Abstract
The involvement of immune cells in prion capture and transport to lymphoid tissues still remains unclear. To investigate the role of dendritic cells (DC), we used DTR+/+ mice, a transgenic model designed to trigger short-term ablation of DC. Transient depletion of DC around the time of intraperitoneal infection delayed prion replication in the spleen, as followed by PrPsc amount, a specific hallmark of prion diseases. Consequently, neuroinvasion and incubation time of prion disease were delayed. In contrast, no differences were observed after oral infection. These results suggest that DC act as vectors for prions from the peripheral entry site to the spleen.
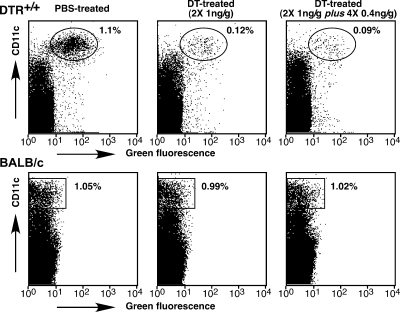
Transmissible spongiform encephalopathies (TSEs) are neurodegenerative diseases caused by the progressive accumulation of the pathological prion protein isoform (PrPsc or PrPres) in brain tissue of affected humans and animals. PrPsc, a pathological conformer of the cellular prion protein (PrPc) was proposed to be the causative agent of TSEs (15). Peripheral prion infections induce a rise of PrPsc deposition and infectivity in lymphoid tissues, notably in spleen, long before the infection spreads to the central nervous system. In intraperitoneally (i.p.)-infected mice, infectivity rises in the spleen within a few days (17). The importance of spleen in prion replication and neuroinvasion was suggested by experiments showing a delay of disease onset in splenectomized mice (8). Follicular dendritic cells (DC) appear to support prion replication in lymphoid follicles (4, 9, 12). However, the precise nature of cell types within the lymphoreticular system supporting TSE agent transport from a peripheral infection site to the spleen remains obscure. B lymphocytes are not essential for trafficking prions within lymphoid organs (1). Beringue and al. demonstrated the involvement of macrophages in the clearance of prions rather than in their transport (3). CD11c+ DC subsets can transport prions from lymphoid organs to the central nervous system in the absence of any other lymphoid element (2). Importantly, DC are active endocytotic and migratory cells, making them good candidates for the transport of prions. The main function of DC is to acquire antigens in peripheral tissues and transport them to secondary lymphoid tissues (19). Therefore, in the present study, we assayed the hypothesis that CD11c+ DC might be involved in the uptake and transport of prions from the infection point to the spleen. To do so, we used a well-characterized diphtheria toxin (DT)-based transgenic mouse model that allows inducible and short-term ablation of DC (7). The strategy is based on the fact that murine cells, unlike primate cells, are resistant to DT. Indeed, the cytotoxicity of DT is strictly dependent on its endocytosis mediated by its cellular receptor (DTR) (13). DT resistance of murine cells results from the low affinity of DT for rodent DTR. Genetic transfer of a primate DTR into mice (BALB/c genetic background) confers DT sensitivity to murine cells. DT sensitivity was targeted to DC by introducing a transgene encoding a simian DTR-green fluorescent protein (GFP) fusion protein under the control of the murine CD11c+ promoter; the resulting transgenic mice were named DTR+/+. CD11c+ is considered to be a pan marker of murine DC regardless of subset. Jung and coworkers have demonstrated that the DT-induced depletion of DC was transient and persisted for 2 days, after which the DC number was gradually restored (7). Because PrPsc inoculated i.p. persisted at least 5 days in the mouse body (10), a minimum of 5 days of depletion of DC was required. To increase the duration of DC depletion until the complete disappearance of the inoculated PrPsc, we designed a protocol of systemic DT injections. DTR+/+ mice were submitted to i.p. injections of DT 2 days apart: two injections of 1 ng of toxin/g of body weight, followed by four extra injections (0.4 ng/g of body weight). Flow cytometry analyses were performed on splenocytes from DT-treated or untreated DTR+/+ mice, focusing on GFP+-CD11c+high DC (Fig. 1). The first two injections of DT (1 ng/g of body weight) led to a significant depletion of CD11c+ DC (89.0% ± 3.5%; n = 7; P < 0.01, Mann-Whitney test) as compared to the level in phosphate-buffered saline (PBS)-treated mice (Fig. 1). The systemic injection of DT used here allowed an important and persistent depletion of DC until 2 days after the last injection of toxin but resulted, however, in 20% lethality in DTR+/+ mice. Neither mortality nor depletion of DC was observed when BALB/c mice were submitted to the same procedure (Fig. 1).
FIG. 1.
