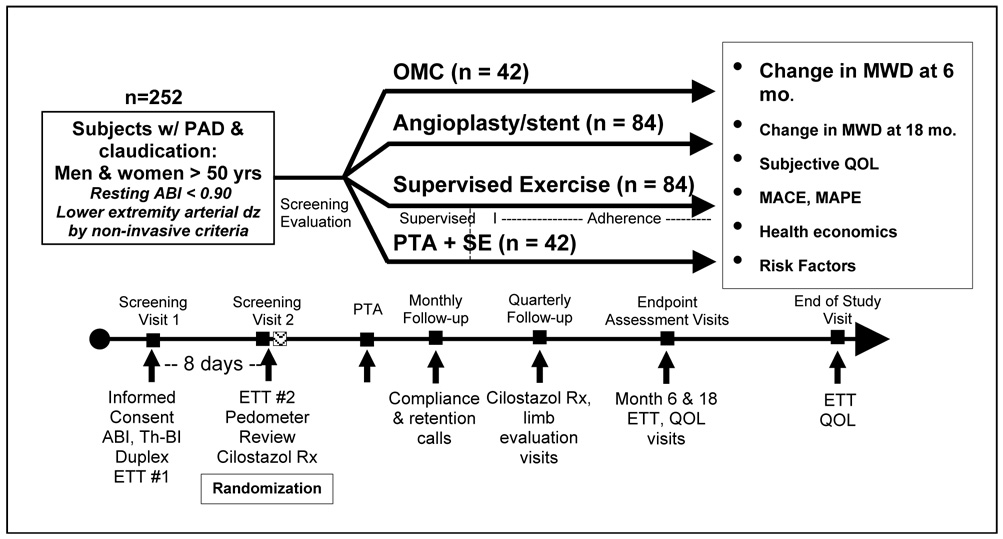
Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) trial timeline and study design.
Author Acknowledgments
Alan T Hirsch, MD, John J Ricotta, MD, Donald E Cutlip, MD, Emile Mohler, MD, Judith G Regensteiner, PhD, Anthony J Comerota, MD, David J Cohen, MD, Joseph Massaro, PhD; Beth A. Lewis, PhD; Michael R. Jaff, DO; George Sopko, MD; Joselyn Cerezo, MD; Niki U. Cotton-Oldenburg, MPH, DrPH; Michael W. Steffes, MD., PhD.; Suzanne H. Goldberg, MSN; and Abby G. Ershow, Sc.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 2.Hiatt WR, Marshall JA, Baxter J, Sandoval R, Hildebrandt W, Kahn LR, et al. Diagnostic methods for peripheral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597–606. doi: 10.1016/0895-4356(90)90164-k. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–489. [PubMed] [Google Scholar]
- 4.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 5.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 6.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Weil E, Wachterman M, McCarthy EP, Davis RB, O'Day B, Iezzoni LI, et al. Obesity among adults with disabling conditions. JAMA. 2002;288:1265–1268. doi: 10.1001/jama.288.10.1265. [DOI] [PubMed] [Google Scholar]
- 8.Sieminski DJ, Gardner AW. The relationship between free-living daily physical activity and the severity of peripheral arterial occlusive disease. Vasc Med. 1997;2:286–291. doi: 10.1177/1358863X9700200402. [DOI] [PubMed] [Google Scholar]
- 9.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23:104–115. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 10.Feinglass J, McCarthy WJ, Slavensky R, Manheim LM, Martin GJ The Chicago Claudication Outcomes Research Group. Effect of lower extremity blood pressure on physical functioning in patients who have intermittent claudication. J Vasc Surg. 1996;24:503–511. doi: 10.1016/s0741-5214(96)70066-6. [DOI] [PubMed] [Google Scholar]
- 11.Housley E. Treating claudication in five words. Br Med J (Clin Res Ed) 1988;296:1483–1484. doi: 10.1136/bmj.296.6635.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffman JD. Intermittent claudication--be conservative. N Engl J Med. 1991;325:577–578. doi: 10.1056/NEJM199108223250810. [DOI] [PubMed] [Google Scholar]
- 13.Hertzer NR. The natural history of peripheral vascular disease. Implications for its management. Circulation. 1991;83:I12–I19. [PubMed] [Google Scholar]
- 14.Murphy TP, Soares GM, Kim HM, Ahn SH, Haas RA. Quality of life and exercise performance after aortoiliac stent placement for claudication. J Vasc Interv Radiol. 2005;16:947–953. doi: 10.1097/01.RVI.0000161140.33944.ED. quiz 954. [DOI] [PubMed] [Google Scholar]
- 15.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med. 2002;347:1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 16.Jelnes R, Gaardsting O, Hougaard Jensen K, Baekgaard N, Tonnesen KH, Schroeder T. Fate in intermittent claudication: outcome and risk factors. Br Med J (Clin Res Ed) 1986;293:1137–1140. doi: 10.1136/bmj.293.6555.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tunis SR, Bass EB, Steinberg EP. The use of angioplasty, bypass surgery, and amputation in the management of peripheral vascular disease. N Engl J Med. 1991;325:556–562. doi: 10.1056/NEJM199108223250806. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation.Circulation 2006113e463–e654. [DOI] [PubMed] [Google Scholar]
