Abstract
Genetic and biochemical studies have provided evidence for an entry/fusion complex (EFC) comprised of at least eight viral proteins (A16, A21, A28, G3, G9, H2, J5, and L5) that together with an associated protein (F9) participates in entry of vaccinia virus (VACV) into cells. The genes encoding these proteins are conserved in all poxviruses, are expressed late in infection, and are components of the mature virion membrane but are not required for viral morphogenesis. In addition, all but one component has intramolecular disulfides that are formed by the poxvirus cytoplasmic redox system. The L1 protein has each of the characteristics enumerated above except that it has been reported to be essential for virus assembly. To further investigate the role of L1, we constructed a recombinant VACV (vL1Ri) that inducibly expresses L1. In the absence of inducer, L1 synthesis was repressed and vL1Ri was unable to form plaques or produce infectious progeny. Unexpectedly, assembly and morphogenesis appeared normal and the noninfectious virus particles were indistinguishable from wild-type VACV as determined by transmission electron microscopy and analysis of the component polypeptides. Notably, the L1-deficient virions were able to attach to cells but the cores failed to penetrate into the cytoplasm. In addition, cells infected with vL1Ri in the absence of inducer did not form syncytia following brief low-pH treatment even though extracellular virus was produced. Coimmunoprecipitation experiments demonstrated that L1 interacted with the EFC and indirectly with F9, suggesting that L1 is an additional component of the viral entry apparatus.
Poxviruses are large, complex DNA viruses that replicate entirely in the cytoplasm of animal cells (19). Vaccinia virus (VACV), the prototypic member of the Poxviridae, has a DNA genome of 195 kbp encoding nearly 200 proteins with diverse functions needed for replication and host interactions. There are two major types of infectious VACV particles: mature virions (MVs) and enveloped virions (EVs) (3). The MVs, which consist of a DNA-protein core surrounded by a lipoprotein membrane, are assembled in cytoplasmic viral factories and contain about 80 polypeptides (9, 26, 41). A subpopulation of MVs is wrapped by modified trans-Golgi or an endosomal cisterna containing additional viral membrane proteins, is transported along microtubules to the cell periphery, and exits the cell through the plasma membrane (21, 33). The EVs are essentially MVs with an additional membrane that is disrupted prior to fusion of the MV with the cell during entry (18). Entry can occur either at the plasma membrane or via a low-pH endosomal pathway (37). Regardless of the route, a complex of eight or more MV membrane proteins activates or mediates the entry/fusion step (6, 16, 22, 28-30, 35, 36). These proteins are also required for cell-cell fusion triggered by low pH or by mutation of the A56 or K2 protein (30, 38). Assembly or stability of the entry/fusion complex (EFC) is apparently dependent on multiple interactions among the A16, A21, A28, G3, G9, H2, J5, and L5 proteins (29). The F9 protein associates with the complex and is also required for viral entry and cell-cell fusion (6). The phenotype of a conditional lethal I2 mutant is similar to that of the other entry protein mutants, though association with the EFC remains to be determined (R. J. Nichols, E. Stanitsa, B. Unger, and P. Traktman, submitted for publication). Although genetic evidence is lacking, an A17-A27 complex has been shown to mediate fusion of transfected cells in the absence of other viral proteins, suggesting a role in entry (17).
Several MV membrane proteins have essential roles in virus morphogenesis as determined by analysis of conditional lethal mutants (10). The L1 MV membrane protein was included in this group based on a report that repression of L1 expression interfered with morphogenesis and prevented the assembly of MVs (25). Additional studies of L1 indicated that it is myristoylated at the N terminus and has three intramolecular disulfide bonds and a C-terminal transmembrane domain (1, 24, 32). The atomic structure of the L1 ectodomain, which was solved to 1.51 Å, exhibits a fold composed of a bundle of α-helices packed against a pair of two-stranded β-sheets (34). We considered that L1 might have a role in entry partly because it is a target of neutralizing antibody and is structurally related to F9 (20, 40). However, because of a report that L1 is required for virion assembly (25), we decided to make an L1 conditional null mutant that could be used to discriminate putative entry and assembly roles by complementation with a panel of point mutants. Unexpectedly, we discovered that the null mutant was able to assemble L1-deficient (L1−) MVs that were indistinguishable in appearance and polypeptide composition from MVs containing L1 (L1+). Moreover, these L1− MVs could attach to cells but were unable to enter or induce membrane fusion. L1 was shown to physically interact with the EFC, suggesting that it acts in concert with the other known entry proteins.
MATERIALS AND METHODS
Cells and viruses.
The Western Reserve (WR) strain of VACV and recombinant viruses derived from this strain were used. Virus amplification, propagation, and titration were performed as previously described (14). The inducible null mutant vL1Ri was made and propagated in medium containing 50 μm isopropyl-β-d-thiogalactopyranoside (IPTG). MVs were purified twice by sedimentation through 36% (wt/vol) sucrose cushions and once on a 25 to 40% (wt/vol) sucrose gradient as described previously (14). The particle/PFU ratio was determined by light scattering at 260 nm (optical density value of 1 = 1.2 × 1010 particles) and plaque titration on BS-C-1 cells.
