Abstract
The hepatitis C virus (HCV) RNA replication complex (RC), which is composed of viral nonstructural (NS) proteins and host cellular proteins, replicates the viral RNA genome in association with intracellular membranes. Two viral NS proteins, NS3 and NS5A, are essential elements of the RC. Here, by using immunoprecipitation and fluorescence resonance energy transfer assays, we demonstrated that NS3 and NS5A interact with tubulin and actin. Furthermore, immunofluorescence microscopy and electron microscopy revealed that HCV RCs were aligned along microtubules and actin filaments in both HCV replicon cells and HCV-infected cells. In addition, the movement of RCs was inhibited when microtubules or actin filaments were depolymerized by colchicine and cytochalasin B, respectively. Based on our observations, we propose that microtubules and actin filaments provide the tracks for the movement of HCV RCs to other regions in the cell, and the molecular interactions between RCs and microtubules, or RCs and actin filaments, are mediated by NS3 and NS5A.
Hepatitis C virus (HCV) is a major causative agent of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. The positive-sense, single-stranded 9.6-kb RNA genome encodes a large polyprotein (>3,000 amino acids), which is processed by host and viral proteases into 10 structural and nonstructural (NS) proteins (17, 34). Most of the NS proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) of HCV were associated with the endoplasmic reticulum (ER) or other subcellular membranes when these proteins were expressed individually or as a polyprotein (23, 24, 40, 52), with the probable exception of NS2, and are involved in HCV RNA replication (4, 35, 43). NS3 is a helicase and a serine protease, with the latter requiring a cofactor, NS4A. It is conceivable that the enzymatic activities of these proteins are key elements of the HCV replication complex (RC). The NS4B protein alone induces the membranous web in a membranous matrix; the newly synthesized HCV RNA also exists in these membranous webs (12, 16). NS5A is a phosphoprotein and an essential component of the HCV RC and plays an indispensable role in viral replication (4). NS5B is an RNA-dependent RNA polymerase. All of these NS proteins and the replicating HCV RNA, together with host proteins, are believed to form a membrane-associated HCV RC. We have further shown that HCV RNA synthesis occurs in a lipid raft membrane structure (2, 47). However, the full content and mechanisms of replication of HCV RC remain unclear.
Our previous studies have shown that vesicle-associated membrane protein-associated protein (VAP) subtype A (VAP-A) and VAP subtype B (VAP-B) bind to both NS5A and NS5B and play a critical role in the formation of HCV RC (14, 21). In addition, both VAP-A and -B are involved in vesicle transport (49) and in the interaction between the microtubule networks (31). Recently, Rab5, an early endosome protein, was found to interact with NS4B and is required for HCV replication (50). Rab5 participates in the regulation of actin dynamics (30). Interestingly, HCV RCs were localized to the distinct speckle-like structures in the cytoplasm of the replicon cell lines (47), usually in the perinuclear region. Following the synthesis of RC in either membranous webs (12, 16) or lipid raft membranes (47), the progeny RCs are transported to reach the lipid droplet for virus assembly (38). However, not much is known about the transport of such large HCV RCs and their subcellular distribution. Because of the high viscosity of the cytoplasm, the movement of large complexes, such as HCV RC, by diffusion is likely to be limited (36). Intracellular microorganisms such as viruses and their macromolecular components overcome the obstacle by utilizing the cytoskeleton as a roadway for trafficking of numerous endogenous cargos throughout the cell (9, 18, 44, 48). Several viral proteins of other viruses have been reported to interact with the cytoskeleton or cytoskeleton-associated proteins. For example, for both Japanese encephalitis virus and Kunjin virus, which, like HCV, belong to the family Flaviviridae, their NS3 proteins are associated with microtubules (10, 42). The structural Gag matrix protein, which is a component of the reverse transcription complex of human immunodeficiency virus type 1, directly interacts with actin (8). The replication complexes of tobacco mosaic virus have also been shown to traffic along actin filaments, possibly through interactions with p126 (33). The cytoskeleton contains three components, including microtubules, actin filaments (microfilaments), and intermediate filaments, all of which contribute to the structural organization of the cytoplasm in eukaryotic cells. Microtubules are polarized cytoskeletal filaments; their polarity is utilized to transport various cargoes, such as membranous organelles and proteins, to specific subcellular regions (20). Actin filament remodeling is involved in cell motility, adhesion, endocytosis, and exocytosis (30). Both microtubules and actin filaments are also implicated in membrane trafficking in mitotic cells (30, 41). Previously, it was shown that the polymerization of both microtubules and actin filaments is required for HCV RNA synthesis (6); however, little is known about the roles of cytoskeletal elements at the molecular level in the HCV life cycle.
In this study, we used an immunoprecipitation-proteomics approach to identify target molecules that are associated with NS3/NS4A. Our approach has identified tubulin and actin as being NS3/NS4A partner proteins. We have further shown that NS5A is also associated with both tubulin and actin in either replicon cells or HCV-infected cells. In addition, the intracellular transport of HCV RCs could be blocked by inhibiting either the microtubule or the actin filament network. These results suggest that the microtubules and actin filaments provide the tracks for the movement of HCV RCs through interacting with the NS3 and NS5A proteins.
MATERIALS AND METHODS
Cells and media.
HEK293T, Huh-7, and Huh-7.5 cells, a mutant line of Huh-7 cells that supports HCV replication at a high efficiency (5), were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Rep 1.1 cells (29) are Huh-7 cells that harbor genotype 1b HCV subgenomic replicons. They were grown in the same medium containing 0.5 mg/ml of G418.
Plasmids.
Plasmids pCI-NS3/4A and pCI-HA-NS3/4A were described previously (29). To generate the hemagglutinin (HA)-tagged NS5A construct (pCI-NS5A-HA), the XhoI-XbaI fragment containing the NS5A cDNA insert of the HCV-Con1 strain was cloned into the XhoI and XbaI sites of vector pCI-HA such that NS5A was fused in frame with a C-terminal HA tag. Vector pCI-HA was constructed by using an annealed oligonucleotide, 5′-gaattccggggtacctctagatatccatatgacgtcccagactatgccTAAgcggccgc-3′ (the EcoRI site is underlined, the KpnI and XbaI sites for cloning are underlined and in boldface type and in boldface and italic type, respectively, the HA tag sequence is in italics and underlined, while the TAA stop codon is in capital letters, followed by a NotI site that is underlined and in boldface italics), which was cloned into EcoRI and NotI sites of vector pCI (Promega). To generate the HA-tagged glutathione S-transferase (GST) constructs, the EcoRV-XbaI fragments containing the GST cDNA insert were cloned into EcoRV and XbaI sites of vector pHA-AT such that the N-terminal HA epitope was fused in frame with GST. Vector pHA-AT was described previously (29). To improve the efficiency of expression, the resulting plasmid, pCI-neo-HA-GST, was digested with XhoI and XbaI and then cloned into XhoI and XbaI sites of vector pCI to generate pCI-HA-GST. Plasmid pJFH1 was kindly provided by Takaji Wakita (National Institute of Infectious Disease, Tokyo, Japan). All plasmids were verified by DNA sequencing.