DT sensitivity of the DC compartment in DTR+/+ transgenic mice. Flow cytometry analysis of freshly isolated splenocytes of DTR+/+ mice 1 day postinjection with PBS or the indicated doses of DT (left). Ovals indicate the GFP+ CD11c+ DC. Representative data from seven independent experiments are presented as dot plots.
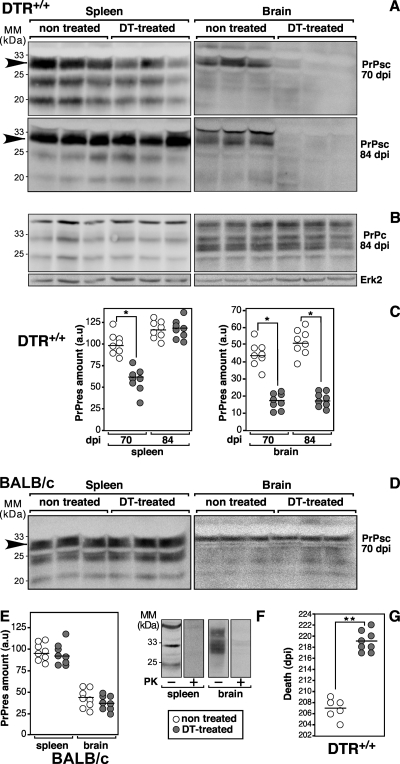
We then investigated the effect of transient depletion of DC on PrPsc formation in the spleen after peripheral prion inoculation. One day after the first two injections of DT, mice were inoculated i.p. with 100 μl of 2% brain homogenate prepared from terminally ill 139A prion-infected BALB/c mice and then the systemic DT treatment was pursued as described above. As a control, PBS-treated DTR+/+ mice were infected under the same experimental conditions. Mice were killed 1 day after the last DT injection (i.e., 8 days postinfection [dpi]) and 70 and 84 dpi, and then spleens and brains were collected and homogenized as described previously (5). For detection of PrPsc, lysates were digested with 100 μg of proteinase K (PK) per mg of total proteins for 45 min at 37°C. PrPc and PrPsc were detected using the anti-PrP monoclonal antibody (MAb) SAF70. The pathological PK-resistant PrP isoform (i.e., PrPsc) was undetectable in spleens and brains of DTR+/+ mice at 8 dpi (data not shown). At 70 dpi, PrPsc was detectable in both organs, indicating the beginning of PrPsc accumulation as estimated by Western blotting (Fig. 2A). The amount of PrPsc was drastically reduced in spleens and brains of DT-treated DTR+/+ mice compared to PBS-treated mice. A marked difference in the amounts of PrPsc remained in the brain of DT-treated versus nontreated mice at 84 dpi, whereas no difference was observed in the spleen (Fig. 2A). Neither the amount nor the glycosylation profiles of PrPc were modified by the DT treatment (Fig. 2B). By plotting the quantified results of blots, we determined that the amount of PrPsc in the spleen was significantly lower in DT-treated mice than in control animals at 70 dpi (n = 8; P < 0.01, Mann-Whitney test [Fig. 2C]), whereas there was no significant difference at 84 dpi. A significant difference in the amounts of cerebral PrPsc was measured between DT-treated and untreated animals at both times postinfection (n = 8; P < 0.01, Mann-Whitney test [Fig. 2C]). As a control, BALB/c mice were submitted to the DT treatment and infected with prions following the same procedure. At 70 dpi, the PrPsc formation was estimated by Western blotting on PK-digested spleen and brain homogenates (Fig. 2D). The amounts of PrPsc were similar in tissues from untreated and DT-treated mice (Fig. 2D and E), indicating that in BALB/c mice, DT per se, had no effect on both PrPsc formation and prion neuroinvasion. After PK digestion, no PrP-like immunoreactivity was detectable in tissue homogenates from healthy BALB/c mice, demonstrating the efficacy of the PK digestion and the specificity of the anti-PrP MAb (Fig. 2F).
FIG. 2.
