Abstract
Herpes simplex virus type 1 (HSV-1) produces oral lesions, encephalitis, keratitis, and severe infections in the immunocompromised host. HSV-1 is almost as common as HSV-2 in causing first episodes of genital herpes, a disease that is associated with an increased risk of human immunodeficiency virus acquisition and transmission. No approved vaccines are currently available to protect against HSV-1 or HSV-2 infection. We developed a novel HSV vaccine strategy that uses a replication-competent strain of HSV-1, NS-gEnull, which has a defect in anterograde and retrograde directional spread and cell-to-cell spread. Following scratch inoculation on the mouse flank, NS-gEnull replicated at the site of inoculation without causing disease. Importantly, the vaccine strain was not isolated from dorsal root ganglia (DRG). We used the flank model to challenge vaccinated mice and demonstrated that NS-gEnull was highly protective against wild-type HSV-1. The challenge virus replicated to low titers at the site of inoculation; therefore, the vaccine strain did not provide sterilizing immunity. Nevertheless, challenge by HSV-1 or HSV-2 resulted in less-severe disease at the inoculation site, and vaccinated mice were totally protected against zosteriform disease and death. After HSV-1 challenge, latent virus was recovered by DRG explant cocultures from <10% of vaccinated mice compared with 100% of mock-vaccinated mice. The vaccine provided protection against disease and death after intravaginal challenge and markedly lowered the titers of the challenge virus in the vagina. Therefore, the HSV-1 gEnull strain is an excellent candidate for further vaccine development.
Herpes simplex virus type 1 (HSV-1) and HSV-2 are closely related alphaherpesviruses that cause lifelong infections for which there is no cure. HSV-1 generally causes oral lesions, while HSV-2 remains the most common cause of genital ulcers; however, the epidemiology of genital herpes is changing in that 35% to 50% of new cases are now caused by HSV-1 (40). The life cycles of HSV-1 and HSV-2 are similar. After replication in epithelial cells, HSV enters local sensory nerve endings of the peripheral nervous system and spreads in a retrograde direction to neuronal cell bodies. HSV then spreads to adjacent neurons in ganglia, where a lifelong latent infection is established (43). During recurrences, HSV travels in the anterograde direction along axon fibers from infected neuronal cell bodies to skin or mucosal surfaces, resulting in asymptomatic virus shedding or symptomatic vesicles and ulcers.
More than 70% of people in the United States are seropositive for HSV-1 or HSV-2 by age 49, with HSV-1 being more prevalent (44). Worldwide, the prevalence of infection is generally higher than in the United States (34). Complications of HSV infection include meningitis, encephalitis, esophagitis, disseminated disease in neonates and immunocompromised individuals, and herpes stromal keratitis, which can lead to blindness. Additionally, HSV-2 infection increases human immunodeficiency virus (HIV) acquisition and transmission rates more than twofold, enhances HIV replication, and speeds progression to AIDS (7, 18, 37). Despite the significant morbidity and mortality associated with HSV infections, no effective vaccines are available.
Efforts to develop HSV vaccines have included inactivated whole-virus vaccines, subunit glycoprotein preparations, DNA plasmids, and live attenuated viruses (32, 35). The vaccine farthest along in human trials is a subunit glycoprotein vaccine developed by GlaxoSmithKline that uses HSV-2 glycoprotein D (gD) as an immunogen with alum and 2-O-deacylated monophosphoryl lipid A as adjuvants (4). In two large, placebo-controlled clinical trials, women who were seronegative for both HSV-1 and HSV-2 were protected against genital ulcer disease. However, no significant protection was detected against infection, as measured by seroconversion, and the vaccine failed to protect men or HSV-1-seropositive women (36).
A precedent exists for developing live virus vaccines for alphaherpesviruses, since a modified live virus vaccine is commercially available to prevent pseudorabies virus in pigs 3 days old or older (Schering-Plough Animal Health Corp.), and an attenuated live varicella-zoster virus vaccine is available for the prevention of chickenpox and shingles (31, 39). In this study, we used an HSV-1 gE deletion strain, NS-gEnull, as a live attenuated vaccine. This strain has a large deletion within the gE gene (US8) and fails to express a functional gE protein (29). Previous studies using NS-gEnull demonstrated that HSV-1 gE is required for efficient cell-to-cell spread in polarized epithelial cells; anterograde transport of viral capsid, tegument, and glycoproteins from the neuron cell body to the axon terminus; and the spread of virus in the retrograde direction from epithelial cells to neuronal cell bodies in dorsal root ganglia (DRG) (11, 33, 41). This result differs from that seen with pseudorabies virus gE, which is required for anterograde but not retrograde spread (6, 8). The NS-gEnull impairment in retrograde spread is an important safety feature for the vaccine, since this strain has reduced capacity for reaching DRG, the site of latency. The anterograde-spread defect of the virus is an added safety feature in that even if limited quantities of vaccine virus reach the DRG by retrograde spread, anterograde transport should not occur back to the skin or mucosa to cause recurrent infections.
In this study, we used the mouse flank model to evaluate the safety and efficacy of the NS-gEnull vaccine. The results demonstrated that after flank scarification, the vaccine strain did not cause lesions and no virus was recovered from DRG. Upon challenge with wild-type HSV-1, vaccinated mice were completely protected against zosteriform disease and death, and virus was not reactivated from DRG by explant coculture in >90% of mice. The NS-gEnull vaccine candidate also protected against HSV-1 challenge intravaginally and provided cross-protection against HSV-2 after flank challenge. Therefore, NS-gEnull has excellent potential for development as a replication-competent, attenuated live virus vaccine.
MATERIALS AND METHODS
Virus strains.
HSV-1(NS) was isolated from the respiratory tract of a child and was used as a low-passage-number wild-type virus for challenge experiments (15). NS-gEnull has a deletion within the region of the US8 gene encoding gE amino acids 124 to 512, while a repaired virus, rNS-gEnull, restores this region and gE functions (29). Other HSV-1 strains included KOS (321), F, and 17. HSV-2(2.12) is a low-passage-number isolate obtained from a genital lesion of an 18-year-old woman (19). All viruses were grown and titered on Vero cells using Dulbecco's modified Eagle medium (DMEM) and 5% fetal bovine serum (FBS).
Growth curves.