Abs.
The NS3-specific mouse monoclonal antibody (Ab) (MAb) used for immunofluorescence staining and electron microscopy was purchased from Vector Laboratories (Burlingame, CA), while mouse anti-NS3 MAb, which was used for immunoblotting, was obtained from Novocastra Laboratories (Newcastle, United Kingdom). Mouse MAb against NS5A was purchased from Biodesign (Saco, ME), whereas rabbit polyclonal Ab against NS5A was purchased from ViroGen (Watertown, MA). Mouse anti-NS5 MAb, directed against both NS5A and NS5B, which was used for immunofluorescence staining in HCV-infected cells, was purchased from Austral Biologicals (San Ramon, CA). Mouse MAbs against the core protein were purchased from Affinity Bioreagents Inc. (Golden, CO). Anti-α-tubulin was obtained from Abcam Inc. (Cambridge, MA). Mouse MAbs against bromodeoxyuridine Ab and Cy3-conjugated primary Ab to β-tubulin were obtained from Sigma-Aldrich (St. Louis, MO), whereas rabbit MAb against β-tubulin and Alexa Fluor 555 conjugates were obtained from Cell Signaling Technology (Beverly, MA). Anti-actin, -vimentin, and -calnexin Abs were purchased from Chemicon (Temecula, CA). Alexa Fluor 568-conjugated phalloidin and anti-rabbit and -mouse secondary Abs were purchased from Invitrogen Molecular Probes (Eugene, OR). Anti-pan-cadherin and anti-calpain-1 Abs were purchased from Calbiochem, EMD Biosciences Inc. (La Jolla, CA). Anti-HA antibody was purchased from Roche Diagnostics (Indianapolis, IN). Goat anti-moue 12-nm colloidal gold conjugate was purchased from Jackson ImmunoResearch Inc. (West Grove, PA).
Labeling of de novo-synthesized viral RNA.
Cell labeling with 5-bromouridine 5′-triphosphate (BrUTP) was performed according to methods described previously (1), with some modifications. Huh-7 and Rep 1.1 cells were grown on four-well chamber slides. One day after seeding, cells were incubated with actinomycin D (10 μg/ml) for 30 min. BrUTP was then transfected into cells using Fugene 6 transfection reagent according to the manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN). We added 20 μl of a BrUTP-Fugene 6 mixture to each well containing 500 μl medium. After 1 h of incubation at 37°C, cells were fixed and processed for immunofluorescence staining as described below.
Immunofluorescence staining.
Cells were gown on glass chamber slides (Lab-Tek II). Cells were fixed with either cold methanol or 4% paraformaldehyde in phosphate-buffered saline (PBS) and then permeabilized in either 0.2% Tween 20-PBS or cold acetone. Samples were blocked in 3% bovine serum albumin-PBS for 30 min. Primary Abs were diluted in 3% bovine serum albumin-PBS and incubated with cells for 1 h at 37°C. After three washes in PBS, fluorescein- and/or Cy3- and Alexa Fluor 555- and 568-conjugated secondary Abs were added to cells for 1 h at 37°C. Nuclear staining by 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich) was performed by mixing DAPI (0.5 μg/ml) with the secondary Ab. After staining, slides were washed in PBS and mounted with ProLong Antifade (Invitrogen Molecular Probes). Photographs of the cells were taken with a confocal microscope (Zeiss LSM 510 confocal laser scanning microscope). Image analysis was performed using the standard system-operating software provided with the microscope. To allow direct comparisons, all images were captured using the same parameters.
Coimmunoprecipitations.
HEK293T cells were transfected with various expression plasmids. The preparation of total lysates and immunoprecipitation were performed according to instructions provided with the Profound Mammalian HA-Tag IP/Co-IP kit (Pierce). The immunoprecipitated proteins were run on a 10% sodium dodecyl sulfate-polyacrylamide gel and detected by immunoblotting.
In-gel enzymatic digestion and mass spectrometry.
The protein bands were excised from a Sypro ruby (Molecular Probes, Eugene, OR)-stained polyacrylamide gel (shown in Fig. 1). Sample preparation for in-gel digestion, liquid chromatography-nanoelectrospray ionization tandem mass spectrometry (MS/MS) analysis for protein identification, and database search parameters were performed according to methods described previously by Lee et al. (32). Criteria for the acceptance of positive hits were defined by a minimum MASCOT score of 25 comprising at least a peptide match.
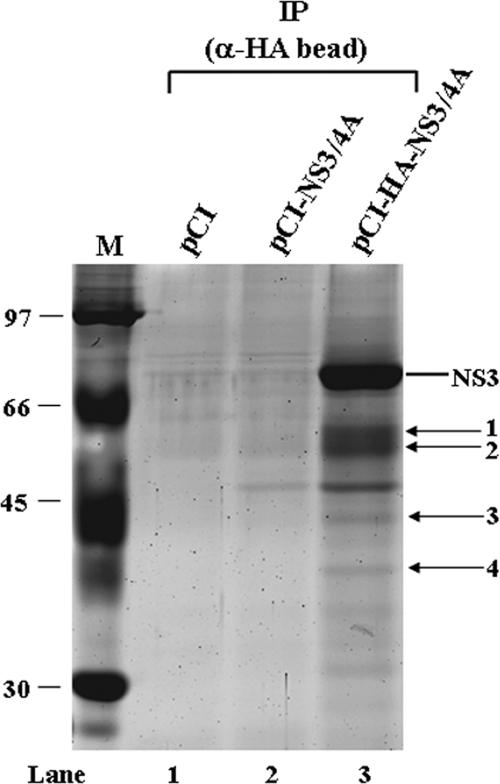
FIG. 1.
Cellular proteins coimmunoprecipitated with anti-HA beads from HA-NS3/NS4A-transfected cells. HEK293T cells were transfected with NS3/NS4A, HA-NS3/NS4A, or empty vector. At 48 h after transfection, cell lysates were immunoprecipitated (IP) with anti-HA beads. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide) followed by Sypro ruby staining. Bands 1 to 4 were excised and subjected to proteomic analysis by MS/MS. The identities of proteins in each band are shown in Table 1. M, molecular weight marker (in thousands).
FRET assay.
