The adaptive immune system is responsible for the final clearance and long lasting immunological memory of invading pathogens. Major histocompatibility complex class I (MHC-I) molecules play a central role in this defense as reporters of cellular content by presenting peptides derived from interior proteins in the cell. When recirculating cytotoxic T lymphocytes recognize MHC-I-presented peptides as foreign (e.g., derived from viral proteins), the presenting cell is killed by cytotoxic T lymphocytes, thereby hindering the spread of the virus. Thus, the key to efficient viral clearance by cytotoxic T lymphocytes lies within both the quality of the T-cell-receptor repertoire and the efficient processing and presentation of MHC-I-bound peptides. From the initial synthesis and folding to the final presentation on the cell surface, the MHC-I molecule gradually matures through multiple steps, most of which take place inside the endoplasmic reticulum (ER). The final stage of maturation for most MHC-I molecules takes place in the peptide-loading complex. The immune system and pathogens have evolved side by side for millions of years, and invading pathogens have developed several escape mechanisms to cripple the immune system. Among them are viral proteins that interfere with antigen presentation (VIPRs), which target both MHC-I and MHC-II antigen processing in order to skew or totally inhibit a functional immune response toward the virus. In this review, we discuss the main discoveries and latest developments concerning VIPRs that target the MHC-I peptide-loading complex.
NORMAL PHYSIOLOGY OF MHC-I ANTIGEN PROCESSING AND THE PEPTIDE-LOADING COMPLEX
MHC-I antigen processing starts with the degradation of intracellular proteins into small peptides. This is mainly accomplished by the proteasome in the cytosol, and the peptides are then transported into the ER by the transporter associated with antigen processing (TAP) in order to be loaded onto peptide-receptive MHC-I molecules. The MHC-I molecule is made up of a transmembrane-spanning heavy chain non-covalently bound to β2-microglobulin. The nascent heavy chain is synthesized directly into the ER, where it is initially bound by the general ER chaperones immunoglobulin-binding protein (BiP) and calnexin. After BiP is released, β2-microglobulin binds to the heavy chain and the soluble lectin calreticulin replaces calnexin (41) (Fig. 1A). Subsequently, the MHC-I molecule is integrated into the peptide-loading complex (17, 41, 60, 82). An essential part of the peptide-loading complex is the heterodimeric TAP composed of the TAP1 and TAP2 subunits, both containing an N-terminal transmembrane domain and a C-terminal cytosolic nucleotide binding domain. TAP1 and TAP2 have 10 and 9 transmembrane helices, respectively, where the 6 C-terminal helices from each subunit build together to form the so-called 6 + 6 TM core complex, which has been shown to be essential and sufficient for ER targeting, assembly of the heterodimer, binding of peptides, and peptide translocation (33). The translocation is a multistep process, beginning with the association of peptides with TAP in an ATP-independent manner (4, 48, 76). Peptides with a length of 8 to 16 amino acids are preferentially bound to TAP (76). Peptides with 8 to 12 amino acids are transported most efficiently, although peptides longer than 40 amino acids are also transported, albeit with a lower level of efficiency (4, 35). The C-terminal amino acid and the first three N-terminal residues of the peptide have been shown to play key roles in TAP recognition (68). Peptides with basic or hydrophobic amino acids at the C terminus are particularly preferred by human TAP. Peptide binding to TAP is followed by a slow isomerization of the TAP complex that triggers an ATP-dependent peptide translocation across the ER membrane (3, 47, 70).
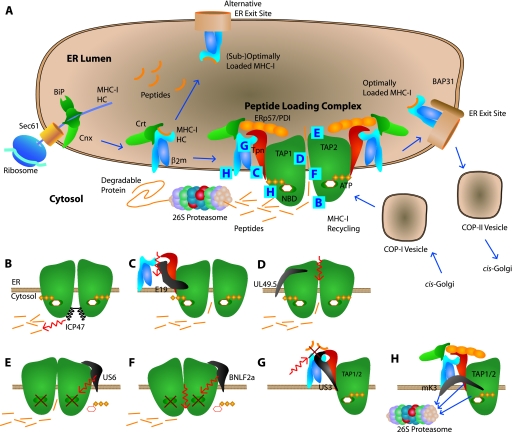
FIG. 1.
MHC-I maturation and virus proteins interfering with the peptide-loading complex. (A) Maturation of MHC-I in the ER starts in a specific way for all MHC-I molecules. The nascent MHC-I heavy chain is translated into the ER lumen through the Sec61 translocon. BiP and calnexin (Cnx) assist in the initial folding of the MHC-I heavy chain, allowing it to bind β2-microglobulin (β2m). After β2-microglobulin binding, the MHC-I molecule binds to calreticulin (Crt). At this intermediate processing stage, the MHC-I molecule may have already acquired a peptide able to induce final maturation; alternatively, the MHC-I may be from a HLA allele less prone to binding the peptide-loading complex or may be unable to bind the peptide-loading complex due to VIPR action, resulting in ER exit in either case. Other MHC-I molecules bind to tapasin (Tpn) and are allowed to mature in the peptide-loading complex, which consists of at least TAP, tapasin, calreticulin, ERp57, and protein disulfide isomerase (PDI). In the peptide-loading complex, tapasin mediates quality control, which ensures the loading of optimal peptides on MHC-I. A proportion of immature tapasin-associated MHC-I molecules escape ER retention but are transported back to the ER from the Golgi compartment in COP-I vesicles. Exit of optimally loaded MHC-I from the ER takes place at specialized ER exit sites. The MHC-I molecules are transported along the secretory pathway in COP-II vesicles and finally egress to the cell surface. (B) Herpes simplex virus ICP47 prevents peptides from binding to TAP. (C) Adenovirus E19 blocks the MHC-I-tapasin interaction and thereby prevents its integration into the peptide-loading complex. (D) Bovine herpes virus UL49.5 allows ATP and peptide binding to TAP but still inhibits the translocation of peptides into the ER. (E) Human cytomegalovirus US6 is an ER luminal protein that prevents ATP binding to TAP on the cytosolic nucleotide binding domain (NBD). (F) Epstein-Barr virus BNFL2a prevents both ATP and peptide binding to TAP. (G) Human cytomegalovirus US3 interacts directly with tapasin and interferes with tapasin-mediated quality control in the peptide-loading complex. (H) Herpes simplex virus type 68 mK3 integrates into a structurally intact peptide-loading complex, thereby mediating the ubiquitination of MHC-I, tapasin, and TAP, leading to proteasomal degradation.
