Abstract
Kaposi's sarcoma (KS) is the most common tumor of AIDS patients worldwide. KS-associated herpesvirus (KSHV) is the infectious cause of this highly vascularized skin tumor. The main cell type found within a KS lesion, the spindle cell, is latently infected with KSHV and has markers of both blood and lymphatic endothelial cells. During development, lymphatic endothelial cells differentiate from preexisting blood endothelial cells. Interestingly, KSHV infection of blood endothelial cells induces lymphatic endothelial cell differentiation. Here, we show that KSHV gene expression is necessary to maintain the expression of the lymphatic markers vascular endothelial growth factor receptor 3 (VEGFR-3) and podoplanin. KSHV infection activates many cell signaling pathways in endothelial cells and persistently activates STAT3 through the gp130 receptor, the common receptor of the interleukin 6 family of cytokines. We find that KSHV infection also activates the phosphatidylinositol 3-OH-kinase (PI3K)/Akt cell signaling pathway in latently infected endothelial cells and that gp130 receptor signaling is necessary for Akt activation. Using both pharmacological inhibitors and small interfering RNA knockdown, we show that the gp130 receptor-mediated activation of both the JAK2/STAT3 and PI3K/Akt cell signaling pathways is necessary for KSHV-induced lymphatic reprogramming of endothelial cells. The induction of the lymphatic endothelial cell-specific transcription factor Prox1 is also involved in KSHV-induced lymphatic reprogramming. The activation of gp130 receptor signaling is a novel mechanism for the differentiation of blood endothelial cells into lymphatic endothelial cells and may be relevant to the developmental or pathological differentiation of lymphatic endothelial cells as well as to KSHV pathogenesis.
Kaposi's sarcoma (KS) is a highly vascularized tumor that is the most frequent neoplasm in AIDS patients and the most common cancer in the central parts of Africa (4). The main cell type in a KS tumor is the spindle cell, a cell of endothelial origin (4). KS tumor spindle cells display markers of the lymphatic endothelium, including vascular endothelial growth factor receptor 3 (VEGFR-3), podoplanin, and Prox1 (26, 50, 60). The gene expression profile of KS tumors also matches that of isolated lymphatic endothelial cells (LECs) (22, 58).
Blood endothelial cells (BECs) and LECs make up the two major vascular systems and differentially express over 300 genes (20). The lymphatic vasculature, comprised primarily of LECs, is responsible for the uptake of activated immune cells and fluids leaked into peripheral tissues and the return of this lymph to the circulation of blood via transport through the lymph nodes and the thoracic duct. The separate blood vascular system of veins and arteries, comprised of BECs, delivers oxygen and nutrients throughout the body (reviewed in reference 1). During embryonic development, the lymphatic vasculature is derived from the blood vascular system, whereby unknown signals induce the expression of the homeobox transcription factor Prox1 in a subset of cells in the cardinal vein (21, 23, 37). Prox1 functions as both a transcriptional repressor and an activator to upregulate the expression of LEC-specific markers, such as VEGFR-3 and podoplanin, and to downregulate the expression of BEC-specific markers (21, 37). VEGFR-3 is necessary for the initial budding and formation of the lymphatic vasculature and subsequent lymphatic vessel growth in response to the binding of its ligand VEGF-C (56). Podoplanin, a surface glycoprotein, may contribute to LEC adhesion and migration in later stages of development (47).
The etiologic agent of KS is KS-associated herpesvirus (KSHV, also known as human herpesvirus 8) (3, 8). KSHV is a gammaherpesvirus, subclassified as a rhadinovirus (33, 42). KSHV is found in KS spindle cells, where it is predominantly in a latent state, though a small percentage of spindle cells contain lytic KSHV (3, 53). Of the 90 genes borne by KSHV, only a few are expressed during latency in KS tumors and KSHV-infected endothelial cells: latency-associated nuclear antigen (LANA), viral cyclin, viral FLICE inhibitory protein (vFLIP) (13, 53), kaposins (44), and 12 microRNAs (6, 38, 46). Both latent and lytic genes initiate cell signaling pathways that can alter the growth and survival of endothelial cells. In addition, the lytic genes potentially contribute to KS pathogenesis in either an autocrine or a paracrine fashion from the small percentage of spindle cells with lytic KSHV (25). Potential lytic genes that could provide a paracrine signal include a constitutively active viral G protein-coupled receptor (vGPCR); K1, which signals through a constitutive immunoreceptor tyrosine-based activation motif; viral interleukin 6 (vIL-6), a viral homolog of human IL-6; and K15, a membrane protein with SH2 and SH3 protein binding domains (5, 12, 29, 30).
Latent KSHV infection has been shown to activate many cell signaling pathways in endothelial cells, including the NF-κB (9, 43), β-catenin (14), PI3K/Akt, and the mitogen-activated protein kinase (MAPK) pathways (43). In addition, we have shown that JAK2/STAT3 signaling is persistently activated by KSHV infection of endothelial cells through the activation of the gp130 receptor (40). The gp130 receptor is the common receptor of the IL-6 family of cytokines, which include human IL-6 (19), vIL-6 (30), oncostatin M (16), leukemia inhibitory factor (16), IL-27 (39), IL-11 (62), cardiotrophin-1 (36), and ciliary neurotrophic factor (54). The individual cytokines bind gp130 and/or a cytokine-specific receptor to transduce intracellular signaling, including the JAK2/STAT3 pathway. Additional cell signaling pathways are activated downstream of the gp130 receptor, including Src-family kinase, PI3K/Akt, and MAPK pathways (15, 48).
Because KS spindle cells express markers of lymphatic endothelium, it has been postulated that KSHV infects LECs in vivo to establish KS tumors (26, 50, 60). However, KSHV can drive the differentiation of BECs into LECs in vitro (7, 22, 58). KSHV infection induces many lymphocyte-specific genes, including Prox1, VEGFR-3, LYVE-1, and podoplanin, in both primary BECs and telomerase-immortalized microvascular endothelial (TIME) cells, which resemble BECs in gene expression profiles (7, 22, 58). All endothelial cells latently infected with KSHV express lymphatic markers, suggesting that latent viral gene expression contributes to lymphatic differentiation (7). This raises the possibility that KSHV infects blood or circulating endothelial cells and drives them to differentiate into lymphatic endothelium as they become spindle cells. This may be of major importance to KS tumor formation.
In this report, we examine the mechanism of KSHV-induced lymphatic reprogramming by dissecting cell signaling pathways altered by KSHV infection of endothelial cells. We show that the PI3K/Akt pathway is activated in latent KSHV-infected endothelial cells through gp130 receptor activation of JAK2 and that it is necessary for the induction of Prox1 and for lymphatic reprogramming of KSHV-infected endothelial cells. This mechanism of KSHV-induced lymphatic reprogramming could provide insight into the signaling pathways involved in the induction of lymphatic differentiation during development.
MATERIALS AND METHODS
Reagents and antibodies.