Vero cells grown in 12-well dishes were infected at a multiplicity of infection of 5 with NS, NS-gEnull, or rNS-gEnull. In some wells, the inoculum was collected immediately after it was placed on the cells as the zero-hour time point. In other wells, virus was adsorbed for 1 h at 37°C, the inoculum was removed, and cells were washed once with citrate buffer (pH 3.0) to inactivate virus that had not yet entered cells and twice with DMEM containing 5% FBS (1). Cells and supernatant fluids were harvested at this time point, which was considered 1 h postinfection, and at 2, 4, 8, 24, and 30 h. Cells and supernatant fluids were frozen at −80°C and were later thawed, sonicated, and titered on Vero cells.
Murine vaccinations.
Vaccinations were performed on 6- to 8-week-old female BALB/c mice (National Cancer Institute). Mice were anesthetized prior to all procedures by using 100 μl xylazine (14.3 mg/ml) (Vedco, Inc.) and ketamine (1.8 mg/ml) (Hospira, Inc.) in phosphate-buffered saline (PBS). Mice were vaccinated either by flank scarification, subcutaneously (SubQ) in the neck scruff, or intramuscularly (i.m.) in the right rear thigh. Fifty microliters of inoculum was used for SubQ and i.m. vaccinations, while for flank scarification, hair was first removed on the right flank by shaving and applying Nair. The following day, 10 μl containing NS-gEnull was placed on the denuded flank and inoculated using a 26 5/8-gauge needle to make 60 gentle scratches, each approximately 5 mm in length (23). Mock-vaccinated animals were scratch inoculated with an equivalent volume of uninfected cell lysate in DMEM with 5% FBS. Mice were scored for lesion severity at both the inoculation and zosteriform sites. Inoculation site disease was scored as 0 for no disease, 1 for redness or swelling, 2 for skin erosions, 3 for ulcers, and 4 for necrosis. Lesions outside the zone of inoculation were considered zosteriform disease, which was scored as 0 for no lesions, 1 for one or more discrete lesions, 2 for coalesced lesions, 3 for ulcerated lesions, and 4 for necrosis.
Murine challenge experiments.
Flank challenge was performed at least 28 days after vaccination by scarification on the flank opposite the one used for immunization. For vaginal challenge, mice received a SubQ injection of 2 mg of medroxyprogesterone (Sicor Pharmaceuticals, Inc., Irvine, CA) in 0.9% NaCl-10 mM HEPES 23 days after flank vaccination (21). Mice were challenged 5 days later with 105 PFU HSV-1(NS) in 5 μl by vaginal instillation. Vaginal specimens were collected daily using dry calcium alginate swabs. The swab tips were cut off and placed in 0.5 ml DMEM with 5% FBS; then they were vortexed and frozen at −80°C until titration on Vero cells.
Titration of virus in tissue.
Mice were euthanized prior to removal of DRG and skin at the inoculation site. DRG samples were minced with small scissors and pulverized using a pestle, and half the sample was titered on Vero cells. The remainder of the DRG sample was evaluated by real-time quantitative PCR (RT qPCR). Skin samples were divided in half. One part was processed using scissors and a pestle and was titered on Vero cells. The other part of the skin sample was processed for immunohistochemistry.
Immunohistochemistry.
Skin samples were placed in 4% paraformaldehyde in PBS for 24 h and transferred to PBS until they were paraffin embedded and sectioned. The Pathology Core Facility at the Children's Hospital of Philadelphia processed the tissue samples, which were stained sequentially for 30 min at room temperature with polyclonal anti-HSV-1 immunoglobulin G (IgG) (1:1,500; Dako), biotinylated anti-rabbit IgG (1:200; Vector Laboratories), and avidin-biotin (1:200) and were then counterstained with hematoxylin.
RT qPCR.
Each sample was amplified for HSV-1 US9 DNA and mouse adipsin DNA. PCRs were performed in 96-well qPCR dishes using 2× FAST Taqman master mix (Applied Biosystems). The primers for detection of US9 DNA were as follows: forward, 5′-ACGGCCTCGCCAGTTTC-3′ (melting temperature [Tm], 58°C); reverse, 5′-TTGGCCGCCTCGTCTTC-3′ (Tm, 59°C). The primers for detection of murine adipsin DNA were as follows: forward, 5′-GCAGTCGAAGGTGTGGTTACG-3′ (Tm, 59°C); reverse, 5′-GGTATAGACGCCCGGCTTTT-3′ (Tm, 59°C). The probes were 5′-TCGAAGCCTACTACTCG-3′ (Tm, 70°C) for US9 and 5′-CTGTGGCAATGGC-3′ (Tm, 70°C) for adipsin. The reporter dye was 6-carboxyfluorescein (5′), while BHQ (3′) was used as the quencher. All reactions were carried out in a FAST 7500 qPCR machine (Applied Biosystems). Standard curves were derived using 5 to 107 copies of HSV-1 DNA and 5 to 105 copies of murine cellular DNA (Biochain, Hayward, CA). RT qPCR results were expressed as the HSV-1 DNA copy number per 5 × 105 copies of murine adipsin.
Explant cocultures of DRG for recovery of latent HSV-1.
At least 28 days after challenge, mice were sacrificed, and the DRG were removed, minced with scissors, and placed on Vero cells in DMEM containing 5% FBS supplemented with 25 μg/ml vancomycin (38). Cultures were observed for virus reactivation for 20 days.
Neutralization assays.
Serum was obtained from mice by the submandibular route using the Goldenrod animal lancet (Medipoint, Inc.) 21 days after vaccination. The serum was heat inactivated for 30 min at 56°C and then diluted 1:10 to 1:320 in DMEM containing 10% FBS, and 50 μl was added to an equal volume containing 100 PFU of HSV-1(NS). After 1 h at 37°C, 300 μl of DMEM containing 10% FBS was added, and 200 μl was inoculated onto duplicate wells of Vero cells in 12-well tissue culture dishes. Plates were incubated for 1 h at 37°C with gentle rocking every 10 min. The inoculum was removed; cells were washed twice with PBS, overlaid with medium containing 0.6% agarose, and incubated for 72 h at 37°C; and plaques were counted (17).
Statistics.
The mean and standard error of the mean (SEM) were calculated for each data point. P values were calculated using GraphPad Prism software. Statistical tests were t tests except that the data in Fig. 1C, 2C, 4C, and 5C were analyzed using a Kruskal-Wallis test, and we used analysis of variance with Tukey's adjustment, which allows for all possible pairwise comparisons, for Fig. 1B, 2B, 4B, and 5B. Survival curves were analyzed using the log rank (Mantel-Cox) test.