Construction of recombinant VACV.
vL1Ri was constructed from vT7LacOI, a recombinant VACV that has a bacteriophage T7 RNA polymerase gene under the transcriptional control of the Escherichia coli lac operator and a constitutively expressed lac repressor gene (39). The recombinant vL1Ri was constructed essentially as described previously (31). The final PCR product used for transfection of cells infected with vT7lacOI consisted of 506 bp of the L1R open reading frame (nucleotides 77025 to 77331) adjacent to the lac operator-regulated T7 promoter, the enhanced green fluorescent protein (EGFP) gene under the control of a VACV synthetic early-late promoter (8), and 548 bp of G9R open reading frame (nucleotides 77024 to 76476). The recombinant virus was cloned by three rounds of plaque purification in the presence of 50 μm IPTG. Gene insertions and modifications were confirmed by DNA sequencing. The recombinant VACV vA28i/F9TAP was provided by Timothy Wagenaar (NIAID).
Antibodies.
Mouse monoclonal antibodies (MAbs) 7D11 (40) and AB1.1 (23) recognized L1 and D8, respectively. Rabbit polyclonal antibodies to VACV proteins and peptides were to A4 (12), A21 (36), L5 (35), A28 (G. Nelson and B. Moss, unpublished data), A17 (4), H3 (11), and A3 (p4b/4b) (27). Mouse MAb to D8, rabbit polyclonal antibody to A4, and rabbit polyclonal antibodies to F9 and L1 were kindly provided by G. L. Smith (Imperial College, London, United Kingdom), M. Esteban (CSI, Madrid, Spain), and G. Cohen (University of Pennsylvania), respectively. Mouse anti-V5 MAb, clone V5-10, conjugated to agarose, was from Sigma (St. Louis, MO). Alexa Fluor 488 goat anti-rabbit immunoglobulin G (IgG) and Alexa Fluor 594 goat anti-mouse IgG were purchased from Invitrogen (Carlsbad, CA).
SDS-PAGE and Western blotting.
Cells were lysed in 50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitor cocktail tablets (Roche Applied Science, Indianapolis, IN) at 4°C for 30 min. Proteins were solubilized in lithium dodecyl sulfate sample buffer with reducing agent (Invitrogen), heated to 70°C for 10 min, and resolved by SDS-polyacrylamide gel electrophoresis (PAGE) on a 4 to 12% NuPage Bis-Tris gel (Invitrogen). For Western blot analysis, the proteins were transferred to a nitrocellulose membrane and blocked with 5% nonfat dry milk in phosphate-buffered saline, pH 7.5. Membranes were incubated with an appropriate primary antibody, washed with 0.05% Tween, and incubated with a 1:10,000 dilution of horseradish peroxidase-linked secondary antibody (GE Healthcare, Pittsburgh, PA) and Super Signal chemiluminescence reagents (Pierce, Rockford, IL).
Immunopurification.
Lysates prepared as described above were incubated overnight at 4°C with a 1:250 dilution of appropriate antibody or anti-V5 agarose or streptavidin-Sepharose conjugate. The antigen-antibody complexes were mixed by rotation with protein G-Sepharose beads for 2 h at 4°C. The beads were washed, and the bound proteins were subjected to SDS-PAGE and Western blotting as described above. Protein bound to streptavidin-Sepharose was eluted with biotin. Before SDS-PAGE the eluate was concentrated by 10% trichloroacetic acid precipitation.
Cell binding and core penetration assay.
HeLa cells were infected with 10 PFU per cell of purified L1+ virions or with the corresponding optical density at 260 nm of L1− virions at 4°C for 1 h in the presence of 300 μg of cycloheximide (Sigma) per ml. The cells were washed and incubated for 2 h at 37°C in the presence of cycloheximide before being fixed with 4% paraformaldehyde. Autofluorescence was quenched with 2% glycine, and cells were permeabilized with 0.1% Triton X-100. After being blocked with 10% fetal bovine serum, the cells were incubated with a 1:100 dilution of anti-D8 mouse MAb and anti-A4 rabbit polyclonal antibody. After extensive washes, the cells were incubated with Alexa Fluor 594 anti-mouse IgG and Alexa Fluor 488 anti-rabbit IgG at a dilution of 1:100 and later with diamidino-2-phenylindole dihydrochloride (DAPI) from Invitrogen at a dilution of 1:2,000. Images were collected with a Leica laser scanning confocal microscope.
Cell-cell fusion.
For “fusion from within,” which is dependent on the presence of fusion-competent progeny virus on the cell surface, BS-C-1 cells were infected with 5 PFU per cell of vL1Ri in the presence or absence of 50 μM IPTG. After 18 h, cells were exposed to pH 5.3 or pH 7.4 buffer for 3 min at 37°C. The buffers were then replaced with complete Eagle's minimum essential medium and incubated for 3 h at 37°C, fixed with 4% paraformaldehyde, permeabilized with 0.05% Triton X-100, stained with DAPI, and visualized by phase-contrast and fluorescence microscopy.
Electron microscopy.
BS-C-1 cells were grown in dishes with 60-mm-diameter wells and infected with 10 PFU per cell of purified virus. At 18 h after infection, cells were prepared for conventional transmission electron microscopy by being fixed with 2% glutaraldehyde and embedded in EmBed-182 resin (Electron Microscopy Sciences, Hatfield, PA). The specimens were viewed with an FEI-CM100 transmission electron microscope (Hillsboro, OR).