A Zeiss LSM510 Meta-NLO confocal microscope with a 63×, 1.4-numerical-aperture oil immersion Plan-Apochromate objective was employed for fluorescence resonance energy transfer (FRET) analysis using the acceptor photobleaching method as described previously (25, 26), with some modifications. The following settings were used throughout the experiments. Fluorescein isothiocyanate (FITC) was excited with an Argon laser line at 488 nm (1.6% laser intensity) and was detected by using a band pass filter of 500 to 550 nm. Cy3 was excited with an Argon laser line at 561 nm (20.7% laser intensity), and emission was collected with a long-pass filter at 575 nm. First, two images of prephotobleach FITC and Cy3 were acquired. A region of interest (ROI) in the cytoplasm was rendered by bleaching of Cy3 by scanning 40 times (0.54 s/scan) at 20.7% laser intensity with the 561-nm laser line. Four images of postphotobleach FITC and Cy3 were acquired in time series. Exposing single-labeled FITC cells to 561 nm light for equivalent times did not alter the amount of fluorescein emission. An unbleached cell in the same field was used as a background control. After correction for background, the average fluorescence intensities of the donor were measured before and after bleaching. The FRET efficiencies (E) in the ROI were calculated by using the formula E = (FITCmax − FITCmin)/FITCmax, where FITCmin is the average intensity of the prephotobleach FITC and FITCmax is the maximum increased intensity of FITC for the estimated total bleaching of Cy3. The change in donor fluorescence was assessed in 100 ROIs, with 10 cells for each experiment.
Immunoelectron microscopy.
Cells were detached from the dish with a cell scraper after fixation in 4% paraformaldehyde-PBS for 24 h and washed in PBS. Cells were dehydrated using an ethanol series, and LR Gold (London Resin Company, London, United Kingdom) was used for infiltration and UV polymerization at −20°C. Ultrathin sections (100 nm) were labeled with primary Abs (anti-NS3 and anti-NS5A) and colloidal gold particles (12 nm) conjugated to anti-mouse immunoglobulin G. Samples were stained with uranyl acetate and lead citrate and examined with a Tecnai Spirit transmission electron microscope (FEI Co.) at 120 kV.
RNA synthesis, transfection, and HCV infection.
In vitro synthesis of HCV RNA and electroporation were performed by the methods described previously by Wakita et al. (55), with minor modifications. Cells were mixed with in vitro-transcribed RNA and pulsed at 220 V and 975 μF using a BTX ECM630 electroporator. Culture medium was harvested 13 days after transfection. Collected medium was cleared by low-speed centrifugation at 2,000 rpm for 10 min, passed through a filter with a 0.45-μm pore size (Millipore), and then concentrated 25-fold using an Amicon Ultra-15 centrifugal filter with Ultracel-100K membrane (Millipore) and used for infection. Huh-7.5 cells were seeded at a density of 5 × 104 cells/well in a four-well chamber slide (Lab-Tek II). After 24 h, cells were incubated with 150 μl of the concentrated medium for 3 h, washed, and then added to complete medium. Immunofluorescence staining was performed 2 days after infection.
RESULTS
Identification of NS3/NS4A-associated proteins by mass spectrometry.
To identify cellular proteins in association with NS3 and NS4A, we performed an immunoisolation by incubating anti-HA beads with cell homogenates in which HA epitope-tagged NS3/NS4A was expressed. As controls, cells transfected with a nontagged NS3/NS4A expression vector or an empty vector were used. Since the transfection efficiency of HEK293T cells is higher than that of Huh-7 cells, we chose HEK293T cells for transfection to achieve better expression of the viral proteins. Figure 1 shows a Sypro ruby-stained gel of proteins from transfected HEK293T cells coimmunoprecipitated with anti-HA beads. In addition to NS3, several cellular proteins smaller than 66 kDa were visible (Fig. 1, lane 3), which were detected only in pCI-HA-NS3/4A-transfected cells precipitated by anti-HA beads (Fig. 1). Four bands were excised and digested with trypsin. The identities of the proteins were determined by MS/MS and the MASCOT program (Table 1). One of these proteins, elongation factor 1A, is known to interact with NS4A (28). Five other proteins are heterogeneous nuclear ribonucleoproteins (hnRNPs). Although hnRNPs are abundant proteins in cells, they are not common background proteins found in affinity purifications using agarose-Sepharose-based resins (27, 46, 53). Therefore, these proteins are specific candidate NS3/NS4A-interacting partners. Particularly, hnRNP C1/C2 interacts with the 3′-untranslated region of the HCV RNA genome (15), and NS3 binds to this region (3), suggesting the potential importance of this interaction. The most prominent proteins are tubulin and actin, with MASCOT scores of 834 and 596, respectively, and 21 and 19 matching peptides, respectively, indicating the accurate identification of these two proteins. These results were reproducible in multiple coimmunoprecipitation experiments. Tubulin and actin were chosen for further studies since the cytoskeleton has been reported to be involved in the replication of HCV (6).
TABLE 1.
Cellular proteins that copurified with HA-NS3/NS4A
| Band | Protein | GenBank accession no. |
|---|---|---|
| 1 | Tubulin, β-2 | P07437 |
| Tubulin, α-8 | P68371 | |
| Heterogeneous nuclear ribonucleoprotein H | P31943 | |
| Elongation factor 1Aa | P68104 | |
| 2 | Heterogeneous nuclear ribonucleoprotein F | P52597 |
| TAR DNA-binding protein 43 | Q13148 | |
| RNase inhibitor | P13489 | |
| 3 | β-Actin | P60709 |
| Heterogeneous nuclear ribonucleoprotein E1 | Q15365 | |
| Heterogeneous nuclear ribonucleoprotein D0 | Q14103 | |
| 4 | Heterogeneous nuclear ribonucleoprotein C1/C2b | P07910 |
| Annexin A2 | P07355 |
HCV NS3 and NS5A in speckle-like structures are associated with microtubules and actin filaments.
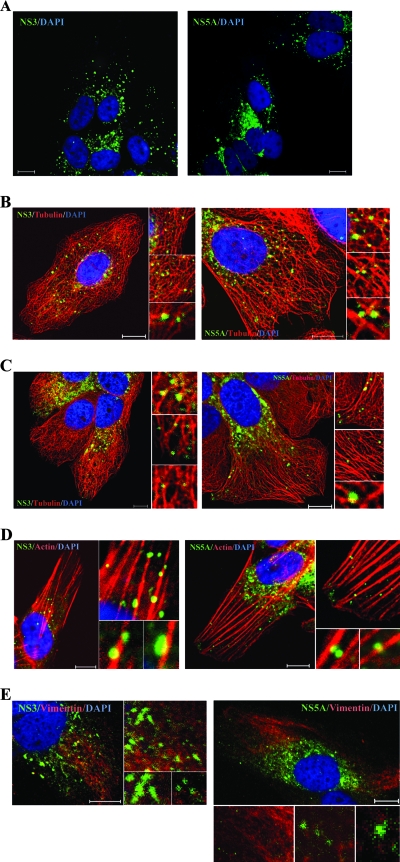
Tubulin and actin are major cytoskeleton components. Tubulin has three distinct forms, designated α-, β-, and γ-tubulins. α-Tubulin and β-tubulin form heterodimers, which multimerize to from microtubule filaments. Actin monomers polymerize into actin filaments. Thus, we investigated whether NS3 colocalized with microtubules or actin filaments in the cells harboring an HCV subgenomic replicon (29). Immunofluorescence staining revealed that the NS3-labeled speckles were distributed in two different patterns in different replicon cells, viz., speckles either dispersed in the cytoplasm or clustered in the perinuclear region (Fig. 2A, left). Irrespective of the distribution pattern, many NS3 speckles colocalized with tubulin or actin (Fig. 2B to D, left). In contrast, NS3 in speckles did not colocalize with vimentin (Fig. 2E, left).