Phospho-Akt (pAkt; Ser473), pan Akt, phospho-STAT3 (pSTAT3; Y705), and pan STAT3 antibodies (Cell Signaling Technology) were used as specified by the manufacturer. Antibodies to β-actin (Sigma), open reading frame 59 (ORF59) (Advanced Biotechnologies, Inc.), LANA (a kind gift of D. Ganem), gp130, VEGFR-3 (Santa Cruz Biotechnologies), VEGFR-1, and podoplanin (Abcam) were used in an immunoblot or immunofluorescence analysis as outlined below. The kinase inhibitors AG490, LY294002, SU6656, BAY 11-7082, SP600125 (Jun N-terminal protein kinase inhibitor II), GF109203X (bisindolylmaleimide-l), and KRN633 (VEGFR tyrosine kinase inhibitor III; Calbiochem); wortmannin (Biosource, Inc.); and PD98059 (Cell Signaling Technology) were reconstituted in dimethyl sulfoxide (DMSO) and used at the concentrations specified in Fig. 2 and 4.
FIG. 2.
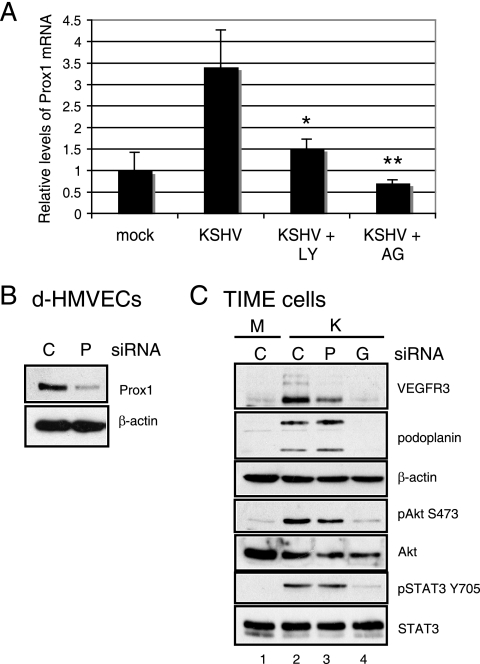
KSHV-mediated lymphatic reprogramming requires the activation of PI3K/Akt and JAK2/STAT3 signaling pathways. (A) TIME cells were mock (lane M) or KSHV (lane K) infected, and either DMSO was used as a vehicle control or pharmacological inhibitors were added at the indicated concentrations at 24 hpi and again at 40 hpi. At 48 hpi, cell extracts were analyzed by immunoblot analysis with the indicated antibodies. The inhibitors used were the PI3K inhibitor LY294002 (LY), the JAK2 inhibitor AG490 (AG), and the MEK1 inhibitor PD98057 (PD). PI3K inhibition blocks KSHV induction of VEGFR-3 and podoplanin in TIME cells, while JAK2 inhibition blocks VEGFR-3 induction. (B) Mock- or KSHV-infected TIME cells were fixed at 48 hpi and stained either for LANA and pAkt (Ser473) (top two panels) or for ORF59 and pAkt (Ser473) (bottom panel). pAkt is present in both ORF59-positive (arrow) and -negative cells, indicating that pAkt is present in both lytically and latently KSHV-infected cells.
FIG. 4.
Prox1 is induced by KSHV through the PI3K/Akt and JAK2/STAT3 signaling pathways and is involved in VEGFR-3 induction. (A) TIME cells were either mock or KSHV infected and then treated with either DMSO, 40 μM LY294002 (LY), or 100 μM AG490 (AG) for 24 h. Relative abundances of Prox1 mRNA were analyzed by one-step real-time RT-PCR. Student's t test was used to assess the differences between Prox1 mRNA levels in the KSHV-plus-DMSO and the KSHV-plus-inhibitor samples. *, P < 0.01; **, P < 0.001. Error bars give the standard errors of the means of four replicates for each treatment. (B) To demonstrate the knockdown of Prox1 protein expression by siRNA, primary d-HMVECs were transfected with 3 μg negative-control (lane C) or Prox1 (lane P) siRNA and subsequently harvested at 72 h posttransfection. Cell lysates were assayed by immunoblot analysis with antibodies to Prox1 and β-actin. (C) TIME cells were transfected with 3 μg of negative-control (lanes C), Prox1 (lane P), or gp130 (lane G) siRNA, and after 24 h, cells were either mock (M lane) or KSHV (K lanes) infected. Cell lysates were assayed 48 hpi by immunoblot analysis with the indicated antibodies. Prox1 siRNA blocked VEGFR-3 induction but had no effect on the phosphorylation of Akt or STAT3 (lane 3).
Cells.
TIME cells were maintained as monolayer cultures in EGM-2 medium (Lonza) (28, 57). Primary dermal human microvascular endothelial cells (d-HMVECs) and primary human dermal BECs were purchased from Lonza and were maintained in EGM-2 medium. BCBL-1 cells (41) and BJAB cells were maintained in RPMI 1640 medium (Cellgro, Mediatech, Inc.) supplemented with 10% fetal bovine serum, penicillin, streptomycin, l-glutamine, and β-mercaptoethanol. BJAB cells harboring vIL-6-positive and -negative recombinant KSHV (10, 11) were maintained in the same media with the addition of 10 μg/ml puromycin.
Viruses and infection.
KSHV inoculum, from BCBL-1 cells, was used to infect TIME cells and primary BECS as previously described (28, 40). KSHV infections of TIME cells and primary BECs were performed in serum-free EBM-2 medium for 3 h, after which the medium was replaced with complete EGM-2 medium containing serum and supplements. Infection rates were assessed by immunofluorescence using antibodies against LANA and the lytic protein ORF59. vIL-6-positive and -negative recombinant KSHV was induced in cells with 3,000 particles per cell of Ad50 (adenovirus expressing the lytic switch protein ORF50 [kind gift of D. Ganem]) and 3 M sodium butyrate and isolated as previously described (10, 11).
Transfection of siRNA.
siRNA specific to gp130, Akt1, STAT3, Prox1, and negative-control oligonucleotides were designed and synthesized by Ambion (Austin, TX). The following oligonucleotide sequences were used: gp130 (Ambion identification [ID] no. 106709; sense, 5′-GGC AUA CCU UAA ACA AGC UdTdT-3′), Akt1 (ID no. 633; sense, 5′-GGG CAC UUU CGG CAA GGU GdTdT-3′), STAT3 (ID no. 116558; sense 5′-GCA CAA UCU ACG AAG AAU CdTdT-3′), Prox1 (ID no. 106879; sense, 5′-GGG AAU UUG UUA ACG AUG CdTdT-3′), VEGFR-1A (ID no. 190; 5′-GGU UCA AAA UUA AAA GAU CdTdT-3′), VEGFR-1B (ID no. 191; 5′-GGA AAA GGA AAA AAA GCA AdTdT-3′), VEGFR-1C (ID no. 192; 5′-GGA UCU AGU UCA GGU UCA AdTdT-3′), VEGFR-3 (ID no. 194; 5′-GGA UGA AGA CAU UUG AGG AdTdT-3′), and negative-control siRNA (sense, 5′-AGU ACU GCU UAC GAU ACG GdTdT-3′). TIME cells or primary d-HMVECs and BECs were transfected with 1.5 μg or 3 μg siRNA using Amaxa's Nucleofector kit (Cologne, Germany) according to the manufacturer's protocol. The transfection efficiency for siRNA was approximately 90% when it was assessed with 6-carboxyfluorescein-labeled negative-control siRNA. At 24 h posttransfection, cells were mock or KSHV infected and subsequently harvested for analysis after an additional 2 days.