RESULTS
L1 is essential for VACV replication.
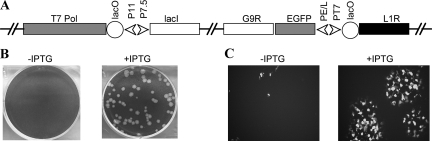
The genetic modifications of the recombinant vL1Ri, with an IPTG-inducible L1R gene, are shown in Fig. 1A. The inserted DNA consists of (i) the E. coli lac repressor gene regulated by a VACV early/late promoter; (ii) the bacteriophage T7 RNA polymerase gene regulated by the lac operator and VACV late promoter; (iii) the L1R gene regulated by the T7 promoter and lac operator, and (iv) the reporter EGFP gene regulated by a VACV early/late promoter. The lac operator regulates expression of both the T7 RNA polymerase and L1R genes, thereby providing stringent repression in the absence of IPTG. In the presence of the inducer IPTG, the lac repressor is inactivated, resulting in sequential transcription of the T7 RNA polymerase and L1R genes. The purpose of the EGFP gene was to allow the clonal isolation of vL1Ri by picking green fluorescent plaques several times in succession in the presence of IPTG.
FIG. 1.
Construction and characterization of an L1-inducible virus. (A) Diagram of vL1Ri. The relevant segment of the viral genome is shown. Abbreviations: T7 Pol, bacteriophage T7 RNA polymerase open reading frame; lacO, E. coli lac operator; lacI, E. coli lac repressor open reading frame; P11, VACV late promoter; PE/L and P7.5, VACV early/late promoters; PT7, T7 promoter; EGFP, EGFP open reading frame. (B) Plaque formation by vL1Ri in the absence and presence of IPTG. BS-C-1 cells were infected with vL1Ri in the absence (−) or presence (+) of 50 μM IPTG. After 48 h, monolayers were fixed and stained with crystal violet. (C) Cells were infected as for panel B except that at 24 h the plate was examined with a fluorescence microscope for EGFP expression.
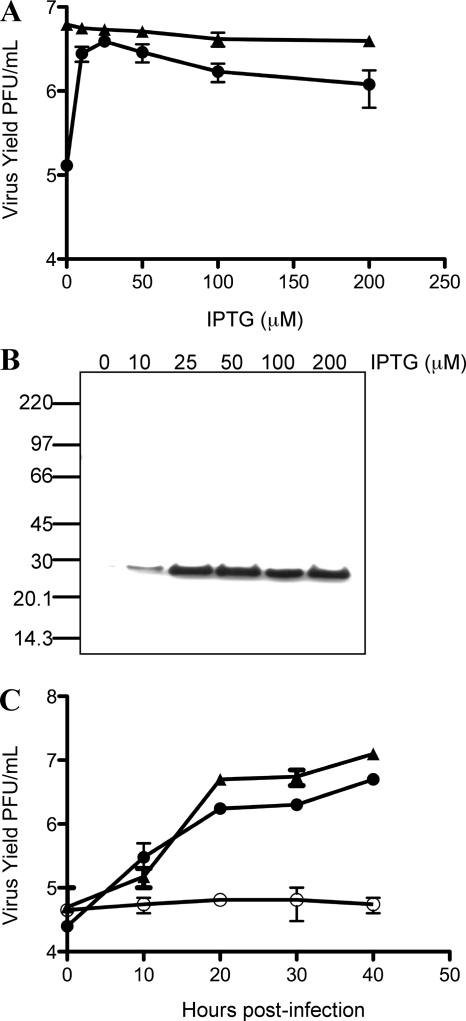
vL1Ri was propagated in the presence of IPTG and characterized by infecting cells in the absence or presence of inducer. The conditional lethal phenotype of vL1Ri was indicated by an IPTG requirement for plaque formation (Fig. 1B). In the absence of IPTG only individual EGFP-positive cells were detected by fluorescence microscopy, showing the inability of the mutant virus to spread (Fig. 1C). A replication block was demonstrated by infecting cells under one-step growth conditions. Virus yield increased with IPTG concentration reaching a maximum at 25 μM (Fig. 2A), suggesting that L1 overexpression might be slightly inhibitory. The repression of L1 in the absence of IPTG and the concentration-dependent increase in L1 synthesis were determined by Western blotting (Fig. 2B). At 50 μM the kinetics of virus replication was similar to that of the parental virus, but no increase in the yield of vL1Ri was detected in the absence of IPTG (Fig. 2C).
FIG. 2.
Replication of vL1Ri. (A) Effect of IPTG concentrations on virus yield. BS-C-1 cells were infected with 5 PFU per cell of vT7lacOI (triangles) or vL1Ri (circles) in the presence of 0 to 200 μM IPTG. At 24 h postinfection, virus yields were determined by plaque assay on BS-C-1 cells in the presence of 50 μM IPTG. (B) Expression of L1. At 24 h, in the presence of indicated concentrations of IPTG, L1 was measured by Western blotting with anti-L1 MAb followed by horseradish peroxidase-linked secondary antibody. The masses in kDa of mobility marker proteins are shown on the left. (C) One-step growth curve. BS-C-1 cells were infected with 5 PFU per cell of vT7lacOI (triangles) or vL1Ri in the presence (solid circles) or absence (open circles) of IPTG. Cells were harvested at the indicated times, and the virus titers were determined by plaque assay as described above.