TAP binds to the MHC-I-dedicated chaperone tapasin, and together they form the core of the peptide-loading complex. The TAP-tapasin complex interacts with MHC-I, calreticulin, ERp57, and protein disulfide isomerase to form a fully functional peptide-loading complex capable of loading peptides into the peptide-receptive MHC-I binding groove (53) (Fig. 1A). Tapasin is a type I transmembrane protein with a large N-terminal ER luminal region, a single transmembrane-spanning domain, and a short cytoplasmic tail. The precise site at which it binds to TAP has not yet been mapped, but it has been suggested that the first N-terminal transmembrane helix of TAP binds to the transmembrane domain of tapasin (34), which is supported by the fact that soluble human tapasin variants are defective in TAP association, resulting in impaired MHC-I surface expression (73). In the absence of tapasin, MHC-I is not found in association with TAP (66). Tapasin-deficient cells have decreased levels of cell surface expression of MHC-I, with alterations in their presented peptide repertoire and an impaired cytotoxic-T-lymphocyte response (20, 22, 61, 66). The ensurance that high-stability mature MHC-I molecules will be transported to the cell surface in wild-type cells has generally been attributed to the retention of immature MHC-I molecules in the ER, and indeed, results from different experimental systems have shown that tapasin retains MHC-I molecules in the ER until optimal peptides have been loaded (22, 67). In addition, a mechanism that recycles MHC-I molecules from late secretory compartments back to the ER has been suggested by several studies (14, 29, 52, 55). Vesicles with a protein coat composed of coatomer and ARF1 (COP-I-coated vesicles) recognize and bind to C-terminal KKXX motifs in membrane proteins that function as ER retrieval signals for proteins (16). That tapasin contains a C-terminal KKXX motif (55) and has been shown to have prolonged association with non-optimally loaded MHC-I molecules (14, 54, 55) led to the investigation of tapasin involvement in COP-I transport. Indeed, tapasin was demonstrated to bind to COP-I via its KKXX motif (54). In cells expressing tapasin with the KKXX motif mutated to AAXX, neither tapasin nor MHC-I was detected in association with COP-I, indicating a direct role for the tapasin KKXX motif in mediating the MHC-I transport by COP-I-coated vesicles. In the same cells, cell surface expression of MHC-I molecules was significantly increased, but MHC-I molecule degradation was also increased, suggesting that immature MHC-I molecules escape to the cell surface.
In the presence of tapasin, MHC-I molecules have been shown to improve their peptide cargo over time, both quantitatively and qualitatively (reviewed in reference 56). Tapasin has been proposed to act as a peptide editor that alters the conformation of the peptide binding pocket in MHC-I. This alteration improves and facilitates the efficient loading with optimal peptides that confers high conformational stability and a long half-life at the cell surface. Different MHC-I alleles depend to different degrees on tapasin for efficient peptide loading (Fig. 1A). The amino acid of human leukocyte antigen class I (HLA-I, human MHC-I) at position 114 has been shown to be of crucial importance to tapasin dependence: the higher the acidity of this amino acid, the higher the tapasin dependence (51). Both HLA-B*2705 and HLA-B*2702 have histidine at position 114, and both have been shown to be tapasin independent, while HLA-B*4402 and HLA-B*3501 have aspartic acid at residue 114 and have been demonstrated to be dependent on tapasin (51, 58).
Several studies have indicated that complete oxidation of the MHC-I heavy chain is a prerequisite for binding to the peptide-loading complex. ERp57 is a thiol oxidoreductase that forms a disulfide conjugate with tapasin within the peptide-loading complex (57). Studies have shown that this interaction is crucial for protection from reduction of the α2 disulfide bond in the peptide-binding groove on MHC-I (32, 79). Cooperation of ERp57 with tapasin has been suggested to significantly boost the efficiency whereby tapasin promotes MHC-I peptide binding (79). Recently, Ahn and coworkers presented the protein disulfide isomerase as the newest member of the peptide-loading complex, with a role in regulating the oxidation of the α2 disulfide bond of MHC-I (53).
Viruses have evolved strategies to prevent the generation and presentation of antigenic peptides, resulting in their ability to escape from the immune system. Viruses have evolved to target many crucial steps of antigen processing simultaneously, and many of the VIPRs directly assault the peptide-loading complex per se.
PREVENTION OF PEPTIDE BINDING TO TAP BY INDUCTION OF A CONFORMATIONAL ARREST AND DIRECT BLOCKAGE OF THE PEPTIDE BINDING SITE
In order for a peptide to be transported from the cytosol to the ER by TAP, it has to bind to the peptide binding site on TAP (6). Preventing this binding will inevitably result in a reduced pool of peptides in the ER available for association with MHC-I, ultimately resulting in a reduced level of MHC-I cell surface expression in accordance with the results found in TAP-deficient cells. Herpes simplex virus type 1 encodes an 88-amino-acid-long cytoplasmic protein called ICP47, which was originally observed to retain MHC-I in the ER (27). It was observed that these MHC-I molecules lack peptide, and it was suggested that ICP47 somehow blocks the translocation of peptides into the ER (83), and later, TAP was identified by immunoprecipitation as the ICP47 target (19). By using recombinant ICP47 and microsomes, it was shown that ICP47 binds strongly to TAP and remains stably associated (1, 74). Furthermore, the same study showed that ICP47 competes with the binding of peptides to TAP, suggesting that ICP47 binds to the same TAP peptide binding site or partly overlaps it (Fig. 1B).