Immunofluorescence.
Prior to the harvesting of cells for immunoblot analysis, an aliquot of mock- or KSHV-infected TIME cells was seeded on a four-well chamber slide and fixed with 4% (vol/vol) paraformaldehyde in phosphate-buffered saline or 100% methanol. Immunofluorescence was performed as described previously for ORF59 and LANA (28) or as specified by the manufacturer (pAkt). Briefly, cells were incubated with primary antisera at a dilution of 1:100 (anti-pAkt) or 1:1,000 (rabbit or rat anti-LANA, mouse anti-ORF59). Cells were then incubated with fluor-conjugated secondary antibodies (goat anti-rabbit Alexa Fluor 488, goat anti-mouse Alexa Fluor 594, or goat anti-rat Alexa Fluor 488 [Molecular Probes/Invitrogen]). Cells were mounted in Vectashield containing DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories, Inc.) before being viewed under a Nikon Eclipse E400 fluorescence microscope (Nikon, Inc.). Images were captured with the Micropublisher RTV camera using QCapture Suite (QImaging).
Immunoblot analysis.
Cells were harvested and resuspended in RIPA lysis buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 40 mM β-glycerophosphate, and Complete Mini protease inhibitor tablet [Roche]), and cell debris was removed after a 20-min incubation by centrifugation. Protein concentrations were determined by the bicinchoninic acid assay (Pierce), and 10 μg protein was fractionated on a sodium dodecyl sulfate-polyacrylamide gel, transferred to a polyvinyl difluoride membrane, blotted with the appropriate antibody (dilutions were 1:1,000 for anti-pAkt, anti-Akt, anti-pSTAT3, anti-STAT3, anti-gp130, and anti-VEGFR-3; 1:2,500 for anti-podoplanin; and 1:10,000 for anti-β-actin), and subsequently probed with horseradish peroxidase-conjugated goat anti-mouse or -rabbit immunoglobulin G (Jackson ImmunoResearch Laboratories). Immunoreactive proteins were visualized by chemiluminescence using Amersham ECL Plus.
RNA isolation and quantitative RT-PCR.
Total RNA was isolated from TIME cells using the TRIzol method (Invitrogen) and then was treated with Turbo DNA-free (Ambion). Two hundred fifty or 500 ng of total RNA was used in a SuperScript III, Platinum SYBR green, one-step, quantitative reverse transcription PCR (RT-PCR) (Invitrogen) according to manufacturer's protocols with the primers for either GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (forward, 5′-AAG GTG AAG GTC GGA GTC AAC G-3′; reverse, 5′-TGG AAG ATG GTG ATG GGA TTT C-3′) or Prox1 (forward, 5′-AGT GCA ATG CAG GAA GGA TT-3′; reverse, 5′-CCA CTT GAT GAG CTG AGA GG-3′). Relative abundances of Prox1 mRNA were normalized by the delta threshold cycle method to the abundance of GAPDH, with mock-infected TIME cells set to 1. Error bars reflect standard errors of the means (four experiments).
RESULTS
KSHV gene expression is necessary to maintain induced lymphatic reprogramming of endothelial cells.
We previously determined that the lymphatic genes for Prox1, podoplanin, LYVE-1, and VEGFR-3 were induced in latently infected TIME cells (7). In vitro passaging of KS tumor cells or cell lines infected with KSHV results in the progressive loss of the KSHV viral episome (18). To test if lymphatic reprogramming was maintained during the loss of the viral episome, TIME cells were infected and harvested during an extended passage. As seen in Fig. 1, podoplanin expression was induced 24 h postinfection (hpi) (27 and 41 kDa, corresponding to the precursor and mature glycosylated forms), while VEGFR-3 was induced by 48 hpi (125, 175, and 195 kDa, corresponding to the precursor and glycosylated forms). The protein expression of both VEGFR-3 and podoplanin decreased upon the loss of the KSHV episome, as determined by a decrease in the number of cells exhibiting positive KSHV LANA immunofluorescence. This result indicates that KSHV gene expression is necessary to maintain the expression of lymphatic markers. Reinfection of the cells at day 21, when KSHV gene expression is at 10%, led to a reinduction of VEGFR-3 and podoplanin expression to levels comparable to those at the initial infection (Fig. 1, lane 8).
FIG. 1.
The maintenance of KSHV-mediated lymphatic reprogramming requires KSHV gene expression. TIME cells were mock or KSHV infected and passaged at 2, 6, 10, 14, and 20 days postinfection (dpi). At 21 dpi, cells were again either KSHV or mock infected. Cell lysates were harvested on the indicated dpi and then subjected to immunoblot analysis with the indicated antibodies. Immunofluorescence for the latent KSHV LANA gene (% infected) was performed to monitor KSHV episome loss. VEGFR-3 and podoplanin expression correlates with KSHV episome loss. ND, not determined.
JAK2 and PI3K are necessary for VEGFR-3 and/or podoplanin induction in KSHV-infected TIME cells.
Previously, we demonstrated that KSHV infection of TIME cells induces the persistent phosphorylation of STAT3 through the nonreceptor tyrosine kinase JAK2 (40). Here we found that the JAK2 inhibitor AG490 also blocked the KSHV-mediated induction of VEGFR-3 but not of podoplanin, as determined by immunoblot analysis (Fig. 2A, lane 6). We verified that AG490 inhibited JAK2 activity by blocking STAT3 phosphorylation at Tyr705 (pSTAT3) (lane 6). KSHV induction of both VEGFR-3 and podoplanin was blocked in a dose-dependent fashion with the PI3K inhibitor LY294002 (Fig. 2A, lanes 3 and 4). This result was confirmed with another PI3K inhibitor, wortmannin (data not shown). We observed no effect on the KSHV-mediated induction of VEGFR-3 or podoplanin when we treated with either a MEK1 inhibitor, PD98059 (lanes 7 and 8), or pharmacological inhibitors of Jun N-terminal protein kinase (SP600125), Src family kinases (SU6656), protein kinase C (GF109203X), or NF-κB (BAY 11-7082 [data not shown]).
Akt is phosphorylated in cells latently infected with KSHV.
Akt is phosphorylated at Ser473 (pAkt) in KSHV-infected TIME cells at 48 hpi (Fig. 2A, lanes 1 and 2), and this phosphorylation is blocked by LY294002 (lanes 2 to 4). Similar results were also reported for KSHV-infected primary d-HMVECs at 24 hpi (43). Two lytic KSHV proteins, vGPCR and K1, have been shown to activate Akt in endothelial cells (51, 59). To determine if Akt is also activated in latently infected endothelial cells, TIME cells were infected with KSHV for 48 h, with the last 24 hpi in serum-free medium, and stained with antibodies against pAkt (Ser473) and either the KSHV latent protein LANA or lytic protein ORF59 (Fig. 2B). Heterogeneous staining of pAkt was observed in cultures, with greater than 90% of the cells staining for LANA and around 1% staining for ORF59. While positive pAkt staining was observed in ORF59-positive cells (Fig. 2B, bottom panels), pAkt staining is also clearly found in surrounding cells latently infected with KSHV. This is the first demonstration of Akt activation in de novo endothelial cells latently infected with KSHV. Importantly, high levels of active pAkt are detected in most spindle cells in KS tumors in vivo, where 95% of the cells are latently infected and 5% lytically infected (51). Interestingly, JAK2 inhibition with AG490 blocked the phosphorylation of Akt (Fig. 2A, lane 6), indicating that JAK2 activity is required for the activation of the PI3K/Akt pathway in KSHV-infected TIME cells.