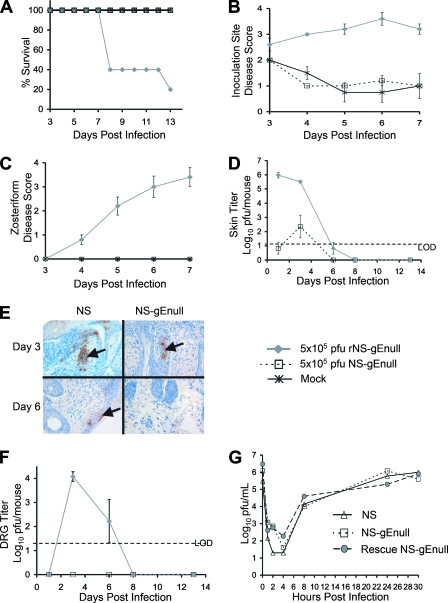
FIG. 1.
Safety studies of NS-gEnull as a vaccine candidate. (A to C) Mice were monitored for survival, inoculation site disease, and zosteriform disease (P = 0.01 in panel A for comparison of NS-gEnull or mock vaccination with rNS-gEnull; P < 0.01 in panels B and C for comparison of these groups). There were five mice in each group. (D) Skin samples were harvested for viral titers at the inoculation site (P < 0.01 on days 1 and 3; P > 0.05 on day 6). There were three mice in each group. (E) Skin samples were evaluated by immunohistochemistry to detect HSV-1 antigens (arrows). (F) DRG were evaluated for viral titers (P < 0.01 on day 3; P = 0.4 on day 6). There were three mice in each group. (G) Single-step growth curves of NS, NS-gEnull, and rNS-gEnull in Vero cells demonstrated comparable replication of the viruses. Results in panels B, C, D, and F are means ± SEMs. LOD refers to the limit of detection for the assay, which was 20 PFU.
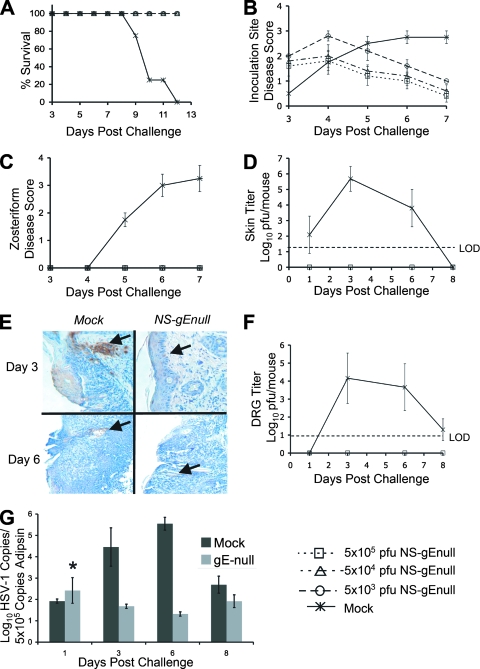
FIG. 2.
NS-gEnull vaccination protects against flank challenge with 105 PFU HSV-1(NS). (A to C) Mice were monitored for survival, inoculation site disease, and zosteriform disease (P = 0.003 in panel A for comparison of each vaccinated group with mock-vaccinated mice; P < 0.001 in panels B and C for comparison of these groups). There were five mice in each group. (D) Skin titers after challenge of mice vaccinated with 5 × 105 PFU of NS-gEnull or mock vaccinated (P > 0.05 on day 1; P = 0.02 on day 3; P > 0.05 on day 6). Three mice were included for each data point. (E) Immunohistochemistry of skin for HSV-1 antigens (arrows). (F) Viral titers in DRG of mice vaccinated with 5 × 105 PFU or mock vaccinated (P = 0.02 on days 3 and 6; P = 0.4 on day 8). There were three mice in each group. (G) HSV-1 viral DNA copies per 5 × 105 copies of adipsin within the DRG of challenged mice (P = 0.03 on day 3; P = 0.02 on day 6; P = 0.4 on day 8). The asterisk indicates the average log10 copy number including an outlier of 104 PFU, possibly due to contamination, on day 1 (P = 0.48 with the day 1 outlier; P = 0.42 without the outlier). There were three mice in each group. Data points in panels B, C, D, F, and G are means ± SEMs. LOD refers to the limit of detection for the assay, which was 20 PFU.
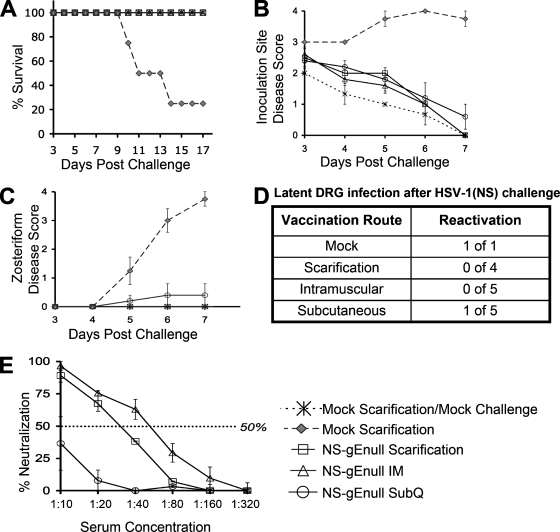
FIG. 4.
NS-gEnull vaccination is protective when administered by scarification, i.m., or SubQ. (A) Mice were monitored for survival (P = 0.02 for comparison of mock vaccination with each immunization route). (B) Inoculation site disease (P < 0.001 for comparison of disease in mock-vaccinated mice with that for each immunization route). (C) Zosteriform disease (P = 0.03 for comparison of mock vaccination with each immunization group). (D) Twenty-eight days after challenge, DRG explant cultures were performed. (E) HSV-1 neutralizing antibody titers (P = 0.03 for comparison of scarification or the i.m. route with the SubQ route; P > 0.05 for comparison of the i.m. route and scarification). There were five mice in each group, except for the epidermal scarification group, which had four mice. Results in panels B, C, and E are means ± SEMs.
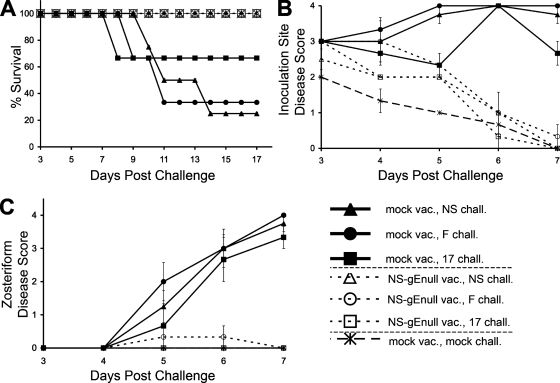
FIG. 5.