FIG. 2.
Colocalization of NS3 and NS5A with microtubules and actin filaments in HCV replicon cells. Rep 1.1 cells were stained with anti-NS3 (green) (A, left) or anti-NS5A (green) (A, right) or costained with anti-NS3 or -NS5A (green) and anti-β-tubulin Alexa Fluor 555 conjugate (red) (B and C) or Alexa Fluor 568-phalloidin (red) (D) or anti-vimentin (red) (E). Cellular DNA was labeled with DAPI (blue). Images shown were collected sequentially with a confocal laser scanning microscope and merged to demonstrate colocalization (yellow merge fluorescence). Enlarged views of parts of the left image are shown (inset). Scale bars, 10 μm.
Previous experiments showed that NS5A colocalized with NS3 and other NS proteins in speckles (47). We therefore investigated whether NS5A in speckles was also associated with microtubules and actin filaments. The distribution pattern of NS5A-labeled speckles (Fig. 2A, right) was similar to that of NS3-labeled speckles (Fig. 2A, left). Similar to NS3, many NS5A speckles colocalized with tubulin and actin (Fig. 2B to D, right) but not with vimentin (Fig. 2E, right). Taken together, these results indicated that NS3 and NS5A speckles are associated with microtubules and actin filaments but not with intermediate filament.
HCV NS3 and NS5A directly interact with tubulin and actin.
Next, we confirmed the interaction of tubulin and actin with NS3/NS4A by coimmunoprecipitation-Western blotting analyses. Total lysates from NS3/NS4A- or HA-NS3/NS4A-transfected HEK293T cells were precipitated with anti-HA beads and then analyzed by immunoblotting using MAbs against tubulin, actin, or NS3. In order to exclude the possibility of background contaminants, we also used Abs against vimentin; a cell membrane protein, cadherin; and a cytosolic protein, calpain-1. The results showed that NS3/4A specifically coprecipitated tubulin and actin (Fig. 3A, lane 4) but not vimentin, cadherin, or calpain-1. Furthermore, NS3 alone, without NS4A, could coprecipitate both tubulin and actin (data not shown), indicating that NS4A is not required for this interaction. A control immunoprecipitation using HEK293T cells transfected with the nontagged NS3/NS4A (Fig. 3A, Lane 3) did not precipitate tubulin and precipitated very small amounts of actin. Also, HA-GST did not coprecipitate tubulin or actin when anti-HA beads were used for immunoprecipitation (data not shown). These results combined indicate the specificity of the observed NS3-tubulin and -actin interactions.
FIG. 3.
Coimmunoprecipitation of NS3 and NS5A with tubulin and actin. HEK293T cells were transfected with NS3/NS4A or HA-NS3/NS4A (A) or with NS5A or HA-GST or NS5A-HA (B) and then immunoprecipitated (IP) with anti-HA beads. The immunoprecipitates and 1/20 of the total cell lysates were blotted with anti-NS3, anti-HA, anti-tubulin, anti-actin, anti-vimentin, anti-pan-cadherin, and anti-calpain-1 Abs. WB, Western blot.
Similar results were obtained with HCV NS5A. NS5A was coprecipitated with tubulin and actin protein (Fig. 3B, lane 6) but not with vimentin. Taken together, these findings indicate that HCV NS3 and NS5A proteins interact with both tubulin and actin.
HCV RNA in speckle-like structures is associated with microtubules and actin filaments.
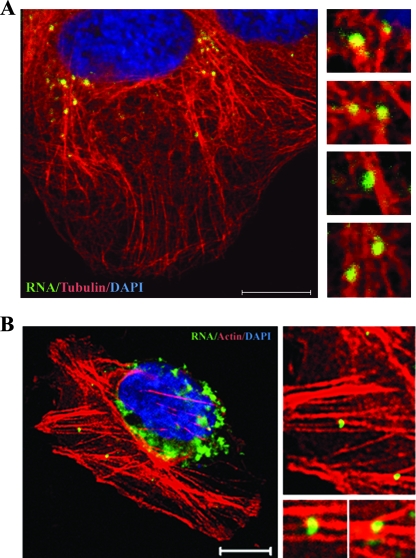
The colocalization of de novo-synthesized HCV RNA and NS proteins in speckles has been established by previous reports (13, 47) indicating that the speckles represent HCV RCs. Therefore, we next investigated whether HCV RNA was associated with microtubules and actin filaments. HCV RNA was metabolically labeled with BrUTP. Immunofluorescence staining showed that many BrUTP-labeled speckles, which represent newly synthesized RNA, were associated with microtubules (Fig. 4A) and actin filaments (Fig. 4B). As a negative control, no signal was detected in naïve Huh-7 cells (data not shown). The BrUTP staining pattern is similar to the speckles of the NS3 and NS5A proteins (Fig. 2). Since NS3, NS5A, and RNA are key elements of the HCV RC and colocalize at the replication site, these results indicate that the speckles represent HCV RCs, consistent with data from previous reports (13, 47). These results indicate that HCV RCs are associated with microtubules and actin filaments.
FIG. 4.
Colocalization of HCV RNA with microtubules (A) and actin filaments (B) in HCV replicon cells. Rep 1.1 cells were transfected with BrUTP in the presence of actinomycin D for 1 h and then stained with mouse MAb against bromodeoxyuridine (green) and rabbit MAb against β-tubulin Alexa Fluor 555 conjugates (red) or Alexa Fluor 568-conjugated phalloidin (red). Cellular DNA was labeled with DAPI (blue). Scale bars, 10 μm.
Molecular interaction between NS3, NS5A, and tubulin is validated by FRET.