DT systemic treatment delays splenic and cerebral PrPsc formation of DTR+/+ mice after i.p. prion infection. Representative Western blots were performed on brain (right) or spleen (left) homogenates of three DTR+/+ (A and C) and wild-type BALB/c (D and E) mice treated twice with DT (1 ng/g of body weight), i.p. inoculated with prions 1 day later, and treated again with four extra injections of DT at 0.4 ng/g every 2 days. The nontreated group of animals was injected with the saline buffer alone. Mice were sacrificed at 70 and 84 dpi, and spleens and brains were homogenized in lysis buffer. MM, molecular mass markers. PrPsc (A and D) and PrPc (B) were prepared from PK-digested and undigested homogenates, respectively. Four hundred micrograms (A and D) or 25 μg (B) of total proteins was loaded per lane, submitted to electrophoresis, and blotted, and PrP isoforms were detected using the anti-PrP MAb SAF70. Alternatively, brain and spleen homogenates of healthy BALB/c mice were analyzed with or without PK digestion (F). To determine the correction factor of load, blots performed with nondigested proteins were reprobed with the anti-Erk2 antibody (B). Blots were revealed using an enhanced chemiluminescence system (Amersham) with a LAS3000 detector (Fuji). Densitometry analyses were performed with National Institutes of Health Image software on blots corresponding to eight animals per group (C and E). The results of the densitometry analysis performed on immunopositive bands indicated with an arrow were calculated as follows: (mean intensity/surface × correction factor of load) and expressed in arbitrary units (a.u.). (G) Effect of DT treatment on the incubation time of prion-infected DTR+/+ mice. Mice were i.p. infected and treated with DT as described above. Mice were observed daily and killed when death was considered as imminent (i.e., weight loss, motionless, loss of righting reflex, and eye discharge), defining the incubation time of the disease. Statistical analyses were performed using the nonparametric Mann-Whitney test *, P < 0.01; **, P < 0.001.
In order to determine whether transient DC depletion could delay the prion disease incubation time, DTR+/+ mice were infected with the 139A scrapie strain (100 μl of 2% brain homogenate i.p.) and treated with DT or not treated as described above (Fig. 2G). Systemic DT treatment significantly increased the survival time of the infected mice as compared to the vehicle-treated group (P < 0.001, Mann-Whitney test). However, all DTR+/+ mice finally developed clinical signs of prion disease, such as ataxia, tremor, and swaying gait and PrPsc was detected in their brains at the terminal illness stage (data not shown). When inoculated with prions directly into the brain, all control and DT-treated DTR+/+ mice developed clinical signs of disease and died with similar incubation times (i.e., 148 ± 5.0 dpi and 145 ± 4.0 dpi, respectively).
In summary, short-term depletion of DC before prion infection decreased the kinetics of newly formed PrPsc accumulation in the spleen, after i.p. infection. Consequently, the cerebral formation of PrPsc and the incubation time to death were both delayed. Together, these results strongly suggest that CD11c+ DC may partly control prion infection in peripheral tissues by sequestration and transport of the TSE agent from the peripheral infection site to the spleen.
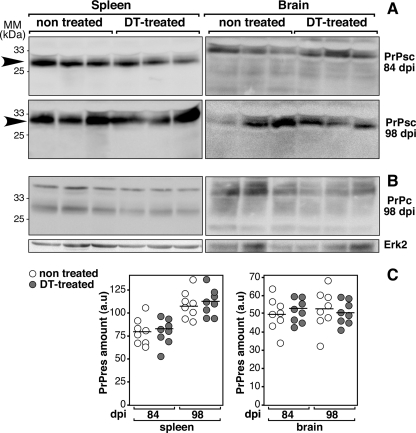
There is substantial evidence suggesting that the lymphoreticular requirements for i.p. and oral uptake of prions differ from each other (18). The effect of DC depletion after oral contamination with prions was studied using the systemic toxin treatment described above, and the inoculum (100 μl of 2% brain homogenate of 139A scrapie strain) was administered intragastrically using a feeding needle. After intragastric exposure, no distinct difference in amounts of PrPsc was observed in spleens and brains of DT-treated untreated DTR+/+ mice (Fig. 3). Thus, in contrast to the i.p. route of infection, transient depletion of DC had no effect on the kinetics of PrPsc accumulation in the spleen after oral infection. These results suggest that CD11c+ DC were not required in the uptake and transport of prions from the gut to the spleen. Our results are in line with previous reports indicating differences in lymphoreticular requirements between oral and i.p. prion infections (14, 18). Regarding the oral route of prion infection, the role of DC remains uncertain. It is conceivable that the oral route uses primarily efferent nerve fibers for neuroinvasion, with a minor role being played by mobile immune cells (11). In agreement with our results, Sethi et al. have shown that a defect of CD8+ CD11c+ DC impedes neuroinvasion after i.p. but not oral infection with prions (18), whereas it has been proposed by others that DC could play an important role in the transport of prions (6). Recently, Raymond et al. have reported that 2 days of depletion of DC impaired the neuroinvasion of prions in ∼50% of DTR+/+ mice under a C57BL6 genetic background (16). Since, in our model, DC depletion was stronger (i.e., 89% versus 60%) and longer (i.e., 8 dpi versus 2 dpi), these discrepancies could be explained by the different methods of oral inoculations of the infectious agent (feeding versus intragastric exposure). Alternatively, the involvement of DC might depend on the strain of mouse.
FIG. 3.
No change in the splenic and cerebral PrPsc formation of DT-treated DTR+/+ mice after oral prion inoculation. Western blot analyses were performed on brain (right) or spleen (left) homogenates of orally prion-infected DTR+/+ mice DT treated or not. Detection of PrPsc (A) and PrPc (B) was performed on brain and spleen of DTR+/+ mice at 84 and 98 dpi. MM, molecular mass markers. (C) Densitometry analyses were performed on blots with eight animals per group.