High-affinity peptides are known to stabilize the TAP heterodimer (75). Based on chemical cross-linking of the two TAP subunits, it was demonstrated that peptides stabilize the TAP heterodimer whereas ICP47 actually causes its destabilization (39). The destabilizing effect offers an explanation for an additional mechanism by which ICP47 prevents peptide binding to TAP, since a functional TAP heterodimer is required for peptide binding and translocation (6, 75). The peptide-loading complex components are able to form around only one of the TAP subunits (5, 62, 65), so ICP47 is not expected to prevent formation of the peptide-loading complex: due to the fact that it destabilizes the TAP heterodimer, it merely prevents peptide binding to TAP by the previously described mechanism. Furthermore, it was shown that the six core transmembrane helices of each TAP subunit are sufficient for peptide translocation and also for ICP47 blocking (33). Since only the first N-terminal membrane-spanning helix of TAP is necessary for tapasin binding (34) and tapasin and other peptide-loading complex components are observed to bind to TAP simultaneously with ICP47 (19), it can be inferred that ICP47 modulates TAP only in such a way that it becomes incapable of binding peptides. A complete analysis of the impact of ICP47 on the functional integrity of the peptide-loading complex remains to be done.
INHIBITING TAP-DEPENDENT PEPTIDE TRANSLOCATION BY PREVENTING ATP BINDING TO TAP
TAP depends on ATP-derived energy for the translocation of peptides into the ER, and the disruption of ATP binding to the nucleotide binding domain on TAP represents another way of inhibiting TAP function by VIPRs. The human cytomegalovirus US6 gene product was first identified in 1997 as a 2-kDa, ER-restricted glycoprotein with the major part consisting of an ER luminal domain but which also contains a transmembrane and a cytosolic domain (25, 40). It was also observed that cells expressing this protein produced peptide-deficient MHC-I molecules and that US6 alone is sufficient to inhibit TAP-dependent peptide translocation into the ER. Later, it was shown that US6 effectively inhibits ATP, but not ADP, from binding to TAP by arresting TAP in a conformation able to bind peptide and ADP but not ATP (26, 38) (Fig. 1E). By using recombinant US6, it was also demonstrated that the ER luminal domain is sufficient for TAP inhibition, suggesting that no transmembrane domain-transmembrane domain interactions between US6 and TAP are necessary. However, the Lehner and Tampé studies did not totally agree on the detailed mechanism. The Lehner group (26) found that US6 prevents ATP binding only to the TAP1 subunit and actually promotes ATP binding to TAP2. Tampé and coworkers found that US6 prevented ATP binding to both the TAP1 and TAP2 subunits (38). However, a similar functional outcome was achieved by both models, since by preventing ATP from binding to TAP, US6 cuts off the energy source required for structural rearrangements and the following peptide translocation. US6 interaction sites on TAP were mapped to C-terminal transmembrane domain loops on TAP1 and an N-terminal loop on TAP2 (24). By immunoprecipitation experiments, it was also shown that US6 coprecipitated the peptide-loading complex components TAP, tapasin, calreticulin, MHC-I heavy chain, and β2-microglobulin (25, 40). However, they also showed that US6 does not need MHC-I heavy chain and tapasin to effectively block TAP. Further experiments need to be carried out to see if US6 interacts only with TAP or if it also interacts with or influences other components of the peptide-loading complex.
ABROGATING BOTH PEPTIDE AND ATP BINDING TO TAP
The lytic cycle of Epstein-Barr virus was previously found to be associated with decreased MHC-I cell surface expression due to a diminished peptide pool in the ER (23, 31). The steady-state level of TAP was unaffected by Epstein-Barr virus transformation, and it was suggested that VIPRs downmodulate TAP at the functional level (63). Very recently, a more detailed mechanism was elucidated. It was found that the Epstein-Barr virus lytic cycle BNLF2a protein coimmunoprecipitated TAP, tapasin, and MHC-I heavy chain (28). The same report also demonstrated by peptide cross-linking and ATP-agarose binding that BNLF2a abrogates the binding of both peptides and ATP to TAP, thereby combining the actions of both ICP47 and US6 and thus ensuring efficient inhibition of peptide translocation (Fig. 1F). A further characterization of BNLF2a will be interesting, including studies of possible additional mechanisms targeting other peptide-loading complex components.
DYSFUNCTIONAL TAP THAT STILL CAN BIND PEPTIDES AND ATP
Bovine herpesvirus type 1 was initially observed to downregulate cell surface-expressed MHC-I and interfere with TAP-dependent peptide translocation into the ER (37). Bovine herpesvirus type 1 encodes a viral envelope protein termed UL49.5 (8, 64) that was recently found to have an inhibitory effect on TAP (36). The same study also showed by cross-linking peptides and binding to ATP-agarose that UL49.5 does not prevent the binding of peptides or ATP to TAP (Fig. 1D). This finding suggests an inhibitory mechanism different from that of BNFL2a, but the mechanism that inhibits TAP function is still unknown. New findings have shown that bovine herpesvirus type 1 glycoprotein M binds directly to UL49.5, which results in less UL49.5 available for TAP inhibition (43). However, the same study showed that since UL49.5 is normally produced in excessive amounts, there is still a reduction of TAP activity during bovine herpesvirus type 1 infection.