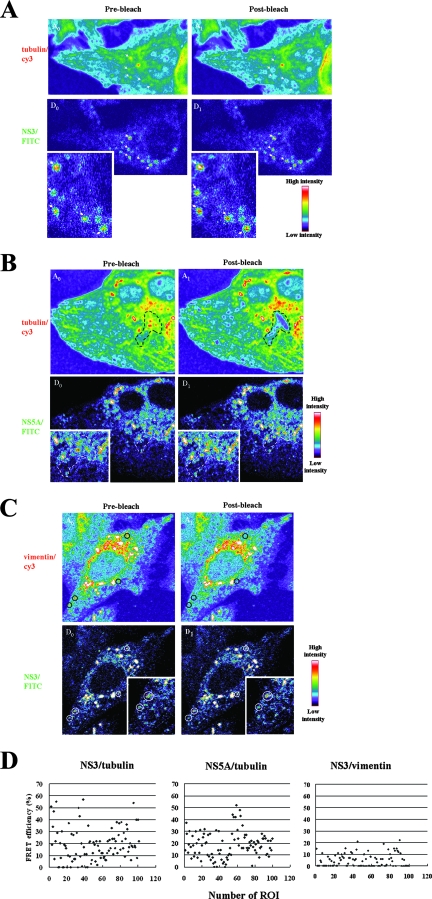
To further confirm the molecular interaction between NS3, NS5A, and tubulin, a FRET assay was performed in a replicon cell line, Rep 1.1. For the detection of FRET between the proteins, cells were immunostained with anti-NS5A, -NS3, -tubulin, and -vimentin; NS5A and NS3 were then labeled by FITC-conjugated Ab, whereas tubulin and vimentin were labeled by Cy3-conjugated Ab. The amount of FRET was calculated as the percentage of increase in donor fluorescence (FITC) intensity after receptor (Cy3) photobleaching (Fig. 5A to C). When the Cy3 acceptor fluorophore was selectively bleached, an increase in the fluorescence signal in the donor FITC in the ROI was observed in either NS3 (Fig. 5A)- or NS5A (Fig. 5B)-stained cells, indicating the occurrence of FRET. As a negative control, FRET was assessed using cells labeled with MAbs against NS3 and a polyclonal anti-vimentin Ab; no FRET was observed (Fig. 5C). These results are consistent with those seen with immunofluorescence staining (Fig. 2). In addition, the FRET efficiency was determined by statistics with 100 ROIs/10 cells, which were randomly selected with speckles in the cytoplasm. The results showed that the FRET efficiency displaying more than 20% was 39 ROIs and 43 ROIs in either NS3-tubulin or NS5A-tubulin, respectively, whereas NS3/vimentin was found in only 2 ROIs (Fig. 5D). These results indicated that the FRET signal is detectable between FITC-NS3 or -NS5A and Cy3-tubulin, further confirming the molecular interaction between NS3 or NS5A and tubulin.
FIG. 5.
FRET assay of molecular interactions between tubulin and NS3 or NS5A. Rep 1.1 cells were costained with anti-tubulin (Cy3) and anti-NS3 (FITC) (A) or anti-NS5A (FITC) (B) as the FRET acceptor and donor, respectively, and analyzed for FRET by confocal microscopy using the acceptor photobleaching method. This method is based on the principle that energy transfer is eliminated when the acceptor is bleached, thereby causing an increase in donor fluorescence. Intensity maps of tubulin-Cy3 (A0 and A1) and NS3-FITC or NS5A-FITC (D0 and D1) in the cytoplasm of a cell are shown. The FRET intensity calculated from the difference between donor prephotobleaching (D0) and postphotobleaching (D1) intensities is shown in pseudocolor. A0 indicates the fluorescence intensity distribution of tubulin-Cy3 excited at 561 nm. A1 indicates tubulin-Cy3 after photobleaching of the acceptor. The bleached regions are indicated by a white arrow (A), a black dashed box (B, top), and a white dashed box (B, bottom). (C) As a negative control, the intensity maps of vimentin-Cy3 (A0 and A1) and NS3-FITC (D0 and D1) in the cytoplasm of a cell were also determined by the same method. Enlarged views of parts of the donors' image are shown (inset). (D) FRET efficiency (percent) between tubulin and NS3 or NS5A and a negative control between vimentin and NS3. One hundred ROIs were evaluated, and the data are displayed in x-y scatter plots.
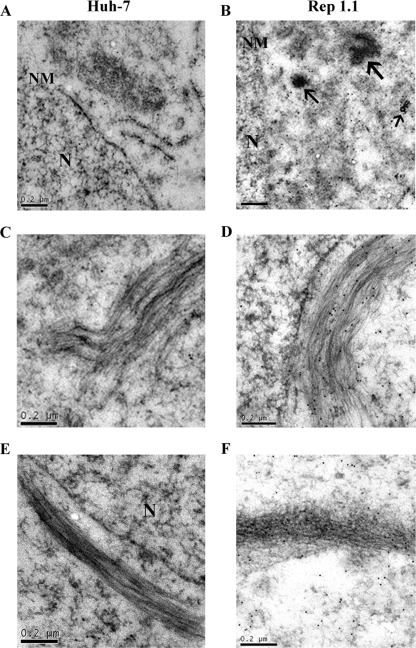
Association of NS3 and NS5A with microtubules and actin bundles as demonstrated by immunoelectron microscopy.
Immunoelectron microscopy was used to further examine the interaction of NS3 or NS5A with microtubules and actin filaments. Naïve Huh-7 and Rep 1.1 cells were labeled with anti-NS3 or anti-NS5A Abs, followed by colloidal gold particles (12 nm) conjugated to anti-mouse immunoglobulin G. As shown in Fig. 6B, most of colloid gold-NS5A particles clustered in the perinuclear region of Rep 1.1 cells. Many of the gold particles were associated directly with microtubules and actin bundles (Fig. 6D and F) or in their vicinity. A similar pattern was observed for NS3 (data not shown). In naïve Huh-7 cells (Fig. 6A, C, and E), very few gold particles were observed. The results suggested that the HCV replication complex aligned along both microtubules and actin filament tracks.
FIG. 6.
Immunoelectron microscopy of NS5A-associated microtubules and actin filaments. Huh-7 (A, C, and E) or Rep 1.1 (B, D, and F) cells labeled with Ab against NS5A are shown. NS5A was tethered to 12-nm gold particles. Shown are views of the perinuclear region in Huh-7 (A) and Rep 1.1 (B) cells. Arrows show NS5A in electron-dense granules in the perinuclear region of Rep 1.1 cells. Also shown are views of microtubule bundles in Huh-7 (C) and Rep 1.1 (D) cells and views of actin bundles in Huh-7 (E) and Rep 1.1 (F) cells. N, nucleus. NM, nuclear membrane. Bars, 0.2 μm.
Transport of the HCV replication complexes requires microtubules and actin filaments.