NS-gEnull vaccination protects against flank challenge (chall.) with HSV-1(NS), HSV-1(F), and HSV-1(17) at 105 PFU. (A) Mice were monitored for survival (P = 0.08 for strain NS, P = 0.08 for strain F, and P = 0.3 for strain 17 for comparison of the survival of immunized and mock-immunized mice). vac., vaccination. (B) Inoculation site disease (P < 0.001 for comparison of the same groups as in panel A). (C) Zosteriform disease (P < 0.001 for comparison of these groups). Three mice were challenged with strain F or 17, and four mice were challenged with strain NS. The results in panels B and C are means ± SEMs.
RESULTS
Safety of HSV-1 NS-gEnull as a vaccine in mice.
A mouse flank scarification model was used to evaluate the safety of the NS-gEnull live virus vaccine candidate. Comparisons included the repaired virus, rNS-gEnull, and mock scarification, which served as a control for lesions produced during the scratch immunization. All mice survived after NS-gEnull infection at 5 × 105 PFU; however, only 20% survived after infection with rNS-gEnull (Fig. 1A). rNS-gEnull caused severe lesions at the inoculation site, while NS-gEnull disease was indistinguishable from that with mock scarification (Fig. 1B). Zosteriform disease develops when virus spreads from the DRG back to the skin. rNS-gEnull caused severe zosteriform disease (Fig. 1C), appearing as a necrotic band spanning the entire right flank. No zosteriform lesions were seen in mice inoculated with NS-gEnull.
rNS-gEnull grew to high titers in the skin, while only low titers of NS-gEnull were isolated (Fig. 1D). The skin titer results were corroborated by immunohistochemistry in that viral antigens were readily detected on day 3 following infection with HSV-1(NS) while only scant antigen was noted with NS-gEnull (Fig. 1E). Importantly, NS-gEnull was not recovered from DRG on day 1, 3, 6, or 8 postinfection (Fig. 1F). In contrast, rNS-gEnull was isolated from DRG, reaching titers of 104 PFU on day 3. Additionally, no NS-gEnull was recovered from explant cocultures of right and left sacral ganglia performed 1 year after the vaccination of five mice with 5 × 105 PFU of NS-gEnull (data not shown). NS-gEnull replicated comparably to wild-type and rescued strains in single-step growth curves performed in Vero cells (Fig. 1G), indicating that reduced skin and DRG titers were not caused by defective replication.
NS-gEnull vaccination protects against HSV-1(NS) flank challenge.
Mice were vaccinated by scarification on the left flank and were challenged 28 days later with 105 PFU of HSV-1(NS) by scarification on the opposite flank. None of the mock-vaccinated mice survived, while all mice vaccinated with 5 × 103, 5 × 104, or 5 × 105 PFU of NS-gEnull survived the challenge and appeared healthy (Fig. 2A). Mock-vaccinated mice had significantly more disease than vaccinated mice at the inoculation site (Fig. 2B). The mock-vaccinated animals developed ulcers, whereas the inoculation site disease of the vaccinated mice healed rapidly. All mock-vaccinated mice developed severe zosteriform disease, while none of the NS-gEnull-vaccinated mice developed zosteriform lesions (Fig. 2C).
Viral skin titers at the inoculation site and DRG titers were determined after challenge of mock-vaccinated mice or mice vaccinated with 5 × 105 PFU of NS-gEnull. Titers of NS-gEnull-vaccinated mice were below the limit of detection for the assay (20 PFU), while 106 PFU was detected in mock-vaccinated mice (Fig. 2D). Immunohistochemistry revealed only trace amounts of viral antigen in NS-gEnull-vaccinated mice on days 3 and 6 postchallenge, compared with abundant antigen in mock-vaccinated mice (Fig. 2E). DRG viral titers reached 104 PFU in mock-vaccinated mice on day 3 postchallenge, while no virus was recovered from the DRG of vaccinated mice (Fig. 2F). RT qPCR of viral genomes in the DRG demonstrated differences of 3 to 4 log10 copies of HSV DNA between mock- and NS-gEnull-vaccinated mice on days 3 and 6 postchallenge (Fig. 2G). Therefore, no infectious virus was recovered from DRG after the challenge of NS-gEnull-vaccinated mice; levels of viral DNA in DRG were greatly reduced; and animals were totally protected from zosteriform disease and death.
Additional challenge studies were performed using HSV-1(NS) at doses of 106 and 107 PFU. Mice vaccinated with 5 × 105 PFU of NS-gEnull were protected from severe inoculation site disease, zosteriform lesions, and death, even after challenge at these higher doses (data not shown).
NS-gEnull vaccination reduces the recovery of latent challenge virus from DRG explants.
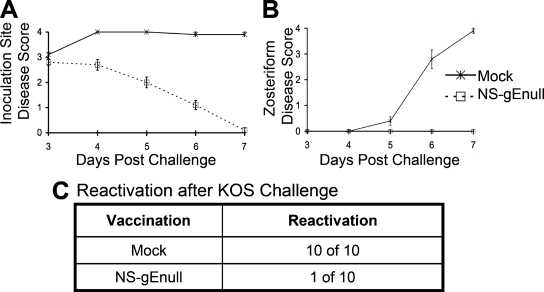
We evaluated whether the vaccine protects the DRG of mice from infection as assessed by virus reactivation from explant cocultures. Mice were either mock vaccinated or vaccinated with 5 × 105 PFU of NS-gEnull by flank scarification. Twenty-eight days later, mice were challenged with 105 PFU of HSV-1(KOS) by scarification of the opposite flank. Strain KOS was used because it does not kill mice at this dose after flank inoculation; therefore, mock-immunized mice survived, permitting evaluation of DRG for recovery of latent virus.
KOS produced severe inoculation and zosteriform site disease in mock-immunized mice, while challenge of NS-gEnull-vaccinated mice produced less inoculation site disease and no zosteriform disease (Fig. 3A and B). Virus was reactivated from explant cultures of all 10 DRG from mock-vaccinated mice, while only 1 of 10 DRG from NS-gEnull-vaccinated mice yielded virus after challenge (Fig. 3C). Therefore, the vaccine protected 90% of the mice from latent DRG infection after KOS challenge, as evaluated by explant cocultures.