ABROGATION OF MHC-I/TAPASIN INTERACTION: PREVENTING MHC-I INTEGRATION INTO THE PEPTIDE-LOADING COMPLEX
The adenovirus expresses a set of early transcription unit 3 (E3) proteins during replication (reviewed in reference 81). The E3-19 kDa protein, also termed E19, is an ER resident protein with VIPR properties. E19 was initially shown to bind MHC-I in the ER and downmodulate MHC-I cell surface expression (2, 15, 18, 69). Recently, amino acid position 56 located on the MHC-I α1-helix of the peptide binding groove has been shown to be critical for E19 binding to the MHC-I and thus represents a putative binding site (44). Several years after the initial discoveries, it was found by coimmunoprecipitation that E19 is also able to bind to TAP (9). By using MHC-I- and tapasin-deficient cell lines, the same study showed that the TAP-E19 interaction is independent of the presence of MHC-I and tapasin. Furthermore, the study also showed no difference in the association of tapasin with TAP in the presence of E19 but a clearly abrogated TAP-MHC-I interaction, which could most likely be attributed to disruption of the tapasin-MHC-I interaction, since tapasin has been shown to bridge MHC-I to TAP in the peptide-loading complex (59, 73) (Fig. 1C). The absence of tapasin has been shown to result in a decreased half-life for TAP (21, 49, 62), but since E19 does not abrogate the tapasin-TAP interaction, we speculate here that E19 does not destabilize TAP.
INTERFERENCE WITH TAPASIN-MEDIATED QUALITY CONTROL IN THE PEPTIDE-LOADING COMPLEX
Human cytomegalovirus encodes an US3 ER-expressed glycoprotein, and by using flow cytometry it was observed that US3 results in MHC-I downregulation; pulse-chase experiments further established that the reason is degradation of the MHC-I heavy chain (30). Subsequently, it was shown that US3 disrupts the tapasin-mediated peptide-loading quality control process (50) (Fig. 1G). A direct interaction between tapasin and US3 was demonstrated, and the study showed that the US3-mediated downregulation was MHC-I allele specific, targeting only the tapasin-dependent MHC-I alleles that are integrated into the peptide-loading complex to a high degree. It was demonstrated that the presence of US3 decreases the thermostability of tapasin-dependent MHC-I molecules, indicating that tapasin-mediated quality control in the peptide-loading complex is negatively affected by US3. The exact mechanism by which tapasin exerts this quality control is still under debate, but further studies of the quality control process in combination with US3 might shed some more light on the underlying tapasin optimization mechanism.
VIPR-INDUCED PEPTIDE-LOADING COMPLEX DEGRADATION
Many VIPRs, such as human cytomegalovirus US2 and US11, target MHC-I for degradation outside the peptide-loading complex (7). However, murine gamma-2 herpesvirus 68 produces a VIPR called mK3, which targets only peptide-loading complex-incorporated MHC-I for degradation (71, 72). Interestingly, mK3 has a PHD/LAP finger motif, which ubiquitinates MHC-I and thereby targets it for proteasomal degradation (12, 71, 84). One study showed that the mK3 PHD/LAP finger motif is required for MHC-I ubiquitination but not for mK3 association with MHC-I (12). Subsequently, it was shown that the mK3-MHC-I association takes place only in the peptide-loading complex, suggesting that peptide-loading complex chaperones are required for MHC-I ubiquitination by mK3 (84) (Fig. 1H). Later, it was revealed that both TAP and tapasin interact with mK3 and are required for MHC-I ubiquitination (46). The same study also showed that mK3 associates only with β2-microglobulin-associated MHC-I heavy chains, probably because only β2-microglobulin-associated MHC-I heavy chains are integrated into the peptide-loading complex. By introducing the T134K mutation in MHC-I, the interaction with tapasin is abrogated, and only tapasin-interacting MHC-I was found to be degraded in the presence of mK3 (46), which further supports the requirement of tapasin interactions for mK3 to exert ubiquitination. An effect of mK3 MHC-I ubiquitination is that tapasin and TAP also become ubiquitinated and subsequently degraded (10). It was suggested by Wang et al. and Wearsch et al. that mK3 integration into the peptide-loading complex orients mK3 in such a way that it becomes able to ubiquitinate MHC-I (77, 78). These studies suggested that mK3 interacts with the C-terminal domains of TAP and tapasin and the N-terminal domain of MHC-I. These structural requirements are proposed to result in proper orientation of the mK3 ubiquitination domain with respect to MHC-I. Further studies supporting this theory showed that intact tapasin is required to degrade MHC-I, whereas only the TM and cytosolic regions of tapasin are required to degrade TAP (11). As described in the previous section, human cytomegalovirus encodes the US3 protein which interferes directly with tapasin-mediated quality control. In addition to its functional interference with tapasin, it was recently found that US3 mediates the degradation of the newly discovered peptide-loading complex component protein disulfide isomerase (53). Protein disulfide isomerase has been proposed to be important for disulfide isomerization of the disulfide bond located in the MHC-I peptide binding groove. The US3-mediated degradation of protein disulfide isomerase is thus another way of interfering with optimal peptide loading in the peptide-loading complex.