HCV replication has been shown to occur on the intracellular membrane structures in the perinuclear region (39, 47). We hypothesize that after replication, the replication complexes are transported along microtubules and actin filaments. To test this possibility, Rep 1.1 cells were treated with drugs affecting the stabilization of microtubules and the polymerization of actin filaments, namely, colchicine and cytochalasin B, respectively. In the absence of inhibitors, HCV NS5A showed the typical speckled pattern in the perinuclear region and also the dispersed distribution throughout the cytoplasm. NS5A was colocalized with the ER marker protein calnexin in the perinuclear region (Fig. 7A), in agreement with data from a previous report (47). In the distal part of the cytoplasm, NS5A was not colocalized with calnexin. In the presence of either 10 μM cytochalasin B or 25 μM colchicine, NS5A clustered almost exclusively in the perinuclear region and colocalized with calnexin (Fig. 7B and C). These results suggested that microtubules and actin filaments are required for the transport of RCs in the cytoplasm. The morphology of the ER was not affected by the microtubule- and microfilament-depolymerizing agents, consistent with data from a previous report (51).
FIG. 7.
Effects of cytochalasin B (Cyto B) and colchicine (Col) on movement of HCV replication complexes. Rep 1.1 cells were treated with cytochalasin B, colchicine, or dimethyl sulfoxide (control) for 7 h. The cells were costained with MAb against an ER marker, calnexin, and rabbit polyclonal Ab against NS5A. Immunofluorescence images show the distribution of RCs in an untreated cell (A) and a cell treated with cytochalasin B (B) or colchicine (C). Scale bars, 10 μm.
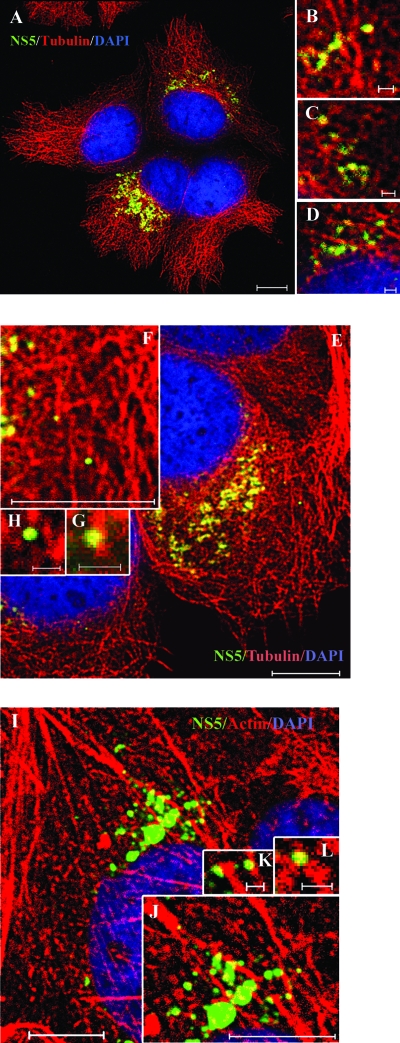
In HCV-infected cells, HCV replication complexes were associated with microtubules and actin filaments.
To demonstrate that the findings reported above are relevant to HCV replication during natural viral infection, we repeated immunofluorescence staining in JFH1 virus-infected cells. Virus infection was confirmed by staining with anti-core MAb (data not shown). Since Abs against NS3 of JFH1 were not available, we used mouse MAbs against NS5 of JFH1 for immunofluorescence staining. High-magnification imaging by a confocal laser scanning microscopy revealed that NS5-labeled speckles were distributed in patterns similar to those seen in the replicon cells (Fig. 8A). Most of the NS5A speckles colocalized with the microtubules and actin filaments (Fig. 8). We conclude that HCV RCs are tethered or docked with microtubules and actin filaments in HCV-infected cells.
FIG. 8.
Colocalization of NS5 with microtubules and actin filaments in HCV-infected cells. Cells were infected with JFH1. At 2 days postinfection, cells were fixed and costained with anti-NS5 (green) and anti-β-tubulin Alexa Fluor 555 conjugate (red) (A to H) or Alexa Fluor 568-phalloidin (red) (I to L). (B to D, F to H, and J to L) Higher-magnification images of parts of A, E, and I, respectively. Cellular DNA was labeled with DAPI (blue). Scale bars, 10 μm (A, E, F, I, and J) and 1 μm (B to D, G, H, K, and L).
DISCUSSION
In this study, by performing in vitro coimmunoprecipitation experiments, we showed that both NS3/NS4A and NS5A closely interacted with tubulin and actin (Fig. 3). This interaction was confirmed by FRET analysis; a specific FRET signal was detected between NS3 or NS5A and tubulin in HCV replicon cells. Significantly, the numbers of ROI with >20% FRET efficiency in both NS3-tubulin and NS5A-tubulin interactions were very similar, i.e., 39 ROIs or 43 ROIs, respectively (Fig. 5D). We speculate that NS3 and NS5A first form an RC, which, acting as a whole, become associated with microtubules, resulting in similar FRET efficiencies. However, there were some ROIs with <10% FRET efficiency in either NS3-tubulin or NS5A-tubulin. The lower FRET efficiency may have come from cells with lower RNA replication activity.
As shown in Fig. 2 and 8, immunofluorescence analysis using Abs against NS3, NS5A, or NS5 (a mixture of NS5A and NS5B) showed heterogeneously sized speckles tethered or docked with both microtubules and actin filaments in either replicon cells or HCV-infected cells. However, either NS3 or NS5A expressed alone did not show the speckle-like structure (data not shown) (29). These speckles contain BrUTP-labeled RNA (Fig. 4) (13, 47). Therefore, these speckles most likely represent HCV RCs. Previous studies showed that HCV RNA replication takes place on the membranous web derived from the ER or other membranes (12, 16, 39) or detergent-resistant membrane structures, most likely a lipid raft membrane structure (2, 47). The morphogenesis of the membranous web is still unknown, but the ER is a dynamic network of interconnected membrane tubules that pervades almost the entire cytoplasm. Microtubules are required for the maintenance of the ER (54) and probably membranous webs as well. Lipid rafts have a highly dynamic nature and are involved in vesicle membrane trafficking (7). In addition, microtubules and actin filaments are required for the morphogenesis of the lipid raft (22). The inhibition of actin and/or microtubules reduced levels of HCV replicon RNA (6). In addition, after the treatment of HCV replicon cells with either colchicine or cytochalasin B, no NS5A-containing speckle-like structure was observed, and all NS5A clustered in the perinuclear region (Fig. 7). We therefore hypothesize that microtubules and actin filaments are major determinants for the assembly and functioning of the HCV RC and also for their characteristic subcellular distribution. The transport of HCV RC along microtubules and actin filaments is likely important for the translation and replication of HCV RNA and also for the assembly of virus particles.