L1 is not required for VACV morphogenesis.
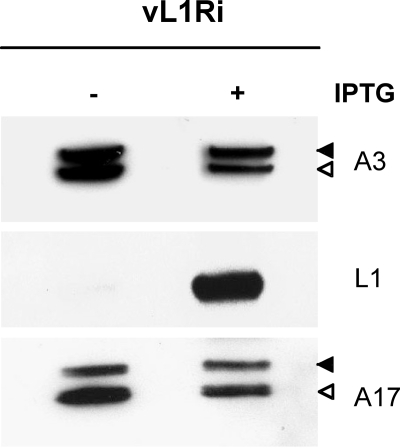
Previous studies had shown that the processing of the membrane protein A17 and the core protein A3 by the I7 protease occurs during early and late stages in morphogenesis, respectively (2, 7). Since L1 was thought to be required for morphogenesis, we analyzed lysates from cells infected with vL1Ri in the presence or absence of IPTG. Precursor and product forms of A17 and A3 were detected regardless of whether inducer was present or not, indicating that synthesis and processing of both proteins occurred in the absence of L1 (Fig. 3). In a parallel control experiment we confirmed that rifampin, a known inhibitor of morphogenesis, blocked processing of the A3 protein but had no effect on processing of A17 (not shown).
FIG. 3.
Synthesis and processing of viral proteins. BS-C-1 cells were infected with 5 PFU of vL1Ri per cell in the absence or presence of 50 μM IPTG. At 24 h postinfection, cells were harvested, lysed, and subjected to SDS-PAGE followed by Western blotting with anti-A3, anti-L1, or anti-A17 antibody followed by horseradish peroxidase-linked secondary antibody. Solid and open arrowheads point to precursor and product polypeptides, respectively.
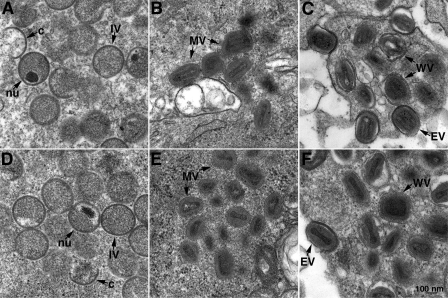
Transmission electron microscopy was used to further investigate a role of L1 in morphogenesis. In the absence of IPTG, all forms of virus particles including immature, mature, and enveloped virions were seen at 12 h (Fig. 4A to C) and 20 h (Fig. 4D to F) after infection. Indeed, no difference was discerned between images of cells infected in the absence and those of cells infected in the presence (not shown) of IPTG. Thus, both biochemical and microscopic studies indicated no effect on VACV morphogenesis despite stringent repression of L1 as shown in Fig. 1 and 2.
FIG. 4.
Transmission electron microscopy of the cells infected with vL1Ri in the absence of IPTG. BS-C-1 cells were infected with 5 PFU per cell of vL1Ri in the absence of IPTG. At 12 h (A to C) or 20 h (D to F) the cells were fixed, embedded, sectioned, and examined by transmission electron microscopy. Abbreviations: c, crescent; IV, immature virion; nu, nucleoid within an immature virion; WV, wrapped virion; EV, extracellular virion. Bars, 100 nm.
Characterization of purified L1− virions.
To compare the infectivities of MVs lacking and containing L1, virions were purified by sucrose gradient sedimentation from cells infected with vL1Ri in the absence or presence of IPTG. The number of virus particles was determined from the optical density. Data from two determinations are shown in Table 1. The average particle/PFU ratios for virions made in the presence and absence of IPTG were approximately 65 and 4,800, respectively. Thus, there was about a 75-fold difference in infectivity. The measurable infectivity of the virions made in the absence of IPTG could have resulted from residual inoculum virus.
TABLE 1.
Specific infectivities of L1+ and L1− virions
| Expt no. and agent | Yield
|
Particle/ PFU ratio | |
|---|---|---|---|
| No. of particles | PFU | ||
| 1 | |||
| vL1Ri with IPTG | 8.0 × 1010 | 1.3 × 109 | 62 |
| vL1Ri without IPTG | 13.3 × 1010 | 3.3 × 107 | 4,000 |
| 2 | |||
| vL1Ri with IPTG | 8.3 × 1010 | 1.2 × 109 | 69 |
| vL1Ri without IPTG | 14.0 × 1010 | 2.5 × 107 | 5,600 |
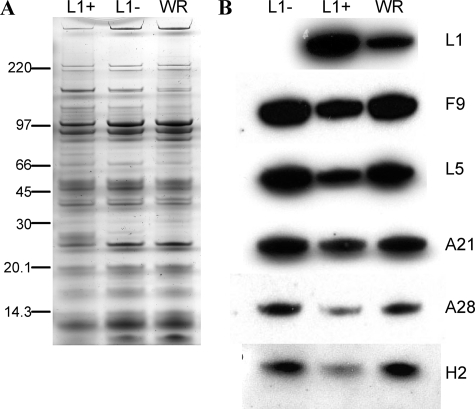
Equal numbers of virus particles purified from cells infected with vL1Ri in the presence and absence of IPTG or with VACV WR were solubilized with SDS and analyzed by PAGE. The polypeptide compositions, determined by direct staining (Fig. 5A) and Western blotting (Fig. 5B), were similar except for the absence of L1 in L1− MVs and an excess of L1 in L1+ virions relative to the amount in WR. The excess of L1 in L1− virions seemed to be compensated by lower amounts of other MV membrane proteins (Fig. 5B), which could account for the slight toxicity of 50 μM IPTG (Fig. 2A). Of more relevance, the Western blot revealed that the amounts of F9 and representative EFC polypeptides in L1− MVs were similar to the amounts in WR, suggesting that L1 was not required for the insertion of these proteins into the viral membrane (Fig. 5B).