PERSPECTIVES
Downregulation of cell surface-expressed MHC-I during viral infection and replication is a frequently used viral strategy to avoid immune recognition. This renders the infected cell invisible to cytotoxic T lymphocytes but in some cases still leaves it susceptible to NK cells (13), although a diverse set of strategies to prevent NK cell recognition has also evolved. Viruses have different ways of interfering with the MHC-I antigen-processing machinery by transcriptional downregulation of vital antigen-processing machinery components (reviewed in references 42 and 45), but as we have described in this review, VIPRs have also evolved to directly target the functionality of the peptide-loading complex at the protein level. Peptide availability in the ER is a key requirement for subsequent MHC-I antigen presentation at the cell surface. Limiting the peptide pool in the ER results in reduced and altered antigen presentation, and indeed, the major peptide supplier to the ER, TAP, has been shown to be the target of a number of different VIPRs. Another key component for efficient antigen presentation is tapasin. Deficient tapasin function results in both qualitatively and quantitatively altered MHC-I peptide presentation, and hence tapasin is an obvious target for viral interference, as illustrated by the discovery of a number of VIPRs acting on tapasin. Interference with TAP or tapasin thus prevents virus epitopes from being presented for recognition by cytotoxic T lymphocytes. Even though all MHC-I molecules, including those classified as tapasin independent, are to some degree optimized in the peptide-loading complex (80), the effect of many VIPRs that target the peptide-loading complex is exclusively on the so called tapasin-dependent alleles. Therefore, HLA polymorphism and codominant expression are likely to play a major role in our capability to fight many viral infections. A detailed understanding of the evolutionary pressures on both the host and the viruses targeting the MHC-I peptide-loading complex will be of great benefit to understanding antigen processing and presentation, as well as other cellular processes such as intracellular transport. Similarly to tapasin, the adenoviral protein E19, for example, has been suggested to mediate COP-I recycling of MHC-I molecules back to the ER. A further characterization of E19 might both elucidate the mechanism for MHC-I optimization by tapasin and bring light to the complex field of intracellular transport. Continued research in the field of peptide-loading complex VIPRs has obvious implications for developing medical strategies to fight viruses as well as to prevent immune recognition in autoimmune diseases, transplantation, and viral vector-based gene therapies.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (grant diarie 2006-6500) and the Alfred Benzon, Novo Nordisk, and Lundbeck foundations.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Ahn, K., T. H. Meyer, S. Uebel, P. Sempe, H. Djaballah, Y. Yang, P. A. Peterson, K. Fruh, and R. Tampe. 1996. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 153247-3255. [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, M., S. Paabo, T. Nilsson, and P. A. Peterson. 1985. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell 43215-222. [DOI] [PubMed] [Google Scholar]
- 3.Androlewicz, M. J., K. S. Anderson, and P. Cresswell. 1993. Evidence that transporters associated with antigen processing translocate a major histocompatibility complex class I-binding peptide into the endoplasmic reticulum in an ATP-dependent manner. Proc. Natl. Acad. Sci. USA 909130-9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Androlewicz, M. J., and P. Cresswell. 1994. Human transporters associated with antigen processing possess a promiscuous peptide-binding site. Immunity 17-14. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou, A. N., S. Ford, E. S. Pilley, N. Blake, and S. J. Powis. 2002. Interactions formed by individually expressed TAP1 and TAP2 polypeptide subunits. Immunology 106182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora, S., P. E. Lapinski, and M. Raghavan. 2001. Use of chimeric proteins to investigate the role of transporter associated with antigen processing (TAP) structural domains in peptide binding and translocation. Proc. Natl. Acad. Sci. USA 987241-7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barel, M. T., G. C. Hassink, S. van Voorden, and E. J. Wiertz. 2006. Human cytomegalovirus-encoded US2 and US11 target unassembled MHC class I heavy chains for degradation. Mol. Immunol. 431258-1266. [DOI] [PubMed] [Google Scholar]
- 8.Barker, D. E., and B. Roizman. 1992. The unique sequence of the herpes simplex virus 1 L component contains an additional translated open reading frame designated UL49.5. J. Virol. 66562-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett, E. M., J. R. Bennink, J. W. Yewdell, and F. M. Brodsky. 1999. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J. Immunol. 1625049-5052. [PubMed] [Google Scholar]
- 10.Boname, J. M., B. D. de Lima, P. J. Lehner, and P. G. Stevenson. 2004. Viral degradation of the MHC class I peptide loading complex. Immunity 20305-317. [DOI] [PubMed] [Google Scholar]
- 11.Boname, J. M., J. S. May, and P. G. Stevenson. 2005. The murine gamma-herpesvirus-68 MK3 protein causes TAP degradation independent of MHC class I heavy chain degradation. Eur. J. Immunol. 35171-179. [DOI] [PubMed] [Google Scholar]
- 12.Boname, J. M., and P. G. Stevenson. 2001. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity 15627-636. [DOI] [PubMed] [Google Scholar]