The gp130 receptor is required for the KSHV-mediated activation of STAT3 and Akt and the induction of lymphatic markers in endothelial cells.
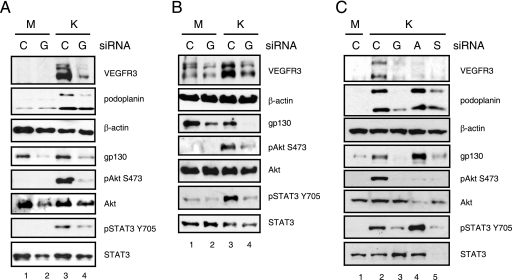
The gp130 receptor is required for STAT3 activation in KSHV-infected endothelial cells (40). To determine if activation of the gp130 receptor is required for PI3K/Akt activation, TIME cells were transfected with siRNA to gp130 or a negative-control siRNA, followed by mock or KSHV infection. gp130 siRNA led to a >90% knockdown of gp130 protein expression in both mock- and KSHV-infected TIME cells (Fig. 3A, lanes 2 and 4). KSHV-induced phosphorylation of both Akt and STAT3 was blocked by the gp130 siRNA, while total Akt and STAT3 protein levels were unaffected (lane 4). Infection rates, as monitored by LANA immunofluorescence, were the same (90%) for both the gp130- and negative-control-siRNA-transfected cells. KSHV induction of both VEGFR-3 and podoplanin was also blocked by gp130 siRNA (Fig. 3A, lane 4), suggesting that cell signaling downstream of the gp130 receptor is responsible for KSHV's induction of lymphatic markers. Similar results were found with primary BECs. While the primary BECS expressed low levels of VEGFR-3 (Fig. 3B, lane 1), VEGFR-3 protein expression was strongly induced upon KSHV infection but remained at basal levels upon KSHV infection of cells transfected with the gp130 siRNA (Fig. 3B, lanes 3 and 4). In addition, the phosphorylation of both Akt and STAT3 was induced in KSHV-infected BECs and was blocked by gp130 siRNA (lane 4). No induction of podoplanin was observed upon KSHV infection of these primary BECs (data not shown). These primary BECs were sorted from primary d-HMVECs by negative selection for podoplanin expression, which may preclude podoplanin induction (see Discussion).
FIG. 3.
KSHV-induced lymphatic reprogramming requires gp130 receptor-mediated activation of both the PI3K/Akt and JAK2/STAT3 intracellular signaling pathways. (A) TIME cells were transfected with 1.5 μg negative-control (lane C) or gp130 (lane G) siRNA. After 24 h, cells were mock (M lanes) or KSHV (K lanes) infected and harvested at 48 hpi. Cell lysates were subjected to immunoblot analysis with the indicated antibodies. gp130 siRNA blocked the phosphorylation of STAT3 and Akt and blocked the induction of VEGFR-3 and podoplanin in KSHV-infected TIME cells (lane 4). (B) Primary BECs were assayed as described for panel A, with similar results. (C) TIME cells were transfected with 3 μg of negative-control, gp130, Akt1 (lane A), or STAT3 (lane S) siRNA. After 24 h, cells were either mock (M lane) or KSHV (K lanes) infected for 48 h. Cell lysates were subjected to immunoblot analysis with the indicated antibodies. Akt1 siRNA blocked VEGFR-3 induction and knocked down pAkt and total Akt in KSHV-infected cells (lane 4). STAT3 siRNA blocked the induction of VEGFR-3, podoplanin, and gp130 in KSHV-infected cells. STAT3 siRNA knocked down pSTAT3 and total STAT3, as well as pAkt (lane 5).
KSHV-induced STAT3 activation is required for the activation of Akt and subsequent LEC reprogramming.
While the pharmacological inhibitors that we used are very specific, the potential for off-target effects formally exists. To confirm the precise role of Akt and lymphatic STAT3 in KSHV reprogramming, we transfected TIME cells with siRNA against STAT3 or Akt1, the dominant Akt isoform in endothelial cells, and then infected them with KSHV (52). Akt1 siRNA knocked down both pAkt and total Akt expression and also blocked the induction of VEGFR-3 but not of podoplanin (Fig. 3C, lane 4). Total STAT3 and pSTAT3 levels were unaffected by Akt1 siRNA. STAT3 siRNA knocked down the levels of both pSTAT3 and total STAT3 protein, as well as the KSHV-mediated induction of VEGFR-3 and podoplanin (lane 5). STAT3 siRNA also blocked Akt phosphorylation without affecting total Akt expression. Previously, we showed an induction of the gp130 receptor by KSHV infection of TIME cells (7, 40). KSHV induction of gp130 expression is blocked by the STAT3 siRNA in TIME cells (Fig. 3C, lane 5), indicating that the knockdown of STAT3 blocks gp130 upregulation by KSHV. The gp130 promoter contains DNA-binding sites responsive to STAT3 and STAT1 homo- and heterodimers, and activation of the gp130 receptor and thus of STAT3 can lead to the induction of gp130 protein expression in a positive-feedback loop (34).
KSHV induction of Prox1 is involved in the induction of lymphatic markers.
We and others previously reported that KSHV infection of TIME cells or primary BECs induced the LEC-specific transcription factor Prox1 (7, 22). To determine whether Prox1 is induced by the PI3K/Akt and JAK2/STAT3 signaling pathways, we quantitatively measured KSHV induction of Prox1 mRNA by real-time RT-PCR in TIME cells with and without pharmacological inhibitors. There is a 3.5-fold induction of Prox1 mRNA at 24 hpi (Fig. 4A), which was knocked down to 1.5-fold and 0.8-fold by treatment of the cultures with the PI3K inhibitor LY294002 and the JAK2 inhibitor AG490, respectively. We were unable to detect Prox1 protein induction by KSHV in TIME cells by Western blotting or immunofluorescence, presumably because protein levels were below the limit of detection by the Prox1 antibody that we used.
Prox1 siRNA was used to confirm the role of Prox1 in the induction of lymphatic markers by KSHV infection of TIME cells. In KSHV-infected TIME cells, Prox1 siRNA blocked the induction of VEGFR-3 to around 50% of the level induced with negative-control siRNA, while podoplanin levels were unchanged (Fig. 4C, lanes 1 to 3). There was no change in the level of phosphorylation of Akt or STAT3, indicating that these cell signaling pathways are upstream of the Prox1 induction site. The Prox1 siRNA knocked down Prox1 mRNA by 85.8% ± 6.0% in transfected TIME cells, as determined by quantitative real-time RT-PCR. Since we were unable to detect Prox1 by Western blotting of TIME cells, we verified Prox1 protein knockdown by Prox1 siRNA transfection into primary d-HMVECs, which are a mixture of BECs and LECS and have Prox1 detectable by Western blotting. We observed an approximately 80% decrease in Prox1 protein expression at 72 h posttransfection compared to the level of expression with negative-control siRNA in the primary d-HMVECs (Fig. 4B). Another Prox1 siRNA knocked down Prox1 mRNA expression by 81% ± 4.2% and also inhibited the KSHV induction of VEGFR-3, but not podoplanin, in TIME cells (data not shown). Our results are consistent with previously published data showing that Prox1 siRNA blocked KSHV's induction of LEC-specific genes in primary BECs (22), indicating that the induction of Prox1 is involved in lymphatic reprogramming by KSHV.
vIL-6 is not essential for KSHV-induced lymphatic reprogramming of TIME cells.