FIG. 5.
Polypeptide composition of purified virions. Virions were purified through two sucrose cushions and a gradient from lysates of RK13 cells infected with VACV or vL1Ri in the presence and absence of IPTG. (A) Equal amounts of purified L1+, L1−, and VACV WR virions were analyzed by SDS-PAGE and silver staining. Numbers at left are molecular masses in kilodaltons. (B) Following SDS-PAGE, Western blotting with MAb 7D11 to L1 and polyclonal antibodies to F9, L5, A21, A28, and H2 was followed by horseradish peroxidase-linked secondary antibody.
Cell binding and penetration.
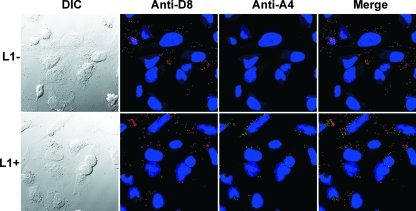
The finding that L1− MVs had low infectivity suggested a defect in virus attachment or entry. To investigate these steps, HeLa cells were incubated with purified L1− or L1+ virions at 4°C for 1 h to allow binding but not penetration. The cells were then washed to remove loosely bound or unbound virus and incubated for an additional 2 h at 37°C to permit entry. Cycloheximide, a protein synthesis inhibitor, was present to prevent uncoating of cores and allow their accumulation in the cytoplasm. After fixation and permeabilization, the cells were incubated with antibodies to the viral membrane protein D8 and core protein A4. Both L1− and L1+ MVs were bound to the cells as shown by the anti-D8 antibody staining (Fig. 6). In contrast, cores stained by anti-A4 antibody were detected only in cells incubated with L1+ MVs (Fig. 6). Thus, L1 was needed for penetration of the virus cores into cells.
FIG. 6.
Cell binding of virions and entry of cores. HeLa cells were infected with 5 PFU per cell of L1+ or the equivalent numbers of L1− virions in the presence of cycloheximide for 1 h at 4°C and then for 2 h at 37°C. The cells were then fixed, permeabilized, stained red with a mouse MAb to the D8 MV membrane protein and Alexa Fluor 594-conjugated goat anti-mouse secondary antibody, and stained green with a rabbit antibody to the A4 core protein and Alexa Fluor 498-conjugated goat anti-rabbit secondary antibody. DNA was stained with DAPI, and the cells were examined by confocal microscopy. Dark-field (DIC [differential interference contrast]), anti-D8, anti-L1, and a merge of anti-D8 and anti-L1 images are labeled.
L1 is required to induce low-pH-triggered cell-cell fusion.
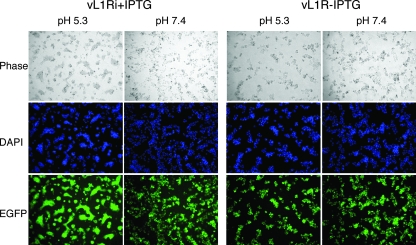
VACV-infected cells can be triggered to form syncytia by brief low-pH treatment (13, 15). Despite the name “fusion from within,” this phenomenon actually depends on the presence of progeny EVs on the cell surface (5, 20) and expression of the previously described EFC polypeptides (6, 16, 22, 28-30, 35, 36). Since L1 is required for entry, we predicted that L1 would also be needed for syncytium formation. BS-C-1 cells were infected with vL1Ri in the presence or absence of IPTG for 18 h, briefly exposed to low pH, and further incubated for 3 h in normal medium. Large syncytia were observed after cells infected with vL1Ri in the presence of IPTG were exposed to low pH but not neutral pH (Fig. 7). In contrast, syncytia were not detected after low- or neutral-pH exposure of cells infected with vL1Ri in the absence of IPTG (Fig. 7). This result indicates that fusion depends on the expression of L1.
FIG. 7.
Low pH triggered cell-cell fusion. BS-C-1 monolayers were infected with 5 PFU per cell of vL1Ri in the presence (+) or absence (−) of IPTG. At 18 h, the cells were treated for 3 min with pH 5.3 or pH 7.4 buffer before replacement with normal medium. After 3 h, the cells were fixed, permeabilized, stained with DAPI, and visualized by phase-contrast and fluorescence microscopy.
L1 interacts with the EFC.