- 13.Bottley, G., O. G. Watherston, Y. L. Hiew, B. Norrild, G. P. Cook, and G. E. Blair. 2008. High-risk human papillomavirus E7 expression reduces cell-surface MHC class I molecules and increases susceptibility to natural killer cells. Oncogene 271794-1799. [DOI] [PubMed] [Google Scholar]
- 14.Bresnahan, P. A., L. D. Barber, and F. M. Brodsky. 1997. Localization of class I histocompatibility molecule assembly by subfractionation of the early secretory pathway. Hum. Immunol. 53129-139. [DOI] [PubMed] [Google Scholar]
- 15.Burgert, H. G., and S. Kvist. 1985. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell 41987-997. [DOI] [PubMed] [Google Scholar]
- 16.Cosson, P., and F. Letourneur. 1994. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 2631629-1631. [DOI] [PubMed] [Google Scholar]
- 17.Culina, S., G. Lauvau, B. Gubler, and P. M. van Endert. 2004. Calreticulin promotes folding of functional human leukocyte antigen class I molecules in vitro. J. Biol. Chem. 27954210-54215. [DOI] [PubMed] [Google Scholar]
- 18.Flomenberg, P., J. Szmulewicz, E. Gutierrez, and H. Lupatkin. 1992. Role of the adenovirus E3-19k conserved region in binding major histocompatibility complex class I molecules. J. Virol. 664778-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Früh, K., K. Ahn, H. Djaballah, P. Sempe, P. M. van Endert, R. Tampe, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375415-418. [DOI] [PubMed] [Google Scholar]
- 20.Garbi, N., P. Tan, A. D. Diehl, B. J. Chambers, H. G. Ljunggren, F. Momburg, and G. J. Hammerling. 2000. Impaired immune responses and altered peptide repertoire in tapasin-deficient mice. Nat. Immunol. 1234-238. [DOI] [PubMed] [Google Scholar]
- 21.Garbi, N., N. Tiwari, F. Momburg, and G. J. Hammerling. 2003. A major role for tapasin as a stabilizer of the TAP peptide transporter and consequences for MHC class I expression. Eur. J. Immunol. 33264-273. [DOI] [PubMed] [Google Scholar]
- 22.Grandea, A. G., III, T. N. Golovina, S. E. Hamilton, V. Sriram, T. Spies, R. R. Brutkiewicz, J. T. Harty, L. C. Eisenlohr, and L. Van Kaer. 2000. Impaired assembly yet normal trafficking of MHC class I molecules in Tapasin mutant mice. Immunity 13213-222. [DOI] [PubMed] [Google Scholar]
- 23.Guerreiro-Cacais, A. O., M. Uzunel, J. Levitskaya, and V. Levitsky. 2007. Inhibition of heavy chain and β2-microglobulin synthesis as a mechanism of major histocompatibility complex class I downregulation during Epstein-Barr virus replication. J. Virol. 811390-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halenius, A., F. Momburg, H. Reinhard, D. Bauer, M. Lobigs, and H. Hengel. 2006. Physical and functional interactions of the cytomegalovirus US6 glycoprotein with the transporter associated with antigen processing. J. Biol. Chem. 2815383-5390. [DOI] [PubMed] [Google Scholar]
- 25.Hengel, H., J. O. Koopmann, T. Flohr, W. Muranyi, E. Goulmy, G. J. Hammerling, U. H. Koszinowski, and F. Momburg. 1997. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6623-632. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt, E. W., S. S. Gupta, and P. J. Lehner. 2001. The human cytomegalovirus gene product US6 inhibits ATP binding by TAP. EMBO J. 20387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill, A. B., B. C. Barnett, A. J. McMichael, and D. J. McGeoch. 1994. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J. Immunol. 1522736-2741. [PubMed] [Google Scholar]
- 28.Hislop, A. D., M. E. Ressing, D. van Leeuwen, V. A. Pudney, D. Horst, D. Koppers-Lalic, N. P. Croft, J. J. Neefjes, A. B. Rickinson, and E. J. Wiertz. 2007. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J. Exp. Med. 2041863-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu, V. W., L. C. Yuan, J. G. Nuchtern, J. Lippincott-Schwartz, G. J. Hammerling, and R. D. Klausner. 1991. A recycling pathway between the endoplasmic reticulum and the Golgi apparatus for retention of unassembled MHC class I molecules. Nature 352441-444. [DOI] [PubMed] [Google Scholar]
- 30.Jun, Y., E. Kim, M. Jin, H. C. Sung, H. Han, D. E. Geraghty, and K. Ahn. 2000. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 164805-811. [DOI] [PubMed] [Google Scholar]
- 31.Keating, S., S. Prince, M. Jones, and M. Rowe. 2002. The lytic cycle of Epstein-Barr virus is associated with decreased expression of cell surface major histocompatibility complex class I and class II molecules. J. Virol. 768179-8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kienast, A., M. Preuss, M. Winkler, and T. P. Dick. 2007. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat. Immunol. 8864-872. [DOI] [PubMed] [Google Scholar]
- 33.Koch, J., R. Guntrum, S. Heintke, C. Kyritsis, and R. Tampe. 2004. Functional dissection of the transmembrane domains of the transporter associated with antigen processing (TAP). J. Biol. Chem. 27910142-10147. [DOI] [PubMed] [Google Scholar]
- 34.Koch, J., R. Guntrum, and R. Tampe. 2006. The first N-terminal transmembrane helix of each subunit of the antigenic peptide transporter TAP is essential for independent tapasin binding. FEBS Lett. 5804091-4096. [DOI] [PubMed] [Google Scholar]
- 35.Koopmann, J. O., M. Post, J. J. Neefjes, G. J. Hammerling, and F. Momburg. 1996. Translocation of long peptides by transporters associated with antigen processing (TAP). Eur. J. Immunol. 261720-1728. [DOI] [PubMed] [Google Scholar]