FIG. 3.
NS-gEnull vaccination protects against disease and reduces the recovery of latent virus from DRG after flank challenge with HSV-1(KOS) at 105 PFU. (A and B) Mice were monitored for inoculation site disease (P = 0.03) and zosteriform disease (P = 0.04 on day 5; P < 0.001 on days 6 and 7). The data points are means ± SEMs. (C) DRG explant cocultures were performed 28 days after challenge (P < 0.001). There were 10 mice in each group.
NS-gEnull vaccination is effective when administered i.m. or SubQ.
The effectiveness of NS-gEnull vaccination with 5 × 105 PFU was compared using epidermal scarification, the i.m. route, or the SubQ route. Control mice were vaccinated by mock epidermal scarification. Twenty-eight days later, mice were challenged on the right flank using 105 PFU HSV-1(NS).
All mice vaccinated with NS-gEnull survived the challenge regardless of the route of vaccination, while 20% of mock-vaccinated animals survived (Fig. 4A). Mice vaccinated with NS-gEnull by any route developed only mild inoculation site disease, which healed rapidly (Fig. 4B). Mock-vaccinated mice developed severe zosteriform disease, while mice vaccinated by the i.m. or epidermal route were completely protected from zosteriform disease. One of five mice vaccinated SubQ had mild zosteriform disease (Fig. 4C).
Explant cocultures were performed on DRG to monitor for reactivation of infection 28 days following challenge. A single mock-vaccinated survivor served as a positive control (Fig. 4D). All mice vaccinated with NS-gEnull i.m. or by scarification failed to reactivate virus from DRG explant cocultures; however, the DRG from one of five mice vaccinated SubQ reactivated the virus.
Antibody neutralization titers were evaluated 3 weeks postimmunization. Neutralizing antibody titers induced by i.m. or epidermal scarification vaccination were more robust than those for the SubQ route (Fig. 4E); however, all immunization methods were protective.
NS-gEnull vaccination protects against epidermal challenge with heterologous HSV-1 strains.
In experiments shown in Fig. 1 to 3, protection provided by NS-gEnull immunization was determined by challenge with the same strain that was used to produce the vaccine virus, or with a less virulent HSV-1 strain, KOS. We next evaluated protection using two additional HSV-1 strains, F and 17. Mice were vaccinated with 5 × 105 PFU of NS-gEnull by epidermal scarification and were challenged 28 days later by epidermal scarification on the opposite flank with 105 PFU of HSV-1(NS), HSV-1(F), or HSV-1(17). All the NS-gEnull-vaccinated mice survived, while the survival of mock-vaccinated mice was 25% (1 of 4) for strain NS, 33% (1 of 3) for strain F, and 67% (2 of 3) for strain 17 (Fig. 5A).
The challenge viruses caused inoculation site disease in vaccinated mice that was more severe than that produced by the scratch alone but less severe than that in mock-vaccinated mice (Fig. 5B). Vaccinated mice were completely protected from zosteriform disease caused by strain 17 or NS, while one of three vaccinated mice challenged with strain F developed mild zosteriform disease on day 5 that healed by day 7 (Fig. 5C).
NS-gEnull vaccination cross-protects against HSV-2 challenge.
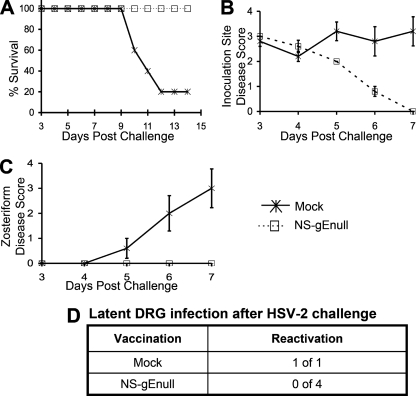
Mice were either mock vaccinated or vaccinated with 5 × 105 PFU of NS-gEnull by flank scarification. Twenty-eight days later, mice were challenged with 105 PFU of HSV-2(2.12) by scarification on the opposite flank. All vaccinated mice survived, while only 20% of mock-vaccinated mice survived (Fig. 6A). Vaccinated mice developed disease at the inoculation site; however, the lesions healed more rapidly than those in mock-vaccinated mice (Fig. 6B). All mock-vaccinated mice developed severe zosteriform disease, while none of the NS-gEnull-vaccinated mice developed zosteriform lesions (Fig. 6C). One year following challenge, DRG were evaluated for latent virus by explant cocultures from the four vaccinated mice and the one surviving mock-vaccinated mouse. The DRG from the mock-vaccinated mouse reactivated the virus, while no virus was recovered from the DRG of NS-gEnull-vaccinated mice (Fig. 6D). Therefore, NS-gEnull cross-protected mice against HSV-2 challenge.
FIG. 6.
NS-gEnull vaccination cross-protects against HSV-2(2.12) challenge at 105 PFU. (A) Mice were monitored for survival (P < 0.05). (B) Inoculation site disease (P > 0.05 on day 1; P = 0.01, P = 0.03, and P = 0.001 on days 5 to 7, respectively). (C) Zosteriform disease (P > 0.05 on days 3 and 4; P = 0.01, P = 0.02, and P = 0.005 on days 5 to 7, respectively). There were five mice in each group. Data points in panels B and C are means ± SEMs. (D) One year after challenge, explant cultures of DRG were monitored for virus reactivation.
NS-gEnull vaccination protects against vaginal HSV-1 challenge.
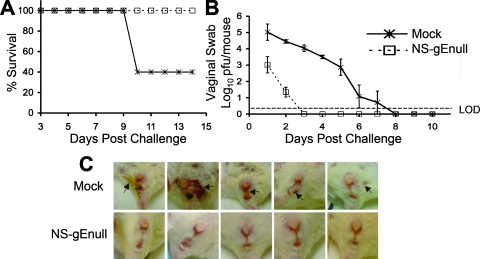
The flank model is useful as a surrogate for epidermal infection of the male genitalia. To evaluate the protection provided by NS-gEnull scarification against mucosal challenge, mice were either mock vaccinated or vaccinated with 5 × 105 PFU of NS-gEnull by epidermal scarification, and 28 days later, they were challenged by vaginal infection with 105 PFU of HSV-1(NS). All mice vaccinated with NS-gEnull survived, while only 40% of mock-vaccinated mice survived (Fig. 7A). Vaginal titers on day 1 in vaccinated mice were 2 log10 units lower than those in mock-vaccinated mice, and by day 2, titers were approximately 3 log10 units lower. By day 3, titers were undetectable in NS-gEnull-vaccinated mice, while titers remained positive until day 8 in mock-vaccinated mice (Fig. 7B). All mock-vaccinated mice showed signs of vaginal disease, but none of the NS-gEnull-vaccinated mice had disease (Fig. 7C). Therefore, NS-gEnull vaccination protected mice against disease and death after vaginal HSV-1 challenge and accelerated the time to clearance of the challenge virus from the vagina.