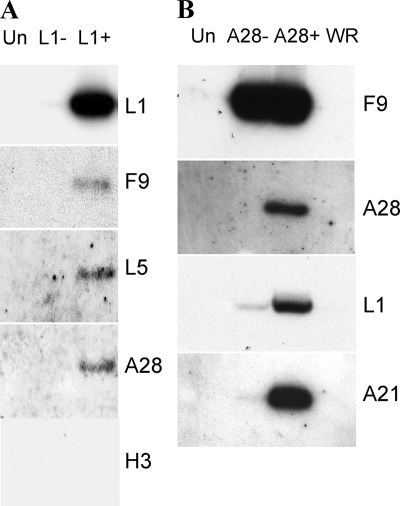
Since L1 is required for entry and cell-cell fusion, we considered that L1 might interact with F9 or proteins of the entry/fusion complex. Cells were mock infected or infected with vL1Ri in the absence or presence of IPTG and harvested after 24 h. The detergent-treated lysates were incubated with mouse MAb 7D11, which specifically recognizes native L1 (40), and then with protein G Sepharose beads. The bound proteins were dissociated and resolved by SDS-PAGE followed by Western blotting with polyclonal antibodies to representative entry proteins. F9, L5, and A28 (Fig. 8A) and A21 (not shown) were associated with L1 from cells infected with vL1Ri in the presence of IPTG. The latter proteins were not detected when IPTG was omitted and L1 was not expressed (Fig. 8A). The specificity of L1 for interaction with entry proteins was confirmed by the inability to detect the VACV MV membrane protein H3, which is not required for entry or fusion, by Western blotting of the proteins captured with L1 (Fig. 8A).
FIG. 8.
Interaction of L1 with the EFC. (A) BS-C-1 cells were uninfected (Un) or infected with vL1Ri in the absence (L1−) or presence (L1+) of IPTG for 24 h. The lysates were incubated overnight with MAb 7D11 (anti-L1), and the antigen-antibody complexes were immobilized on protein G Sepharose. The bound proteins were then resolved by SDS-PAGE followed by Western blotting with polyclonal antibodies to L1, F9, L5, A28, and H3 and secondary antibody conjugated to horseradish peroxidase. (B) BS-C-1 cells were infected with vA28i/F9TAP in the absence (A28−) or presence (A28+) of IPTG or VACV WR (WR). The lysates were incubated overnight with streptavidin beads, and the bound proteins were eluted with biotin and analyzed by Western blotting using polyclonal antibodies to F9, A28, L1, and A21. Un, uninfected cells (control).
Of the proteins that interacted with L1 in the above experiment, three (A21, A28, and L5) are among the eight integral components of the EFC, whereas F9 interacts more peripherally with the complex (6). Previous studies demonstrated that the EFC does not assemble or is destabilized when A28 or other integral components are not expressed (29). This feature allowed us to design an experiment to determine whether L1 interacts primarily with F9 or the EFC. Cells in the presence or absence of IPTG were infected with vA28i/F9TAP, a recombinant VACV that expresses A28 inducibly and tandem affinity purification (TAP)-tagged F9 (F9TAP) constitutively. Following infection, F9TAP and any associated proteins were purified from detergent-treated lysates with streptavidin beads. The bound proteins were then eluted and analyzed by SDS-PAGE and Western blotting with antibodies to F9, A28, A21, and L1. In the presence of IPTG, the three proteins copurified with F9TAP (Fig. 8B), confirming their association. However, when IPTG was omitted and A28 was not expressed, only trace amounts of L1 and A21 were detected in association with F9TAP (Fig. 8B). This result suggested that L1 interacts with A28 or some other component of the EFC rather than with F9 directly.
L1 was not required for stability of the EFC.
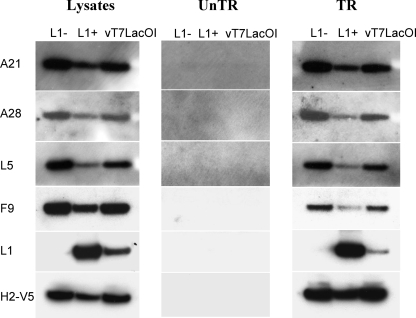
The next experiment was designed to determine whether L1 has a peripheral association with the EFC or is an integral EFC component that is required for the formation or stability of the complex. Cells were infected with vL1Ri in the presence or absence of IPTG or with the parental virus vT7lacOI and then transfected with a plasmid encoding the V5 epitope-tagged H2 component of the EFC regulated by its natural promoter. Analysis of the cell lysates by Western blotting indicated the absence of L1 when IPTG was omitted and overexpression of L1 when 50 μM IPTG was present (Fig. 9). In the absence of L1, the amounts of the EFC components (A21, A28, L5, and H2) and of F9 were similar to that of the vT7lacOI control, whereas in the presence of IPTG the EFC components and F9 were reduced somewhat. The lysates from the transfected cells and untransfected control cells were incubated with immobilized antibody to V5, and the bound proteins were analyzed by Western blotting. Expression of L1 was not required for the association of H2 with other components of the EFC, suggesting that L1 associates peripherally with the complex (Fig. 9). The specificity of binding was confirmed by the absence of any bands from the untransfected cells (Fig. 9).
FIG. 9.