- 36.Koppers-Lalic, D., E. A. Reits, M. E. Ressing, A. D. Lipinska, R. Abele, J. Koch, M. Marcondes Rezende, P. Admiraal, D. van Leeuwen, K. Bienkowska-Szewczyk, T. C. Mettenleiter, F. A. Rijsewijk, R. Tampe, J. Neefjes, and E. J. Wiertz. 2005. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc. Natl. Acad. Sci. USA 1025144-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koppers-Lalic, D., M. Rychlowski, D. van Leeuwen, F. A. Rijsewijk, M. E. Ressing, J. J. Neefjes, K. Bienkowska-Szewczyk, and E. J. Wiertz. 2003. Bovine herpesvirus 1 interferes with TAP-dependent peptide transport and intracellular trafficking of MHC class I molecules in human cells. Arch. Virol. 1482023-2037. [DOI] [PubMed] [Google Scholar]
- 38.Kyritsis, C., S. Gorbulev, S. Hutschenreiter, K. Pawlitschko, R. Abele, and R. Tampé. 2001. Molecular mechanism and structural aspects of transporter associated with antigen processing inhibition by the cytomegalovirus protein US6. J. Biol. Chem. 27648031-48039. [DOI] [PubMed] [Google Scholar]
- 39.Lacaille, V. G., and M. J. Androlewicz. 1998. Herpes simplex virus inhibitor ICP47 destabilizes the transporter associated with antigen processing (TAP) heterodimer. J. Biol. Chem. 27317386-17390. [DOI] [PubMed] [Google Scholar]
- 40.Lehner, P. J., J. T. Karttunen, G. W. Wilkinson, and P. Cresswell. 1997. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. USA 946904-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis, J. W., and T. Elliott. 1998. Evidence for successive peptide binding and quality control stages during MHC class I assembly. Curr. Biol. 8717-720. [DOI] [PubMed] [Google Scholar]
- 42.Lilley, B. N., and H. L. Ploegh. 2005. Viral modulation of antigen presentation: manipulation of cellular targets in the ER and beyond. Immunol. Rev. 207126-144. [DOI] [PubMed] [Google Scholar]
- 43.Lipińska, A. D., D. Koppers-Lalic, M. Rychlowski, P. Admiraal, F. A. Rijsewijk, K. Bieńkowska-Szewczyk, and E. J. Wiertz. 2006. Bovine herpesvirus 1 UL49.5 protein inhibits the transporter associated with antigen processing despite complex formation with glycoprotein M. J. Virol. 805822-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, H., J. Fu, and M. Bouvier. 2007. Allele- and locus-specific recognition of class I MHC molecules by the immunomodulatory E3-19K protein from adenovirus. J. Immunol. 1784567-4575. [DOI] [PubMed] [Google Scholar]
- 45.Loch, S., and R. Tampe. 2005. Viral evasion of the MHC class I antigen-processing machinery. Pflugers Arch. 451409-417. [DOI] [PubMed] [Google Scholar]
- 46.Lybarger, L., X. Wang, M. R. Harris, H. W. Virgin IV, and T. H. Hansen. 2003. Virus subversion of the MHC class I peptide-loading complex. Immunity 18121-130. [DOI] [PubMed] [Google Scholar]
- 47.Neefjes, J. J., F. Momburg, and G. J. Hammerling. 1993. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science 261769-771. [DOI] [PubMed] [Google Scholar]
- 48.Neumann, L., and R. Tampe. 1999. Kinetic analysis of peptide binding to the TAP transport complex: evidence for structural rearrangements induced by substrate binding. J. Mol. Biol. 2941203-1213. [DOI] [PubMed] [Google Scholar]
- 49.Papadopoulos, M., and F. Momburg. 2007. Multiple residues in the transmembrane helix and connecting peptide of mouse tapasin stabilize the transporter associated with the antigen-processing TAP2 subunit. J. Biol. Chem. 2829401-9410. [DOI] [PubMed] [Google Scholar]
- 50.Park, B., Y. Kim, J. Shin, S. Lee, K. Cho, K. Fruh, S. Lee, and K. Ahn. 2004. Human cytomegalovirus inhibits tapasin-dependent peptide loading and optimization of the MHC class I peptide cargo for immune evasion. Immunity 2071-85. [DOI] [PubMed] [Google Scholar]
- 51.Park, B., S. Lee, E. Kim, and K. Ahn. 2003. A single polymorphic residue within the peptide-binding cleft of MHC class I molecules determines spectrum of tapasin dependence. J. Immunol. 170961-968. [DOI] [PubMed] [Google Scholar]
- 52.Park, B., S. Lee, E. Kim, S. Chang, M. Jin, and K. Ahn. 2001. The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity 15213-224. [DOI] [PubMed] [Google Scholar]
- 53.Park, B., S. Lee, E. Kim, K. Cho, S. R. Riddell, S. Cho, and K. Ahn. 2006. Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. Cell 127369-382. [DOI] [PubMed] [Google Scholar]
- 54.Paulsson, K. M., M. Jevon, J. W. Wang, S. Li, and P. Wang. 2006. The double lysine motif of tapasin is a retrieval signal for retention of unstable MHC class I molecules in the endoplasmic reticulum. J. Immunol. 1767482-7488. [DOI] [PubMed] [Google Scholar]
- 55.Paulsson, K. M., M. J. Kleijmeer, J. Griffith, M. Jevon, S. Chen, P. O. Anderson, H. O. Sjogren, S. Li, and P. Wang. 2002. Association of tapasin and COPI provides a mechanism for the retrograde transport of major histocompatibility complex (MHC) class I molecules from the Golgi complex to the endoplasmic reticulum. J. Biol. Chem. 27718266-18271. [DOI] [PubMed] [Google Scholar]
- 56.Paulsson, K. M., and P. Wang. 2004. Quality control of MHC class I maturation. FASEB J. 1831-38. [DOI] [PubMed] [Google Scholar]
- 57.Peaper, D. R., P. A. Wearsch, and P. Cresswell. 2005. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 243613-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peh, C. A., S. R. Burrows, M. Barnden, R. Khanna, P. Cresswell, D. J. Moss, and J. McCluskey. 1998. HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity 8531-542. [DOI] [PubMed] [Google Scholar]
- 59.Peh, C. A., N. Laham, S. R. Burrows, Y. Zhu, and J. McCluskey. 2000. Distinct functions of tapasin revealed by polymorphism in MHC class I peptide loading. J. Immunol. 164292-299. [DOI] [PubMed] [Google Scholar]