FIG. 7.
NS-gEnull vaccination protects against vaginal challenge with HSV-1(NS) at 105 PFU. (A) Mice were monitored for survival (P = 0.01). (B) Vaginal titers (P < 0.01 on day 1; P < 0.001 on days 2 to 5; P > 0.05 on days 6 and 7). Data points are means ± SEMs. LOD refers to the limit of detection for the assay, which was 2 PFU. (C) Vaginal disease in each of five mice on day 8 after challenge. There were five mice in each group, except that vaginal swabs on days 1 to 4 were from nine mice. Arrows indicate lesions.
DISCUSSION
We described a live virus vaccine candidate using NS-gEnull, a gE-defective strain that is replication competent but impaired in anterograde and retrograde spread and cell-to-cell spread (33, 41). An important indicator of an effective HSV vaccine is the ability to prevent recurrences by protecting the DRG from infection. Since spontaneous recurrences do not occur in mice, we used DRG explant cocultures as a surrogate for recurrences. Our results demonstrated that NS-gEnull was both safe and effective in mice, since it produced no observable disease and greatly reduced the recovery of latent challenge virus from DRG explant cultures.
Other HSV-1 live-virus vaccines have been evaluated in animal models, including a strain defective in γ34.5, a neurovirulence gene, and mutants R7017 and R7020. HSV-1 γ34.5 mutant 1716 protected mice in a zosteriform model from skin disease and death; however, protection of ganglia was not reported (27). This mutant strain is currently under evaluation as a gene therapy vector for brain tumors but not as a vaccine (24). R7017 and R7020 have deletions of the thymidine kinase gene and a 14.5-kb fragment extending across the inverted-repeat segments. HSV-2 glycoproteins D, G, I, and a truncated form of E were inserted into R2017 at the site of the 14.5-kb deletion, while thymidine kinase was also inserted into R2020 (25). R2017 and R2020 prevented recovery of latent virus from the trigeminal ganglia of 30% and 60% of mice, respectively, that were challenged with 105 PFU of HSV-1(F) using a corneal infection model (25). Although the vaccine strains were safe when tested in owl monkeys, R7020 was poorly immunogenic in phase I human clinical trials, a result that is perhaps attributable to impaired inoculation site replication of this strain (26, 35).
An HSV-2 gH pseudotype virus, referred to as a disabled infectious single-cycle (DISC) virus, has been evaluated as a live-virus vaccine (10). The gene encoding gH was deleted, and the mutant strain was grown on gH-complementing cells. The vaccine virus is capable of only a single round of replication, since gH is required for virus entry. In humans, the DISC vaccine induced humoral and cellular immunity but failed to modify recurrence rates when used as a therapeutic vaccine in subjects with genital herpes.
HSV-2 dl5-29 is a replication-defective live-virus vaccine candidate containing deletions in the helicase-primase complex (UL5) and single-stranded DNA binding protein (UL29) genes (9). This vaccine protected guinea pigs in a vaginal challenge model, leading to lower viral titers during acute infection, >10-fold reduction of latent viral DNA loads, and fewer recurrences (20). Protection was similar to that provided by an HSV-2 gD subunit vaccine; however, the live-virus vaccine induced higher titers of neutralizing antibodies and a more robust HSV-2-specific CD8+ T-cell response.
NS-gEnull is replication competent; however, the vaccine strain is impaired in cell-to-cell spread, as evidenced by its small plaque phenotype (33). This spread defect likely contributes to the limited disease, to the low virus titers detected at the skin inoculation site, and to reduced infection of DRG. Despite the spread defect, NS-gEnull replication at the inoculation site is sufficient to induce neutralizing antibodies and protect against challenge by virulent HSV-1 and HSV-2 strains. This strain also has an anterograde-spread defect that likely will reduce the risk of recurrent skin or mucosal lesions and person-to-person transmission if it is used as a human vaccine.
HSV-1(F) challenge at 105 PFU resulted in breakthrough zosteriform lesions, although few in number, while NS challenge at 107 PFU failed to produce zosteriform lesions. These results suggest that protection is more effective against the strain used to produce the vaccine than against other HSV-1 isolates. We made no adjustments to administer identical 50% lethal doses (LD50) in the challenge experiments; however, for the small number of animals evaluated, survival was comparable in mock-immunized mice challenged with 105 PFU of strain NS, F, or 17 (25%, 33%, and 67%, respectively), suggesting little variation in the LD50 for these three virus strains. Future studies will need to evaluate the protection provided by the vaccine strain against a wider range of isolates challenged at comparable LD50.
Viral DNA was detected in the DRG by RT qPCR following challenge of vaccinated mice; however, no infectious virus was detected by DRG cultures. The RT qPCR does not distinguish the vaccine virus from the challenge virus, since both viruses contain the US9 region used for amplification. The DRG were harvested from the right side of the body, which was the side of the challenge and opposite the side used for vaccination, suggesting that the viral DNA detected was more likely the challenge strain than the vaccine strain. DRG DNA levels in vaccinated mice did not increase from day 1 to day 8 postchallenge, suggesting that much of the DNA was noninfectious; however, explant cultures 28 days or later after challenge were positive for HSV-1 in 2/24 (8%) mice, suggesting that the challenge virus reached the DRG and established latency in some mice.
HSV-1 gE functions as an immune evasion molecule that interacts with gI to form a high-affinity Fcγ receptor that binds the Fc domain of human IgG and blocks Fc-mediated events, such as complement activation and antibody-dependent cellular cytotoxicity (3, 12). HSV-1 NS-gEnull lacks this immune evasion potential, which, in addition to its inability to spread, is another safety feature of the vaccine strain. Deletion of gE results in the loss of this glycoprotein as an immunogen; however, studies that assessed the immunization properties of gE indicated that this glycoprotein provides only modest protection against challenge when used alone (5, 16, 28, 30).