- 19.Savage P, Ricci MA, Lynn M, Gardner A, Knight S, Brochu M, et al. Effects of home versus supervised exercise for patients with intermittent claudication. J Cardiopulm Rehabil. 2001;21:152–157. doi: 10.1097/00008483-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. J Vasc Surg. 1997;25:312–318. doi: 10.1016/s0741-5214(97)70352-5. discussion 318–319. [DOI] [PubMed] [Google Scholar]
- 21.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc. 2001;49:755–762. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 22.Ekroth R, Dahllof AG, Gundevall B, Holm J, Schersten T. Physical training of patients with intermittent claudication: indications, methods, and results. Surgery. 1978;84:640–643. [PubMed] [Google Scholar]
- 23.Clifford PC, Davies PW, Hayne JA, Baird RN. Intermittent claudication: is a supervised exercise class worth while? Br Med J. 1980;280:1503–1505. doi: 10.1136/bmj.280.6230.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE, Jr, Bortey EB, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Arch Intern Med. 1999;159:2041–2050. doi: 10.1001/archinte.159.17.2041. [DOI] [PubMed] [Google Scholar]
- 25.Larsen OA, Lassen NA. Effect of daily muscular exercise in patients with intermittent claudication. Lancet. 1966;2:1093–1096. doi: 10.1016/s0140-6736(66)92191-x. [DOI] [PubMed] [Google Scholar]
- 26.Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000990. CD000990. [DOI] [PubMed] [Google Scholar]
- 27.Fowkes FG, Gillespie IN. Angioplasty (versus non surgical management) for intermittent claudication. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000017. CD000017. [DOI] [PubMed] [Google Scholar]
- 28.Perkins JM, Collin J, Creasy TS, Fletcher EW, Morris PJ. Exercise training versus angioplasty for stable claudication. Long and medium term results of a prospective, randomised trial. Eur J Vasc Endovasc Surg. 1996;11:409–413. doi: 10.1016/s1078-5884(96)80171-7. [DOI] [PubMed] [Google Scholar]
- 29.Whyman MR, Fowkes FG, Kerracher EM, Gillespie IN, Lee AJ, Housley E, et al. Is intermittent claudication improved by percutaneous transluminal angioplasty? A randomized controlled trial. J Vasc Surg. 1997;26:551–557. doi: 10.1016/s0741-5214(97)70052-1. [DOI] [PubMed] [Google Scholar]
- 30.Creasy TS, Fletcher EW. Angioplasty for intermittent claudication. Clin Radiol. 1991;43:81–83. doi: 10.1016/s0009-9260(05)81582-x. [DOI] [PubMed] [Google Scholar]
- 31.Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291–300. doi: 10.1177/000331979704800402. [DOI] [PubMed] [Google Scholar]
- 32.Pernow B, Zetterquist S. Metabolic evaluation of the leg blood flow in claudicating patients with arterial obstructions at different levels. Scand J Clin Lab Invest. 1968;21:277–287. doi: 10.3109/00365516809076995. [DOI] [PubMed] [Google Scholar]
- 33.Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–609. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 34.Anonymous. [accessed 30 March 2007];Bethesda, MD: National Heart, Lung, and Blood Institute; The DASH Eating Plan. NIH Publication No. 06-4082. National Institutes of Health, US Department of Health and Human Services. 2006 Abstract http://www.nhlbi.nih.gov/health/public/heart/hbp/dash/new_dash.pdf.
- 35.Dolan NC, Liu K, Criqui MH, Greenland P, Guralnik JM, Chan C, et al. Peripheral artery disease, diabetes, and reduced lower extremity functioning. Diabetes Care. 2002;25:113–120. doi: 10.2337/diacare.25.1.113. [DOI] [PubMed] [Google Scholar]
- 36.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]