- 60.Peterson, J. R., A. Ora, P. N. Van, and A. Helenius. 1995. Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Mol. Biol. Cell 61173-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Purcell, A. W., J. J. Gorman, M. Garcia-Peydro, A. Paradela, S. R. Burrows, G. H. Talbo, N. Laham, C. A. Peh, E. C. Reynolds, J. A. Lopez De Castro, and J. McCluskey. 2001. Quantitative and qualitative influences of tapasin on the class I peptide repertoire. J. Immunol. 1661016-1027. [DOI] [PubMed] [Google Scholar]
- 62.Raghuraman, G., P. E. Lapinski, and M. Raghavan. 2002. Tapasin interacts with the membrane-spanning domains of both TAP subunits and enhances the structural stability of TAP1·TAP2 complexes. J. Biol. Chem. 27741786-41794. [DOI] [PubMed] [Google Scholar]
- 63.Ressing, M. E., S. E. Keating, D. van Leeuwen, D. Koppers-Lalic, I. Y. Pappworth, E. J. Wiertz, and M. Rowe. 2005. Impaired transporter associated with antigen processing-dependent peptide transport during productive EBV infection. J. Immunol. 1746829-6838. [DOI] [PubMed] [Google Scholar]
- 64.Romanelli, M. G., P. Mavromara-Nazos, D. Spector, and B. Roizman. 1992. Mutational analysis of the ICP4 binding sites in the 5′ transcribed noncoding domains of the herpes simplex virus 1 UL49.5 γ2 gene. J. Virol. 664855-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rufer, E., R. M. Leonhardt, and M. R. Knittler. 2007. Molecular architecture of the TAP-associated MHC class I peptide-loading complex. J. Immunol. 1795717-5727. [DOI] [PubMed] [Google Scholar]
- 66.Sadasivan, B., P. J. Lehner, B. Ortmann, T. Spies, and P. Cresswell. 1996. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity 5103-114. [DOI] [PubMed] [Google Scholar]
- 67.Schoenhals, G. J., R. M. Krishna, A. G. Grandea III, T. Spies, P. A. Peterson, Y. Yang, and K. Fruh. 1999. Retention of empty MHC class I molecules by tapasin is essential to reconstitute antigen presentation in invertebrate cells. EMBO J. 18743-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schölz, C., and R. Tampe. 2005. The intracellular antigen transport machinery TAP in adaptive immunity and virus escape mechanisms. J. Bioenerg. Biomembr. 37509-515. [DOI] [PubMed] [Google Scholar]
- 69.Sester, M., and H. G. Burgert. 1994. Conserved cysteine residues within the E3/19K protein of adenovirus type 2 are essential for binding to major histocompatibility complex antigens. J. Virol. 685423-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shepherd, J. C., T. N. Schumacher, P. G. Ashton-Rickardt, S. Imaeda, H. L. Ploegh, C. A. Janeway, Jr., and S. Tonegawa. 1993. TAP1-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell 74577-584. [DOI] [PubMed] [Google Scholar]
- 71.Stevenson, P. G., S. Efstathiou, P. C. Doherty, and P. J. Lehner. 2000. Inhibition of MHC class I-restricted antigen presentation by γ2-herpesviruses. Proc. Natl. Acad. Sci. USA 978455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stevenson, P. G., J. S. May, X. G. Smith, S. Marques, H. Adler, U. H. Koszinowski, J. P. Simas, and S. Efstathiou. 2002. K3-mediated evasion of CD8+ T cells aids amplification of a latent γ-herpesvirus. Nat. Immunol. 3733-740. [DOI] [PubMed] [Google Scholar]
- 73.Tan, P., H. Kropshofer, O. Mandelboim, N. Bulbuc, G. J. Hammerling, and F. Momburg. 2002. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. J. Immunol. 1681950-1960. [DOI] [PubMed] [Google Scholar]
- 74.Tomazin, R., A. B. Hill, P. Jugovic, I. York, P. van Endert, H. L. Ploegh, D. W. Andrews, and D. C. Johnson. 1996. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 153256-3266. [PMC free article] [PubMed] [Google Scholar]
- 75.van Endert, P. M. 1999. Role of nucleotides and peptide substrate for stability and functional state of the human ABC family transporters associated with antigen processing. J. Biol. Chem. 27414632-14638. [DOI] [PubMed] [Google Scholar]
- 76.van Endert, P. M., R. Tampe, T. H. Meyer, R. Tisch, J. F. Bach, and H. O. McDevitt. 1994. A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity 1491-500. [DOI] [PubMed] [Google Scholar]
- 77.Wang, X., R. Connors, M. R. Harris, T. H. Hansen, and L. Lybarger. 2005. Requirements for the selective degradation of endoplasmic reticulum-resident major histocompatibility complex class I proteins by the viral immune evasion molecule mK3. J. Virol. 794099-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang, X., L. Lybarger, R. Connors, M. R. Harris, and T. H. Hansen. 2004. Model for the interaction of gammaherpesvirus 68 RING-CH finger protein mK3 with major histocompatibility complex class I and the peptide-loading complex. J. Virol. 788673-8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wearsch, P. A., and P. Cresswell. 2007. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat. Immunol. 8873-881. [DOI] [PubMed] [Google Scholar]
- 80.Williams, A. P., C. A. Peh, A. W. Purcell, J. McCluskey, and T. Elliott. 2002. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity 16509-520. [DOI] [PubMed] [Google Scholar]
- 81.Windheim, M., A. Hilgendorf, and H. G. Burgert. 2004. Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways. Curr. Top. Microbiol. Immunol. 27329-85. [DOI] [PubMed] [Google Scholar]
- 82.Wright, C. A., P. Kozik, M. Zacharias, and S. Springer. 2004. Tapasin and other chaperones: models of the MHC class I loading complex. Biol. Chem. 385763-778. [DOI] [PubMed] [Google Scholar]
- 83.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77525-535. [DOI] [PubMed] [Google Scholar]
- 84.Yu, Y. Y., M. R. Harris, L. Lybarger, L. A. Kimpler, N. B. Myers, H. W. Virgin IV, and T. H. Hansen. 2002. Physical association of the K3 protein of gamma-2 herpesvirus 68 with major histocompatibility complex class I molecules with impaired peptide and β2-microglobulin assembly. J. Virol. 762796-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]