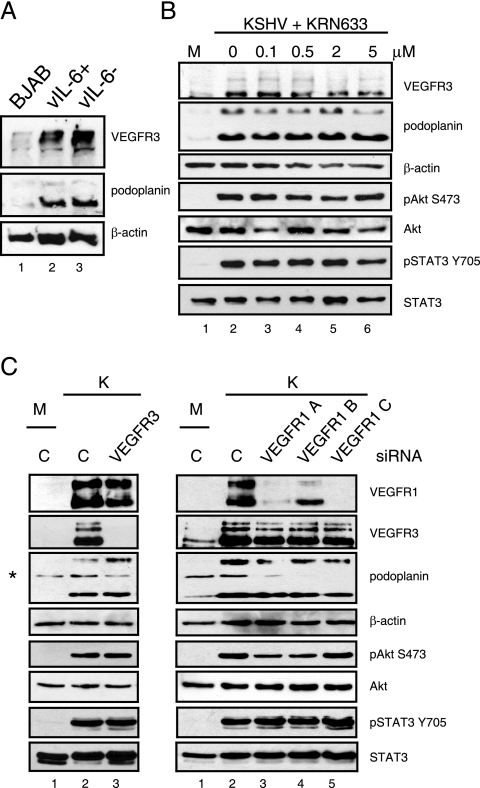
Multiple cytokines of the IL-6 family bind to the gp130 receptor as a common subunit of their cytokine receptors, including the KSHV-encoded IL-6 homolog, vIL-6 (30, 31, 40). We previously showed that the gp130 receptor cytokines vIL-6, human IL-6, and oncostatin M are not responsible for KSHV's activation of STAT3 (40). To determine if vIL-6 is involved in LEC marker induction in the context of KSHV infection, we used a recombinant virus where the ORFK2 gene encoding vIL-6 is deleted (11). TIME cells infected with vIL-6-positive or -negative recombinant KSHV isolates (11, 40) induced equivalent levels of VEGFR-3 and podoplanin expression (Fig. 5A, lanes 2 and 3), indicating that vIL-6 is not essential for lymphatic reprogramming in the context of KSHV infection. The KSHV recombinants also encode green fluorescent protein under a constitutive cytomegalovirus promoter (11, 40); therefore, green fluorescent protein fluorescence was used to ensure equivalent TIME cell infection rates by the recombinant vIL-6-positive and -negative KSHV isolates.
FIG. 5.
vIL-6 expression and VEGFR-1, -2, and -3 activation are not required for KSHV-induced lymphatic reprogramming. (A) TIME cells were infected with recombinant vIL-6-positive (vIL-6+) and vIL-6-negative (vIL-6−) KSHV for 48 h, and cell lysates were assayed by immunoblot analysis with the indicated antibodies. VEGFR-3 and podoplanin expression were induced by both recombinant viruses (lanes 2 and 3) but not in infected control BJAB cells (lane 1). (B) At 24 and 40 hpi, KSHV-infected TIME cells were treated with the indicated doses of the VEGFR Tyr kinase inhibitor KRN633, and cell lysates were harvested at 48 hpi for immunoblot analysis with the indicated antibodies. VEGFR-1, -2, and -3 activation is not required for the phosphorylation of Akt and STAT3 or KSHV-mediated lymphatic reprogramming. Lane M, mock-infected cells. (C) TIME cells were transfected with 3 μg of negative-control siRNA (lanes C) or the indicated VEGFR-1 or VEGFR-3 siRNA. After 24 h, cells were mock (M lanes) or KSHV (K lanes) infected and harvested at 48 hpi. Cell lysates were subjected to immunoblot analysis with the indicated antibodies. VEGFR-3 siRNA did not block KSHV's induction of VEGFR-1 and podoplanin or the phosphorylation of STAT3 and Akt (left panels, lane 3). VEGFR-1 siRNAs did not block KSHV's induction of VEGFR-3 and podoplanin or the phosphorylation of STAT3 and Akt (right panels, lanes 3 to 5). *, cross-reactive band of the anti-podoplanin antibody.
VEGFR-1, -2, or -3 activation is not required for KSHV-induced lymphatic reprogramming.
VEGFR-1, -2, and -3 are class III receptor tyrosine kinases and can activate the PI3K/Akt pathway in response to binding their ligands (35). Upon KSHV infection of different endothelial cell populations, the expression of all VEGFRs are induced, as is the expression of their ligands VEGF-A and VEGF-C (7, 49, 55). Furthermore, VEGF-C treatment of TIME cells alone can induce the expression of Prox1 (49). To show that activation of the VEGF receptors is not necessary for the activation of Akt or the induction of lymphatic differentiation in KSHV-infected endothelial cells, we blocked VEGFR-1, -2, and -3 tyrosine kinase activities with the chemical inhibitor KRN633 (32). Increasing doses of KRN633 (up to 5 μM) did not block the KSHV-mediated induction of VEGFR-3 and podoplanin, nor did KRN633 block the KSHV-mediated activation of pAkt and pSTAT3 in TIME cells (Fig. 5B). This drug concentration is 500-fold over the concentration needed to completely inhibit receptor signaling (10 nM) and 100-fold higher than the concentration needed to inhibit VEGF-A-mediated endothelial-cell proliferation (100 nM [32]). We confirmed that KRN633 inhibited VEGF-A's activation of Akt at less than 10 nM in TIME cells (data not shown). To further confirm that VEGF receptor signaling is not involved, we knocked down VEGFR-1 and VEGFR-3 expression with specific siRNAs and found no block in the KSHV-mediated activation of pAkt or pSTAT3 (Fig. 5C). In addition, VEGFR-3 or VEGFR-1 siRNA knockdown did not block KSHV's induction of podoplanin (Fig. 5C), and VEGFR-1 siRNA knockdown did not block VEGFR-3 induction (Fig. 5C, right panels, lanes 3 to 5). Furthermore, VEGFR-3 siRNA knockdown did not block KSHV's induction of VEGFR-1. These results indicate that pAkt and pSTAT3 activation do not require the upregulation of VEGFR-3 expression but rather that VEGFR-3 expression is induced in response to Akt and STAT3 activation.