In summary, we clearly demonstrated that CD11c+ DC may act as carriers for prions very early after i.p. infection. Owing to the fact that DC are present in a wide range of epithelia, such as skin, oral mucosa, and gastrointestinal tract, the role of DC in the transport of prions following any other possible infection routes (scarification, blood transfusion, intraocular, and dental) remains to be established.
Acknowledgments
We are indebted to F. Bihl and G. Lauvau for supplying us with DTR+/+ mice and J. Grassi for the anti-PrP MAb.
This work was supported by a grant from the Agence Nationale de la Recherche. S.C.-D. was the recipient of a fellowship from the Fondation pour la Recherche Médicale.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Aguzzi, A., F. L. Heppner, M. Heikenwalder, M. Prinz, K. Mertz, H. Seeger, and M. Glatzel. 2003. Immune system and peripheral nerves in propagation of prions to CNS. Br. Med. Bull. 66141-159. [DOI] [PubMed] [Google Scholar]
- 2.Aucouturier, P., F. Geissmann, D. Damotte, G. P. Saborio, H. C. Meeker, R. Kascsak, R. I. Carp, and T. Wisniewski. 2001. Infected splenic dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie. J. Clin. Investig. 108703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beringue, V., M. Demoy, C. I. Lasmezas, B. Gouritin, C. Weingarten, J. P. Deslys, J. P. Andreux, P. Couvreur, and D. Dormont. 2000. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J. Pathol. 190495-502. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. L., K. Stewart, D. L. Ritchie, N. A. Mabbott, A. Williams, H. Fraser, W. I. Morrison, and M. E. Bruce. 1999. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat. Med. 51308-1312. [DOI] [PubMed] [Google Scholar]
- 5.Dirikoc, S., S. A. Priola, M. Marella, N. Zsürger, and J. Chabry. 2007. Nonpsychoactive cannabidiol prevents prion accumulation and protects neurons against prion toxicity. J. Neurosci. 279537-9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang, F. P., C. F. Farquhar, N. A. Mabbott, M. E. Bruce, and G. G. MacPherson. 2002. Migrating intestinal dendritic cells transport PrP(Sc) from the gut. J. Gen. Virol. 83267-271. [DOI] [PubMed] [Google Scholar]
- 7.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimberlin, R. H., and C. A. Walker. 1989. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Res. 12201-211. [DOI] [PubMed] [Google Scholar]
- 9.Mabbott, N. A., F. Mackay, F. Minns, and M. E. Bruce. 2000. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat. Med. 6719-720. [DOI] [PubMed] [Google Scholar]
- 10.Maignien, T., C. I. Lasmezas, V. Beringue, D. Dormont, and J. P. Deslys. 1999. Pathogenesis of the oral route of infection of mice with scrapie and bovine spongiform encephalopathy agents. J. Gen.Virol. 803035-3042. [DOI] [PubMed] [Google Scholar]
- 11.McBride, P. A., W. J. Schulz-Schaeffer, M. Donaldson, M. Bruce, H. Diringer, H. A. Kretzschmar, and M. Beekes. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 759320-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montrasio, F., R. Frigg, M. Glatzel, M. A. Klein, F. Mackay, A. Aguzzi, and C. Weissmann. 2000. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 2881257-1259. [DOI] [PubMed] [Google Scholar]
- 13.Naglich, J. G., J. E. Metherall, D. W. Russell, and L. Eidels. 1992. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 691051-1061. [DOI] [PubMed] [Google Scholar]
- 14.Prinz, M., G. Huber, A. J. Macpherson, F. L. Heppner, M. Glatzel, H. P. Eugster, N. Wagner, and A. Aguzzi. 2003. Oral prion infection requires normal numbers of Peyer's patches but not of enteric lymphocytes. Am. J. Pathol. 1621103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 9513363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raymond, C. R., P. Aucouturier, and N. A. Mabbott. 2007. In vivo depletion of CD11c+ cells impairs scrapie agent neuroinvasion from the intestine J. Immunol. 1797758-7766. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein, R., P. A. Merz, R. J. Kascsak, C. L. Scalici, M. C. Papini, R. I. Carp, and R. H. Kimberlin. 1991. Scrapie-infected spleens: analysis of infectivity, scrapie-associated fibrils, and protease-resistant proteins. J. Infect. Dis. 16429-35. [DOI] [PubMed] [Google Scholar]
- 18.Sethi, S., K. M. Kerksiek, T. Brocker, and H. Kretzschmar. 2007. Role of the CD8+ dendritic cell subset in transmission of prions. J. Virol. 814877-4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman, R. M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9271-296. [DOI] [PubMed] [Google Scholar]