How NS3 or NS5A can interact with both microtubules and actin at the same time is an interesting question. By comparison, murine sarcoma virus-associated protein kinase also possesses the ability to interact with both actin and tubulin (45). A variety of cellular proteins such as microtubule-associated protein 2c (11) that mediate the interaction between microtubule and actin filaments have been identified. Our preliminary results suggest that NS3 and NS5A contain both actin- and microtubule-binding sites.
The association of HCV RCs with microtubules and actin filaments was demonstrated not only in HCV replicon cells but also in HCV-infected cells. More recently, Miyanari et al. demonstrated that HCV assembly occurs in close proximity to the lipid droplet (38). The lipid droplet is derived from discrete regions of the ER and is associated with microtubules (19, 37). Future work will be necessary to confirm that both microtubules and actin filaments provide tracks for the transport of HCV RCs to reach the lipid droplet, where virus assembly occurs.
Acknowledgments
We thank Takaji Wakita of the National Institute of Infectious Disease, Tokyo, Japan, for plasmid pJFH1; Charles M. Rice from the Center for the Study of Hepatitis C, Rockefeller University, New York, NY, for Huh-7.5 cells; Sue-Ping Lee from the Imaging Core of Institute of Molecular Biology, Academia Sinica, Taiwan, for help in immunoelectron microscopy; and Vikas Saxena, from our laboratory, for plasmid pCI-NS5A-HA.
Footnotes
Published ahead of print on 18 June 2008.
REFERENCES
- 1.Aizaki, H., K. S. Choi, M. Liu, Y. J. Li, and M. M. Lai. 2006. Polypyrimidine-tract-binding protein is a component of the HCV RNA replication complex and necessary for RNA synthesis. J. Biomed. Sci. 13469-480. [DOI] [PubMed] [Google Scholar]
- 2.Aizaki, H., K. J. Lee, V. M. Sung, H. Ishiko, and M. M. Lai. 2004. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology 324450-461. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, R., and A. Dasgupta. 2001. Specific interaction of hepatitis C virus protease/helicase NS3 with the 3′-terminal sequences of viral positive- and negative-strand RNA. J. Virol. 751708-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 2901972-1974. [DOI] [PubMed] [Google Scholar]
- 5.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 7613001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bost, A. G., D. Venable, L. Liu, and B. A. Heinz. 2003. Cytoskeletal requirements for hepatitis C virus (HCV) RNA synthesis in the HCV replicon cell culture system. J. Virol. 774401-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugger, B., R. Sandhoff, S. Wegehingel, K. Gorgas, J. Malsam, J. B. Helms, W. D. Lehmann, W. Nickel, and F. T. Wieland. 2000. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J. Cell Biol. 151507-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukrinskaya, A., B. Brichacek, A. Mann, and M. Stevenson. 1998. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J. Exp. Med. 1882113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, E. M., and T. J. Hope. 2005. Gene therapy progress and prospects: viral trafficking during infection. Gene Ther. 121353-1359. [DOI] [PubMed] [Google Scholar]
- 10.Chiou, C. T., C. C. Hu, P. H. Chen, C. L. Liao, Y. L. Lin, and J. J. Wang. 2003. Association of Japanese encephalitis virus NS3 protein with microtubules and tumour susceptibility gene 101 (TSG101) protein. J. Gen. Virol. 842795-2805. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham, C. C., N. Leclerc, L. A. Flanagan, M. Lu, P. A. Janmey, and K. S. Kosik. 1997. Microtubule-associated protein 2c reorganizes both microtubules and microfilaments into distinct cytological structures in an actin-binding protein-280-deficient melanoma cell line. J. Cell Biol. 136845-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger, D., B. Wolk, R. Gosert, L. Bianchi, H. E. Blum, D. Moradpour, and K. Bienz. 2002. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 765974-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Hage, N., and G. Luo. 2003. Replication of hepatitis C virus RNA occurs in a membrane-bound replication complex containing nonstructural viral proteins and RNA. J. Gen. Virol. 842761-2769. [DOI] [PubMed] [Google Scholar]
- 14.Gao, L., H. Aizaki, J. W. He, and M. M. Lai. 2004. Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft. J. Virol. 783480-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gontarek, R. R., L. L. Gutshall, K. M. Herold, J. Tsai, G. M. Sathe, J. Mao, C. Prescott, and A. M. Del Vecchio. 1999. hnRNP C and polypyrimidine tract-binding protein specifically interact with the pyrimidine-rich region within the 3′NTR of the HCV RNA genome. Nucleic Acids Res. 271457-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosert, R., D. Egger, V. Lohmann, R. Bartenschlager, H. E. Blum, K. Bienz, and D. Moradpour. 2003. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J. Virol. 775487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grakoui, A., C. Wychowski, C. Lin, S. M. Feinstone, and C. M. Rice. 1993. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 671385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greber, U. F., and M. Way. 2006. A superhighway to virus infection. Cell 124741-754. [DOI] [PubMed] [Google Scholar]
- 19.Gross, S. P., M. A. Welte, S. M. Block, and E. F. Wieschaus. 2000. Dynein-mediated cargo transport in vivo. A switch controls travel distance. J. Cell Biol. 148945-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gundersen, G. G. 2002. Evolutionary conservation of microtubule-capture mechanisms. Nat. Rev. Mol. Cell Biol. 3296-304. [DOI] [PubMed] [Google Scholar]
- 21.Hamamoto, I., Y. Nishimura, T. Okamoto, H. Aizaki, M. Liu, Y. Mori, T. Abe, T. Suzuki, M. M. Lai, T. Miyamura, K. Moriishi, and Y. Matsuura. 2005. Human VAP-B is involved in hepatitis C virus replication through interaction with NS5A and NS5B. J. Virol. 7913473-13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Head, B. P., H. H. Patel, D. M. Roth, F. Murray, J. S. Swaney, I. R. Niesman, M. G. Farquhar, and P. A. Insel. 2006. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J. Biol. Chem. 28126391-26399. [DOI] [PubMed] [Google Scholar]