DISCUSSION
In this report, we delineate the signaling pathways necessary for KSHV-induced lymphatic reprogramming. We show that the gp130 receptor is required for KSHV-induced lymphatic reprogramming of endothelial cells and relies on the activation of the JAK2/STAT3 and PI3K/Akt pathways (Fig. 6). Active pAkt is present in endothelial cells both latently and lytically infected with KSHV, as detected by immunofluorescence. We propose that Akt activation results in the induction of the lymphatic transcription factor Prox1, which is necessary for the induction of VEGFR-3. STAT3 activation results in a positive-feedback loop of the overall gp130 signaling pathway, increasing the expression of the gp130 receptor likely through the STAT3-responsive elements in the gp130 promoter (34).
FIG. 6.
Proposed model of KSHV-induced lymphatic reprogramming of endothelial cells. KSHV infection of endothelial cells induces the secretion of a soluble factor that activates the gp130 receptor. Activation of the gp130 receptor activates JAK2, which directly phosphorylates and activates STAT3. Downstream of gp130, JAK2 also promotes the activation of PI3K to phosphorylate Akt. Prox1 is induced downstream of the PI3K/Akt pathway and regulates VEGFR-3 induction and podoplanin expression. STAT3 regulates gp130 induction, and its persistent activation controls a positive autoregulatory loop of the entire signaling pathway. Arrows with solid lines, direct regulation; arrows with dashed lines, indirect regulation of the target.
KSHV induction of podoplanin, another marker of LEC differentiation, also requires gp130 receptor activation. We cannot rule out the involvement of Akt and Prox1 in podoplanin induction due to the incomplete knockdown of Akt1 and Prox1 by their respective siRNAs (Fig. 3C and 4C). Podoplanin may be a more sensitive indicator of lymphatic differentiation since KSHV induces podoplanin expression at 24 hpi while VEGFR-3 is induced later, at 48 hpi (Fig. 1). Therefore, smaller perturbations in signaling may have greater effects on VEGFR-3 expression than on podoplanin expression. Since Prox1 is a transcription factor, incomplete siRNA knockdown may still leave low levels sufficient for the transcription of certain genes. In addition, Prox1 overexpression in TIME cells and primary BECs results in the induction of both podoplanin and VEGFR-3 expression, indicating that Prox1 is sufficient to induce lymphatic markers (21, 37; V. A. Morris and M. Lagunoff, unpublished results). Prox1 overexpression in nonendothelial cells does not upregulate LEC-specific genes, indicating the involvement of endothelial-cell-specific cofactors for lymphatic differentiation (37). Therefore, an alternative explanation of our results is that Prox1 and additional endothelial-cell-specific factors are induced by gp130 receptor signaling to regulate lymphatic reprogramming. Prox1 mRNA induction peaks at 24 hpi, and then levels fall back to mock levels by 48 hpi, suggesting the involvement of additional factors in the long-term lymphatic differentiation induced by KSHV (49; data not shown).
A singular definition of lymphatic reprogramming remains somewhat unclear because cultured LECs and BECs have heterogeneous expression of their cell-specific markers, likely depending on which cell surface marker is used to isolate the separate cell populations (reviewed in reference 45). Depending on the isolation method of primary BECs, KSHV induced different sets of lymphatic genes; in cells sorted for CD34 expression (a BEC gene), KSHV induced Prox1 and LYVE-1 (22), and in cells sorted against podoplanin expression (a LEC gene), KSHV induced VEGFR-3 and podoplanin (58). In the current study, the purchased primary BECs were isolated by negative podoplanin expression and expressed low levels of VEGFR-3, yet KSHV infection induced VEGFR-3 but not podoplanin (Fig. 3B). In contrast, KSHV infection of TIME cells induces all four lymphatic markers: VEGFR-3, LYVE-1, Prox1, and podoplanin (7). Nevertheless, our results indicate that the gp130 signaling pathway controls the KSHV induction of the major LEC-specific markers in both TIME cells and primary BECs.
What is the role of this pathogen-induced pathway in the developmental differentiation of lymphatic endothelium? Major vascular anomalies have not been described for mice with conditional deletions of either the gp130 receptor or STAT3 in endothelial cells, ruling out a role for these pathways in developmental LEC differentiation (27, 61). However, it remains possible that gp130 receptor signaling and/or the JAK2/STAT3 pathway may play a role in the pathological induction of lymphatic reprogramming or new-vessel formation through lymphangiogenesis. In adults, lymphangiogenesis is stimulated by inflammation and immune responses, tumorigenesis, and factors that damage the lymphatic vasculature (reviewed in reference 24). Further work is needed to address the gp130 receptor signaling pathway in these processes.
The PI3K/Akt signaling pathway may be involved in developmental lymphatic differentiation. Several cytokines have been shown to induce lymphatic markers in endothelial cells, including IL-3 and IL-7 (2, 17). Both of these interleukins can signal through the PI3K/Akt pathway, and PI3K/Akt signaling is required for IL-7's induction of Prox1 (2, 17). However, we found that recombinant IL-3 and IL-7 did not induce VEGFR-3 or podoplanin expression in TIME cells, making it unlikely that these cytokines are involved in KSHV-induced lymphatic reprogramming (V. A. Morris and M. Lagunoff, unpublished results). The indirect activation of VEGFR-3 by its ligand VEGF-C or VEGF-D is responsible for the induction of lymphatic markers by IL-7 (2, 17). In addition, VEGF-C alone is sufficient to induce Prox1 expression in TIME cells (49). However, our results with the pharmacological inhibition of all three VEGFRs (VEGFR-1, -2, and -3) and siRNA knockdown of VEGFR-1 and VEGFR-3 indicate that VEGFR signaling is not necessary for the induction of lymphatic markers in the context of KSHV infection (Fig. 5B).
A number of lytic KSHV proteins can activate the signaling pathways that are necessary for KSHV-induced LEC differentiation (25). Although vIL-6 can activate STAT3 through the gp130 receptor (30), our results indicate that vIL-6 is not essential for KSHV-mediated lymphatic reprogramming in the context of viral infection (Fig. 5A). Expression of the lytic KSHV proteins vGPCR and K1 can activate PI3K/Akt signaling (51, 59). In addition, vGPCR overexpression can activate Akt in a paracrine fashion (51, 59). Recently, it was reported that both vIL-6 and vGPCR induce the LEC-specific gene angiopoietin-2 in KSHV-infected primary LECs through the induction of paracrine factors, which then activate the MEK MAPK pathway (55). However, the evidence for the involvement of either gene in the context of infection was only correlative. Nevertheless, vGPCR may induce a compensatory secreted factor to activate the gp130 pathway. Although a paracrine factor is involved in the activation of the gp130 receptor by KSHV, we have observed constitutive pAkt and pSTAT3 activation only in latently infected cells and not in surrounding uninfected cells by immunofluorescence (40). In addition, KSHV-mediated upregulation of lymphatic markers is observed only in latently infected cells and not in the surrounding uninfected cells (7, 22, 58). This suggests that separate sets of KSHV genes could be involved in inducing versus maintaining the cell signaling pathways: for example, a viral gene to induce the paracrine factor and a different viral gene to maintain the signal in latent cells.