A recent report compared HSV-1 gD subunit and DNA vaccines with a live-virus preparation, ANGpath/gE3-3 (13). This mutant virus is both gE null and syncytial because of a mutation in gB (42). Protection by the live virus and the gD DNA preparations were comparable in a murine intraperitoneal challenge model, and both were superior to the gD glycoprotein vaccine. The results with the ANGpath/gE3-3 strain are consistent with our findings that a gE deletion mutant virus is highly protective in a murine model.
The GlaxoSmithKline HSV-2 gD subunit human vaccine trial showed partial protection in seronegative women but no protection in HSV-1-seropositive women or men (36). The gender difference in protection remains unexplained, since vaccines for other pathogens have not shown gender specificity. Studies that evaluate protection by the human papillomavirus vaccine in men and women will help clarify whether males are less readily protected against sexually transmitted infections than women (2). Perhaps the gender disparity in protection is related to differences between mucosal and epidermal immunity (14). The vagina is comprised of nonkeratinized mucosal epithelial cells, while the penis contains keratinized epidermal cells. Cellular and humoral immune components may infiltrate into mucosal vaginal tissues more readily than into keratinized skin. The mouse flank scarification challenge more closely mimics male genital infection, while the mouse vaginal challenge resembles infection of the human female genital tract. We were encouraged that NS-gEnull protected mice in both epidermal and vaginal challenge models.
More information is available about the molecular pathogenesis of HSV-1 than about that of HSV-2, although vaccine efforts to date have focused largely on prevention of HSV-2 infection. NS-gEnull vaccination of mice provided substantial protection against disease following HSV-2 challenge. Epidemiologic studies of humans indicate that subjects previously infected with HSV-1 remain at least partially susceptible to HSV-2; therefore, we expect that protection against HSV-2 will require a vaccine prepared in an HSV-2 background (22). HSV-2 gE shares 73% amino acid identity with HSV-1, suggesting that a similar approach using HSV-2 gEnull as a vaccine candidate may be effective. An HSV-1 vaccine is worth pursuing even if a separate HSV-2 vaccine is also required, since encephalitis, keratitis, and many newly acquired genital herpes infections are attributed to HSV-1 (40). An effective HSV-1 live-virus vaccine may reduce the incidence of multiple serious diseases, including some cases of genital ulcer disease, thereby reducing the rates of HIV acquisition and transmission (37).
Acknowledgments
This work was funded by NIH grant RO1 AI33063, NIH postdoctoral training grant T32 AI07634, and Merck and Co., Inc.
We thank Dan Martinez and the Pathology Core Facility at the Children's Hospital of Philadelphia for processing skin samples for immunohistochemistry and Sarah J. Radcliffe from the University of Pennsylvania Department of Biostatistics and Epidemiology for advice on statistical analysis.
Footnotes
Published ahead of print on 18 June 2008.
REFERENCES
- 1.Awasthi, S., J. M. Lubinski, R. J. Eisenberg, G. H. Cohen, and H. M. Friedman. 2008. An HSV-1 gD mutant virus as an entry-impaired live virus vaccine. Vaccine 261195-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr, E., and G. Tamms. 2007. Quadrivalent human papillomavirus vaccine. Clin. Infect. Dis. 45609-617. [DOI] [PubMed] [Google Scholar]
- 3.Basu, S., G. Dubin, M. Basu, V. Nguyen, and H. M. Friedman. 1995. Characterization of regions of herpes simplex virus type 1 glycoprotein E involved in binding the Fc domain of monomeric IgG and in forming a complex with glycoprotein I. J. Immunol. 154260-267. [PubMed] [Google Scholar]
- 4.Bernstein, D. I., F. Y. Aoki, S. K. Tyring, L. R. Stanberry, C. St-Pierre, S. D. Shafran, G. Leroux-Roels, K. Van Herck, A. Bollaerts, and G. Dubin. 2005. Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clin. Infect. Dis. 401271-1281. [DOI] [PubMed] [Google Scholar]
- 5.Blacklaws, B. A., S. Krishna, A. C. Minson, and A. A. Nash. 1990. Immunogenicity of herpes simplex virus type 1 glycoproteins expressed in vaccinia virus recombinants. Virology 177727-736. [DOI] [PubMed] [Google Scholar]
- 6.Brittle, E. E., A. E. Reynolds, and L. W. Enquist. 2004. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J. Virol. 7812951-12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, E. L., A. Wald, J. P. Hughes, R. A. Morrow, E. Krantz, K. Mayer, S. Buchbinder, B. Koblin, and C. Celum. 2006. High risk of human immunodeficiency virus in men who have sex with men with herpes simplex virus type 2 in the EXPLORE study. Am. J. Epidemiol. 164733-741. [DOI] [PubMed] [Google Scholar]
- 8.Ch'ng, T. H., and L. W. Enquist. 2005. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J. Virol. 798835-8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Costa, X., M. F. Kramer, J. Zhu, M. A. Brockman, and D. M. Knipe. 2000. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J. Virol. 747963-7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bruyn, G., M. Vargas-Cortez, T. Warren, S. K. Tyring, K. H. Fife, J. Lalezari, R. C. Brady, M. Shahmanesh, G. Kinghorn, and K. R. Beutner. 2006. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine 24914-920. [DOI] [PubMed] [Google Scholar]
- 11.Dingwell, K. S., C. R. Brunetti, R. L. Hendricks, Q. Tang, M. Tang, A. J. Rainbow, and D. C. Johnson. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 657046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durmanova, V., T. Mosko, M. Sapak, J. Kosovsky, I. Rezuchova, M. Buc, and J. Rajcani. 2006. Efficacy of recombinant herpes simplex virus 1 glycoprotein D candidate vaccines in mice. Acta Microbiol. Immunol. Hung. 53459-477. [DOI] [PubMed] [Google Scholar]
- 14.Friedman, H. M. 2006. Keratin, a dual role in herpes simplex virus pathogenesis. J. Clin. Virol. 35103-105. [DOI] [PubMed] [Google Scholar]
- 15.Friedman, H. M., E. J. Macarak, R. R. MacGregor, J. Wolfe, and N. A. Kefalides. 1981. Virus infection of endothelial cells. J. Infect. Dis. 143266-273. [DOI] [PubMed] [Google Scholar]