- 23.Hijikata, M., H. Mizushima, Y. Tanji, Y. Komoda, Y. Hirowatari, T. Akagi, N. Kato, K. Kimura, and K. Shimotohno. 1993. Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc. Natl. Acad. Sci. USA 9010773-10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang, S. B., K. J. Park, Y. S. Kim, Y. C. Sung, and M. M. Lai. 1997. Hepatitis C virus NS5B protein is a membrane-associated phosphoprotein with a predominantly perinuclear localization. Virology 227439-446. [DOI] [PubMed] [Google Scholar]
- 25.Kenworthy, A. K. 2001. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods 24289-296. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita, A., C. M. Whelan, C. J. Smith, I. Mikhailenko, G. W. Rebeck, D. K. Strickland, and B. T. Hyman. 2001. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J. Neurosci. 218354-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kocks, C., R. Maehr, H. S. Overkleeft, E. W. Wang, L. K. Iyer, A. M. Lennon-Dumenil, H. L. Ploegh, and B. M. Kessler. 2003. Functional proteomics of the active cysteine protease content in Drosophila S2 cells. Mol. Cell Proteomics 21188-1197. [DOI] [PubMed] [Google Scholar]
- 28.Kou, Y. H., S. M. Chou, Y. M. Wang, Y. T. Chang, S. Y. Huang, M. Y. Jung, Y. H. Huang, M. R. Chen, M. F. Chang, and S. C. Chang. 2006. Hepatitis C virus NS4A inhibits cap-dependent and the viral IRES-mediated translation through interacting with eukaryotic elongation factor 1A. J. Biomed Sci. 13861-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai, C. K., K. S. Jeng, K. Machida, Y. S. Cheng, and M. M. Lai. 2008. Hepatitis C virus NS3/4A protein interacts with ATM, impairs DNA repair and enhances sensitivity to ionizing radiation. Virology 370295-309. [DOI] [PubMed] [Google Scholar]
- 30.Lanzetti, L. 2007. Actin in membrane trafficking. Curr. Opin. Cell Biol. 19453-458. [DOI] [PubMed] [Google Scholar]
- 31.Lapierre, L. A., P. L. Tuma, J. Navarre, J. R. Goldenring, and J. M. Anderson. 1999. VAP-33 localizes to both an intracellular vesicle population and with occludin at the tight junction. J. Cell Sci. 1123723-3732. [DOI] [PubMed] [Google Scholar]
- 32.Lee, C. L., H. H. Hsiao, C. W. Lin, S. P. Wu, S. Y. Huang, C. Y. Wu, A. H. Wang, and K. H. Khoo. 2003. Strategic shotgun proteomics approach for efficient construction of an expression map of targeted protein families in hepatoma cell lines. Proteomics 32472-2486. [DOI] [PubMed] [Google Scholar]
- 33.Liu, J. Z., E. B. Blancaflor, and R. S. Nelson. 2005. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 1381853-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohmann, V., J. O. Koch, and R. Bartenschlager. 1996. Processing pathways of the hepatitis C virus proteins. J. Hepatol. 2411-19. [PubMed] [Google Scholar]
- 35.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285110-113. [DOI] [PubMed] [Google Scholar]
- 36.Luby-Phelps, K. 2000. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 192189-221. [DOI] [PubMed] [Google Scholar]
- 37.Martin, S., and R. G. Parton. 2006. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7373-378. [DOI] [PubMed] [Google Scholar]
- 38.Miyanari, Y., K. Atsuzawa, N. Usuda, K. Watashi, T. Hishiki, M. Zayas, R. Bartenschlager, T. Wakita, M. Hijikata, and K. Shimotohno. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 91089-1097. [DOI] [PubMed] [Google Scholar]
- 39.Moradpour, D., F. Penin, and C. M. Rice. 2007. Replication of hepatitis C virus. Nat. Rev. Microbiol. 5453-463. [DOI] [PubMed] [Google Scholar]
- 40.Mottola, G., G. Cardinali, A. Ceccacci, C. Trozzi, L. Bartholomew, M. R. Torrisi, E. Pedrazzini, S. Bonatti, and G. Migliaccio. 2002. Hepatitis C virus nonstructural proteins are localized in a modified endoplasmic reticulum of cells expressing viral subgenomic replicons. Virology 29331-43. [DOI] [PubMed] [Google Scholar]
- 41.Mundy, D. I., T. Machleidt, Y. S. Ying, R. G. Anderson, and G. S. Bloom. 2002. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J. Cell Sci. 1154327-4339. [DOI] [PubMed] [Google Scholar]
- 42.Ng, M. L., and S. S. Hong. 1989. Flavivirus infection: essential ultrastructural changes and association of Kunjin virus NS3 protein with microtubules. Arch. Virol. 106103-120. [DOI] [PubMed] [Google Scholar]
- 43.Pietschmann, T., V. Lohmann, G. Rutter, K. Kurpanek, and R. Bartenschlager. 2001. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J. Virol. 751252-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radtke, K., K. Dohner, and B. Sodeik. 2006. Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell. Cell. Microbiol. 8387-400. [DOI] [PubMed] [Google Scholar]
- 45.Sen, A., and G. J. Todaro. 1979. A murine sarcoma virus-associated protein kinase: interaction with actin and microtubular protein. Cell 17347-356. [DOI] [PubMed] [Google Scholar]
- 46.Shevchenko, A., D. Schaft, A. Roguev, W. W. Pijnappel, and A. F. Stewart. 2002. Deciphering protein complexes and protein interaction networks by tandem affinity purification and mass spectrometry: analytical perspective. Mol. Cell Proteomics. 1204-212. [DOI] [PubMed] [Google Scholar]
- 47.Shi, S. T., K. J. Lee, H. Aizaki, S. B. Hwang, and M. M. Lai. 2003. Hepatitis C virus RNA replication occurs on a detergent-resistant membrane that cofractionates with caveolin-2. J. Virol. 774160-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8465-472. [DOI] [PubMed] [Google Scholar]
- 49.Soussan, L., D. Burakov, M. P. Daniels, M. Toister-Achituv, A. Porat, Y. Yarden, and Z. Elazar. 1999. ERG30, a VAP-33-related protein, functions in protein transport mediated by COPI vesicles. J. Cell Biol. 146301-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone, M., S. Jia, W. D. Heo, T. Meyer, and K. V. Konan. 2007. Participation of Rab5, an early endosome protein, in hepatitis C virus RNA replication machinery. J. Virol. 814551-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terasaki, M., L. B. Chen, and K. Fujiwara. 1986. Microtubules and the endoplasmic reticulum are highly interdependent structures. J. Cell Biol. 1031557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tu, H., L. Gao, S. T. Shi, D. R. Taylor, T. Yang, A. K. Mircheff, Y. Wen, A. E. Gorbalenya, S. B. Hwang, and M. M. Lai. 1999. Hepatitis C virus RNA polymerase and NS5A complex with a SNARE-like protein. Virology 26330-41. [DOI] [PubMed] [Google Scholar]
- 53.Vasilescu, J., X. Guo, and J. Kast. 2004. Identification of protein-protein interactions using in vivo cross-linking and mass spectrometry. Proteomics 43845-3854. [DOI] [PubMed] [Google Scholar]
- 54.Vedrenne, C., and H. P. Hauri. 2006. Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic 7639-646. [DOI] [PubMed] [Google Scholar]
- 55.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]