Several hypotheses have been made about the functional role of KSHV-induced lymphatic differentiation. Cells expressing VEGFR-3 are slightly more infectible by KSHV, suggesting that the upregulation of lymphatic markers could enhance viral infection or reinfection, which may be required for the maintenance of KSHV latency (58, 63). However, VEGFR-3 is neither necessary nor sufficient for viral entry. KSHV glycoprotein B (gB) was found to bind both VEGFR-3 and α3β1 integrin to induce VEGFR-3 intracellular signaling pathways for cell proliferation and migration (63). This suggests that the virus could have growth-stimulatory effects on VEGFR-3-expressing cells without viral entry. Finally, infected lymphatic cells may be better suited for seeding of a tumor and subsequent tumor growth. Unlike BECs, LECs lack the expression of basal lamina proteins (1), which could allow infected endothelial cells to invade surrounding tissues and expand. By expressing VEGFR-1, -2 and -3, KS tumor cells can respond to all VEGFR ligands (VEGF-A, -B, -C, and -D) to promote growth, survival, and migration of the KSHV-infected cells, potentially playing a significant role in KS tumor formation.
Acknowledgments
We thank Sheri Dellos-Nolan, Patrick Carroll, and Christopher Wells for technical assistance.
V.A.M. was supported by a National Science Foundation graduate research fellowship. A.S.P. was supported by a postdoctoral training grant from the National Cancer Institute (T32CA09229). M.L. was supported by a research scholar grant from the American Cancer Society and a grant from the National Cancer Institute (R01 CA097934-01A1).
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Adams, R. H., and K. Alitalo. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8464-478. [DOI] [PubMed] [Google Scholar]
- 2.Al-Rawi, M. A., G. Watkins, R. E. Mansel, and W. G. Jiang. 2005. The effects of interleukin-7 on the lymphangiogenic properties of human endothelial cells. Int. J. Oncol. 27721-730. [PubMed] [Google Scholar]
- 3.Aluigi, M. G., A. Albini, S. Carlone, L. Repetto, R. De Marchi, A. Icardi, M. Moro, D. Noonan, and R. Benelli. 1996. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi's sarcoma. Res. Virol. 147267-275. [DOI] [PubMed] [Google Scholar]
- 4.Antman, K., and Y. Chang. 2000. Kaposi's sarcoma. N. Engl. J. Med. 3421027-1038. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann, M. M., M. Glenn, L. Rainbow, A. Kieser, C. Henke-Gendo, and T. F. Schulz. 2003. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 779346-9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai, X., S. Lu, Z. Zhang, C. M. Gonzalez, B. Damania, and B. R. Cullen. 2005. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. USA 1025570-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll, P. A., E. Brazeau, and M. Lagunoff. 2004. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 3287-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 2661865-1869. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary, P. M., A. Jasmin, M. T. Eby, and L. Hood. 1999. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene 185738-5746. [DOI] [PubMed] [Google Scholar]
- 10.Chen, L., and M. Lagunoff. 2005. Establishment and maintenance of Kaposi's sarcoma-associated herpesvirus latency in B cells. J. Virol. 7914383-14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, L., and M. Lagunoff. 2007. The KSHV viral interleukin-6 is not essential for latency or lytic replication in BJAB cells. Virology 359425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couty, J. P., and M. C. Gershengorn. 2004. Insights into the viral G protein-coupled receptor encoded by human herpesvirus type 8 (HHV-8). Biol. Cell 96349-354. [DOI] [PubMed] [Google Scholar]
- 13.Dittmer, D., M. Lagunoff, R. Renne, K. Staskus, A. Haase, and D. Ganem. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 728309-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimuro, M., F. Y. Wu, C. apRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of β-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9300-306. [DOI] [PubMed] [Google Scholar]
- 15.Fukada, T., Y. Yoshida, K. Nishida, T. Ohtani, T. Shirogane, M. Hibi, and T. Hirano. 1999. Signaling through Gp130: toward a general scenario of cytokine action. Growth Factors 1781-91. [DOI] [PubMed] [Google Scholar]
- 16.Gearing, D. P., M. R. Comeau, D. J. Friend, S. D. Gimpel, C. J. Thut, J. McGourty, K. K. Brasher, J. A. King, S. Gillis, B. Mosley, et al. 1992. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science 2551434-1437. [DOI] [PubMed] [Google Scholar]
- 17.Groger, M., R. Loewe, W. Holnthoner, R. Embacher, M. Pillinger, G. S. Herron, K. Wolff, and P. Petzelbauer. 2004. IL-3 induces expression of lymphatic markers Prox-1 and podoplanin in human endothelial cells. J. Immunol. 1737161-7169. [DOI] [PubMed] [Google Scholar]
- 18.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 113124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinrich, P. C., I. Behrmann, G. Muller-Newen, F. Schaper, and L. Graeve. 1998. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334297-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirakawa, S., Y. K. Hong, N. Harvey, V. Schacht, K. Matsuda, T. Libermann, and M. Detmar. 2003. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 162575-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong, Y. K., and M. Detmar. 2003. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 31485-92. [DOI] [PubMed] [Google Scholar]
- 22.Hong, Y. K., K. Foreman, J. W. Shin, S. Hirakawa, C. L. Curry, D. R. Sage, T. Libermann, B. J. Dezube, J. D. Fingeroth, and M. Detmar. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36683-685. [DOI] [PubMed] [Google Scholar]
- 23.Hong, Y. K., N. Harvey, Y. H. Noh, V. Schacht, S. Hirakawa, M. Detmar, and G. Oliver. 2002. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 225351-357. [DOI] [PubMed] [Google Scholar]
- 24.Hosking, B., and T. Makinen. 2007. Lymphatic vasculature: a molecular perspective. Bioessays 291192-1202. [DOI] [PubMed] [Google Scholar]
- 25.Jarviluoma, A., and P. M. Ojala. 2006. Cell signaling pathways engaged by KSHV. Biochim. Biophys. Acta 1766140-158. [DOI] [PubMed] [Google Scholar]
- 26.Jussila, L., R. Valtola, T. A. Partanen, P. Salven, P. Heikkila, M. T. Matikainen, R. Renkonen, A. Kaipainen, M. Detmar, E. Tschachler, R. Alitalo, and K. Alitalo. 1998. Lymphatic endothelium and Kaposi's sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 581599-1604. [PubMed] [Google Scholar]
- 27.Kano, A., M. J. Wolfgang, Q. Gao, J. Jacoby, G. X. Chai, W. Hansen, Y. Iwamoto, J. S. Pober, R. A. Flavell, and X. Y. Fu. 2003. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J. Exp. Med. 1981517-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 762440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagunoff, M., R. Majeti, A. Weiss, and D. Ganem. 1999. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 965704-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molden, J., Y. Chang, Y. You, P. S. Moore, and M. A. Goldsmith. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 27219625-19631. [DOI] [PubMed] [Google Scholar]