L1 was not required for assembly of the EFC. BS-C-1 cells were infected with vL1Ri in the absence (L1−) or presence (L1+) of IPTG or with control vT7LacOI and either untransfected (UnTR) or transfected (TR) with a plasmid expressing H2 with a C-terminal V5 tag controlled by its native promoter. Approximately 5% of soluble lysates were analyzed directly by SDS-PAGE followed by Western blotting with antibodies to A21, A28, L5, F9, L1, and V5. The Western blot of the lysate from transfected cells is shown on the left. The Western blot of the lysate from the untransfected cells was identical and is not shown. The remaining portions of the cell lysates from the untransfected (middle panel) and transfected (right panel) cells were incubated with immobilized anti-V5 antibody, and bound proteins were analyzed by Western blotting.
DISCUSSION
Our conjecture that L1 might be involved in cell entry was based on the following reasoning: (i) L1 is a target of neutralizing antibody and is therefore exposed on the MV surface (40); (ii) the L1 amino acid sequence is approximately 24% identical to that of F9, which is required for entry and fusion (6); and (iii) L1 has intramolecular disulfide bonds formed by the poxvirus cytoplasmic redox system (32), and the other known substrate proteins all have roles in entry and membrane fusion (6, 22, 28-30, 35, 36). At variance with this idea, however, was a report that L1 is required for assembly and morphogenesis (25). Nevertheless, we found that L1-deficient virions assembled under nonpermissive conditions and that those virions were indistinguishable from wild type in ultrastructure and polypeptide composition as determined by transmission electron microscopy and SDS-PAGE, respectively. We cannot explain this difference from the previous report (25) as that L1-inducible virus, which was constructed 15 years ago, is no longer available for comparison (D. Hruby, personal communication). Although the L1-deficient MVs were able to bind to cells, the cores could not enter the cytoplasm, accounting for their lack of infectivity. Furthermore, cells infected with the conditional lethal mutant could not be triggered to form syncytia by low-pH treatment despite the presence of progeny extracellular virions. In these respects, the phenotype of the conditional lethal L1 null mutant closely resembled that of other EFC-null mutants. Repression of L1 did not diminish the amounts of other entry proteins in the MV membrane or prevent the formation of the EFC complex, suggesting a specific role in entry.
Including L1, 10 proteins that are conserved in all poxviruses have been shown by genetic means to have nonredundant roles in VACV entry and membrane fusion (16, 22, 28, 30, 35, 36; Nichols et al., submitted). An 11th protein, J5, is probably also involved in entry because of its association with the EFC components (28), though so far we have been unable to delete J5 or make a stringent conditional lethal mutant to verify this role (S. Ojeda and B. Moss, unpublished data). The eight EFC components (A16, A21, A28, G3, G9, H2, J5, and L5) form a stable complex that was purified by affinity chromatography and analyzed by mass spectroscopy (29). Neither F9 nor L1 was identified as an EFC component by the mass spectroscopy analysis, although an association was detected by sensitive Western blotting. Because F9 and L1 are relatively abundant proteins, the small amounts suggest that they interact with the EFC nonstoichiometrically or more weakly. The assembly of the EFC, even when F9 (T. Senkevich, personal communication) or L1 was not expressed, also suggested a peripheral association with the complex. In contrast the EFC does not assemble or is destabilized when individual components are not expressed, suggesting multiple interactions (29). Therefore, we tentatively classify F9 and L1 as EFC-associated proteins rather than integral components. The atomic structure of L1 does not resemble that of other known fusion proteins (34), and further work is needed to determine whether L1 is involved in receptor interactions or some aspect of the fusion process.
Acknowledgments
We thank Mariano Esteban, Geoffrey Smith, and Gary Cohen for their generosity in providing antibodies; Norman Cooper and Catherine Cotter for tissue culture cells; Timothy R. Wagenaar for vA28i/F9TAP virus; Arban Domi for help in confocal microscopy and the entry assay; and Tatiana G. Senkevich for useful discussions.
The study was supported by the Division of Intramural Research, NIAID, NIH.
Footnotes
Published ahead of print on 2 July 2008.
REFERENCES
- 1.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 2005. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 34159-71. [DOI] [PubMed] [Google Scholar]
- 2.Ansarah-Sobrinho, C., and B. Moss. 2004. Role of the I7 protein in proteolytic processing of vaccinia virus membrane and core components. J. Virol. 786335-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleyard, G., A. J. Hapel, and E. A. Boulter. 1971. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 139-17. [DOI] [PubMed] [Google Scholar]
- 4.Betakova, T., E. J. Wolffe, and B. Moss. 1999. Membrane topology of the vaccinia virus A17L envelope protein. Virology 261347-356. [DOI] [PubMed] [Google Scholar]
- 5.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J. Virol. 655910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, E., T. G. Senkevich, and B. Moss. 2006. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related L1 protein. J. Virol. 809455-9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd, C. M., T. C. Bolken, and D. E. Hruby. 2002. The vaccinia virus I7L gene product is the core protein proteinase. J. Virol. 768973-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 211904-1907. [DOI] [PubMed] [Google Scholar]
- 9.Chung, C. S., C. H. Chen, M. Y. Ho, C. Y. Huang, C. L. Liao, and W. Chang. 2006. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 802127-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condit, R. C., N. Moussatche, and P. Traktman. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 6631-124. [DOI] [PubMed] [Google Scholar]
- 11.da Fonseca, F. G., A. Weisberg, E. J. Wolffe, and B. Moss. 2000. Characterization of the vaccinia virus H3L envelope protein: topology and posttranslational membrane insertion via the C-terminal hydrophobic tail. J. Virol. 747508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demkowicz, W. E., J. S. Maa, and M. Esteban. 1992. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J. Virol. 66386-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doms, R. W., R. Blumenthal, and B. Moss. 1990. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J. Virol. 644884-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 1998. Generation of recombinant vaccinia viruses, p.16.17.1-16.17.19. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates & Wiley Interscience, New York, NY.