- 16.Ghiasi, H., R. Kaiwar, A. B. Nesburn, S. Slanina, and S. L. Wechsler. 1994. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J. Virol. 682118-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, S. L., I. Frank, A. Yee, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 1990. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J. Infect. Dis. 162331-337. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg, S. D., J. A. Stewart, A. R. Gerber, R. H. Byers, F. K. Lee, P. M. O'Malley, and A. J. Nahmias. 1988. Prior herpes simplex virus type 2 infection as a risk factor for HIV infection. JAMA 2591048-1050. [PubMed] [Google Scholar]
- 19.Hook, L. M., J. M. Lubinski, M. Jiang, M. K. Pangburn, and H. M. Friedman. 2006. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J. Virol. 804038-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshino, Y., S. K. Dalai, K. Wang, L. Pesnicak, T. Y. Lau, D. M. Knipe, J. I. Cohen, and S. E. Straus. 2005. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 79410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaushic, C., A. A. Ashkar, L. A. Reid, and K. L. Rosenthal. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 774558-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langenberg, A. G., L. Corey, R. L. Ashley, W. P. Leong, and S. E. Straus, for the Chiron HSV Vaccine Study Group. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. N. Engl. J. Med. 3411432-1438. [DOI] [PubMed] [Google Scholar]
- 23.Lubinski, J., L. Wang, D. Mastellos, A. Sahu, J. D. Lambris, and H. M. Friedman. 1999. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J. Exp. Med. 1901637-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markert, J. M., J. N. Parker, D. J. Buchsbaum, W. E. Grizzle, G. Y. Gillespie, and R. J. Whitley. 2006. Oncolytic HSV-1 for the treatment of brain tumours. Herpes 1366-71. [PubMed] [Google Scholar]
- 25.Meignier, B., R. Longnecker, and B. Roizman. 1988. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J. Infect. Dis. 158602-614. [DOI] [PubMed] [Google Scholar]
- 26.Meignier, B., B. Martin, R. J. Whitley, and B. Roizman. 1990. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus). J. Infect. Dis. 162313-321. [DOI] [PubMed] [Google Scholar]
- 27.Mercadal, C. M., M. Slaoui, S. M. Brown, and B. T. Rouse. 1993. Efficacy of the herpes simplex virus types 1 and 2 mutant viruses to confer protection against zosteriform spread in mice. Viral Immunol. 635-42. [DOI] [PubMed] [Google Scholar]
- 28.Miriagou, V., R. Argnani, A. Kakkanas, U. Georgopoulou, R. Manservigi, and P. Mavromara. 1995. Expression of the herpes simplex virus type 1 glycoprotein E in human cells and in Escherichia coli: protection studies against lethal viral infection in mice. J. Gen. Virol. 763137-3143. [DOI] [PubMed] [Google Scholar]
- 29.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 725351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nass, P. H., K. L. Elkins, and J. P. Weir. 1998. Antibody response and protective capacity of plasmid vaccines expressing three different herpes simplex virus glycoproteins. J. Infect. Dis. 178611-617. [DOI] [PubMed] [Google Scholar]
- 31.Oxman, M. N., M. J. Levin, G. R. Johnson, K. E. Schmader, S. E. Straus, L. D. Gelb, R. D. Arbeit, M. S. Simberkoff, A. A. Gershon, L. E. Davis, A. Weinberg, K. D. Boardman, H. M. Williams, J. H. Zhang, P. N. Peduzzi, C. E. Beisel, V. A. Morrison, J. C. Guatelli, P. A. Brooks, C. A. Kauffman, C. T. Pachucki, K. M. Neuzil, R. F. Betts, P. F. Wright, M. R. Griffin, P. Brunell, N. E. Soto, A. R. Marques, S. K. Keay, R. P. Goodman, D. J. Cotton, J. W. Gnann, Jr., J. Loutit, M. Holodniy, W. A. Keitel, G. E. Crawford, S. S. Yeh, Z. Lobo, J. F. Toney, R. N. Greenberg, P. M. Keller, R. Harbecke, A. R. Hayward, M. R. Irwin, T. C. Kyriakides, C. Y. Chan, I. S. Chan, W. W. Wang, P. W. Annunziato, and J. L. Silber. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 3522271-2284. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran, S., and P. R. Kinchington. 2007. Potential prophylactic and therapeutic vaccines for HSV infections. Curr. Pharm. Des. 131965-1973. [DOI] [PubMed] [Google Scholar]
- 33.Saldanha, C. E., J. Lubinski, C. Martin, T. Nagashunmugam, L. Wang, H. van der Keyl, R. Tal-Singer, and H. M. Friedman. 2000. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J. Virol. 746712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, J. S., and N. J. Robinson. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186S3-S28. [DOI] [PubMed] [Google Scholar]
- 35.Stanberry, L. R., A. L. Cunningham, A. Mindel, L. L. Scott, S. L. Spruance, F. Y. Aoki, and C. J. Lacey. 2000. Prospects for control of herpes simplex virus disease through immunization. Clin. Infect. Dis. 30549-566. [DOI] [PubMed] [Google Scholar]
- 36.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin, for the GlaxoSmithKline Herpes Vaccine Efficacy Study Group. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 3471652-1661. [DOI] [PubMed] [Google Scholar]
- 37.Suligoi, B., M. Dorrucci, I. Uccella, M. Andreoni, and G. Rezza. 2003. Effect of multiple herpesvirus infections on the progression of HIV disease in a cohort of HIV seroconverters. J. Med. Virol. 69182-187. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, R. L., and N. M. Sawtell. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J. Virol. 756660-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, and M. B. Pensaert. 2003. Antibody-induced internalization of viral glycoproteins and gE-gI Fc receptor activity protect pseudorabies virus-infected monocytes from efficient complement-mediated lysis. J. Gen. Virol. 84939-947. [DOI] [PubMed] [Google Scholar]
- 40.Wald, A. 2006. Genital HSV-1 infections. Sex. Transm. Infect. 82189-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, F., W. Tang, H. M. McGraw, J. Bennett, L. W. Enquist, and H. M. Friedman. 2005. Herpes simplex virus type 1 glycoprotein E is required for axonal localization of capsid, tegument, and membrane glycoproteins. J. Virol. 7913362-13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weise, K., H. C. Kaerner, J. Glorioso, and C. H. Schroder. 1987. Replacement of glycoprotein B gene sequences in herpes simplex virus type 1 strain ANG by corresponding sequences of the strain KOS causes changes of plaque morphology and neuropathogenicity. J. Gen. Virol. 681909-1919. [DOI] [PubMed] [Google Scholar]
- 43.Whitley, R. J., and B. Roizman. 2002. Herpes simplex viruses: is a vaccine tenable? J. Clin. Investig. 110145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296964-973. [DOI] [PubMed] [Google Scholar]