- 31.Murakami, M., D. Kamimura, and T. Hirano. 2004. New IL-6 (gp130) family cytokine members, CLC/NNT1/BSF3 and IL-27. Growth Factors 2275-77. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura, K., A. Yamamoto, M. Kamishohara, K. Takahashi, E. Taguchi, T. Miura, K. Kubo, M. Shibuya, and T. Isoe. 2004. KRN633: a selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase that suppresses tumor angiogenesis and growth. Mol. Cancer Ther. 31639-1649. [PubMed] [Google Scholar]
- 33.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 714187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien, C. A., and S. C. Manolagas. 1997. Isolation and characterization of the human gp130 promoter. Regulation by STATS. J. Biol. Chem. 27215003-15010. [DOI] [PubMed] [Google Scholar]
- 35.Otrock, Z. K., J. A. Makarem, and A. I. Shamseddine. 2007. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol. Dis. 38258-268. [DOI] [PubMed] [Google Scholar]
- 36.Pennica, D., K. J. Shaw, T. A. Swanson, M. W. Moore, D. L. Shelton, K. A. Zioncheck, A. Rosenthal, T. Taga, N. F. Paoni, and W. I. Wood. 1995. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J. Biol. Chem. 27010915-10922. [DOI] [PubMed] [Google Scholar]
- 37.Petrova, T. V., T. Makinen, T. P. Makela, J. Saarela, I. Virtanen, R. E. Ferrell, D. N. Finegold, D. Kerjaschki, S. Yla-Herttuala, and K. Alitalo. 2002. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 214593-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeffer, S., A. Sewer, M. Lagos-Quintana, R. Sheridan, C. Sander, F. A. Grasser, L. F. van Dyk, C. K. Ho, S. Shuman, M. Chien, J. J. Russo, J. Ju, G. Randall, B. D. Lindenbach, C. M. Rice, V. Simon, D. D. Ho, M. Zavolan, and T. Tuschl. 2005. Identification of microRNAs of the herpesvirus family. Nat. Methods 2269-276. [DOI] [PubMed] [Google Scholar]
- 39.Pflanz, S., L. Hibbert, J. Mattson, R. Rosales, E. Vaisberg, J. F. Bazan, J. H. Phillips, T. K. McClanahan, R. de Waal Malefyt, and R. A. Kastelein. 2004. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 1722225-2231. [DOI] [PubMed] [Google Scholar]
- 40.Punjabi, A. S., P. A. Carroll, L. Chen, and M. Lagunoff. 2007. Persistent activation of STAT3 by latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells. J. Virol. 812449-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2342-346. [DOI] [PubMed] [Google Scholar]
- 42.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 9314862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadagopan, S., N. Sharma-Walia, M. V. Veettil, H. Raghu, R. Sivakumar, V. Bottero, and B. Chandran. 2007. Kaposi's sarcoma-associated herpesvirus induces sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J. Virol. 813949-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadler, R., L. Wu, B. Forghani, R. Renne, W. Zhong, B. Herndier, and D. Ganem. 1999. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi's sarcoma-associated herpesvirus. J. Virol. 735722-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saharinen, P., T. Tammela, M. J. Karkkainen, and K. Alitalo. 2004. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 25387-395. [DOI] [PubMed] [Google Scholar]
- 46.Samols, M. A., J. Hu, R. L. Skalsky, and R. Renne. 2005. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. J. Virol. 799301-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schacht, V., M. I. Ramirez, Y. K. Hong, S. Hirakawa, D. Feng, N. Harvey, M. Williams, A. M. Dvorak, H. F. Dvorak, G. Oliver, and M. Detmar. 2003. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 223546-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaeffer, M., M. Schneiderbauer, S. Weidler, R. Tavares, M. Warmuth, G. de Vos, and M. Hallek. 2001. Signaling through a novel domain of gp130 mediates cell proliferation and activation of Hck and Erk kinases. Mol. Cell. Biol. 218068-8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sivakumar, R., N. Sharma-Walia, H. Raghu, M. V. Veettil, S. Sadagopan, V. Bottero, L. Varga, R. Levine, and B. Chandran. 2008. Kaposi's sarcoma-associated herpesvirus induces sustained levels of vascular endothelial growth factors A and C early during in vitro infection of human microvascular dermal endothelial cells: biological implications. J. Virol. 821759-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skobe, M., L. F. Brown, K. Tognazzi, R. K. Ganju, B. J. Dezube, K. Alitalo, and M. Detmar. 1999. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J. Investig. Dermatol. 1131047-1053. [DOI] [PubMed] [Google Scholar]
- 51.Sodhi, A., S. Montaner, V. Patel, J. J. Gomez-Roman, Y. Li, E. A. Sausville, E. T. Sawai, and J. S. Gutkind. 2004. Akt plays a central role in sarcomagenesis induced by Kaposi's sarcoma herpesvirus-encoded G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 1014821-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somanath, P. R., O. V. Razorenova, J. Chen, and T. V. Byzova. 2006. Akt1 in endothelial cell and angiogenesis. Cell Cycle 5512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taga, T., M. Narazaki, K. Yasukawa, T. Saito, D. Miki, M. Hamaguchi, S. Davis, M. Shoyab, G. D. Yancopoulos, and T. Kishimoto. 1992. Functional inhibition of hematopoietic and neurotrophic cytokines by blocking the interleukin 6 signal transducer gp130. Proc. Natl. Acad. Sci. USA 8910998-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vart, R. J., L. L. Nikitenko, D. Lagos, M. W. Trotter, M. Cannon, D. Bourboulia, F. Gratrix, Y. Takeuchi, and C. Boshoff. 2007. Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6 and G-protein-coupled receptor regulate angiopoietin-2 expression in lymphatic endothelial cells. Cancer Res. 674042-4051. [DOI] [PubMed] [Google Scholar]
- 56.Veikkola, T., L. Jussila, T. Makinen, T. Karpanen, M. Jeltsch, T. V. Petrova, H. Kubo, G. Thurston, D. M. McDonald, M. G. Achen, S. A. Stacker, and K. Alitalo. 2001. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 201223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venetsanakos, E., A. Mirza, C. Fanton, S. R. Romanov, T. Tlsty, and M. McMahon. 2002. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp. Cell Res. 27321-33. [DOI] [PubMed] [Google Scholar]
- 58.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36687-693. [DOI] [PubMed] [Google Scholar]
- 59.Wang, L., D. P. Dittmer, C. C. Tomlinson, F. D. Fakhari, and B. Damania. 2006. Immortalization of primary endothelial cells by the K1 protein of Kaposi's sarcoma-associated herpesvirus. Cancer Res. 663658-3666. [DOI] [PubMed] [Google Scholar]
- 60.Weninger, W., T. A. Partanen, S. Breiteneder-Geleff, C. Mayer, H. Kowalski, M. Mildner, J. Pammer, M. Sturzl, D. Kerjaschki, K. Alitalo, and E. Tschachler. 1999. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi's sarcoma tumor cells. Lab. Investig. 79243-251. [PubMed] [Google Scholar]
- 61.Yao, L., T. Yokota, L. Xia, P. W. Kincade, and R. P. McEver. 2005. Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood 1064093-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin, T., T. Taga, M. L. Tsang, K. Yasukawa, T. Kishimoto, and Y. C. Yang. 1993. Involvement of IL-6 signal transducer gp130 in IL-11-mediated signal transduction. J. Immunol. 1512555-2561. [PubMed] [Google Scholar]
- 63.Zhang, X., J. F. Wang, B. Chandran, K. Persaud, B. Pytowski, J. Fingeroth, and J. E. Groopman. 2005. Kaposi's sarcoma-associated herpesvirus activation of vascular endothelial growth factor receptor 3 alters endothelial function and enhances infection. J. Biol. Chem. 28026216-26224. [DOI] [PubMed] [Google Scholar]