- 15.Gong, S. C., C. F. Lai, and M. Esteban. 1990. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology 17881-91. [DOI] [PubMed] [Google Scholar]
- 16.Izmailyan, R. A., C. Y. Huang, S. Mohammad, S. N. Isaacs, and W. Chang. 2006. The envelope G3L protein is essential for entry of vaccinia virus into host cells. J. Virol. 808402-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochan, G., D. Escors, J. M. Gonzalez, J. M. Casasnovas, and M. Esteban. 2008. Membrane cell fusion activity of the vaccinia virus A17-A27 protein complex. Cell. Microbiol. 101149-1164. [DOI] [PubMed] [Google Scholar]
- 18.Law, M., G. C. Carter, K. L. Roberts, M. Hollinshead, and G. L. Smith. 2006. Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. USA 1035989-5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss, B. 2007. Poxviridae: the viruses and their replication, p. 2905-2946. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 20.Moss, B. 2006. Poxvirus entry and membrane fusion. Virology 34448-54. [DOI] [PubMed] [Google Scholar]
- 21.Moss, B., and B. M. Ward. 2001. High-speed mass transit for poxviruses on microtubules. Nat. Cell Biol. 3E245-E246. [DOI] [PubMed] [Google Scholar]
- 22.Ojeda, S., T. G. Senkevich, and B. Moss. 2006. Entry of vaccinia virus and cell-cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene. J. Virol. 8051-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkinson, J. E., and G. L. Smith. 1994. Vaccinia virus gene A36R encodes a Mr 43-50 K protein on the surface of extracellular enveloped virus. Virology 204376-390. [DOI] [PubMed] [Google Scholar]
- 24.Ravanello, M. P., and D. E. Hruby. 1994. Characterization of the vaccinia virus L1R myristylprotein as a component of the intracellular virion envelope. J. Gen. Virol. 751479-1483. [DOI] [PubMed] [Google Scholar]
- 25.Ravanello, M. P., and D. E. Hruby. 1994. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virus assembly. J. Virol. 686401-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resch, W., K. K. Hixon, R. J. Moore, M. S. Lipton, and B. Moss. 2007. Protein composition of the vaccinia virus mature virion. Virology 358233-247. [DOI] [PubMed] [Google Scholar]
- 27.Resch, W., A. S. Weisberg, and B. Moss. 2005. Vaccinia virus nonstructural protein encoded by the A11R gene is required for formation of the virion membrane. J. Virol. 796598-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senkevich, T. G., and B. Moss. 2005. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 794744-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senkevich, T. G., S. Ojeda, A. Townsley, G. E. Nelson, and B. Moss. 2005. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. USA 10218572-18577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senkevich, T. G., B. M. Ward, and B. Moss. 2004. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 782357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senkevich, T. G., A. Weisberg, and B. Moss. 2000. Vaccinia virus E10R protein is associated with the membranes of intracellular mature virions and has a role in morphogenesis. Virology 278244-252. [DOI] [PubMed] [Google Scholar]
- 32.Senkevich, T. G., C. L. White, E. V. Koonin, and B. Moss. 2002. Complete pathway for protein disulfide bond formation encoded by poxviruses. Proc. Natl. Acad. Sci. USA 996667-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 832915-2931. [DOI] [PubMed] [Google Scholar]
- 34.Su, H. P., S. C. Garman, T. J. Allison, C. Fogg, B. Moss, and D. N. Garboczi. 2005. The 1.51-A structure of the poxvirus L1 protein, a target of potent neutralizing antibodies. Proc. Natl. Acad. Sci. USA 1024240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsley, A., T. G. Senkevich, and B. Moss. 2005. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion. J. Virol. 7910988-10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsley, A., T. G. Senkevich, and B. Moss. 2005. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 799458-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsley, A. C., A. S. Weisberg, T. R. Wagenaar, and B. Moss. 2006. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J. Virol. 808899-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagenaar, T. R., and B. Moss. 2007. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J. Virol. 816286-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward, G. A., C. K. Stover, B. Moss, and T. R. Fuerst. 1995. Stringent chemical and thermal regulation of recombinant gene expression by vaccinia virus vectors in mammalian cells. Proc. Natl. Acad. Sci. USA 926773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolffe, E. J., S. Vijaya, and B. Moss. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 21153-63. [DOI] [PubMed] [Google Scholar]
- 41.Yoder, J. D., T. S. Chen, C. R. Gagnier, S. Vemulapalli, C. S. Maier, and D. E. Hruby. 2006. Pox proteomics: mass spectrometry analysis and identification of Vaccinia virion proteins. Virol. J. 310. [DOI] [PMC free article] [PubMed] [Google Scholar]